Abstract
The bicarbonate-buffered anoxic vent waters at Chocolate Pots hot springs in Yellowstone National Park are 51–54°C, pH 5.5–6.0, and are very high in dissolved Fe(II) at 5.8–5.9 mg/L. The aqueous Fe(II) is oxidized by a combination of biotic and abiotic mechanisms and precipitated as primary siliceous nanophase iron oxyhydroxides (ferrihydrite). Four distinct prokaryotic photosynthetic microbial mat types grow on top of these iron deposits. Lipids were used to characterize the community composition of the microbial mats, link source organisms to geologically significant biomarkers, and investigate how iron mineralization degrades the lipid signature of the community. The phospholipid and glycolipid fatty acid profiles of the highest-temperature mats indicate that they are dominated by cyanobacteria and green nonsulfur filamentous anoxygenic phototrophs (FAPs). Diagnostic lipid biomarkers of the cyanobacteria include midchain branched mono- and dimethylalkanes and, most notably, 2-methylbacteriohopanepolyol. Diagnostic lipid biomarkers of the FAPs (Chloroflexus and Roseiflexus spp.) include wax esters and a long-chain tri-unsaturated alkene. Surprisingly, the lipid biomarkers resisted the earliest stages of microbial degradation and diagenesis to survive in the iron oxides beneath the mats. Understanding the potential of particular sedimentary environments to capture and preserve fossil biosignatures is of vital importance in the selection of the best landing sites for future astrobiological missions to Mars. This study explores the nature of organic degradation processes in moderately thermal Fe(II)-rich groundwater springs—environmental conditions that have been previously identified as highly relevant for Mars exploration. Key Words: Lipid biomarkers—Photosynthesis—Iron—Hot springs—Mars. Astrobiology 14, 502–521.
1. Introduction
1.1. Biogeochemistry of iron hot springs
Terrestrial hydrothermal systems have long been recognized as habitats for microbial life on Earth (Brock, 1967, 1978; Castenholz, 1969). The composition of the hydrothermal fluids, which dictate which microbial populations are present, is controlled by a complex set of interactions between the magmatic gases, the deep parent brine, mineral dissolution of the wall rock, deposition of secondary minerals, underground boiling, vapor loss, dilution, and mixing with fluids of different origin (Giggenbach, 1988). In general, the rhyolite and basalt-hosted hydrothermal systems in Yellowstone National Park are characterized by two geochemically distinct end members: (1) alkaline-chloride silica-depositing hot springs and (2) acid-sulfate boiling pools, mud pots, and fumaroles (Fournier, 1989). Mixing of these two geochemical end members generates a spectrum of acid-sulfate-chloride (ASC) springs (Fournier, 1989). Depending on the host rock and pH, some of these ASC springs can be high in iron.
The ASC waters at Chocolate Pots hot springs, ∼5 km southwest of Norris Geyser Basin, are pH 5.5–6.0, 51–54°C, and contain 5.8–5.9 mg/L dissolved Fe(II) (Pierson et al., 1999; Pierson and Parenteau, 2000; Trouwborst et al., 2007; Parenteau and Cady, 2010). As a result of buffering by high levels of bicarbonate (Parenteau and Cady, 2010), the vent waters are slightly acidic, which allows the Fe(II) to remain in solution. The pH is also just above the lower limit for prokaryotic phototrophs such as cyanobacteria, which do not tolerate pH values below ∼4.5 (Castenholz, 1988). This pH range allows cyanobacteria and green nonsulfur filamentous anoxygenic phototrophs (FAPs) (e.g., Chloroflexus and Roseiflexus spp.) to grow in the presence of high levels of Fe(II). To better understand the evolution of oxygenic photosynthesis, our group studied the physiological ecology of this ecosystem and searched for cyanobacteria that use Fe(II) as an electron donor for photosynthesis (Pierson et al., 1999). We have also quantified the impact of cyanobacterial oxygen production on Fe(II) oxidation as a model for Precambrian Fe(II) oxidation (Trouwborst et al., 2007).
1.2. Biosignatures in hot springs
We are currently examining the production and early preservation of microbial biosignatures (biofabrics, microfossils, and lipid biomarkers) by iron oxides in this system. The preservation of microbial biosignatures in terrestrial hot springs via the rapid entombment of the microbes by aqueous mineral precipitates was identified long ago as relevant to understanding the processes of microbial fossilization on early Earth and Mars (e.g., Walter, 1972; Walter and Des Marais, 1993; Farmer, 1995; Cady and Farmer, 1996; Farmer and Des Marais, 1999; Cady et al., 2003; Farmer, 2003). Most studies have been directed toward elucidating the mechanisms of silica permineralization in microbial fossil preservation (e.g., Cady and Farmer, 1996; Farmer 1999; Phoenix et al., 2000; Konhauser et al., 2001; Mountain et al., 2003; Benning et al., 2004; Ferris and Magalhaes, 2008), one of the common modes of microfossil preservation in Precambrian cherts (e.g., Walsh and Lowe, 1985). There have also been studies that examined the production and preservation of microbial lipids in modern and subrecent siliceous sinters that have not undergone significant diagenesis (Jahnke et al., 2001, 2004; Pancost et al., 2005, 2006; Talbot et al., 2005; Gibson et al., 2008; Kaur et al., 2008, 2011).
In contrast to the silicification studies, preservation of microbial biosignatures by iron oxides is relatively understudied because it is generally thought that iron-rich sediments do not preserve biosignatures well (e.g., Klein, 2005). However, our previous studies have revealed excellent early preservation of cyanobacterial microfossils by nanophase iron oxyhydroxide (ferrihydrite) permineralization (Parenteau and Cady, 2010) and distinct dendritic microbial biofabrics by iron encrustation (Wade et al., 1999; Parenteau and Cady, 2010). Other groups have reported preservation of microfossils by nanophase iron oxides in modern as well as ancient settings in the Río Tinto acid mine drainage system (Fernández-Remolar et al., 2005; Fernández-Remolar and Knoll, 2008), which is more acidic than the Chocolate Pots system.
Examining the capture and retention of microbial biosignatures in modern hot springs is relevant to the search for evidence of past microbial life in analogous deposits on Mars. Hydrothermal activity on Mars is supported by evidence for widespread volcanism and higher heat flow during the Noachian (4.1–3.7 Ga) (e.g., Farmer, 1996; Baratoux et al., 2011). This is supported by the recent discovery of siliceous hydrothermal deposits by the Spirit rover at Home Plate in the Columbia Hills, Gusev Crater, Mars (Ruff et al., 2011). The subsurface could have provided a major habitat for thermophilic life, and warm mineralizing surface springs could have provided optimal conditions for fossil biosignature capture and preservation (Walter and Des Marais, 1993; Farmer and Des Marais, 1999). Chocolate Pots hot springs, which is a surface expression of moderately thermal, slightly acidic Fe(II)-rich groundwater, provides a site at which to study the taphonomy of organic compounds in iron-silica systems and provides useful insights for evaluating the preservation potential of iron-rich sedimentary rocks on Mars.
This study focused on the characterization of lipid biomarkers in the modern iron-mineralized, prokaryotic phototrophic mats in this understudied class of bicarbonate-buffered ASC iron hot springs. Specifically, we used lipid biomarkers to (1) describe the community composition of four types of phototrophic mats and (2) determine how lithification by siliceous 2-line ferrihydrite, the primary iron oxide phase of the sinter, affects the biomarker signature of the communities. The aim was to characterize the taphonomy of the lipid biomarkers in this iron-rich system, namely, which compounds survive microbial degradative processes within the mats and through the earliest stages of diagenesis in the iron deposits beneath the mats. We also examined the survival of organics in a nearby extinct iron-silica spring that has undergone significant postdepositional cementation during diagenesis.
2. Methods
2.1. Field measurements
The temperature and pH of the water that flows over the microbial mats at Chocolate Pots hot springs were measured as described in Parenteau and Cady (2010). The dissolved Fe(II) concentration was measured by using the colorimetric ferrozine assay with a Hach DR/2400 portable spectrophotometer (Pierson et al., 1999; Parenteau and Cady, 2010). Detailed chemical analyses of the thermal waters have been performed several times since 1935; the last two samplings are summarized in Table 1 (Pierson and Parenteau, 2000; Parenteau and Cady, 2010).
Table 1.
Geochemical Composition of Vent Waters at Chocolate Pots Hot Springs
| Species (mg/L) | Pierson and Parenteau, 2000 | Parenteau and Cady, 2010 |
|---|---|---|
| Temperature (°C) | 52.5 | 51.8 |
| pH | 5.9 | 5.7 |
| Aluminum | 0.17 | 0.1 |
| Ammonium nitrogen | <0.1 | nd |
| Bicarbonate | 248 | nd |
| Boron | nd | 0.5 |
| Calcium | 20 | 21 |
| Carbonate | 0 | nd |
| Chloride | 28 | 29 |
| Fluorine | nd | 4.5 |
| Iron(II) | 5.1 | 5.5 |
| Iron(III) | <0.1 | nd |
| Total iron | nd | 5.4 |
| Lithium | nd | 0.8 |
| Magnesium | 3 | 2.0 |
| Manganese | 1.04 | 1.4 |
| Nitrate nitrogen | <0.2 | nd |
| Nitrite nitrogen | <0.2 | nd |
| Potassium | 24 | 23 |
| Phosphorous | 0.01 | <0.1 |
| Silica | 159 | 141 |
| Sodium | 102 | 115 |
| Sulfate | 32 | 25 |
| Sulfide | 0 | nd |
nd, not determined.
2.2. Sample collection
Four distinct phototrophic mat types were identified in previous studies of the in situ physiological ecology of this system (Pierson et al., 1999; Pierson and Parenteau, 2000), including (1) Synechococcus-Chloroflexi, (2) Pseudanabaena spp., (3) Oscillatoria cf. princeps, and (4) narrow Oscillatoria spp. The phylum-level designation of Chloroflexi is used to include the green nonsulfur FAPs Chloroflexus and Roseiflexus, both of which are present in the Synechococcus-Chloroflexi mat. These mat systems, which are briefly reviewed below, provided a focus for the lipid biomarker study reported here.
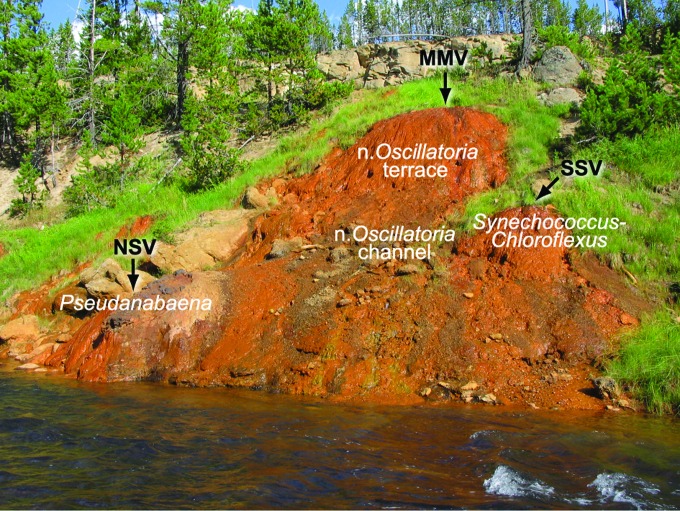
The Synechococcus-Chloroflexi mat and the Pseudanabaena mat were located closest to the vents and consequently experienced the highest temperature and levels of Fe(II) (Figs. 1 and 2a, 2d, Table 2). The Oscillatoria princeps mat occurred in areas of low flow and was associated with a floating reflective mineral/organic film that broke into fragments when handled (Fig. 2e, Table 2). The fourth mat type, narrow Oscillatoria, was the most widely distributed over the iron spring deposits and occurred as two types of mat morphologies: terracette structures on the steep face of the main iron deposit (Figs. 1 and 2c, Table 2) and laminated mats in a rapidly flowing channel (Figs. 1 and 2b, Table 2). These mats were collected in July 2003 and immediately placed on dry ice in the field. Frozen samples were stored on dry ice during transit to the laboratory, where they were subsequently freeze-dried. Freeze-dried samples were stored at 4°C until lipid analyses were performed in August 2003.
FIG. 1.
Distribution of three phototrophic mats examined in this study on the main Chocolate Pots iron deposit. The Pseudanabaena mat occurred at the north satellite vent (NSV). The narrow Oscillatoria mat occurred in a terracette structure on the steep face of the main iron deposit and in the rapidly flowing vent outflow channel. The Synechococcus-Chloroflexi mat occurred at the south satellite vent (SSV). The Oscillatoria princeps mat occurred in areas of low flow (not pictured). MMV, main mound vent.
FIG. 2.
Field images of the four phototrophic mats examined in this study. (a) The Synechococcus-Chloroflexi mat in the vent pool. A core of the mat was removed (upper arrow) and sectioned into the mat, and ferrihydrite beneath the mat. A ferrihydrite core with no conspicuous mat on the surface was also collected (lower arrow). (b) Narrow Oscillatoria mat in rapid outflow channel. (c) Narrow Oscillatoria mat in association with a terracette structure on the steep face of the main iron deposit. (d) Floating streams of the Pseudanabaena mat near the north satellite vent. (e) The Oscillatoria princeps mat (arrow) in association with reflective iron film that fractured upon handling.
Table 2.
Geochemical Measurements of Phototrophic Mats at Chocolate Pots Hot Springs
| Sample | Temperature (°C) | pH | Fe(II) (mg/L) | % TOC |
|---|---|---|---|---|
| Synechococcus-Chloroflexi mat core (a) | 52.8 | 5.9 | 5.9 | 1.3 |
| Ferrihydrite beneath mat (b) | 52.8 | 5.9 | 5.9 | 0.2 |
| Ferrihydrite core adjacent to mat | 51.5 | 6.0 | 5.9 | 0.3 |
| Pseudanabaena mat | 50.4 | 5.5 | 5.8 | 28.8 |
| Oscillatoria cf. princeps mat | 41.4 | 5.8 | nd | 11.6 |
| Narrow Oscillatoria mat terrace | 40.1 | 7.3 | 0.4* | 0.4 |
| Narrow Oscillatoria mat channel | 39.6 | 7.3 | 0.2* | 2.2 |
nd, not determined.
Measured above mat types in 2005.
To characterize the preservation of lipids in the iron oxide sinter, a 1 cm×2.5 cm deep core of the Synechococcus-Chloroflexi mat was removed, frozen, and sectioned with a sterile scalpel into two parts: (1) the top 2 mm of mat and a thin layer (∼0.5 mm) of the iron oxide sinter beneath the mat, and (2) the remaining underlying 2.5–25 mm iron oxide sinter. The maximum core depth was dictated by the conditions of our National Park Service research permit. A similar-size core of iron oxide sinter with no conspicuous mat on the surface was also collected adjacent to the Synechococcus-Chloroflexi mat core site. All the samples were immediately placed on dry ice in the field, freeze-dried in the laboratory, and analyzed within a month of returning them to the lab, as noted above.
A hand sample of fossilized microbial “streamer” fabric (i.e., centimeter-scale tufts of microbes oriented in the direction of water flow) was removed from an extinct iron-silica spring deposit located approximately 700 m north of Chocolate Pots (Fig. 3). The outcrop of sinter was exposed in a cut bank along the Gibbon River. Petrographic thin sections were prepared from the sinter block with the streamer fabric oriented in cross section, or perpendicular to the bedding. Light micrographs were acquired with a Nikon Microphot FXA microscope. Epifluorescence images of the immature kerogen in the microfossils were acquired by using a blue excitation filter (420–490 nm).
FIG. 3.
Field images and micrographs of the extinct iron-silica hot spring located approximately 700 m north of Chocolate Pots. (a) Outcrop of sinter. (b) End-on view of a hand sample of a fossilized streamer biofabric. (c) End-on view of biofabric viewed under a dissecting microscope. Streamers present in iron band on top of sample. Underneath are alternating laminae of opaline silica (upper X) and iron (lower X). (d) Remarkably well-preserved Calothrix filament. Amber-colored kerogen visible in microfossils. (e) More filamentous microfossils in a matrix of silica and iron. (f) Same field of view as in (e), revealing the fluorescence of immature kerogen in the microfossils and dispersed throughout the biofabric.
2.3. Elemental analysis and X-ray diffraction
The freeze-dried phototrophic mats and iron oxide sinter samples were analyzed for total carbon and total organic carbon (TOC) as previously described (Parenteau and Cady, 2010). The extracted mat and iron oxide cores were prepared for powder X-ray diffraction (XRD) as previously described (Parenteau and Cady, 2010). XRD patterns of the extinct iron-silica sinter were obtained with Terra, which is a terrestrial analogue of the CheMin instrument on the Mars Science Laboratory Curiosity rover (Sarrazin et al., 2008). Terra and CheMin are combination XRD/XRF instruments designed for rock and mineral analysis in the field and on Mars, respectively. The peaks on the powder XRD patterns were identified by comparing the measured sample d-spacings to previously published iron oxide and opal-A d-spacings.
2.4. Lipid extraction, separation, and derivatization
Lipids were extracted with a modified Bligh and Dyer procedure (Jahnke et al., 1992) to generate a total lipid extract. Phosphatidylcholine (Sigma) was added as an internal extraction standard, and the total lipid extract was separated into polar and neutral fractions by using a cold acetone precipitation. The polar lipid precipitate was separated into glycolipids, phospholipids, and bacteriohopanepolyols (BHPs) by preparative thin-layer chromatography (TLC) on Silica gel G plates (Merck) with the use of an acetone-benzene-water (91:30:8) solvent system (Pohl et al., 1970). The Sigma standards digalactosyl diglyceride (DG) and phosphatidylglycerol (PG) were used to aid in the identification of the glycolipid and phospholipid zones, respectively. The BHPs were recovered from the spotting zone (Rf=0).
The neutral lipids (acetone supernatant) were separated into hydrocarbons, wax esters, pigments, and glycolipids by preparative TLC on Silica gel G plates (Merck) by subsequent development in methylene chloride and then hexane (Jahnke et al., 2004). The Sigma standards C14–C25 n-alkane mix (Rf=0.95), hexadecyl hexadecanoate (Rf=0.8), and cholestanol (Rf=0.2) were used to identify hydrocarbon, wax ester, and pigment/glycolipid zones, respectively. The (bacterio)chlorophylls and glycolipids were recovered from the lower portion of the plate (Rf=0 to ∼0.2).
The polar fractions (polar glycolipids, phospholipids, and BHPs) and the neutral fractions (pigments and neutral glycolipids) were recovered from the TLC plates by eluting with the Bligh and Dyer procedure. The polar glycolipids, phospholipids, pigments, and neutral glycolipids were treated by using a mild alkaline methanolysis procedure, which generates fatty acid methyl esters (FAMEs) and chlorophyll-derived phytol (Jahnke et al., 2001, 2004). The bond position of the monounsaturated FAMEs was determined by preparing dimethyl disulfide (DMDS) adducts (Yamamoto et al., 1991). In the case of the phospholipid fraction from the iron sinter cores, the sample size was not sufficient, and the proportion of Δ9 and Δ11 C18:1 was determined by retention times relative to methyl ester standards of palmitoleic acid (C18:1Δ9) and vaccenic acid (C18:1Δ11). Hopanols (C31, C32 and 2-methylhomologs) were prepared by treatment of the BHPs with an oxidation-reduction procedure and were analyzed as acetate derivatives (Rohmer et al., 1984; Jahnke et al., 2004). Phytol was hydrogenated prior to gas chromatography analysis (Jahnke et al., 2004). For characterization of Chloroflexus fatty acid double-bond positions, the type strain Chloroflexus aurantiacus J-10-fl was obtained from Beverly Pierson (Professor Emerita, University of Puget Sound) and grown photoheterotrophically at 50°C (Pierson and Castenholz, 1974).
2.5. Gas chromatography
Fatty acid methyl esters, DMDS adducts, BHP hopanol-acetates, hydrogenated phytol, hydrocarbons, and wax esters were analyzed with a HP 6890 gas chromatograph (GC) equipped with a flame ionization detector (FID) and a HP-5 column (30 m×0.25 mm, 0.25 μm film), or an HP 5890 GC equipped with a 5971 mass selective detector and a J&W Scientific DB-5ms column (60 m×0.25 mm, 0.25 μm film). Chromatographic conditions for FAMEs were 50–120°C at 10°C min−1, then at 4°C min−1 to 320°C, held isothermal for 3 min. Conditions for DMDS adducts were 50–120°C at 10°C min−1, then at 4°C min−1 to 300°C, held isothermal for 30 min, then at 5°C min−1 to 320°C. Conditions for BHP hopanol-acetates were 90–200°C at 10°C min−1, then at 4°C min−1 to 320°C, held isothermal for 50 min, then at 4°C min−1 to 340°C. Conditions for hydrogenated phytol were 50–150°C at 10°C min−1, then at 4°C min−1 to 300°C, held isothermal for 10 min, then at 5°C min−1 to 320°C, held isothermal for 5 min. Conditions for hydrocarbons were 50–150°C at 5°C min−1, then at 1°C min−1 to 240°C, then at 4°C min−1 to 320°C, held isothermal for 20 min. Conditions for wax esters were 50–300°C at 10°C min−1, held isothermal for 60 min, then at 4°C min−1 to 320°C, held isothermal for 15 min.
The FAMEs were quantified on the GC-FID by using methyl tricosanoate (Sigma) as an internal standard. The BHP hopanol-acetates were quantified on the gas chromatograph–mass spectrometer (GC-MS) by using cholestanol (Sigma) as an internal standard. The hydrogenated phytols and hydrocarbons were quantified on the GC-MS by using cholestane (Sigma) and methyl tridecane (Sigma) as internal standards, respectively. The wax esters were quantified on the GC-FID by using cholestane (Sigma) as an internal standard. All quantified compounds were reported as micrograms per gram TOC.
2.6. Lipid nomenclature
Fatty acids were named according to the delta convention X:YΔZ, where X is the number of carbon atoms in the chain, Y is the number of unsaturations, and Z is the position of the unsaturation relative to the carboxyl carbon. Methyl branching at C-2 and C-3 is designated relative to the methyl end as iso (i) and anteiso (a), respectively, while midchain branching is specified relative to the carboxyl end (e.g., 10-Me). Straight-chain compounds that lack branches are designated normal (n). Cyclopropyl compounds are indicated by the prefix cy.
3. Results
3.1. Chocolate Pots phototrophic mats
3.1.1. Ester-linked membrane fatty acids
The ester-linked membrane fatty acids of the phototrophic mats at Chocolate Pots were dominated by glycolipids (Table 3). The Synechococcus-Chloroflexi mat and the narrow Oscillatoria mat contained between 85% and 96% neutral and polar glycolipids, and between 4% and 15% phospholipids. The profile of the fatty acid chains in the glycolipid and phospholipid fractions of the Synechococcus-Chloroflexi mat revealed that the n-C16:1, n-C16, n-C18:1, and n-C18 comprised approximately 83% of the fatty acids recovered (Fig. 4). Fatty acids with two double bonds (dienoic fatty acids, n-C18:2) were also present in all three fractions but were highest in the neutral glycolipids (∼10%). The remaining fatty acids were comprised of terminally and midchain branched compounds (0.2–8%).
Table 3.
Basic Quantification of Different Lipid Classes Recovered from Phototrophic Mats, Ferrihydrite beneath Mats, and Ferrihydrite Adjacent to Mats at Chocolate Pots Hot Springs
| Lipid class | Syn-Cf mat core (a) | Ferrihydrite beneath mat (b) | Adjacent ferrihydrite core | Pseudanabaena mat | O. princeps mat | Narrow Oscillatoria mat channel | Narrow Oscillatoria mat terrace |
|---|---|---|---|---|---|---|---|
| Total FAME | 138,736.9 | 3,330.5 | 2,890.5 | nd | nd | 34,988.2 | 1,317.0 |
| Phospholipids | 20,564.0 | 391.1 | 323.7 | nd | nd | 1,250.3 | 68.5 |
| Polar glycolipids | 993.5 | 94.5 | 19.7 | nd | nd | 4,100.1 | 510.0 |
| Neutral glycolipids | 117,179.4 | 2,844.9 | 2,547.1 | nd | nd | 29,637.8 | 194.3 |
| Wax esters | 2,984.5 | 100.1 | 74.9 | 46.0 | - | - | - |
| n-C17 | 123.7 | 56.7 | 23.1 | 3,071.9 | 1,559.1 | 726.0 | 663.1 |
| MMA | 300.0 | 195.7 | 27.0 | 6,383.1 | 641.2 | 1,695.8 | 298.4 |
| DMA | 53.6 | 34.3 | 6.0 | - | - | - | - |
| n-C31:3 | 14.3 | 8.0 | 2.9 | - | - | - | - |
| Hopanepolyol products | 1.9 | 0.01 | 0.08 | 1,001.4 | - | 0.03 | 124.4 |
See Methods text for lipid nomenclature. Units are μg/g TOC.
Syn-Cf, Synechococcus-Chloroflexi mat; O. princeps, Oscillatoria princeps; FAME, fatty acid methyl esters; nd, not determined; -, not detected; MMA, monomethylalkanes; DMA, dimethylalkanes.
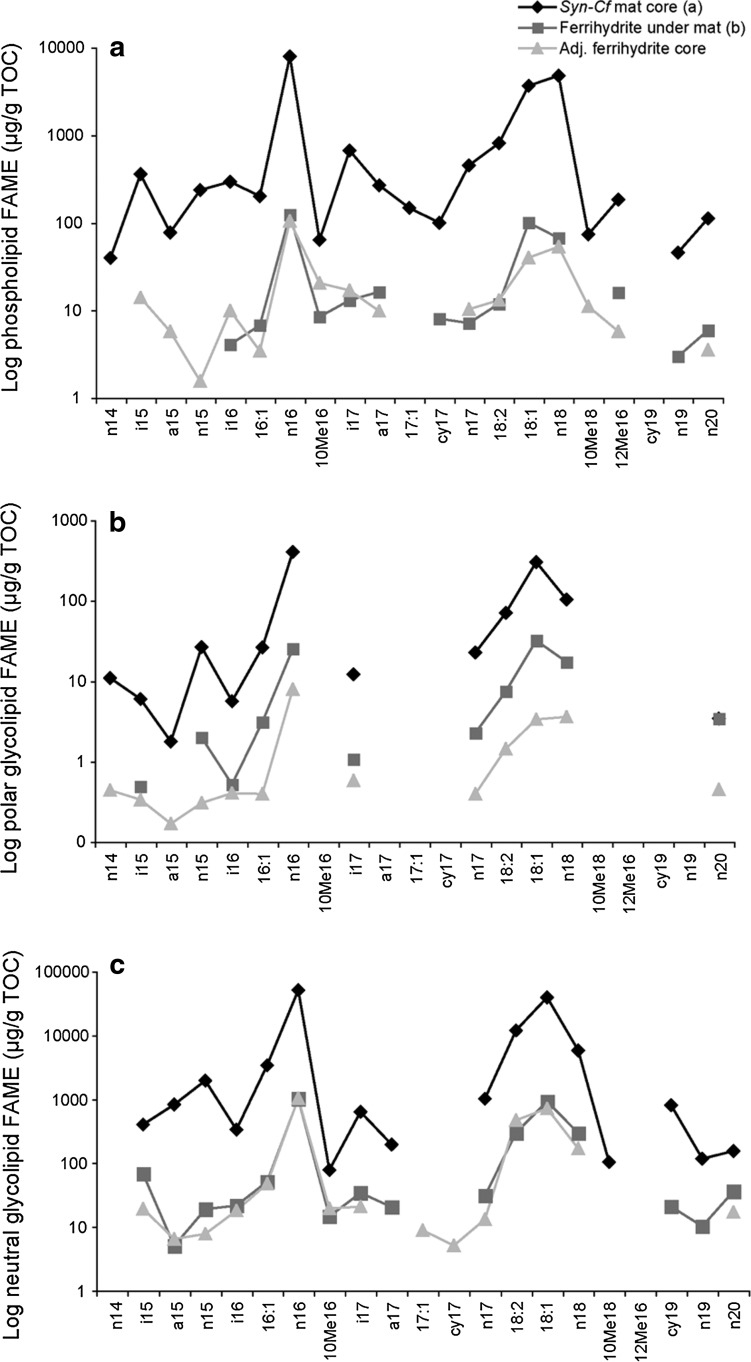
FIG. 4.
(a) Phospholipid, (b) polar glycolipid, and (c) neutral glycolipid FAME profiles of Synechococcus-Chloroflexi mat, ferrihydrite core beneath mat, and adjacent ferrihydrite core lacking a conspicuous mat on the surface. Graphs were plotted on a log scale to be able to compare the subtleties of the trends.
For the Synechococcus-Chloroflexi mat, the neutral glycolipid n-C18:1 contained 97% of the double bonds at the Δ9 position, with only 3% at the Δ11 position. The polar glycolipid contained 89% of the double bonds in the Δ9 position, with 10% at Δ11 and 1% at Δ13.
3.1.2. Wax esters
Wax esters are formed by the combination of a long-chain fatty acid with a long-chain alcohol. The alcohol portion may be formed from the reduction of a fatty acid or by oxidation of a hydrocarbon (Ratledge and Wilkinson, 1988). Straight-chain saturated normal, normal (n,n-) wax esters dominated the wax ester profile of the Synechococcus-Chloroflexi mat (Table 4). The n,n-C34 and n,n-C32 were most abundant, with slightly greater quantities of the C34. Wax esters with one and two iso-methyl moieties as iso, normal- (i,n-) and iso, iso- (i,i-), respectively, were also detected. The Pseudanabaena mat displayed a similar profile, except that in this mat, the n,n-C32 wax ester was more abundant than the C34. Wax esters were not detected in the lower-temperature Oscillatoria princeps or in the narrow Oscillatoria mats.
Table 4.
Diagnostic Lipid Biomarkers Found in Phototrophic Mats, Ferrihydrite beneath Mats, and Ferrihydrite Adjacent to Mats at Chocolate Pots Hot Springs
| Lipid biomarker | Syn-Cf mat core (a) | Ferrihydrite beneath mat (b) | Adjacent ferrihydrite core | Pseudanabaena mat | O. princeps mat | Narrow Oscillatoria mat channel | Narrow Oscillatoria mat terrace |
|---|---|---|---|---|---|---|---|
| Hydrocarbons | |||||||
| n-C15 | 10.8 | 11.5 | 1.1 | 7.8 | 18.3 | 43.2 | 55.9 |
| n-C16 | 29.9 | 34.3 | 6.5 | 70.3 | 77.6 | 192.1 | 90.8 |
| 6Me-C16 | 33.2 | 39.6 | 3.1 | 128.5 | 107.3 | 328.8 | 52.5 |
| 5Me-C16 | 10.2 | 12.2 | - | 28.7 | 0.2 | 66.2 | - |
| 4Me-C16 | 12.9 | 14.9 | 1.4 | 25.3 | 34.2 | 136.1 | - |
| 3Me-C16 | - | - | 1.9 | 58.0 | 42.6 | 165.8 | - |
| 2Me-C16 | 12.9 | 15.4 | 1.6 | 51.5 | 34.6 | 112.5 | - |
| n-C17:1 | - | - | - | - | - | 141.7 | 49.9 |
| n-C17 | 123.7 | 56.7 | 23.1 | 3071.9 | 1559.1 | 726.0 | 663.1 |
| Pristane | 3.2 | 40.8 | 0.6 | 60.0 | 1.3 | 520.6 | 14.0 |
| 7Me-C17 | 137.6 | 44.0 | 8.4 | - | 224.9 | 236.9 | 121.4 |
| 6Me-C17 | 18.3 | 0.6 | 3.0 | 2783.6 | 5.3 | 1.8 | 3.3 |
| 5Me-C17 | 16.9 | 21.7 | 2.7 | 2570.8 | 41.6 | 185.0 | 76.8 |
| 4Me-C17 | 23.0 | 13.4 | 1.9 | 365.1 | 39.2 | 122.4 | 0.1 |
| 3Me-C17 | 16.9 | 13.5 | 3.0 | 116.2 | 41.1 | 162.7 | 44.1 |
| 2Me-C17 | 18.1 | 20.5 | - | 95.7 | 70.2 | 177.6 | 0.1 |
| 7,11DiMe-C17 | 53.6 | 34.3 | 6.0 | - | - | - | - |
| 6Me-C18 | - | - | - | 159.6 | - | - | - |
| n-C18:1 | - | - | - | - | - | 230.1 | - |
| n-C18 | 33.1 | 33.2 | 12.1 | 594.7 | 119.6 | 396.3 | 112.8 |
| Phytane | 21.9 | 26.8 | 3.2 | 258.1 | 107.7 | 389.1 | 85.3 |
| n-C19 | 18.6 | 18.3 | 3.1 | 197.5 | 45.8 | 181.5 | 99.4 |
| n-C31:3 | 14.3 | 8.0 | 2.9 | - | - | - | - |
| Wax Esters | |||||||
| n,n-C29 | 5.0 | 3.6 | 1.4 | 0.4 | - | - | - |
| i,n-C30 | 7.3 | 0.5 | 0.6 | 0.3 | - | - | - |
| n,n-C30 | 51.0 | 1.9 | 1.5 | 2.0 | - | - | - |
| i,n-C31 | 23.0 | 9.4 | 2.6 | 1.3 | - | - | - |
| n,n-C31 | 97.2 | 3.4 | 2.8 | 4.7 | - | - | - |
| i,i-C32 | 5.6 | - | 0.5 | 0.3 | - | - | - |
| i,n-C32 | 114.4 | 4.2 | 3.5 | 3.6 | - | - | - |
| n,n-C32 | 799.8 | 22.5 | 14.3 | 13.6 | - | - | - |
| i,i-C33 | 14.3 | - | 1.3 | - | - | - | - |
| i,n-C33 | 73.2 | 2.8 | 3.6 | 1.9 | - | - | - |
| n,n-C33 | 322.9 | 10.2 | 11.3 | 7.1 | - | - | - |
| i,i-C34 | 7.7 | - | 0.0 | - | - | - | - |
| i,n-C34 | 138.8 | 5.0 | 4.5 | 3.0 | - | - | - |
| n,n-C34 | 965.9 | 26.9 | 19.4 | 7.4 | - | - | - |
| i,i-C35 | 24.3 | - | 0.0 | - | - | - | - |
| i,n-C35 | 31.0 | - | 1.1 | - | - | - | - |
| n,n-C35 | 148.9 | 4.0 | 3.7 | 0.2 | - | - | - |
| i,n-C36 | 20.9 | - | 0.0 | - | - | - | - |
| n,n-C36 | 89.9 | 4.1 | 2.4 | - | - | - | - |
| n,n-C37 | 43.4 | 1.5 | 0.5 | - | - | - | - |
| Phytol | 67.6 | 136.1 | 16.4 | 168.7 | 60.8 | 146.9 | 1.2 |
| Hopanepolyol products | |||||||
| 2-MeC31 | 0.02 | - | - | - | - | - | 10.90 |
| C31 | 0.21 | 0.01 | - | 26.50 | - | - | 7.98 |
| 2-MeC32 | 0.02 | - | - | - | - | - | - |
| C32 | 1.68 | 0.01 | 0.08 | 974.91 | - | 0.03 | 105.51 |
See Methods text for lipid nomenclature. Units are μg/g TOC.
Syn-Cf, Synechococcus-Chloroflexi mat; O. princeps, Oscillatoria princeps; -, not detected.
3.1.3. Alkanes
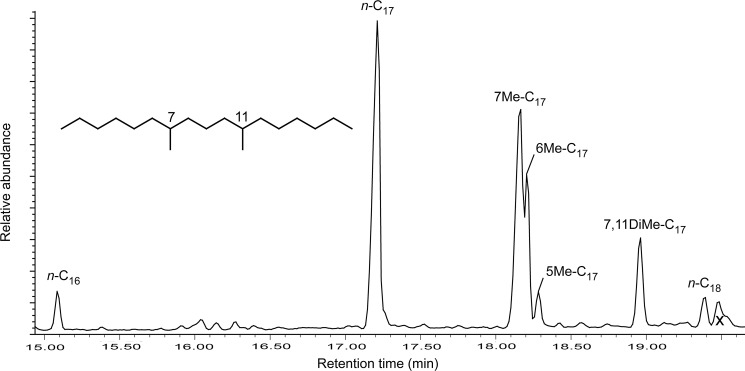
The hydrocarbon fraction of the Synechococcus-Chloroflexi mat was dominated by normal straight-chain alkanes (n-C15 to n-C19) (Table 4). A long-chain tri-unsaturated alkene, n-C31:3, was detected. Two series of short-chain monomethylalkanes (MMAs) and one dimethylalkane (DMA) (7,11-dimethylheptadecane) were also detected and identified based on their retention times relative to the n-alkanes and their mass fragmentation patterns (Table 4, Fig. 5). The Pseudanabaena, O. princeps, and narrow Oscillatoria mats contained short-chain normal alkanes (C15 to C19), but lacked the n-C31:3 alkene (Table 4). These mats also contained the two series of MMAs but lacked any DMA (Table 4).
FIG. 5.
Gas chromatogram of hydrocarbon fraction of Synechococcus-Chloroflexi mat displaying midchain branched monomethylalkanes (7-, 6-, and 5-methylheptadecanes) and one dimethylalkane (7,11-dimethylheptadecane). The x denotes a junk peak that does not correspond to a lipid.
3.1.4. Bacteriohopanepolyols
The greatest structural variety of hopanol products was recovered by the oxidation-reduction procedure from the Synechococcus-Chloroflexi mat (Table 4). A homohopanol (C31) and bishomohopanol (C32) were detected, as well as small amounts of their 2-methyl homologues (2-MeC31 and 2-MeC32). The homohopanol and bishomohopanol were also found in the Pseudanabaena and narrow Oscillatoria mats (Table 4). The narrow Oscillatoria mat in the terracette structure on the face of the main iron deposit also contained the 2-MeC31 BHP product.
3.2. Ferrihydrite core
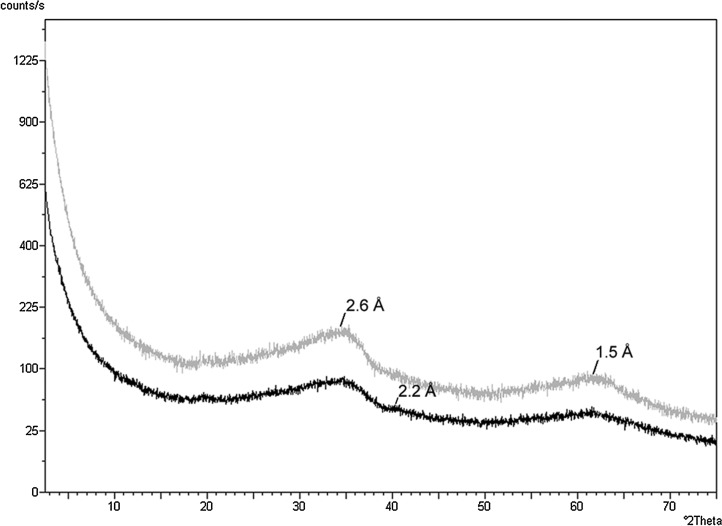
The next phase of our study aimed to characterize the preservation of lipids by iron oxides. The random powder XRD patterns of the ferrihydrite beneath the Synechococcus-Chloroflexi mat, and the adjacent core with no conspicuous mat on the surface, revealed the presence of 2-line and 6-line ferrihydrite (Fig. 6). The two broad amorphous peaks of 2-line ferrihydrite were visible at 2.6 and 1.5 Å. Also present was the 2.2 Å peak of the slightly more ordered 6-line ferrihydrite. The broad amorphous peak of the silica phase opal-A located at ∼4 Å was not present.
FIG. 6.
Random powder XRD patterns of the iron oxide beneath the Synechococcus-Chloroflexi mat (gray) and the adjacent iron oxide core with no conspicuous mat on the surface (black). The two broad amorphous peaks of 2-line ferrihydrite are visible at 2.6 and 1.5 Å. Also visible in the lower pattern is the 2.2 Å peak of the slightly more ordered 6-line ferrihydrite.
The ester-linked fatty acids in the ferrihydrite core beneath the Synechococcus-Chloroflexi mat were remarkably similar to the mat but were 97.6% lower in absolute abundance (Table 3). This indicates that a reasonably high proportion of the fatty acids were remineralized to CO2 over the scale of ∼2 cm. Examination of the TOC revealed a similar trend. The mat contained 1.3 wt % TOC, whereas the ferrihydrite core contained 0.2 wt % TOC (Table 2), which represents an 85% loss of TOC over the scale of ∼2 cm.
There did not appear to be preferential preservation of glycolipids compared to phospholipids in the ferrihydrite cores (Table 3). Both classes of fatty acids experienced 97.6–98.4% destruction. This lack of preferential preservation has also been documented in other environmental studies (Lipp et al., 2008, and references therein).
Of the specific fatty acids that did escape degradation, the phospholipid, polar and neutral glycolipid profiles were again dominated by n-C16 and n-C18 fatty acids, reflecting the same distribution as was observed in the overlying mat (Fig. 4). The proportion of the dienoic fatty acid n-C18:2 was highest in the neutral glycolipid fraction (10 %), as in the surface mat. The n-C18:1 contained 94% of the double bond at the Δ9 position, a slight decrease from that observed in the mat, and 5% and 1% at Δ11 and Δ13, respectively. There was a slight 2.8% increase in the iso-, anteiso-, and midchain branched fatty acids in the ferrihydrite core (Fig. 4). This indicates that proportionally there were slightly more chemotrophs present in the ferrihydrite than in the mat. This is not unexpected, as the lipid signature of the decomposer communities can sometimes overtake that of the primary producers (Harvey et al., 1986, and references therein). However, the high percentage of n-C18:1Δ9 and the presence of n-C18:2 indicate that the signature of the cyanobacteria was strongly preserved in the ferrihydrite.
The adjacent ferrihydrite sinter core with no conspicuous mat on the surface also retained a phototrophic fatty acid signature (Fig. 4), most likely inherited from mats that had colonized the surface of the sinter prior to having been entombed during mineralization. The absolute concentrations of the fatty acids were similar to those found in the ferrihydrite beneath the Synechococcus-Chloroflexi mat. This core was used as a control for the scanning voltammetric microelectrode measurements, and the oxygen profile reflected atmospheric diffusion and not photosynthetic oxygen production (Trouwborst et al., unpublished data). Therefore, this core did not contain a biofilm of active phototrophs, and the phototrophic lipids detected were preserved.
The wax esters also persisted in the ferrihydrite cores. The relative compositions were similar to those of the mat with the exception of the iso-, iso-branched compounds, which were in the lowest abundance in the mat and not detected in the core (Table 4).
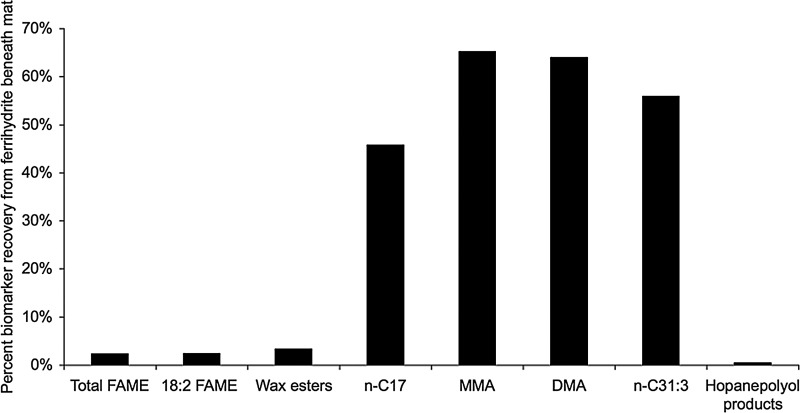
The geologically relevant alkanes detected in the Synechococcus-Chloroflexi mat were also present in the ferrihydrite sinter (Table 4). Most notably, the mono- and dimethylalkanes and the C31:3 alkene survived lithification by ferrihydrite in both cores and displayed the greatest percent recovery (i.e., suffered the least amount of degradation) compared to other lipid classes (Fig. 7).
FIG. 7.
Fraction of lipid biomarkers surviving in the iron oxide (ferrihydrite) beneath the phototrophic mats, expressed as percent recovery.
The other class of geologically relevant lipids, BHPs, not surprisingly, also survived in the ferrihydrite core (Table 4). The C32 hopanol was the most abundant BHP product in the mats and sinter. The 2-methyl homologue (which was produced in the mat) was not detected in the ferrihydrite, probably due to its low relative abundance in the surface mat and the small sample size available for extraction. Compared to the alkanes, the BHPs displayed a relatively low percent recovery (Fig. 7).
3.3. Immature kerogen of permineralized cyanobacterial microfossils in the extinct iron-silica spring
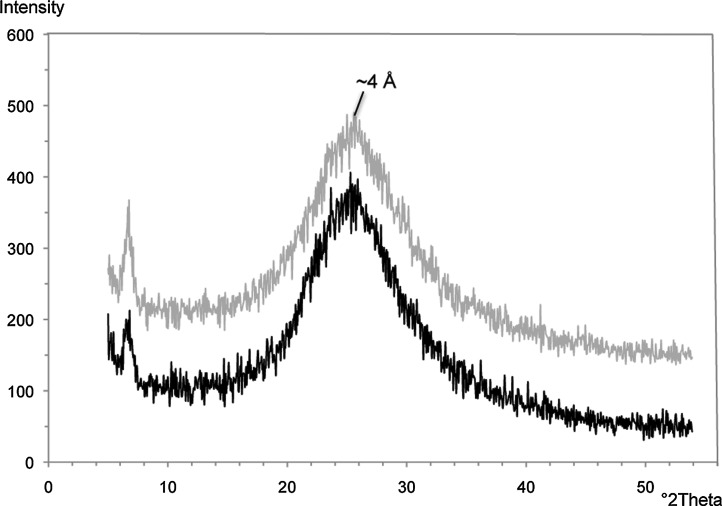
The age of the extinct hydrothermal deposit is unknown, but a soil horizon and vegetation have developed on top of the outcrop (Fig. 3a, 3b). We are currently attempting to determine the age of the organics preserved within the deposit via C-14 dating, as has been performed for other hydrothermal sinter deposits (Lutz et al., 2002). XRD of the upper band of silica in the hand sample (Fig. 3b, 3c) indicates that it is opal-A and has not yet recrystallized to quartz (Fig. 8).
FIG. 8.
Random powder XRD patterns of the mineralized biofabric from the extinct iron-silica spring shown in Fig. 3c. The opaline silica band marked by the X is shown in gray, while the siliceous iron band marked by the other X is shown in black. The broad amorphous peak of opal-A is visible at ∼4 Å.
The petrographic thin sections of the fossilized streamer biofabric revealed abundant amber-colored filamentous microfossils (Fig. 3d, 3e). Some microfossils were clearly identifiable as cyanobacteria, such as the well-preserved Calothrix filament in Fig. 3d. When viewed under ultraviolet light, the amber-colored kerogen in the microfossils fluoresced (Fig. 3f).
4. Discussion
Lipid analysis is a tool used to characterize the structure and function of extant and extinct natural microbial communities. Modern lipid studies are complementary to 16S ribosomal RNA gene analysis, -omics approaches, microscopy, pigment assays, and selective enrichments. Membrane lipids can be used to assess microbial diversity and, while arguably not as sensitive as 16S rRNA, do offer advantages in terms of characterizing how microbes respond to environmental changes (Vestal and White, 1989; Boyd et al., 2011; Kaur et al., 2011). Because intact polar membrane lipids (IPLs) are rapidly degraded upon cell death, they can be used to quantify live microbial biomass (White et al., 1979; Lipp et al., 2008). For studies that extend to the geological record, lipid classes that contain highly branched and polycyclic compounds result in fossil hydrocarbons that can survive for billions of years (Brocks et al., 2003b; Brocks and Summons, 2004; Marshall et al., 2007; Ventura et al., 2008).
4.1. Chocolate Pots phototrophic mats
4.1.1. Ester-linked membrane fatty acids
All bacteria contain a certain amount of phospholipid in their membranes, and these ester-linked fatty acids have historically been used to assess microbial diversity (e.g., White et al., 1993). The fatty acid chain length, number and position of double bonds, cyclopropane rings, and position of methyl branches allow for distinction between aerobes, anaerobes, sulfate-reducing bacteria, cyanobacteria, actinomycetes, fungi, protozoa, plants, and green algae (Vestal and White, 1989, and references therein).
However, the membrane lipids of cyanobacteria and FAPs (e.g., Chloroflexus) are primarily glycolipids, and if only phospholipids are analyzed in natural phototrophic microbial mat samples, much taxonomic information is overlooked. Cyanobacteria predominantly contain monogalactosyl diacylglycerol (MG), digalactosyl diglyceride (DG), and sulfoquinovosyl diacylglycerol (SQ); only one phospholipid, PG, is present (e.g., Fork et al., 1979; Jürgens and Weckesser, 1986; Miller et al., 1988; Ward et al., 1994). Chloroflexus also contains MG, DG, and PG (Kenyon and Gray, 1974; Knudsen et al., 1982, Ward et al., 1994). Our methods were designed to separate the IPLs by their polar head groups into phospholipids (PG), polar glycolipids (DG), and neutral glycolipids (primarily MG and some DG) by preparative TLC (before cleavage and analysis by GC-MS). Separation by polar head groups, coupled with analysis of the acyl chain attributes, allowed for a more detailed assessment of chemotrophic versus phototrophic community members.
In terms of diagnostic fatty acid chain attributes, it should be noted that most bacterial membranes are dominated by the n-C16 and n-C18 saturated fatty acids (Ratledge and Wilkinson, 1988), which do not yield much taxonomic information. Accordingly, the n-C16 and n-C18 also dominate the fatty acid profiles of cyanobacteria and Chloroflexus isolates (Kenyon and Gray, 1974; Fork et al., 1979; Knudsen et al., 1982; Ward et al., 1989). However, a more specific fatty acid biomarker for cyanobacteria is the dienoic fatty acid n-C18:2. Dienoic fatty acids are often found in cyanobacteria (Kenyon, 1972; Kenyon et al., 1972) and are produced by few other prokaryotes, particularly in hot spring environments.
Another fatty acid biomarker that can be considered of cyanobacterial origin is the n-C18:1 with the double bond at the C-9 (Δ9) position, particularly when it is associated with the glycolipid fraction (Sakamoto et al., 1994). Chloroflexus also synthesizes the Δ9 positional isomer (Kenyon and Gray, 1974), which we confirmed by analyzing the type strain Chloroflexus aurantiacus J-10-fl. The determination of double-bond position of the monounsaturated fatty acids aids in the assessment of the relative proportion of cyanobacteria and Chloroflexus sp. to other bacteria in these relatively simple microbial mats due to differences in the biosynthetic pathway that generates the desaturation of the acyl chain. Cyanobacteria contain an oxygen-dependent desaturation mechanism (Bloch, 1971), while other bacteria contain an anaerobic chain-elongation, dehydration system (Cronan and Rock, 1996). These different biosynthetic mechanisms result in n-C18:1Δ9 in cyanobacteria and n-C18:1Δ11 in most other bacteria. So based on the DMDS adducts of the intact fatty acids, cyanobacteria and Chloroflexus sp. were confirmed as the major members (89–97%) of the higher-temperature microbial mats.
Examination of the position of methyl branches on the acyl chain, particularly when the fatty acids are phospholipids, yields information about the chemotrophic community members. The detection of low levels of branched fatty acids suggests the presence of additional bacteria that were not identified in previous studies of the Chocolate Pots phototrophic mats (Fig. 5). Terminally branched iso- and anteiso-fatty acids are common in Gram-positive bacteria and sulfate-reducing bacteria. Midchain branched phospholipid fatty acids such as 10-methylhexadecanoate are considered indicative of Desulfobacter spp. and actinomycetes (Boschker and Middelburg, 2002). However, numerous sulfide and scanning voltammetric microelectrode measurements of these mats in the light and the dark revealed no detectable sulfide (Pierson et al., 1999; Trouwborst et al., 2007). Additionally, no sulfur-containing minerals were detected by XRD or energy-dispersive spectroscopy (Parenteau and Cady, 2010), further suggesting the absence of a detectable sulfur cycle. This was supported by the lack of sulfate-reducing bacteria in the metagenomic data of the Chocolate Pots mat (Klatt et al., 2013). Therefore, actinomycetes are the most probable source of the 10-methyl fatty acids and were likely derived from the soil immediately adjacent to the hot springs. Thermophilic chemolithotrophic iron oxidizers are probably not present in this system; microscopic examination of surface samples taken at the vent, and at 1 m intervals away from the vent, did not reveal obvious morphological evidence of iron oxidizers (Emerson and Weiss, 2004). Attempts at isolating the organisms with gradient and liquid enrichment techniques were also unsuccessful (Emerson and Weiss, 2004).
In terms of other sources of methyl-branched fatty acids, it has been shown that certain species of cyanobacteria from Antarctica synthesize terminally branched iso- and anteiso-fatty acids (Rezanka et al., 2009), which may explain our detection of these compounds given the lack of sulfate-reducing bacteria in this system. To date, it has been shown that Chloroflexus auranticus does not synthesize these branched fatty acids (Knudsen et al., 1982). However, the presence of numerous branched wax esters in Chloroflexus-containing mats throughout the thermal features of Yellowstone (e.g., van der Meer et al., 2003; Jahnke et al., 2004) strongly suggests that some species of Chloroflexus do synthesize branched fatty acids, which are necessary biosynthetic precursors to these compounds.
4.1.2. Wax esters
Wax esters are considered biomarkers for the FAPs Chloroflexus and Roseiflexus (Knudsen et al., 1982; Shiea et al., 1991; van der Meer et al., 1999, 2002). The wax esters and C31:3 alkene (discussed below in the alkane section) support the presence of Chloroflexus in the Synechococcus mat at Chocolate Pots. What is surprising is the lack of the C31:3 and abundance of wax esters in the Pseudanabaena mat, which suggests the presence of Roseiflexus sp. Roseiflexus is probably also likely present in the Synechococcus mat, despite the lack of a characteristic red layer beneath the upper green cyanobacterial layer. This is the first detection of Roseiflexus spp. in a slightly acidic iron-rich hot spring, as they are typically found in alkaline silica-depositing hot springs (Boomer et al., 2000; Hanada and Pierson, 2002; Nübel et al., 2002; Madigan et al., 2005; van der Meer et al., 2005; Klatt et al., 2007).
4.1.3. Alkanes
Normal straight-chain alkanes are the most abundant hydrocarbons in all nonbiodegraded oils and mature bitumens in the rock record (Brocks and Summons, 2004). Many eukaryotes and a few prokaryotes synthesize alkanes, which are derived from fatty acids via an enzymatic decarboxylation mechanism (Albro, 1976; Schirmer et al., 2010). In addition to direct enzymatic production, alkanes can be derived from the diagenesis of other biological precursor lipids and from the catagenesis of kerogens (e.g., Boschker and Middelburg, 2002).
Midchain methyl-branched alkanes, such as the monomethylhexa- and heptadecanes and 7,11-dimethylheptadecane identified here, are considered biomarkers for cyanobacteria (Han and Calvin, 1969; Shiea et al., 1990, 1991; Kenig et al., 1995; Jahnke et al., 2004). MMAs have been documented in a large number of pure cultures of cyanobacteria and in natural cyanobacterial mats (Shiea et al., 1990, and references therein; Zeng et al., 1992; Kenig et al., 1995; Dembitsky et al., 2001; Jahnke et al., 2004). DMAs have been found in cyanobacterial mats throughout the world (Robinson and Eglinton, 1990; Shiea et al., 1990; Kenig et al., 1995; Jahnke et al., 2004), but they have been characterized in only a few cyanobacterial cultures (Summons et al., 1996; Köster et al., 1999; Jahnke et al., 2004). In Yellowstone, DMAs have only been reported in a Phormidium mat from the silica-depositing alkaline chloride springs at Fountain Paint Pots (Jahnke et al., 2004). This report of 7,11-dimethylheptadecane is the first for (1) a Synechococcus-Chloroflexi mat and (2) a slightly acidic iron-depositing hot spring.
The C31:3 alkene is considered a biomarker for Chloroflexus (van der Meer et al., 1999). The detection of this alkene and the wax esters in the higher-temperature Synechococcus-Chloroflexi mat confirms the previous identification of Chloroflexus in this mat by phase and near-infrared epifluorescence microscopy and in vivo pigment analyses (Pierson and Parenteau, 2000).
4.1.4. Bacteriohopanepolyols
Hopanoids are considered the most abundant natural product on Earth (Ourisson and Albrecht, 1992) and are ubiquitous in sedimentary organic matter and petroleum of all geological eras (Brocks and Summons, 2004). The functionalized bacterial precursors of sedimentary hopanoids or hopanes are BHPs. BHPs are pentacyclic triterpanoids with a hopane skeleton linked to a variety of polyhydroxylated side chains (Ourisson et al., 1987; Summons et al., 1999). They are synthesized by a wide variety of cultured aerobic and facultative anaerobic bacteria (Rohmer et al., 1984; Farrimond et al., 2000) and a few strict anaerobes (Sinninghe Damsté et al., 2004; Fischer et al., 2005).
Modification of the hopanoid skeleton by alkyl substituents can occur at either C-2 or C-3. Methylation at C-2 is characteristic of a high proportion of cyanobacteria, particularly those isolated from hot springs, and is considered a biomarker for this group (Summons et al., 1999). This finding was supported by Talbot et al. (2008) in an extended survey of intact BHPs in marine, freshwater, and hydrothermal cyanobacterial cultures and in environmental samples from lakes, hot and cold deserts, and hydrothermal springs. However, 2-methyl(Me)BHP has been found in one nonmarine purple nonsulfur phototroph, Rhodopseudomonas palustris TIE-1 (Rashby et al., 2007), calling into question the utility of this compound as a biomarker for cyanobacteria. But the very low numbers of alphaproteobacterial sequences (and complete lack of purple nonsulfur sequences) retrieved from the Chocolate Pots Synechococcus-Chloroflexi mat metagenomic data and supporting 16S rRNA panels (Klatt et al., 2013) suggest that, in this system, 2-MeBHP is a biomarker for cyanobacteria.
The same 2-MeC31 and 2-MeC32 hopanol products recovered from the Chocolate Pots mats were also found in the Yellowstone Phormidium mats at Fountain Paint Pots and have been identified in a New Zealand hot spring isolate, Oscillatoria amphigranulata (Castenholz and Utkilen, 1984; Jahnke et al., 2004). Interestingly, there were no methylated BHPs found in the Synechococcus-Chloroflexi mat at Octopus hot springs (Talbot et al., 2008).
This examination of modern, functionalized lipids in the Chocolate Pots iron mats compares well to the extensive body of work performed on the phototrophic mats at the alkaline chloride silica-depositing Octopus Spring in Yellowstone (e.g., Ward et al., 1989, 1994; Shiea et al., 1990, 1991; Zeng et al., 1992; Summons et al., 1996; van der Meer et al., 1999, 2002, 2010). The Synechococcus-Chloroflexi mats from both locales are remarkably similar in terms of lipid and community-member composition, despite their vastly different geochemical environments. These observations are supported by a recent metagenomics study, which revealed that the Chocolate Pots Synechococcus sp. were most similar to the Synechococcus sp. B′-like populations observed in Octopus and Mushroom Springs, despite the differences in pH (∼6 vs. >8) (Klatt et al., 2013).
The next phase of our study aimed to characterize the preservation of lipids by iron oxides. There has been substantial work done that has examined the preservation of lipids by silica in New Zealand hot springs, although these studies focused on higher-temperature (∼75°C) communities dominated by bacteria (e.g., Aquificales) and archaea (e.g., Sulfolobales) (Pancost et al., 2005, 2006; Talbot et al., 2005; Gibson et al., 2008; Kaur et al., 2008, 2011). The primary mode of preservation in silica-depositing springs is rapid silicification and entombment within the silica matrix. This mechanism will be contrasted with hypotheses for the iron system.
4.2. Ferrihydrite core
This portion of the study aimed to determine which lipid classes survived microbial degradative processes in the mats. Another goal was to determine how mineralization of the mats by siliceous 2-line ferrihydrite, the primary iron phase of the sinter, affected the lipid signature of the microbial community.
In general, there appears to be some degree (∼15%) of bulk TOC preservation in the ferrihydrite core beneath the cyanobacterial mats at Chocolate Pots. We speculate that iron may play a role in this preservation process. Recently, a mechanism involving iron-enhanced preservation of organic carbon was described in marine sediments (Lalonde et al., 2012). It was shown that iron directly chelates or coprecipitates with the organic carbon, generating a “rusty sink” which functions in the long-term storage of organics. This is in contrast to the prevailing view that iron oxidizes organics and thus destroys them (Klein, 2005).
In addition to the bulk TOC preservation, we unexpectedly recovered labile cyanobacterial and Chloroflexus phospholipids and glycolipids from the ferrihydrite core. These IPLs are rapidly degraded after cell death by the cell's own lytic enzymes or those of decomposers (Harwood and Russell, 1984). As a result, they are used as indicators of cellular viability in natural environments (White et al., 1997; Lipp et al., 2008). We did not anticipate finding any phototrophic IPLs buried in the ferrihydrite core. A possible explanation is that it has been shown that iron may inhibit cellular autolytic enzymes (Herbold and Glaser, 1975; Leduc et al., 1982). Inhibition of the cell's own lytic enzymes, or those of heterotrophs, may promote the survival of lipids during cell death and heterotrophic grazing and thus increase the yield of lipids potentially entering the rock record.
Another possible explanation for the IPL recovery is that our previous microscopic study of microfossil formation in this system revealed that the photosynthetic membrane structures in the cyanobacterial cells were well preserved via ferrihydrite permineralization (Parenteau and Cady, 2010). The ferrihydrite appeared to be physically binding to the membranes, which may make the IPLs unavailable to enzymatic attack.
The geologically relevant phototrophic lipid biomarkers were also preserved in the ferrihydrite core beneath the cyanobacterial mats. Both normal and branched alkanes displayed the greatest percent recovery or least amount of degradation (Fig. 7). Alkanes have proven to be relatively recalcitrant hydrocarbon biomarkers and have been characterized from many ancient sediments, such as Holocene cyanobacterial mats (Kenig et al., 1995) and the 2.78–2.45 Ga shales of the Mount Bruce Supergroup in the Pilbara Craton (Brocks et al., 2003a, 2003b).
Compared to the alkanes, the BHPs displayed a lower percent recovery (Fig. 7). We hypothesize that this may be due to the chemical binding of the polyhydroxylated side chain by iron, which may require more severe chemical treatment to recover. The iron-chelating/coprecipitation mechanism described by Lalonde et al. (2012) may also be functioning in the binding of the BHPs. It has been previously shown that reaction of sulfur with unsaturated and polyfunctional organic compounds (sulfurization) enhances preservation by promoting the incorporation of these compounds into the macromolecular structure of kerogen (Sinninghe Damsté et al., 1989; Sinninghe Damsté and de Leeuw, 1990; Kohnen et al., 1991). We hypothesize that a similar scenario could be happening with the lipid functional groups and the ferrihydrite, and that use of a more destructive method could increase hopanoid recovery.
4.3. Immature kerogen of permineralized cyanobacterial microfossils
The purpose of this portion of the project was to assess organic matter preservation in an extinct iron-silica spring located ∼700 m north of Chocolate Pots. These deposits have undergone later stages of diagenesis. The earliest diagenetic window in the extant microbial mats in the active hot spring encompassed lipid biomarker production and survival through microbial degradation and mineral binding and encrustation onto biological surfaces, and mineral infusion of porous organic materials (i.e., permineralization). We hypothesized that the extinct hydrothermal deposits would allow us to assess the fate of organics upon recrystallization of the primary precipitates to more ordered phases, pore-filling of the mineral matrix, and reaction with later stage redox-reactive fluids.
We anticipated that the silica and iron bands in the extinct sinter would reflect later diagenetic phases such as quartz, goethite, or hematite. However, XRD of the upper band of silica in the hand sample (Fig. 3b, 3c) indicated that the amorphous opal-A has not yet recrystallized to more stable phases like cristobalite, tridymite, or quartz. However, the persistence of opal-A does not necessarily indicate that the deposit is exceptionally young. The oldest known siliceous sinters in Yellowstone are located at Artist Point and are thought to have formed between 600 and 130 ka (White et al., 1988). The silica phases identified in the Artist Point sinters include opal-A, opal-CT, and quartz (Hinman and Walter, 2005). The detection of opal-A in the Artist Point sinters indicates that recrystallization can take several hundred thousand years. So the extinct iron-silica sinter near Chocolate Pots may be as old as the Artist Point sinters, as it does display significant postdepositional cementation.
The organics in the cyanobacterial microfossils in the extinct sinter appeared to be well preserved. Given the high transmissivity and intense fluorescence of the kerogen macerals, it is likely immature liptinite. Other maceral groups such as vitrinite and inertinite do not fluoresce. Liptinite is composed of degraded lipids and other low-molecular-weight compounds that have been incorporated into complex geopolymers by condensation and defunctionalization reactions (Stasiuk, 1994). These geopolymers, along with selectively preserved biopolymers that are resistant to microbial degradation (such as hopanoids) (Tegelaar et al., 1989) and highly aliphatic macromolecules in the cell walls of cyanobacteria (Chalansonnet et al., 1988), are likely important contributors to the immature kerogen in the microfossils and the finely dispersed kerogen in the streamer biofabric. In future studies we will use time-of-flight secondary mass spectroscopy to nondestructively characterize the lipids in the microfossils (e.g., Siljeström et al., 2010).
4.4. Early diagenesis in modern iron-silica springs
This study attempted to characterize the fate of organic material through two diagenetic windows in an iron-silica hydrothermal system: one in the extant microbial mats and the other in subrecent sinter deposits. The first taphonomic window included the production and survival of labile (e.g., fatty acids and wax esters) and refractory, geologically relevant (e.g., branched alkanes and hopanoids) lipids in the Synechococcus-Chloroflexi mat. It is in this window that most of the organic matter is destroyed (i.e., remineralized) via microbial degradation (photoheterotrophy, aerobic respiration, anaerobic respiration, fermentation, and methanogenesis) (Brocks and Summons, 2004). These metabolic processes have been documented to occur in hot spring mats and have been well studied in the Synechococcus-Chloroflexi mat at Octopus and Mushroom Springs (Zeikus et al., 1980; Ward et al., 1998; Dillon et al., 2007). Of these processes, aerobic respiration is probably the most important in organic matter degradation and is controlled by the availability of oxygen.
We expected to see nearly 100% remineralization of labile compounds. Instead, there appeared to be some degree of survival of fatty acids (97.6% loss) and TOC (85% loss). This may be due to the presence of anoxic, Fe(II)-rich waters that bathe the mats. Our scanning voltammetric microelectrode studies have shown that the oxygen produced by the cyanobacteria in the upper layers of the Synechococcus-Chloroflexi mat does not accumulate to supersaturated levels as it does in the Synechococcus-Chloroflexi mats at Octopus (Trouwborst et al., 2007). The oxygen production rate is balanced by consumption by the constant influx of Fe(II) from the vent. The resulting net lower oxygen levels may depress aerobic respiration rates, thereby consuming less organic matter.
Iron may play other roles in enhancing the early preservation of lipids. As mentioned previously, the iron may inhibit the phototrophs' autolytic enzymes that hydrolyze various organic moities upon death, and may inhibit those of heterotrophs as well. Iron can also chemically bind or chelate the organic compounds. In studies of microfossil formation, it has been shown that the electrostatic interaction of the positively charged iron (and potentially other cations) with the predominantly negatively charged cell surface functional groups (e.g., Ferris et al., 1988; Warren and Ferris, 1998; Phoenix et al., 2003) leads to rapid mineralization of the cells. This entombment by iron may make the cells less available to enzymatic attack. It may also facilitate silicification and enhance microfossil preservation by acting as a cation bridge between the negatively charged cell surface and negatively charged silicic acid (e.g., Ferris et al., 1988; Urrutia and Beveridge 1993, 1994; Fortin et al., 1997). The “rapid entombment” mechanism has also been invoked to explain the preservation of lipids in silica-depositing springs in New Zealand (e.g., Kaur et al., 2011).
The preceding discussion identifies ways in which iron may facilitate the preservation of organic material. However, it has also been suggested that iron directly oxidizes organics it comes in contact with and effectively destroys them (Klein, 2005). Because of this, we anticipated no net entry of organic material into the ferrihydrite sinter beneath the mats, or any kerogen in the extinct iron-silica deposits. But it must be noted that primary nanophase iron oxyhydroxide precipitates (ferrihydrite) frequently coprecipitate with silica, which adsorbs to the surface to form siliceous ferrihydrite (e.g., Carlson and Schwertmann, 1981; Schwertmann et al., 1984; Cornell et al., 1987; Parfitt et al., 1992). It has been shown that this silica blocks surface unsaturated Fe-coordination sites (e.g., Carlson and Schwertmann, 1981; Schwertmann et al., 1984; Cornell et al., 1987; Zhao et al., 1994), which effectively makes them unavailable to extract electrons from organic material. This process may enhance early preservation of organics in iron-silica systems. However, it should be noted that ferrihydrite is a metastable phase that will recrystallize to more ordered forms such as hematite or goethite. The adsorbed Si is apparently released at this stage and precipitates as a separate, secondary phase (Harder, 1963). Potentially, during this later diagenetic window, organics may be destroyed as surface Fe sites are once again exposed.
4.5. Relevance to early Earth and Mars
The oldest known subaerial hydrothermal deposits on Earth are found in the Drummond Basin, Australia, and have been dated at 342–298 Ma (Walter et al., 1996). The Verbena sinters contain thin-bedded microfacies with streamer biofabrics that are closely comparable to sinters in Yellowstone (Walter et al., 1996). They are composed of laminae of iron-stained microcrystalline quartz (chert) and appear to be analogous to the extinct iron-silica deposit near Chocolate Pots.
We recognize that the nature of lipid biomarker and kerogen preservation observed in this study occurred early in the diagenetic history of the Chocolate Pots sinter deposits; however, it is clear that at the earliest stage, fossil biosignatures have survived the initial stages of diagenesis. Taphonomic studies of biosignature preservation in modern microbial ecosystems that evaluate the impacts of early diagenesis can lead to an improved understanding of paragenetic histories in iron and silica hydrothermal systems and the impacts on fossil preservation.
This strategy is particularly relevant when searching for evidence of extinct microbial life in similar deposits on Mars, such as the amorphous silica deposits (opal-A) at Home Plate in Gusev Crater. These silica deposits were detected by the NASA Spirit rover and have been interpreted as evidence of a martian hydrothermal spring system (Ruff et al., 2011). Because spectral analyses suggest that the opal-A has not recrystallized to quartz, it makes a case for examining the fate of organics through early diagenesis in modern and subrecent hydrothermal sinters on Earth that also have not undergone extensive recrystallization. The systematic documentation of microbial biosignatures (biofabrics, microfossils, and lipid biomarkers) in modern hydrothermal springs can lay important groundwork in the search for evidence of fossilized microbial life in analogous deposits on Mars.
Acknowledgments
This work was supported by NASA Exobiology Grant NAG5-12328. Additional support was generously provided by a NASA Oregon Space Grant Graduate Fellowship, a NASA Planetary Biology Internship, and a NASA Postdoctoral Program Fellowship to M.N. Parenteau. Part of this work was supported by grants from the NASA Exobiology Program (NNX08AZ47A) to L. Jahnke and the NASA Mars Fundamental Research Program (NNH08ZDA001N) to J.D. Farmer. S.L. Cady acknowledges financial support from the Environmental and Molecular Sciences Laboratory (EMSL), a national scientific user facility sponsored by the Department of Energy's Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory, and from the NASA Exobiology Program (NNX11AR81G). We thank the National Park Service for allowing us to conduct research in Yellowstone National Park and thank Mike Kubo and Tsegereda Embaye, SETI Institute; Kendra Turk, University of California Santa Cruz; and Georg Grathoff, Portland State University for technical assistance. We thank Dave Des Marais and Beverly Pierson for helpful discussions and two anonymous reviewers, who helped strengthen the manuscript.
Author Disclosure Statement
No competing financial interests exist.
Abbreviations
ASC, acid-sulfate-chloride.
BHPs, bacteriohopanepolyols.
DG, digalactosyl diglyceride.
DMA, dimethylalkane.
DMDS, dimethyl disulfide.
FAMEs, fatty acid methyl esters.
FAPs, filamentous anoxygenic phototrophs.
FID, flame ionization detector.
GC, gas chromatograph.
GC-MS, gas chromatograph–mass spectrometer.
IPLs, intact polar membrane lipids.
MG, monogalactosyl diacylglycerol.
MMAs, monomethylalkanes.
PG, phosphatidylglycerol.
TLC, thin-layer chromatography.
TOC, total organic carbon.
XRD, X-ray diffraction.
References
- Albro P.W. (1976) Bacterial waxes. In Chemistry and Biochemistry of Natural Waxes, edited by Kolatukudys P.E., Elsevier, Amsterdam, pp 419–445 [Google Scholar]
- Baratoux D., Toplis M.J., Monnereau M., and Gasnault O. (2011) Thermal history of Mars inferred from orbital geochemistry of volcanic provinces. Nature 472:338–341 [DOI] [PubMed] [Google Scholar]
- Benning L.G., Phoenix V.R., Yee N., and Tobin M.J. (2004) Molecular characterization of cyanobacterial silicification using synchrotron infrared micro-spectroscopy. Geochim Cosmochim Acta 68:729–741 [Google Scholar]
- Bloch K. (1971) B-hydroxydecanoyl thioester dehydrase. In The Enzymes, edited by Boyers P.D., Academic Press, New York, pp 441–464 [Google Scholar]
- Boomer S.M., Pierson B.K., Austinhirst R., and Castenholz R.W. (2000) Characterization of novel bacteriochlorophyll-a-containing red filaments from alkaline hot springs in Yellowstone National Park. Arch Microbiol 174:152–161 [DOI] [PubMed] [Google Scholar]
- Boschker H.T.S. and Middelburg J.J. (2002) Stable isotopes and biomarkers in microbial ecology. FEMS Microbiol Ecol 40:85–95 [DOI] [PubMed] [Google Scholar]
- Boyd E.S., Pearson A., Pi Y., Li W.J., Zhang Y.G., He L., Zhang C.L., and Geesey G.G. (2011) Temperature and pH controls on glycerol dibiphytanyl glycerol tetraether lipid composition in the hyperthermophilic crenarchaeon Acidilobus sulfurireducens. Extremophiles 15:59–65 [DOI] [PubMed] [Google Scholar]
- Brock T.D. (1967) Life at high temperatures. Science 158:1012–1019 [DOI] [PubMed] [Google Scholar]
- Brock T.D. (1978) Thermophilic Microorganisms and Life at High Temperatures, Springer-Verlag, New York [Google Scholar]
- Brocks J.J. and Summons R.E. (2004) Sedimentary hydrocarbons, biomarkers for early life. In Biogeochemistry: Treatise on Geochemistry, Volume 8, edited by Schlesinger W.H., Elsevier Pergamon, Oxford, pp 63–115 [Google Scholar]
- Brocks J.J., Buick R., Logan G.A., and Summons R.E. (2003a) Composition and syngeneity of molecular fossils from the 2.78 to 2.45 billion-year-old Mount Bruce Supergroup, Pilbara Craton, Western Australia. Geochim Cosmochim Acta 67:4289–4319 [Google Scholar]
- Brocks J.J., Buick R., Summons R.E., and Logan G.A. (2003b) A reconstruction of Archean biological diversity based on molecular fossils from the 2.78 to 2.45 billion-year-old Mount Bruce Supergroup, Hamersley Basin, Western Australia. Geochim Cosmochim Acta 67:4321–4335 [Google Scholar]
- Cady S.L. and Farmer J.D. (1996) Fossilization processes in siliceous thermal springs: trends in preservation along thermal gradients. In Evolution of Hydrothermal Ecosystems on Earth (and Mars?), Ciba Foundation Symposium 202, edited by Bock G.R. and Goode J.A., John Wiley and Sons, Chichester, UK, pp 150–173 [DOI] [PubMed] [Google Scholar]
- Cady S.L., Farmer J.D., Grotzinger J.P., Schopf J.W., and Steele A. (2003) Morphological biosignatures and the search for life on Mars. Astrobiology 3:351–368 [DOI] [PubMed] [Google Scholar]
- Carlson L. and Schwertmann U. (1981) Natural ferrihydrites in surface deposits from Finland and their association with silica. Geochim Cosmochim Acta 45:421–429 [Google Scholar]
- Castenholz R.W. (1969) Thermophilic blue-green algae and the thermal environment. Bacteriol Rev 33:476–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castenholz R.W. (1988) Culturing methods for cyanobacteria. Methods Enzymol 167:68–93 [Google Scholar]
- Castenholz R.W. and Utkilen H.C. (1984) Physiology of sulfide tolerance in a thermophilic Oscillatoria. Arch Microbiol 138:299–305 [Google Scholar]
- Chalansonnet S., Largeau C., Casadevall E., Berkaloff C., Peniguel G., and Couderc R. (1988) Cyanobacterial resistant biopolymers. Geochemical implications of the properties of Schizothrix sp. resistant material. Org Geochem 13:1003–1010 [Google Scholar]
- Cornell R.M., Giovanoli R., and Schindler P.W. (1987) Effect of silicate species on the transformation of ferrihydrite into goethite and hematite in alkaline media. Clays Clay Miner 35:12–28 [Google Scholar]
- Cronan J.E. and Rock C.O. (1996) Biosynthesis of membrane lipids. In Escherichia coli and Salmonella: Cellular and Molecular Biology, 2nd ed., edited by Neidhardt F.C., Curtiss R., III, Ingraham J.L., Lin E.C.C., Low K.B., Magasanik B., Reznikoff W.S., Riley M., Schaechter M., and Umbarger H.E.S., ASM Press, Washington, DC, pp 612–636 [Google Scholar]
- Dembitsky V.M., Dor I., Shkrob I., and Aki M. (2001) Branched alkanes and other apolar compounds produced by the cyanobacterium Microcoleus vaginatus from the Negev Desert. Russian Journal of Bioorganic Chemistry 27:110–119 [PubMed] [Google Scholar]
- Dillon J.G., Fishbain S., Miller S.R., Bebout B.M., Habicht K.S., Webb S.M., and Stahl D.A. (2007) High rates of sulfate reduction in a low-sulfate hot spring microbial mat are driven by a low level of diversity of sulfate-respiring microorganisms. Appl Environ Microbiol 73:5218–5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson D. and Weiss J.V. (2004) Bacterial iron oxidation in circumneutral freshwater habitats: findings from the field and the laboratory. Geomicrobiol J 21:405–414 [Google Scholar]
- Farmer J.D. (1995) Mars exopaleontology. Palaios 10:197–198 [PubMed] [Google Scholar]
- Farmer J.D. (1996) Hydrothermal processes on Mars: an assessment of present evidence. In Evolution of Hydrothermal Ecosystems on Earth (and Mars?), Ciba Foundation Symposium 202, edited by Bock G.R. and Goode J.A., John Wiley and Sons, Chichester, UK, pp 273–299 [DOI] [PubMed] [Google Scholar]
- Farmer J.D. (1999) Taphonomic modes in microbial fossilization. In Size Limits of Very Small Microorganisms: Proceedings of a Workshop, National Research Council, The National Academies Press, Washington, DC, pp 94–102 [PubMed] [Google Scholar]
- Farmer J.D. (2003) Exploring for a fossil record of extraterrestrial life. In Paleobiology II, edited by Briggs D.E.G. and Crowther P.R.S., Blackwell Science, Malden, MA, pp 8–13 [Google Scholar]
- Farmer J.D. and Des Marais D.J. (1999) Exploring for a record of ancient martian life. J Geophys Res 104:26977–26995 [DOI] [PubMed] [Google Scholar]
- Farrimond P., Head I.M., and Innes H.E. (2000) Environmental influence on the biohopanoid composition of recent sediments. Geochim Cosmochim Acta 64:2985–2992 [Google Scholar]
- Fernández-Remolar D.C. and Knoll A.H. (2008) Fossilization potential of iron-bearing minerals in acidic environments of Río Tinto, Spain: implications for Mars exploration. Icarus 194:72–85 [Google Scholar]
- Fernández-Remolar D.C., Morris R.V., Gruener J.E., Amils R., and Knoll A.H. (2005) The Río Tinto Basin, Spain: mineralogy, sedimentary geobiology, and implications for interpretation of outcrop rocks at Meridiani Planum, Mars. Earth Planet Sci Lett 240:149–167 [Google Scholar]
- Ferris F.G. and Magalhaes E. (2008) Interfacial energetics of bacterial silicification. Geomicrobiol J 25:333–337 [Google Scholar]
- Ferris F.G., Fyfe W.S., and Beveridge T.J. (1988) Metallic ion binding by Bacillus subtilis: implications for the fossilization of microorganisms. Geology 16:149–152 [Google Scholar]
- Fischer W.W., Summons R.E., and Pearson A. (2005) Targeted genomic detection of biosynthetic pathways: anaerobic production of hopanoid biomarkers by a common sedimentary microbe. Geobiology 3:33–40 [Google Scholar]
- Fork D.C., Murata N., and Sato N. (1979) Effect of growth temperature on the lipid and fatty acid composition, and the dependence on temperature of light-induced redox reactions of cytochrome f and of light energy redistribution in the thermophilic blue-green alga Synechococcus lividus. Plant Physiol 63:524–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin D., Ferris F.G., and Beveridge T.J. (1997) Surface-mediated mineral development by bacteria. Reviews in Mineralogy and Geochemistry 35:161–180 [Google Scholar]
- Fournier R.O. (1989) Geochemistry and dynamics of the Yellowstone National Park hydrothermal system. Annu Rev Earth Planet Sci 17:13–53 [Google Scholar]
- Gibson R.A., Talbot H.M., Kaur G., Pancost R.D., and Mountain B. (2008) Bacteriohopanepolyol signatures of cyanobacterial and methanotrophic bacterial populations recorded in a geothermal vent sinter. Org Geochem 39:1020–1023 [Google Scholar]
- Giggenbach W.F. (1988) Geothermal solute equilibria. Derivation of Na-K-Mg-Ca geoindicators. Geochim Cosmochim Acta 52:2749–2765 [Google Scholar]
- Han J. and Calvin M. (1969) Hydrocarbon distribution of algae and bacteria, and microbiological activity in sediments. Proc Natl Acad Sci USA 64:436–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada S. and Pierson B.K. (2002) The family Chloroflexaceae. In The Prokaryotes: An Evolving Electronic Resource for the Microbiological Community, 3rd ed., Release 3.11, November22, 2002, edited by Dworkins M.E.A., Springer-Verlag, New York [Google Scholar]
- Harder H. (1963) Zur diskussion uber die entstehung der quarzbandererze (itabirite). Neues Jahrbuch fur Mineralogie-Monatshefte 12:303–314 [Google Scholar]
- Harvey H.R., Fallon R.D., and Patton J.S. (1986) The effect of organic matter and oxygen on the degradation of bacterial membrane lipids in marine sediments. Geochim Cosmochim Acta 50:795–804 [Google Scholar]
- Harwood J.L. and Russell N.J. (1984) Lipids in Plants and Microbes, George Allen and Unwin, London [Google Scholar]
- Herbold D.R. and Glaser L. (1975) Bacillus subtilis N-acetylmuramic acid L-alanine amidase. J Biol Chem 250:1676–1682 [PubMed] [Google Scholar]
- Hinman N.W. and Walter M.R. (2005) Textural preservation in siliceous hot spring deposits during early diagenesis: examples from Yellowstone National Park and Nevada, U.S.A. Journal of Sedimentary Research 75:200–215 [Google Scholar]
- Jahnke L.L., Stan-Lotter H., Kato K., and Hochstein L.I. (1992) Presence of methyl sterol and bacteriohopanepolyol in an outer-membrane preparation from Methylococcus capsulatus (bath). J Gen Microbiol 138:1759–1766 [DOI] [PubMed] [Google Scholar]
- Jahnke L.L., Eder W., Huber R., Hope J.M., Hinrichs K.U., Hayes J.M., Des Marais D.J., Cady S.L., and Summons R.E. (2001) Signature lipids and stable carbon isotope analyses of Octopus Spring hyperthermophilic communities compared with those of Aquificales representatives. Appl Environ Microbiol 67:5179–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnke L.L., Embaye T., Hope J., Turk K.A., Van Zuilen M., Des Marais D.J., Farmer J.D., and Summons R.E. (2004) Lipid biomarker and carbon isotopic signatures for stromatolite-forming, microbial mat communities and Phormidium cultures from Yellowstone National Park. Geobiology 2:31–47 [Google Scholar]
- Jürgens U.J. and Weckesser J. (1986) Polysaccharide covalently linked to the peptidoglycan of the cyanobacterium Synechocystis sp. strain PCC6714. J Bacteriol 168:568–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G., Mountain B.W., and Pancost R.D. (2008) Microbial membrane lipids in active and inactive sinters from Champagne Pool, New Zealand: elucidating past geothermal chemistry and microbiology. Org Geochem 39:1024–1028 [Google Scholar]
- Kaur G., Mountain B.W., Hopmans E.C., and Pancost R.D. (2011) Preservation of microbial lipids in geothermal sinters. Astrobiology 11:259–274 [DOI] [PubMed] [Google Scholar]
- Kenig F., Damsté J.S.S., Kock-van Dalen A.C., Rupstra W.I.C., Huc A.Y., and de Leeuw J.W. (1995) Occurrence and origin of mono-, di-, and trimethylalkanes in modern and holocene cyanobacterial mats from Abu Dhabi, United Arab Emirates. Geochim Cosmochim Acta 59:2999–3015 [Google Scholar]
- Kenyon C.N. (1972) Fatty acid composition of unicellular strains of blue-green algae. J Bacteriol 109:827–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C.N. and Gray A.M. (1974) Preliminary analysis of lipids and fatty acids of green bacteria and Chloroflexus aurantiacus. J Bacteriol 120:131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C.N., Rippka R., and Stanier R.Y. (1972) Fatty acid composition and physiological properties of some filamentous blue-green algae. Arch Mikrobiol 83:216–236 [DOI] [PubMed] [Google Scholar]
- Klatt C.G., Bryant D.A., and Ward D.M. (2007) Comparative genomics provides evidence for the 3-hydroxypropionate pathway in filamentous anoxygenic phototrophic bacteria and in hot spring microbial mats. Environ Microbiol 9:2067–2078 [DOI] [PubMed] [Google Scholar]
- Klatt C.G., Inskeep W.P., Herrgard M.J., Jay Z.J., Rusch D.B., Tringe S.G., Parenteau M.N, Ward D.M., Boomer S.M., Bryant D.A., and Miller S.R. (2013) Community structure and function of high-temperature chlorophototrophic microbial mats inhabiting diverse geothermal environments. Front Microbiol 4, doi: 10.3389/fmicb.2013.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C. (2005) Some Precambrian banded iron formations (BIFs) from around the world: their age, geologic setting, mineralogy, metamorphism, geochemistry, and origin. Am Mineral 90:1473–1499 [Google Scholar]
- Knudsen E., Jantzen E., Bryn K., Ormerod J.G., and Sirevag R. (1982) Quantitative and structural characteristics of lipids in Chlorobium and Chloroflexus. Arch Microbiol 132:149–154 [Google Scholar]
- Kohnen M.E.L., Sinninghe Damsté J.S., and de Leeuw J.W. (1991) Biases from natural sulfurization in paleoenvironmental reconstruction based on hydrocarbon biomarker distributions. Nature 349:775–778 [Google Scholar]
- Konhauser K.O., Phoenix V.R., Bottrell S.H., Adams D.G., and Head I.M. (2001) Microbial-silica interactions in Icelandic hot spring sinter: possible analogues for some Precambrian siliceous stromatolites. Sedimentology 48:415–433 [Google Scholar]
- Köster J., Volkman J.K., Rullkötter S.-B.B.M., Rethmeier J., and Fischer U. (1999) Mono-, di- and trimethyl-branched alkanes in cultures of the filamentous cyanobacterium Calothrix scopulorum. Org Geochem 30:1367–1379 [Google Scholar]
- Lalonde K., Mucci A., Ouellet A., and Gelinas Y. (2012) Preservation of organic matter in sediments promoted by iron. Nature 483:198–200 [DOI] [PubMed] [Google Scholar]
- Leduc M., Kasra R., and Van Heijenoort J. (1982) Induction and control of the autolytic system of Escherichia coli. J Bacteriol 152:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp J.S., Morono Y., Inagaki F., and Hinrichs K.U. (2008) Significant contribution of archaea to extant biomass in marine subsurface sediments. Nature 454:991–994 [DOI] [PubMed] [Google Scholar]
- Lutz S.J., Caskey S.J., Mildenhall D.D., Browne P.R.L., and Johnson S.D. (2002) Dating the sinter deposits in northeastern Dixie Valley, Nevada—the paleoseismic record and implications for the Dixie Valley geothermal system [SGP-TR-171]. In Proceedings, Twenty-Seventh Workshop on Geothermal Reservoir Engineering, Stanford University, Stanford, CA [Google Scholar]
- Madigan M.T., Jung D.O., Karr E.A., Sattley W.M., Achenbach L.A., and van der Meer M.T.J. (2005) Diversity of anoxygenic phototrophs in contrasting extreme environments. In Geothermal Biology and Geochemistry in Yellowstone National Park, edited by Inskeep W.P. and McDermott T.R.S, Thermal Biology Institute, Montana State University, Bozeman, MT, pp 203–219 [Google Scholar]
- Marshall C.P., Love G.D., Snape C.E., Hill A.C., Allwood A.C., Walter M.R., Van Kranendonk M.J., Bowden S.A., Sylva S.P., and Summons R.E. (2007) Structural characterization of kerogen in 3.4 Ga Archaean cherts from the Pilbara Craton, Western Australia. Precambrian Res 155:1–23 [Google Scholar]
- Miller M., Pedersen J.Z., and Cox R.P. (1988) Effect of growth temperature on membrane dynamics in a thermophilic cyanobacterium: a spin label study. Biochim Biophys Acta 943:501–510 [DOI] [PubMed] [Google Scholar]
- Mountain B.W., Benning L.G., and Boerema J.A. (2003) Experimental studies on New Zealand hot spring sinters: rates of growth and textural development. Can J Earth Sci 40:1643–1667 [Google Scholar]
- Nübel U., Bateson M.M., Vandieken V., Wieland A., Kuhl M., and Ward D.M. (2002) Microscopic examination of distribution and phenotypic properties of phylogenetically diverse Chloroflexaceae-related bacteria in hot spring microbial mats. Appl Environm Microbiol 68:4593–4603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourisson G. and Albrecht P. (1992) Hopanoids 1. Geohopanoids: the most abundant natural products on Earth? Pure Appl Chem 51:709–729 [Google Scholar]
- Ourisson G., Rohmer M., and Poralla K. (1987) Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Annu Rev Microbiol 41:301–333 [DOI] [PubMed] [Google Scholar]
- Pancost R.D., Pressley S., Coleman J.M., Benning L., and Mountain B.W. (2005) Lipid biomolecules in silica sinters: indicators of microbial biodiversity. Environ Microbiol 7:66–77 [DOI] [PubMed] [Google Scholar]
- Pancost R.D., Pressley S., Coleman J.M., Talbot H.M., Kelly S.P., Farrimond P., Schouten S., Benning L., and Mountain B.W. (2006) Composition and implications of diverse lipids in New Zealand geothermal sinters. Geobiology 4:71–92 [Google Scholar]
- Parenteau M.N. and Cady S.L. (2010) Microbial biosignatures in iron-mineralized phototrophic mats at Chocolate Pots hot springs, Yellowstone National Park, United States. Palaios 25:97–111 [Google Scholar]
- Parfitt R.L., Van der Gaast S.J., and Childs C.W. (1992) A structural model for natural siliceous ferrihydrite. Clays Clay Miner 40:675–681 [Google Scholar]
- Phoenix V.R., Adams D.G., and Konhauser K.O. (2000) Cyanobacterial viability during hydrothermal biomineralization. Chem Geol 169:329–338 [Google Scholar]
- Phoenix V.R., Konhauser K.O., and Ferris F.G. (2003) Experimental study of iron and silica immobilization by bacteria in mixed Fe–Si systems: implications for microbial silicification in hot springs. Can J Earth Sci 40:1669–1678 [Google Scholar]
- Pierson B.K. and Castenholz R.W. (1974) A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch Microbiol 100:5–24 [DOI] [PubMed] [Google Scholar]
- Pierson B.K. and Parenteau M.N. (2000) Phototrophs in high iron microbial mats: microstructure of mats in iron-depositing hot springs. FEMS Microbiol Ecol 32:181–196 [DOI] [PubMed] [Google Scholar]
- Pierson B.K., Parenteau M.N., and Griffin B.M. (1999) Phototrophs in high-iron-concentration microbial mats: physiological ecology of phototrophs in an iron-depositing hot spring. Appl Environ Microbiol 65:5474–5483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl P., Glasl H., and Wagner H. (1970) Zur analytik pflanzlicher glyhko- und phospholipoide und ihrer fettsäuren i. Eine neue dünnschichtchromatograhische methode zur trennung pflanzlicher lipoide und quantitativen bestimmung ihrer fettsäure-zusammñsetzung. J Chromatogr A 49:488–492 [DOI] [PubMed] [Google Scholar]
- Rashby S.E., Sessions A.L., Summons R.E., and Newman D.K. (2007) Biosynthesis of 2-methylbacteriohopanepolyols by an anoxygenic phototroph. Proc Natl Acad Sci USA 104:15099–15104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C. and Wilkinson S.G. (1988) Microbial Lipids, Academic Press, San Diego, CA [Google Scholar]
- Rezanka T., Nedbalová L., Elster J., Cajthaml T., and Sigler K. (2009) Very-long-chain iso an anteiso branched fatty acids in N-acylphosphatidylethanolamines from a natural cyanobacterial mat of Calothrix sp. Phytochemistry 70:655–663 [DOI] [PubMed] [Google Scholar]
- Robinson N. and Eglinton G. (1990) Lipid chemistry of Icelandic hot spring microbial mats. Org Geochem 15:291–298 [Google Scholar]
- Rohmer M., Bouvier-Navre P., and Ourisson G. (1984) Distribution of hopanoid triterpenes in prokaryotes. J Gen Microbiol 130:1137–1150 [Google Scholar]
- Ruff S.W., Farmer J.D., Calvin W.M., Herkenhoff K.E., Johnson J.R., Morris R.V., Rice M.S., Arvidson R.E., Bell J.F., III, Christensen P.R., and Squyres S.W. (2011) Characteristics, distribution, origin, and significance of opaline silica observed by the spirit rover in Gusev Crater, Mars. J Geophys Res 116, doi: 10.1029/2010JE003767 [DOI] [Google Scholar]
- Sakamoto T., Los D.A., Higashi S., Wada H., Nishida I., Ohmori M., and Murata N. (1994) Cloning of ω3 desaturase from cyanobacteria and its use in altering the degree of membrane-lipid unsaturation. Plant Mol Biol 26:249–263 [DOI] [PubMed] [Google Scholar]
- Sarrazin P., Brunner W., Blake D., Gailhanou M., Bish D.L., Vaniman D., Chipera S., Ming D.W., Steele A., Midtkandal I., Amundsen H.E.F., and Peterson R. (2008) Field studies of Mars analog materials using a portable XRD/XRF instrument [abstract 2421]. In 39th Lunar and Planetary Science Conference, Lunar and Planetary Institute, Houston [Google Scholar]
- Schirmer A., Rude M.A., Li X., Popova E., and del Cardayre S.B. (2010) Microbial biosynthesis of alkanes. Science 329:559–562 [DOI] [PubMed] [Google Scholar]
- Schwertmann U., Carlson L., and Fechter H. (1984) Iron oxide formation in artificial groundwaters. Schweizerische Zeitschrift fur Hydrologie 46:185–191 [Google Scholar]
- Shiea J., Brassell S.C., and Ward D.M. (1990) Mid-chain branched mono- and dimethyl alkanes in hot spring cyanobacterial mats: a direct biogenic source for branched alkanes in ancient sediments? Org Geochem 15:223–231 [Google Scholar]
- Shiea J., Brassell S.C., and Ward D.M. (1991) Comparative analysis of extractable lipids in hot spring microbial mats and their component photosynthetic bacteria. Org Geochem 17:309–319 [Google Scholar]
- Siljeström S., Lausmaa J., Sjövall P., Broman C., Thiel V., and Hode T. (2010) Analysis of hopanes and steranes in single oil-bearing fluid inclusions using time-of-flight secondary ion mass spectrometry (TOF-SIMS). Geobiology 8:37–44 [DOI] [PubMed] [Google Scholar]
- Sinninghe Damsté J.S. and de Leeuw J.W. (1990) Analysis, structure, and geochemical significance of organically bound sulfur in the geosphere: state of the art and future research. In Advances in Organic Geochemistry 1989, edited by Durand B. and Behar F.S., Pergamon, Oxford, pp 1077–1101 [Google Scholar]
- Sinninghe Damsté J.S., Rijpstra W.I.C., Kock-van Dalen A.C., de Leeuw J.W., and Schenck P.A. (1989) Quenching of labile functionalized lipids by inorganic sulfur species: evidence for the formation of sedimentary organic sulfur compounds at the early stage of diagenesis. Geochim Cosmochim Acta 53:1343–1355 [Google Scholar]
- Sinninghe Damsté J.S., Rijpstra W.I.C., Schouten S., Fuerst J.A., Jetten M.S.M., and Strous M. (2004) The occurence of hopanoids in planctomycetes: implications for the sedimentary biomarker record. Org Geochem 35:561–566 [Google Scholar]
- Stasiuk L.D. (1994) Fluorescence properties of Palaeozoic oil-prone alginite in relation to hydrocarbon generation, Williston Basin, Saskatchewan, Canada. Marine and Petroleum Geology 11:219–231 [Google Scholar]
- Summons R.E., Jahnke L.L., and Simoneit B.R.T. (1996) Lipid biomarkers for bacterial ecosystems: studies of cultured organisms, hydrothermal environments, and ancient sediments. In Evolution of Hydrothermal Ecosystems on Earth (and Mars?), Ciba Foundation Symposium 202, edited by Bock G.R. and Goode J.A., John Wiley and Sons, Chichester, UK, pp 174–194 [DOI] [PubMed] [Google Scholar]
- Summons R.E., Jahnke L.L., Hope J.M., and Logan G.A. (1999) 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature 400:554–557 [DOI] [PubMed] [Google Scholar]
- Talbot H.M., Farrimond P., Schaeffer P., and Pancost R.D. (2005) Bacteriohopanepolyols in hydrothermal vent biogenic silicates. Org Geochem 36:663–672 [Google Scholar]
- Talbot H.M., Summons R.E., Jahnke L.L., Cockell C.S., Rohmer M., and Farrimond P. (2008) Cyanobacterial bacteriohopanepolyol signatures from cultures and natural environmental settings. Org Geochem 39:232–263 [Google Scholar]
- Tegelaar E.W., de Leeuw J.W., Derenne S., and Largeau C. (1989) A reappraisal of kerogen formation. Geochim Cosmochim Acta 53:3103–3106 [Google Scholar]
- Trouwborst R.E., Johnston A., Koch G., Luther G.W., and Pierson B.K. (2007) Biogeochemistry of Fe(II) oxidation in a photosynthetic microbial mat: implications for Precambrian Fe(II) oxidation. Geochim Cosmochim Acta 71:4629–4643 [Google Scholar]
- Urrutia M.M. and Beveridge T.J. (1993) Mechanism of silicate binding to the bacterial cell wall in Bacillus subtilis. J Bacteriol 175:1936–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia M.M. and Beveridge T.J. (1994) Formation of fine-grained metal and silicate precipitation on a bacterial surface (Bacillus subtilis). Chem Geol 116:261–280 [Google Scholar]
- van der Meer M.T., Schouten S., Ward D.M., Geenevasen J.A., and Sinninghe Damsté J.S. (1999) All-cis hentriaconta-9,15,22-triene in microbial mats formed by the phototrophic prokaryote Chloroflexus. Org Geochem 30:1585–1587 [DOI] [PubMed] [Google Scholar]
- van der Meer M.T., Schouten S., Hanada S., Hopmans E.C., Sinninghe Damsté J.S., and Ward D.M. (2002) Alkane-1,2-diol-based glycosides and fatty glycosides and wax esters in Roseiflexus castenholzii and hot spring microbial mats. Arch Microbiol 178:229–237 [DOI] [PubMed] [Google Scholar]
- van der Meer M.T., Schouten S., Sinninghe Damsté J.S., de Leeuw J.W., and Ward D.M. (2003) Compound-specific isotopic fractionation patterns suggest different carbon metabolisms among Chloroflexus-like bacteria in hot-spring microbial mats. Appl Environ Microbiol 69:6000–6006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer M.T., Schouten S., Bateson M.M., Nübel U., Wieland A., Kuhl M., de Leeuw J.W., Sinninghe Damsté J.S., and Ward D.M. (2005) Diel variations in carbon metabolism by green nonsulfur-like bacteria in alkaline siliceous hot spring microbial mats from Yellowstone National Park. Appl Environ Microbiol 71:3978–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer M.T., Klatt C.G., Wood J., Bryant D.A., Bateson M.M., Lammerts L., Schouten S., Damste J.S., Madigan M.T., and Ward D.M. (2010) Cultivation and genomic, nutritional, and lipid biomarker characterization of Roseiflexus strains closely related to predominant in situ populations inhabiting Yellowstone hot spring microbial mats. J Bacteriol 192:3033–3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura G.T., Kenig F., Reddy C.M., Frysinger G.S., Nelson R.K., Van Mooy B., and Gaines R.B. (2008) Analysis of unresolved complex mixtures of hydrocarbons extracted from Late Archean sediments by comprehensive two-dimensional gas chromatography (GC×GC). Org Geochem 39:846–867 [Google Scholar]
- Vestal R.D. and White D.C. (1989) Lipid analysis in microbial ecology. Quantitative approaches to the study of microbial communities. Bioscience 39:535–541 [PubMed] [Google Scholar]
- Wade M.L., Agresti D.G., Wdowiak T.J., Armendarez L.P., and Farmer J.D. (1999) A Mössbauer investigation of iron-rich terrestrial hydrothermal vent systems: lessons for Mars exploration. J Geophys Res 104:8489–8507 [DOI] [PubMed] [Google Scholar]
- Walsh M.M. and Lowe D.R. (1985) Filamentous microfossils from the 3,500-Myr old Onverwacht Group, Barberton Mountain Land, South Africa. Nature 314:530–532 [DOI] [PubMed] [Google Scholar]
- Walter M.R. (1972) A hot spring analog for the depositional environment of Precambrian iron formations of the Lake Superior Region. Econ Geol 67:965–980 [Google Scholar]
- Walter M.R. and Des Marais D.J. (1993) Preservation of biological information in thermal spring deposits: developing a strategy for the search for a fossil record on Mars. Icarus 10:129–143 [DOI] [PubMed] [Google Scholar]
- Walter M.R., Des Marais D.J., Farmer J.D., and Hinman N.W. (1996) Lithofacies and biofacies of mid-Paleozoic thermal spring deposits in the Drummond Basin, Queensland, Australia. Palaios 11:497–518 [PubMed] [Google Scholar]
- Ward D.M., Shiea J., Zeng Y.B., Dobson G., Brassell S., and Eglinton G. (1989) Lipid biochemical markers and the composition of microbial mats. In Microbial Mats: Physiological Ecology of Benthic Microbial Communities, edited by Cohen Y. and Rosenburgs E., American Society for Microbiology, Washington, DC, pp 439–454 [Google Scholar]
- Ward D.M., Panke S., Kloeppel K.D., Christ R., and Fredrickson H. (1994) Complex polar lipids of a hot spring cyanobacterial mat and its cultivated inhabitants. Appl Environ Microbiol 60:3358–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D.M., Ferris M.J., Nold S.C., and Bateson M.M. (1998) A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiol Mol Biol Rev 62:1353–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L.A. and Ferris F.G. (1998) Continuum between sorption and precipitation of Fe(III) on bacterial cell surfaces. Environ Sci Technol 32:2331–2337 [Google Scholar]
- White D.C., Davis W.M., Nickels J.S., King J.D., and Bobbie R.J. (1979) Determination of the sedimentary microbial biomass by extractable lipid phosphate. Oecologia 40:51–62 [DOI] [PubMed] [Google Scholar]
- White D.C., Meadows P., Eglinton G., and Coleman M.L. (1993) In situ measurement of microbial biomass, community structure, and nutritional status. Philos Trans Phys Sci Eng 344:59–67 [Google Scholar]
- White D.C., Pinkart H.C., and Ringelberg D.B. (1997) Biomass measurement: biochemical approaches. In Manual of Environmental Microbiology, edited by Hursts C.J., ASM Press, Washington, DC, pp 91–101 [Google Scholar]
- White D.E., Hutchinson R.A., and Keith T.E.C. (1988) The Geology and Remarkable Thermal Activity of Norris Geyser Basin, Yellowstone National Park, Wyoming, U.S. Geological Survey Professional Paper 1456, U.S. Geological Survey, Denver, CO [Google Scholar]
- Yamamoto K., Shibahara A., Nakayama T., and Kajimoto G. (1991) Determination of double-bond positions in methylene-interrupted dienoic fatty acids by GC-MS as their dimethyl disulfide adducts. Chem Phys Lipids 60:39–50 [Google Scholar]
- Zeikus J.G., Ben-Bassat A., and Hegge P.W. (1980) Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol 143:432–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y.B., Ward D.M., Brassell S.C., and Eglinton G. (1992) Lipid compositions of Yellowstone (Wyoming, USA) cyanobacterial and Chloroflexus mats. Chem Geol 95:327–345 [Google Scholar]
- Zhao J., Huggins F.E., Feng Z., and Huffman G.P. (1994) Ferrihydrite: surface structure and its effects on phase transformations. Clays Clay Miner 42:737–746 [Google Scholar]