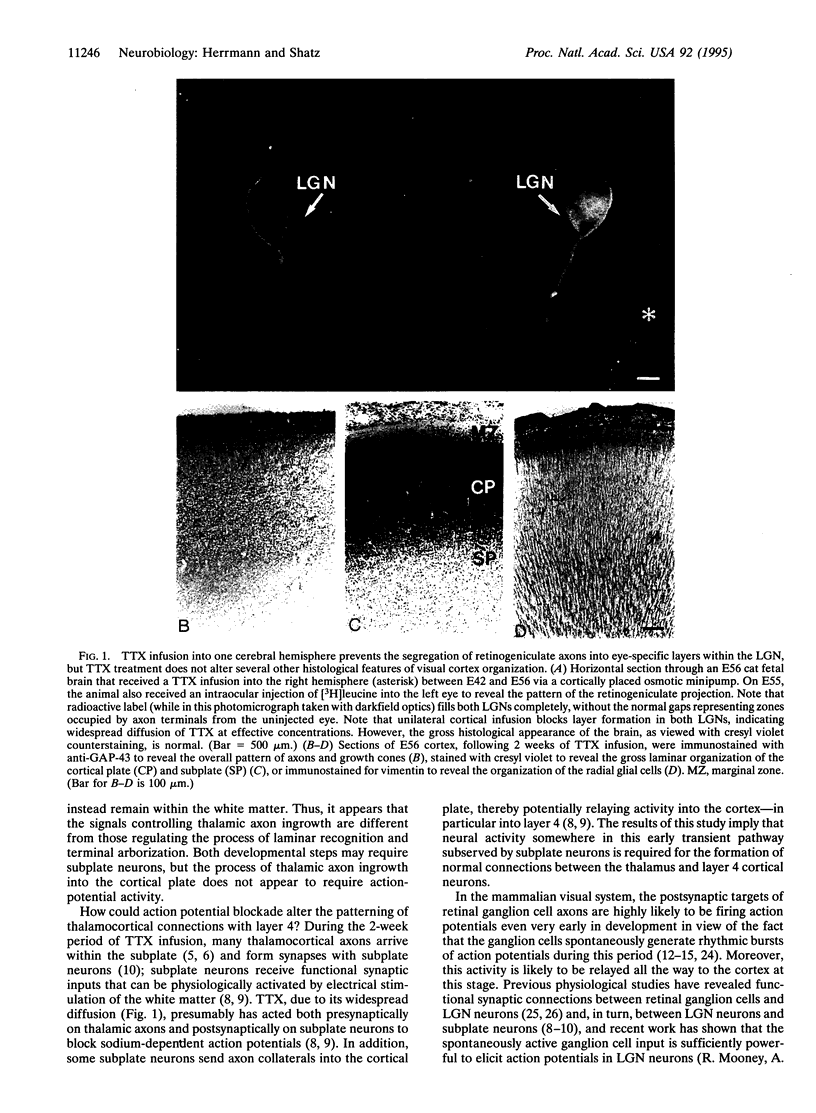
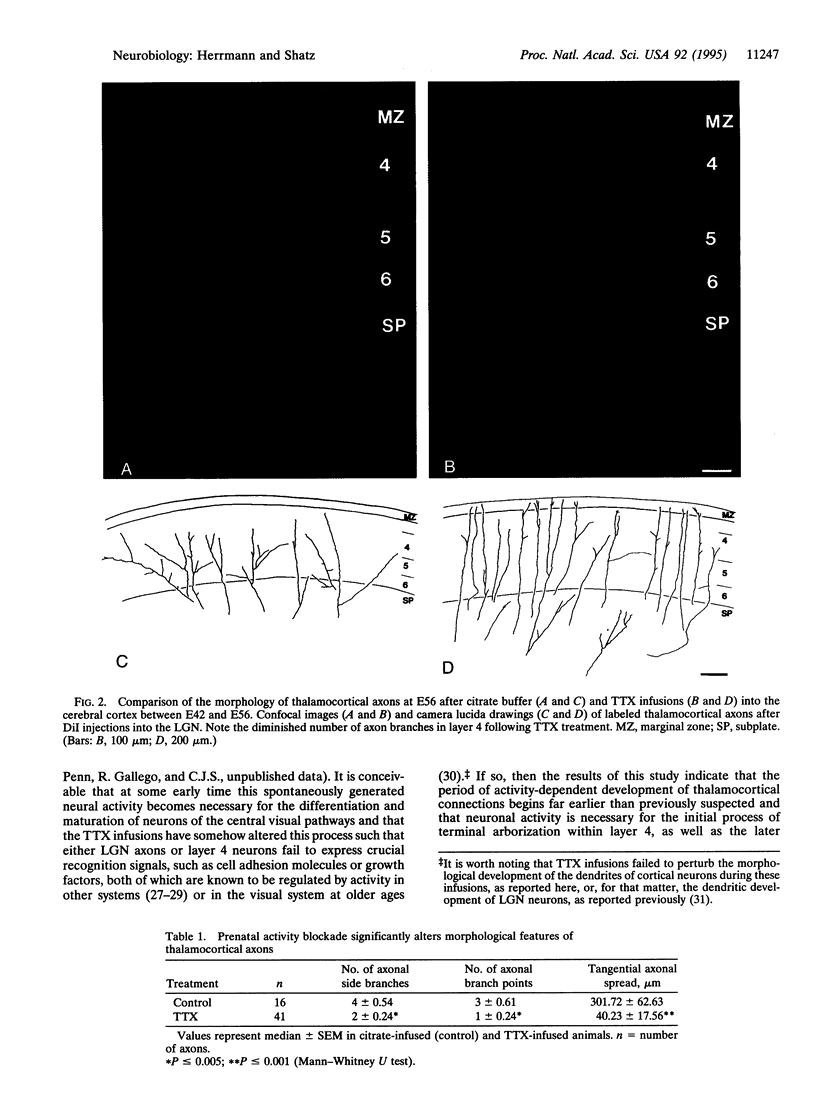
Abstract
In the formation of connections during the development of the nervous system, it is generally accepted that there is an early phase not requiring neural activity and a later activity-dependent phase. The initial processes of axonal pathfinding and target selection are not thought to require neural activity, whereas the later fine-tuning of connections into their final adult patterns does. We report an apparent exception to this rule in which action potential activity seems to be required very early in development for thalamic axons to form appropriate patterns of terminal arborizations with their ultimate target neurons in layer 4 of the cerebral cortex. Blockade of sodium action potentials during the 2-week fetal period when visual thalamic axons initially grow into the primary visual cortex in cats prevents the normally occurring branching of lateral geniculate nucleus axons within layer 4. This observation implies a role for action-potential activity in cerebral cortical development far earlier than previously suspected, weeks before eye-opening and the onset of the well-known process of activity-dependent reorganization of axonal terminal arbors that leads to the formation of ocular dominance columns.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allendoerfer K. L., Shatz C. J. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Chen M., Keller F., Kandel E. R. Serotonin-mediated endocytosis of apCAM: an early step of learning-related synaptic growth in Aplysia. Science. 1992 May 1;256(5057):645–649. doi: 10.1126/science.1585177. [DOI] [PubMed] [Google Scholar]
- Castrén E., Zafra F., Thoenen H., Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J. J., Shatz C. J. The earliest-generated neurons of the cat cerebral cortex: characterization by MAP2 and neurotransmitter immunohistochemistry during fetal life. J Neurosci. 1989 May;9(5):1648–1667. doi: 10.1523/JNEUROSCI.09-05-01648.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva M. B., Ghosh A., Shatz C. J. Independent control of dendritic and axonal form in the developing lateral geniculate nucleus. J Neurosci. 1994 Jun;14(6):3588–3602. doi: 10.1523/JNEUROSCI.14-06-03588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R. E., Connors B. W., Waxman S. G. Rat optic nerve: electrophysiological, pharmacological and anatomical studies during development. Brain Res. 1982 Mar;255(3):371–386. doi: 10.1016/0165-3806(82)90005-0. [DOI] [PubMed] [Google Scholar]
- Fredette B., Rutishauser U., Landmesser L. Regulation and activity-dependence of N-cadherin, NCAM isoforms, and polysialic acid on chick myotubes during development. J Cell Biol. 1993 Dec;123(6 Pt 2):1867–1888. doi: 10.1083/jcb.123.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E., McConnell S. K., Shatz C. J. Functional synaptic circuits in the subplate during fetal and early postnatal development of cat visual cortex. J Neurosci. 1990 Aug;10(8):2601–2613. doi: 10.1523/JNEUROSCI.10-08-02601.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E., Shatz C. J. Changing patterns of synaptic input to subplate and cortical plate during development of visual cortex. J Neurophysiol. 1991 Dec;66(6):2059–2071. doi: 10.1152/jn.1991.66.6.2059. [DOI] [PubMed] [Google Scholar]
- Galli L., Maffei L. Spontaneous impulse activity of rat retinal ganglion cells in prenatal life. Science. 1988 Oct 7;242(4875):90–91. doi: 10.1126/science.3175637. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Antonini A., McConnell S. K., Shatz C. J. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990 Sep 13;347(6289):179–181. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Shatz C. J. A role for subplate neurons in the patterning of connections from thalamus to neocortex. Development. 1993 Mar;117(3):1031–1047. doi: 10.1242/dev.117.3.1031. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Shatz C. J. Pathfinding and target selection by developing geniculocortical axons. J Neurosci. 1992 Jan;12(1):39–55. doi: 10.1523/JNEUROSCI.12-01-00039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K., Antonini A., Shatz C. J. Ultrastructural evidence for synaptic interactions between thalamocortical axons and subplate neurons. Eur J Neurosci. 1994 Nov 1;6(11):1729–1742. doi: 10.1111/j.1460-9568.1994.tb00565.x. [DOI] [PubMed] [Google Scholar]
- LeVay S., Wiesel T. N., Hubel D. H. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980 May 1;191(1):1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Meister M., Wong R. O., Baylor D. A., Shatz C. J. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991 May 17;252(5008):939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Miller K. D., Keller J. B., Stryker M. P. Ocular dominance column development: analysis and simulation. Science. 1989 Aug 11;245(4918):605–615. doi: 10.1126/science.2762813. [DOI] [PubMed] [Google Scholar]
- Mooney R., Madison D. V., Shatz C. J. Enhancement of transmission at the developing retinogeniculate synapse. Neuron. 1993 May;10(5):815–825. doi: 10.1016/0896-6273(93)90198-z. [DOI] [PubMed] [Google Scholar]
- Reiter H. O., Waitzman D. M., Stryker M. P. Cortical activity blockade prevents ocular dominance plasticity in the kitten visual cortex. Exp Brain Res. 1986;65(1):182–188. doi: 10.1007/BF00243841. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Landmesser L. Polysialic acid on the surface of axons regulates patterns of normal and activity-dependent innervation. Trends Neurosci. 1991 Dec;14(12):528–532. doi: 10.1016/0166-2236(91)90006-g. [DOI] [PubMed] [Google Scholar]
- Shatz C. J. Impulse activity and the patterning of connections during CNS development. Neuron. 1990 Dec;5(6):745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Kirkwood P. A. Prenatal development of functional connections in the cat's retinogeniculate pathway. J Neurosci. 1984 May;4(5):1378–1397. doi: 10.1523/JNEUROSCI.04-05-01378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz C. J., Luskin M. B. The relationship between the geniculocortical afferents and their cortical target cells during development of the cat's primary visual cortex. J Neurosci. 1986 Dec;6(12):3655–3668. doi: 10.1523/JNEUROSCI.06-12-03655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz C. J., Stryker M. P. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science. 1988 Oct 7;242(4875):87–89. doi: 10.1126/science.3175636. [DOI] [PubMed] [Google Scholar]
- Shatz C. J. The prenatal development of the cat's retinogeniculate pathway. J Neurosci. 1983 Mar;3(3):482–499. doi: 10.1523/JNEUROSCI.03-03-00482.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan D. W., Shatz C. J., Stryker M. P. Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature. 1988 Dec 1;336(6198):468–471. doi: 10.1038/336468a0. [DOI] [PubMed] [Google Scholar]
- Stryker M. P., Harris W. A. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986 Aug;6(8):2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. O., Chernjavsky A., Smith S. J., Shatz C. J. Early functional neural networks in the developing retina. Nature. 1995 Apr 20;374(6524):716–718. doi: 10.1038/374716a0. [DOI] [PubMed] [Google Scholar]
- Wong R. O., Meister M., Shatz C. J. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993 Nov;11(5):923–938. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]