Abstract
Cardiac and renal disease frequently coexist but have long been difficult to diagnose in a timely manner and treat effectively. Noninvasive and cost-effective biomarkers are needed to help identify cardiac patients who are at risk of acute kidney injury early in the course of disease. Biomarkers can provide insights into underlying mechanisms and lead to a better understanding of complex disease states such as the cardiorenal syndrome, which can lead to better therapies and, ultimately, to improved patient outcomes. The natriuretic peptides are established biomarkers in heart failure and have set the standard for how a well-validated biomarker can be useful for diagnosis/prognosis, monitoring response to therapy and chronic disease management. For patients with acute kidney injury in the setting of cardiac disease, new biomarkers such as neutrophil gelatinase-associated lipocalin, cystatin C, kidney injury molecule-1 and IL-18 are emerging as early signals of renal dysfunction prior to any elevations in serum creatinine. Other promising candidate biomarkers for the early diagnosis of acute kidney injury include osteopontin, N-acetyl-β-D-glucosaminidase, stromal cell-derived factor-1 and exosomes. More research with all of these novel biomarkers is needed; however, the early results are very promising.
Keywords: AKI, cardiorenal syndrome, cystatin C, exosomes, IL-18, KIM-1, NAG, NGAL, osteopontin
Kidney injury in patients with cardiac dysfunction is challenging to treat and has become increasingly recognized as an independent risk factor for morbidity and mortality [1]. The proper function of the heart and kidney are intricately linked through organ cross-talk via neurohumoral feedback mechanisms. Therefore, damage and ensuing dysfunction of one system can often cause concomitant dysfunction of the other [1]. Biomarkers are emerging as important tools to understanding fundamental pathophysiologic mechanisms as well aiding in the early diagnosis of kidney injury. These biomarkers may potentially suggest the optimal timing of treatment initiation and ultimately lead to improved patient management and outcomes.
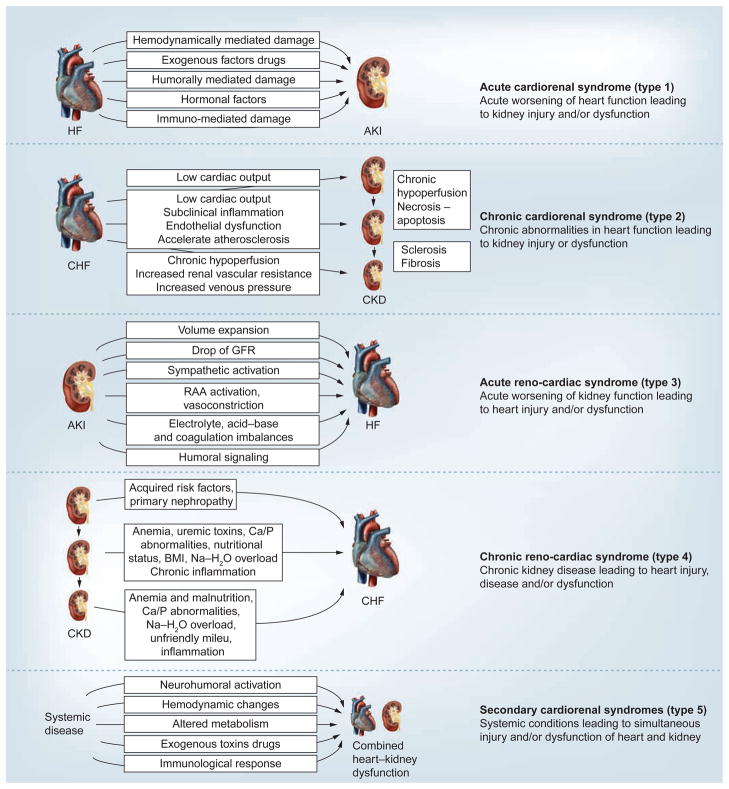
The clinical entity of the cardiorenal syndrome (CRS), which encompasses patients with heart failure (HF), is the most commonly encountered clinical scenario that exemplifies the complex interaction between the heart and the kidney. The five-subtype classification of CRS has recently been proposed to reflect the bidirectional interaction between the heart and kidney, the underlying pathophysiology, the time course of renal injury and to facilitate more focused research in the field (Figure 1). Type 1 CRS is defined as having a primary and acute cardiac substrate, such as acute decompensated (ADHF) that triggers acute kidney injury (AKI) and is very common in the over 1 million HF admissions that occur annually in the USA [1]. In type 1 CRS, the early diagnosis of AKI is difficult but, if made, could lead to treatment modifications that may improve patient outcomes. In fact, impaired renal function has been found to be an independent risk factor for 1-year mortality in patients with acute HF, as well as ST-segment elevation myocardial infarction [1].
Figure 1. Types and pathophysiology of the cardiorenal syndrome.
AKI: Acute kidney injury; Ca/P: Calcium/phosphorous; CHF: Congestive heart failure; CKD: Chronic kidney disease; GFR: Glomerular filtration rate; HF: Heart failure; RAA: Renin–angiotensin–aldosterone.
Adapted from [58] with permission from Oxford University Press.
Type 2 CRS encompasses chronic HF leading to progressive chronic kidney disease (CKD) [1]. Approximately 25% of patients with chronic HF have renal dysfunction. It is important to make the distinction between types 1 and 2 CRS because acute versus chronic HF probably have different mechanisms that cause worsening renal failure. Renal failure in the setting of chronic HF is unexplained by hypoperfusion alone and recent studies show that renal congestion secondary to an elevated right atrial pressure is likely to play a role [1]. The neurohormonal balance between vasoconstrictive and vasodilatory mediators is perturbed and this imbalance may be encouraged through various pharmacologic therapies initiated during the management of these patients [1].
In types 3 and 4 CRS, the primary insult is renal injury with secondary cardiac dysfunction. Type 3 CRS involves acute kidney dysfunction leading to an acute decline in cardiac function while type 4 CRS involves CKD leading to cardiac dysfunction [1]. AKI can stress the heart due to volume overload, hyperkalemia, uremia, acidemia and initiating inflammatory cascades that impact the body as a whole, including the heart. In both types 3 and 4 CRS, clinicians often perceive a need to down titrate medications used in the management of acute or chronic HF such as angiotensin-converting enzyme inhibitors, potentially leading to acute decompensation of HF. Type 5 CRS is distinct from the other types as it reflects a systemic condition (e.g., sepsis) simultaneously causing both cardiac and renal dysfunction.
The majority of the recent biomarker research in the cardiorenal field has been on those in AKI and therefore those will be the main focus of this study. The role of these biomarkers in the multiple aspects of CRS, such as patients with HF and cardiac surgery patients, will be addressed.
Defining AKI
A precise definition of AKI is needed to better understand the role of biomarkers in diagnosis and prognosis of kidney injury and to design clinical trials to evaluate the utility of these biomarkers. AKI is defined using either the Risk, Injury, Failure, Loss and End-stage renal disease (RIFLE) criteria or the Acute Kidney Injury Network (AKIN) criteria. These criteria are summarized in Table 1. The RIFLE criteria classifies patients into three graded levels of severity (risk, injury and failure) based on either the magnitude of elevation in serum creatinine or the decline in urine output. Many studies have shown that the RIFLE criteria are correlated with prognosis [2,3], although they do have some limitations. The main limitation of the RIFILE criteria is the reliance on creatinine measurements, which do not correlate with changes in glomerular filtration rate (GFR) when creatinine levels become significantly elevated in AKI [4].
Table 1.
Comparison of the Risk, Injury, Failure, Loss and End-stage renal disease and Acute Kidney Injury Network criteria for acute kidney injury.
| Classification model | sCr criteria | Urine output criteria |
|---|---|---|
| RIFLE category | ||
| Risk | Increase in sCr ≥1.5× baseline or decrease in GFR ≥25% | <0.5 ml/kg/h for ≥6 h |
| Injury | Increase in sCr ≥2.0× baseline or decrease in GFR ≥50% | <0.5 ml/kg/h for ≥12 h |
| Failure | Increase in sCr ≥3.0× baseline or decrease in GFR ≥75% or an absolute sCr ≥354 μmol/l with an acute rise of at least 44 μmol/l | <0.5 ml/kg/h for ≥24 h or anuria for ≥12 h |
| AKIN criteria | ||
| Stage 1 | Increase in sCr ≥26.2 μmol/l or increase to ≥150–199% (1.5–1.9-fold) from baseline | <0.5 ml/kg/h for ≥6 h |
| Stage 2 | Increase in sCr to 200–299% (>2–2.9-fold) from baseline | <0.5 ml/kg/h for ≥12 h |
| Stage 3 | Increase in sCr to ≥300% (≥three-fold) from baseline or sCr ≥354 μmol/l with an acute rise of at least 44 μmol/l or initiation of RRT | <0.3 ml/kg/h for ≥24 h or anuria for ≥12 h |
AKIN: Acute Kidney Injury Network; GFR: Glomerular filtration rate; RIFLE: Risk, Injury, Failure, Loss and End-stage renal disease; RRT: Renal replacement therapy; sCr: Serum creatinine.
Owing to this limitation, the AKIN proposed a modification of the RIFLE classification, which addressed the diagnostic criteria for AKI as well as a staging system [5]. The diagnostic criteria include a change of an increase in serum creatinine levels ≥0.3 mg/dl from baseline within 48 h and an increase in serum creatinine of ≥50%, or oliguria of <0.5 m/kg/h for longer than 6 h. These criteria are similar to the RIFLE risk criteria with the addition of a change in serum creatinine of ≥0.3 mg/dl based on data showing an 80% increase in mortality risk associated with a change in serum creatinine of as little as 0.3–0.5 mg/dl (Table 1) [6]. Importantly, the AKIN criteria add that the diagnostic criteria should only be applied after optimum volume status is achieved and, if oliguria is used as the only diagnostic criteria, a urinary tract obstruction must first be ruled out. Despite these efforts, there is currently a lack of evidence showing that the AKIN modifications to the RIFLE criteria have improved its ability to predict hospital mortality [7].
Novel biomarkers in the CRS
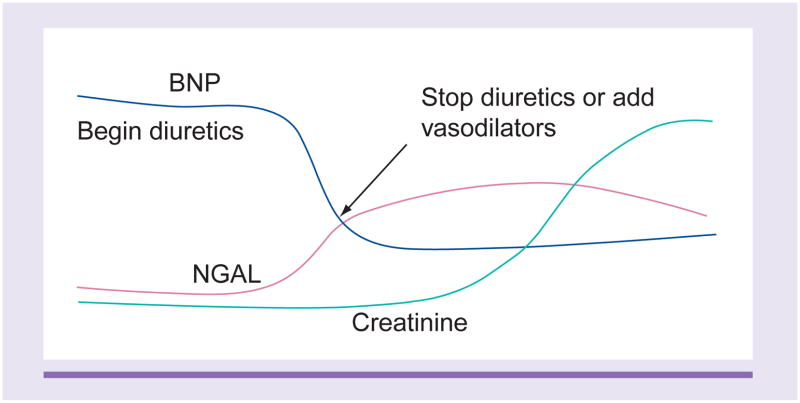
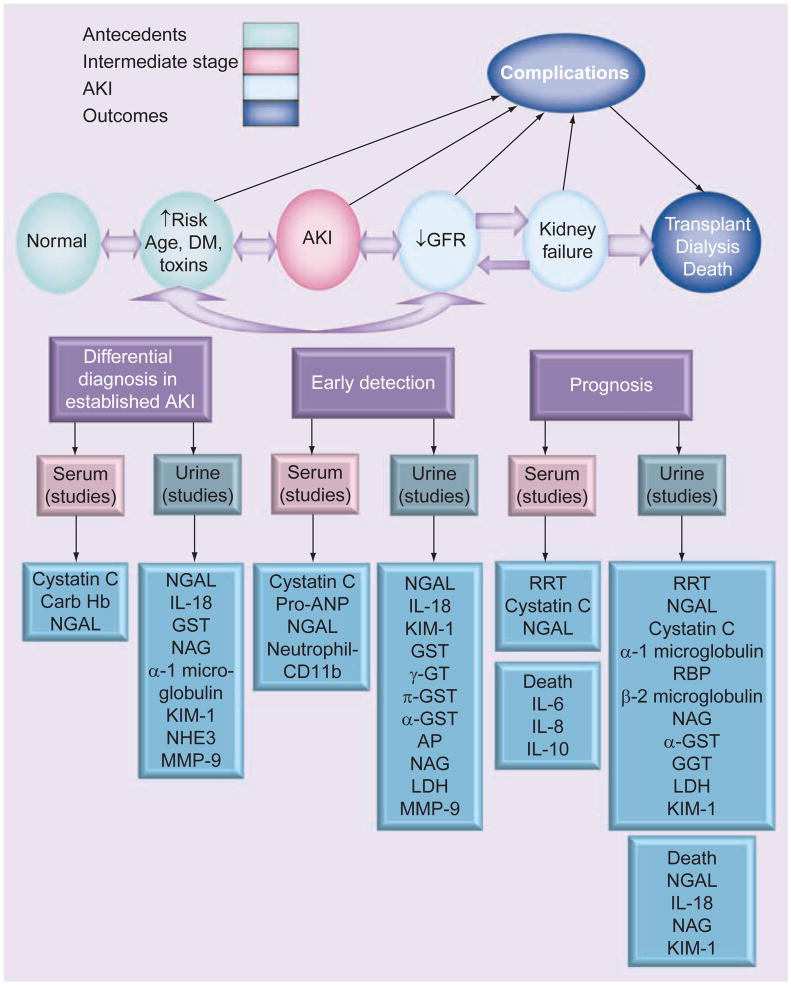
In the complex fields of CRS and AKI, biomarkers can help elucidate the underlying pathophysiology of CRS and lead to a better understanding of this complex disease state [8]. However, the traditional makers of renal injury such as creatinine have a delayed response, only spiking at around 24 h after the event. Serum creatinine, which has traditionally been used in almost all definitions of AKI, is a suboptimal marker immediately following injury when levels are often not truly reflective of GFR. Figure 2 illustrates the delay in rise of serum creatinine compared with biomarkers such as B-type natriuretic peptide (BNP) and neutrophil gelatinase-associated lipocalin (NGAL), which rise earlier and give early clues of impending renal injury. In the setting of AKI, the delay between changes in serum creatinine and changes in GFR inhibits the ability to accurately estimate timing and severity of injury. Thus, there is a pressing need to find biomarkers that will provide an early diagnosis of AKI, and can be used to risk-stratify patients and follow their course of treatment (Figure 3).
Figure 2. Illustrates the time course of biomarker elevation in which neutrophil gelatinase-associated lipocalin and B-type natriuretic peptide levels rise early.
BNP: B-type natriuretic peptide; NGAL: Neutrophil gelatinase-associated lipocalin.
Figure 3. Biomarkers and acute kidney injury.
AKI: Acute kidney injury; AP: Alkaline phosphatase; BNP: B-type natriuretic peptide; DM: Diabetes mellitus; GFR:Glomerular filtration rate; GST: Glutathione-S-transferase; GT: Glutamyl transferase; Hb: Hemoglobin; LDH: Lactate dehydrogenase; KIM-1: Urinary kidney injury molecule-1; MMP: Matrix metalloproteinase; NAG: N-acetyl-β-D-glucosaminidase; NGAL: Neutrophil gelatinase-associated lipocalin; NHE3: Na+/H+ exchanger isoform 3; ProANP: Proatrial natriuretic peptide; RBP: Retinol-binding protein; RRT: Renal replacement therapy.
An ideal biomarker of AKI should identify the primary location of injury (proximal tubule, distal tubule, interstitium or vasculature), address the duration of kidney failure (AKI, CKD or ‘acute-on-chronic’), identify the cause (toxins, sepsis, ischemia or a combination), discern the AKI subtype (prerenal, intrinsic renal or post renal), distinguish AKI from other types of acute renal disease (glomerulonephritis, interstitial nephritis or urinary tract infection), risk-stratify and approximate prognosis (severity and duration of AKI, length of hospital stay, renal replacement therapy [RRT] requirements and mortality), and track the renal injury course and permit the monitoring of intervention response.
Early markers of kidney injury are vital in treatment alteration and in decreasing morbidity and mortality. Biomarker concentration should change in conjunction with the amount of organ damage, even when typical clinical signs are lacking. They should pave the way for early intervention and should be related to treatment response and prognosis. Lastly, the ideal biomarker should be both specific (typical of the organ damage) and sensitive (an early sign of organ injury). Its measurement should have good reproducibility, be relatively cheap and be technically easy. A brief summary of the biomarkers presented here can be found in Table 2.
Table 2.
Review of biomarkers for acute kidney injury.
| Biomarker | Mechanism of release | Key trials |
|---|---|---|
| NGAL | Proximal and distal tubular epithelial cells in response to injury. Also systemically from other organs under stress (i.e., sepsis) | Haase et al. meta-analysis of 2358 patients: NGAL an AUC of 0.815 across all settings to detect AKI [14] |
| Cystatin C | Synthesized and released into plasma by all nucleated cells at a constant rate | Ahlström et al.’s ICU study with 202 patients found an AUC of 0.901 for early detection of AKI [60] |
| IL-18 | Cleaved to mature form in proximal tubular cells after ischemia–reperfusion injury but also general inflammatory states | Parikh et al. showed elevated levels in 52 patients with AKI versus 86 normal individuals [34] |
| KIM-1 | Upregulated in proximal tubular epithelial cells in response to injuries such as ischemia–reperfusion and nephrotoxins | Han et al. shows favorable AUC of 0.90 for the diagnosis of established AKI in 44 patients versus 30 controls [24] |
| BNP | Ventricular myocytes in response to hemodynamic stress | Breathing Not Properly trial of 1586 patients found that the diagnostic accuracy of BNP at 100 pg/ml was 83.4%, with a negative-predictive value of 96% at a cutoff of 50 pg/ml [62] |
| SDF-1 | Constitutively expressed by most organs but upregulated after injury or DNA damage | Togel et al. showed in mice that SDF-1 is a mediator for the migration of CXCR4 (its receptor)-expressing cells to the kidney with possible renoprotective effects as well as renal repair [41] |
| Urinary exosomes | All segments of the nephron as a part of normal signaling; upregulated in response to stress | Zhou et al. found exosomes containing ATF3 in four patients with AKI compared with eight controls [44] |
| Osteopontin | Loop of Henle and distal nephrons in normal kidneys; upregulated in all tubular and glomerular segments following kidney damage | Lorenzen et al. showed it osteopontin be a predictor of mortality with a AUC of 0.82, sensitivity of 100% and specificity of 61% for a cutoff value of 577 ng/ml in 109 critically ill patients [39] |
| NAG | Lysosomal enzyme leaked into renal tubules from damaged proximal tubular cells | Han et al. showed NAG had an AUC of 0.97 in distinguishing established AKI in 44 patients versus 30 controls [25] |
AKI: Acute kidney injury; ATF3: Activating transcription factor 3; AUC: Area under the curve; BNP: B-type natriuretic peptide; ICU: Intensive care unit; KIM-1: Kidney injury molecule-1; NAG: N-acetyl-β-D-glucosaminidase; NGAL: Neutrophil gelatinase-associated lipocalin; SDF-1: Stromal cell-derived factor-1.
Neutrophil gelatinase-associated lipocalin
NGAL is a promising biomarker for the early detection of worsening renal function (WRF). NGAL is detected in human blood and urine at the earliest stages of AKI, 48–72 h before the rise in creatinine. It is a 25-kDa transport protein of low-molecular-weight substances and is expressed by neutrophils and other epithelial cells, including those in the proximal convoluted tubule [9]. Although it is expressed in many human tissues, NGAL was found to be one of the most upregulated transcripts in the kidney early after acute injury, suggesting its role as an early marker of structural tubular damage [10–12]. Although NGAL’s role in detecting AKI early could play a key role in the management of types 1 and 3 CRS, there is also some evidence that it can predict worsening CKD, which subsequently could alter the management of types 2 and 4 CRS [13].
The majority of research into NGAL to date is to do with the detection of AKI. In a study of patients admitted with ADHF, serum NGAL levels measured early during their admission predict the subsequent development of WRF. This was especially true in those with preserved renal function on admission, which is the exact description of type 1 CRS [4]. A rise in serum NGAL may, therefore, lead a clinician to consider renal-sparing therapeutic management to mitigate the risk of subsequent WRF [4]. It is possible that patients with tubular injury at the time of admission (as evidenced by an elevated NGAL level) are more sensitive to the physiologic stress that diuresis may wage on the renal system as a whole [4]. In a recent meta-analysis regarding its ability to predict AKI, NGAL achieved an area under the receiver-operating curve (AUC-ROC) of 0.782 in adults across all settings. However, NGAL is not as powerful a predictor of AKI in critically ill patients, with an AUC-ROC of 0.728 most likely due to various comorbidities and the fact that it can be released by organs other than the kidney [14]. Another meta-analysis focused on NGAL’s ability to detect subclinical AKI in critically ill patients, with normal creatinine versus those with elevated creatinine. They found that those with elevated NGAL and normal creatinine were significantly more likely to require RRT initiation (odds ratio: 16.4; 95% CI: 3.6–76.9; p = 0.001) or die in hospital (odds ratio: 2.8; 95% CI: 1.9–4.1; p = 0.001) than those with normal NGAL and normal creatinine [15]. Interestingly, in their analysis, 43% of patients diagnosed with AKI by NGAL levels would not have been diagnosed as such using creatinine parameters alone. The authors conclude that this subclinical AKI via NGAL leads to poorer outcomes for those affected despite the lack of detection by conventional means [15].
NGAL also may have a role in the chronic aspects of the CRS. A study by Bolignano et al. demonstrated that both serum NGAL and urinary NGAL independently predicted CKD progression (as defined by a doubling of serum creatinine or onset of end-stage renal disease) with AUC-ROCs of 0.70 and 0.78, respectively [13]. This suggests that NGAL could be used to predict which patients are headed for poorer outcomes and allow the clinician to attempt to avoid further renal damage and the subsequent cardiac problems that will arise because of it. As mentioned above, NGAL’s role is not confined to the kidney, as it is expressed both systemically and within the failing myocardium. In fact it has been shown that patients with chronic HF have significantly elevated levels of NGAL compared with control subjects, with the highest levels in New York Heart Association Classes III and IV [16]. The NGAL level also appears to be correlated with the N-terminal prohormone of BNP (NT-proBNP) level, which is a cleavage product of proBNP [16]. These results indicate that NGAL can be a powerfully sensitive biomarker for the CRS, but clinically we may need the addition of other biomarkers to improve diagnostic and prognostic specificity.
Cystatin C
Cystatin C (CysC) is a cationic nonglycosylated low-molecular-weight cysteine protease (13 kd) that is produced by all nucleated cells [17]. As opposed to NGAL, which is a structural marker of cell damage, CysC is a functional marker of GFR along the lines of creatinine. It is freely filtered at the glomerulus and not secreted from the tubules, although it can be reabsorbed and catabolized [18]. However, unlike creatinine, it does not appear to be influenced by gender, race or muscle mass, which makes it a more useful marker or glomerular function. Therefore, it has been suggested that CysC can be utilized in clinical situations where it is difficult to trust creatinine measurements, such as the elderly, cachectic patients or those with numerous comorbidities [19].
In 85 intensive care unit (ICU) patients with normal creatinine at baseline, CysC was able to detect AKI 1–2 days earlier than creatinine with sensitivity and specificity of 82 and 95%, respectively [18]. The same study had an AUC-ROC for predicting the severity of AKI of 0.76, suggesting that CysC may have some value in gauging the degree of renal injury, as well as detecting renal damage earlier than current markers such as creatinine.
CysC has also been shown to have good prognostic value. In 480 patients with acute HF, CysC above the median of 1.30 mg/l was associated with their highest adjusted hazards ratio (HR) of 3.2 (95% CI: 2.0–5.3; p < 0.0001) for all-cause mortality at 12 months [20]. When tertiles were combined with NT-proBNP, the prognostication grew even stronger [20]. In another study, 292 patients admitted for ADHF, CysC was measured on admission and at 48 h. An increase in CysC by >0.3 mg/l was associated with longer duration of hospitalization and increased patient mortality and was also an independent predictor of 90-day mortality [21].
Unfortunately, CysC has also had its share of detractors. One analysis of 1621 middle-aged patients from the general population (excluding coronary or kidney disease) found that it was not a better estimator of GFR than plasma creatinine in this cohort [22]. Obviously, more research needs to be carried out with the marker before a definitive role can be found for it. Still, even though it can be argued than an ideal marker for the CRS or renal injury in general should be a structural one indicative of actual tissue damage (such as troponins for myocardial injury), the results with CysC have suggested that there is still space for a functional marker on any future kidney biomarker panel.
Kidney injury molecule-1
Kidney injury molecule-1 (KIM-1) is a type 1 transmembrane protein that is highly expressed in dedifferentiated proximal tubule epithelial cells after ischemic or toxic injury and is not detectable in normal tissue [23]. Urinary KIM-1 has also been shown to have a role in differentiating between true acute tubular necrosis and other types of renal injury, such as prerenal azotemia and CKD [24].
In a cross-sectional study on 44 patients with various acute and chronic kidney diseases, KIM-1 levels were significantly higher in patients with AKI than in controls or patients with urinary tract infection. KIM-1 had an AUC of 0.90 to detect AKI in these patients [25]. In a study of cardiopulmonary bypass patients, KIM-1 had similar success, outperforming other urinary biomarkers en route to an AUC of 0.78 to detect AKI [26].
Damman et al. recently performed a study on detecting renal tubular injury upon the cessation and reinstitution of diuretic therapy in patients with chronic HF. In this scenario, KIM-1 seemed to outperform other markers of tubular injuries such as NGAL and N-acetyl-β-D-glucosaminidase (NAG). KIM-1 levels increased significantly as early as 8 h after diuretics were stopped, remained elevated throughout the 72-h period and returned to normal levels as early as 4 h after furosemide was resumed [27]. The markers in this study, with KIM-1 leading the way, illustrated how changes in volume status can lead to subclinical tubular injury that may be undetected by traditional biomarkers. Although the early results for KIM-1 as a renal biomarker are promising, larger trials are necessary before a strong endorsement can be made for broader clinical use.
IL-18
IL-18 is an 18-kDa proinflammatory cytokine, which is upregulated during endogenous inflammatory processes and plays an important role in the pathophysiology of sepsis [28]. Urinary IL-18 originates from proximal tubular epithelial cells and has been shown to mediate ischemic acute tubular necrosis in mice, making it a possible candidate as an early marker of AKI through urine measurements [29,30].
Following AKI, IL-18 has been shown to increase within 2 h and remaining elevated for up to 24 h [31]. When used to detect AKI, IL-18 had an 81% sensitivity at 2 h after arrival in the ICU [31]. Furthermore, urinary IL-18 is shown to increase in tandem with NGAL and to continue to increase after NGAL concentrations begin to fall [32].
A review of AKI biomarkers found varied AUCs for the ability of IL-18 to diagnose AKI early on, with values ranging from 0.54 to 0.90 [33]. The authors concluded that, generally, IL-18 has high specificity but low sensitivity for AKI diagnosis and correlated weakly with AKI duration or severity. Furthermore, urinary IL-18 levels were increased significantly in those with AKI at both 24 and 48 h before diagnosis via creatinine; unfortunately the ROC-AUC values were only 0.73 at 24 h and 0.65 for the 48-h time point [34]. Similar to other biomarkers mentioned here, IL-18 has shown inconsistent results overall but remains a viable candidate for a future panel of biomarkers in kidney diseases and could have a key role in the future of CRS treatment.
Natriuretic peptides
The natriuretic peptides (NPs) are established biomarkers in HF and because elevated NP levels can be caused by renal dysfunction as well as congestive heart failure, they probably also reflect renal injury [35]. Thus, NPs are emerging as pleiotropic biomarkers that are useful in the settings of both cardiac and renal dysfunction and have the potential to serve as a valuable diagnostic tool in the diagnosis of types 1 and 2 CRS [1]. In a study of 34 consecutive ICU patients, elevated BNP was able to predict AKI on admission or AKI development during ICU stay with an AUC-ROC of 0.83 [36]. The role of NPs in CRS may also extend to CKD. It has been demonstrated that patients with CKD have higher levels of NPs than normal individuals and, although much of this is attributed to reduced renal clearance, there is most likely some input from the increased myocardial wall stress that accompanies ventricular hypertrophy, hypertension, ischemia and cardiac remodeling [37].
When combined with some of the renal biomarkers mentioned above, NPs can give the clinician a comprehensive look at a patient’s cardiorenal status. This knowledge can, in turn, allow one to diagnose the type of CRS present and thus titrate therapy effectively.
Emerging biomarkers
Osteopontin
Osteopontin is a secreted glycoprotein, which is most highly synthesized in bone and epithelial tissues [38]. It has a variety of roles, which include regulation of osteoclast function, renal calculus formation, accumulation of macrophages, cellular protection from apoptosis and several immune functions [38]. More recently, its role as a biomarker of AKI has been studied. In the kidney, osteopontin is mainly found in the loop of Henle and distal nephrons in normal kidneys and can be upregulated in all tubular and glomerular segments following kidney damage, and may have a role in renal repair [38]. One study on critically ill patients with AKI requiring RRT found were that baseline levels in this cohort were significantly elevated when compared with critically ill controls without AKI [39]. Furthermore, osteopontin levels at the start of RRT were found to be a strong predictor of mortality with an AUC of 0.82, sensitivity of 100% and specificity of 61% for a cutoff value of 577 ng/ml [39]. With future research, it appears that osteopontin could be a powerful prognostic biomarker in patients with AKI due to its activity in the kidney after injury and renoprotective effects.
N-acetyl-β-D-glucosaminidase
NAG is an interesting lysosomal enzyme because its large size (130 kDa) does not allow glomerular filtration and thus urinary NAG is leaked into renal tubules from damaged proximal tubular cells [25]. Despite this promising feature, clinical trials involving NAG have provided mixed results. A cross-sectional study by Han et al. found that NAG had an AUC of 0.97 in distinguishing established AKI from normal patients as well as those with urinary tract infection [25]. Disappointingly, however, NAG failed to have convincing AUC values for cardiac surgery patients with a value of 0.69 at 12 h after bypass and 0.70 at 24 h [25]. In another cardiac surgery study, NAG levels for AKI post-surgery were only significant at the 48-h time point and only achieved an AUC of 0.62 at the 2-h time point [26].
However, in patients with chronic HF, NAG has had a stronger showing as a marker of tubular injury. In a retrospective analysis of the GISSI-HF trial of 2130 patients with chronic HF, Damman et al. found that NAG, KIM-1 and NGAL were all markedly elevated above normal levels and each was independently associated with their combined end point of all-cause mortality and HF readmissions [40]. NAG had the strongest adjusted HR in this group (HR: 1.22; 95% CI: 1.10–1.36; p < 0.001) as compared with KIM-1 (HR: 1.13; 95% CI: 1.02–1.24; p = 0.018) and NGAL (HR: 1.10; 95% CI: 1.00–1.20; p = 0.042) [40]. The strongest HR they found was with the combination of NAG, increased urinary albumin-to-creatinine ratio and impaired enhanced GFR, which yielded a HR of 3.00 (95% CI: 2.29–3.95; p < 0.001) [40]. Although NAG had a promising beginning as a biomarker, currently its value in kidney injury needs further assessment and confirmation.
Stromal cell-derived factor-1
Another renal biomarker of interest in the setting of CRS is stromal cell-derived factor-1 (SDF-1) and its receptor CXCR4. Together they mediate leukocyte homeostasis and have been shown to play a role in the recruitment of leukocytes and bone marrow-derived stem cells to the kidneys after ischemic acute renal failure (as occurs in type 1 CRS), which suggests that SDF-1 may be involved in kidney repair [41]. Both SDF-1 and CXCR4 are upregulated in post-ischemic kidneys and, because SDF-1 is a homeostatic and not an inflammatory chemokine, it may be an important signal of extrinsic renal repair after injury [41]. There is promise that if this signaling pathway can be somehow enhanced in the future, it could have a role in the therapy of mild-to-moderate renal injury.
Exosomes
There is research underway in assessing the utility of urinary exosomes as a diagnostic indicator of WRF in patients with ADHF and thus type 1 CRS. Exosomes are small membrane-bound vesicles that are released into the extracellular environment after fusion with the plasma membrane [42]. They are believed to be released in greater abundance after cellular injury and, because they contain both membrane and intracellular proteins, they can allow a unique and detailed look at cellular pathology [43]. Included in the proteins trapped in exosomes are transcription factors such as activating transcription factor-3, which in one study was detected in the urine of patients with AKI but not in normal individuals or those with CKD [44]. With further research on the horizon, urinary exosomes offer exciting possibilities to not only AKI and CRS diagnosis but also many other forms of renal and systemic pathology.
Application of biomarkers to clinical settings
Acute decompensated HF
ADHF is one of the major precipitants of type 1 CRS. Until the widespread clinical utility of BNP was established, the diagnosis and management of ADHF was extremely difficult for clinicians. NPs have aided with the diagnosis and management of ADHF, but growing knowledge regarding the systemic consequences of HF have opened the door for new biomarkers as adjuncts to NPs.
The prevalence of AKI in patients with ADHF is estimated to be as high as 60% [45]. However the RIFLE and AKIN criteria have not been extensively studied in patient with acute/chronic HF, and thus, the true incidence of AKI in patients with HF is unclear and may be higher.
In patients admitted with acute HF, the NGAL level is useful in predicting renal injury during hospitalization and in prognosis after hospitalization. The GALLANT trial, which examined NGAL levels in patients admitted with HF, showed that patients with an elevated discharge NGAL (134 ng/ml [95% CI: 104–181] vs 84 ng/ml [95% CI: 59–128]; p < 0.001) had higher rates of readmission and mortality at 30 days [46]. However, the role of NGAL in monitoring patients with chronic HF is unclear. In a study of 30 patients with chronic systolic HF, subclinical volume changes after diuretic withdrawal in resulted in elevations in BNP, KIM-1 and NAG, but not NGAL [27]. In another study of patients with stable systolic HF, elevated NGAL did not add any predictive value above GFR for predicting increased risk of death or HF hospitalization. In this cohort, the more promising biomarkers seem to be KIM-1 and NAG, which were associated with an increased risk of death or HF hospitalizations, independent of GFR [47].
Classic cardiac markers such as troponins, as well as novel prognostic markers such as mid-regional proadrenomedullin and ST2, have demonstrated the potential of a multiple-biomarker approach to ADHF [8]. However, much of the morbidity associated with ADHF comes from the association of AKI not just with the pathophysiological mechanisms of type 1 CRS mentioned above but also with aggressive pharmacological treatment. Compounding the problem is the fact that functional renal markers, such as creatinine, require significant time and injury before rising, thus limiting the clinician’s chances of preventing AKI. Markers of actual renal injury (not strictly renal function), such as NGAL, have shown potential for more decreased morbidity as they can allow faster detection of AKI and a subsequent change in management before it is too late. A study of 91 patients admitted with ADHF showed that those who developed WRF had significantly higher median admission serum NGAL levels. In fact, those with levels ≥140 ng/ml were 7.4-times more likely to develop WRF with a sensitivity and specificity of 86 and 54%, respectively [4]. With the combination of novel biomarkers such as NGAL, mid-regional pro-adrenomedullin and ST2, a multimarker approach to HF that gives insights into both the cardiac and renal dysfunction, will probably lead to more precise initiation and withdrawal of therapies and better outcomes.
Cardiac surgery
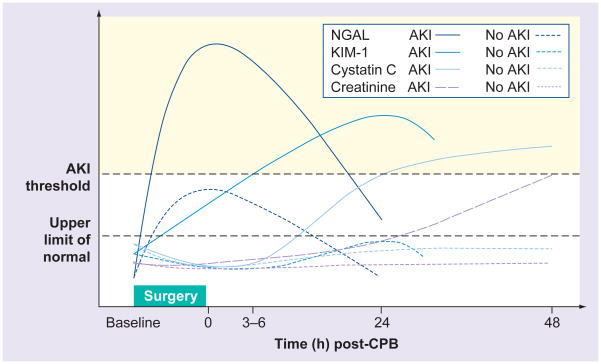
It is logical that if cardiac disease can cause acute or chronic kidney injury, the physiologic stress of cardiac surgery could also cause kidney injury through similar and additional mechanisms. In fact, cardiac surgery with cardiopulmonary bypass (CPB) is the second leading cause of AKI in critically ill patients (with sepsis being the leading cause) and as such can be classified as precipitating type 1 CRS [2]. However, unlike other etiologies of type 1 CRS already discussed, there is no clear understanding of its pathogenesis, nor are there established guidelines of proven prophylaxis or treatment for AKI in the setting of bypass surgery [48]. AKI resulting from cardiac surgery has a complex pathogenesis and includes a variety of injury routes: toxins (exogenous and endogenous), ischemia and reperfusion, oxidative stress, hemodynamic factors and inflammation [48]. Similar to other etiologies of type 1 CRS, for years there were no known biomarkers available to warn clinicians of early renal injury following CPB. However, recent studies illuminate potential biomarkers with high sensitivity and specificity in predicting AKI after CPB [49]. These biomarkers include some of the renal ones mentioned above, as well as others such as hepcidin, α-1 microglobulin and fatty acid-binding proteins (Figure 4).
Figure 4. Predictive time course of common novel renal biomarkers after adult cardiac surgery.
Patterns of change represent ideal circumstances, which have not been consistently demonstrated in clinical studies.
AKI: Acute kidney injury; CBP: Cardiopulmonary bypass; Cystatin C: Serum cystatin C; KIM-1: Urinary kidney injury molecule-1; NGAL: Urinary neutrophil gelatinase-associated lipocalin.
Adapted with permission from [61].
Injury to red blood cells during CPB causes release of free iron in excess of its scavengers, increased systemic vascular resistance, altered coagulation profile, platelet dysfunction, renal tubular damage and increased mortality [50]. Excess free iron causes oxidative stress through reactive oxidation species via the Fenton reaction. Accordingly, during CPB, elevation in plasma-free hemoglobin is associated with early postoperative tubular damage and is independently and significantly related to the consequent loss of kidney function [51].
Both NGAL and hepcidin regulate iron metabolism in renal tubular cells (the former promoting intracellular depletion and the latter intracellular accumulation of iron in proximal tubule cells) and can thus serve as markers of iron-related toxicity [49]. In a recent review, the AUC of NGAL to predict AKI after cardiac surgery was 0.775, with a sensitivity and specificity of 75.5 and 75.1%, respectively [14].
Ho et al. found that hepcidin was upregulated in the urine of patients not developing AKI after cardiac surgery [52]. This is consistent with the observation that urine hepcidin increases during inflammation and declines as inflammation is resolved [53]. α-1 microglobulin is also a biomarker of proximal tubule injury with increased urinary excretion following injury [54]. Consistent with this observation, α-1 microglobulin is markedly increased during the early postoperative phase in patients subsequently developing AKI [52].
Two types of fatty acid-binding proteins (liver [L-FABP] and heart types) are also potential biomarkers of AKI after CPB [55]. Portilla et al. demonstrated that in children undergoing cardiac surgery, L-FABP prognosticates AKI development with 81% accuracy, and levels elevate within 4 h of renal insult [56]. A study on human L-FABP transgenic mice found that urinary L-FABP values permit early and accurate detection of functional and histological injuries in AKI caused by ischemia [57]. As the timing of the renal insult in cardiac surgery cases is better known than in other pathologies, research in this field has the potential to reveal invaluable biomarkers with intricate knowledge regarding the timing of their changes in concentration (Figure 3).
Conclusion
Just as in other modalities, such in the diagnosis of MI, a multimarker and serial sampling strategy will provide the most benefit. Therefore, the most clinically useful biomarkers may be combined in a kidney panel to achieve maximum sensitivity and specificity [1]. Thus, it could be suggested that noninvasive and cost-effective biomarkers of CRS would not only reduce morbidity and mortality, but also healthcare expenditures. Biomarkers in conjunction with stratification of patients based on clinical presentation as is achieved by the CRS definitions are needed in order to better describe the pathophysiology of CRS and thus optimize patient management to improve outcomes.
Expert commentary & five-year view
Within the next few years, the burgeoning field of biomarkers for diagnosis of AKI, particularly in the setting of CRS, will be more advanced and ready for clinical application. Countless studies are currently underway to confirm the clinical utility of the biomarkers reviewed here, as well as many others. Several of the novel markers covered will most likely be in clinical practice and possibly even part of standard of care for AKI. NGAL seems the most likely to enter standard clinical care first as it has the most extensive and promising body of research thus far; however, other markers such as NAG and KIM-1 may not be far behind. The main limitation of these biomarkers is their cost and the accessibility to the laboratory platforms required for their analysis, and it is unclear how they will impact clinical outcomes as these large studies have yet to be conducted. However, based on the current data, these biomarkers appear to have promise to help with the management of AKI and CRS.
Key issues.
Renal injury in patients with cardiac dysfunction is difficult to diagnose and treat in a timely manner.
Increased understanding of the cardiorenal syndrome is making the complex interactions between these organs more clear.
Traditional biomarkers such as blood urea nitrogen and creatinine are not ideal as they are functional and not structural markers and require a substantial drop in the glomerular filtration rate to rise significantly.
Novel biomarkers, such as neutrophil gelatinase-associated lipocalin, cystatin C and exosomes, could soon result in earlier diagnosis of renal damage in patients with cardiac pathologies and allow clinicians to utilize renal-sparing techniques.
These markers could potentially be applied to many common clinical situations that often involve kidney injury, such as acute decompensated heart failure and cardiac surgery.
Footnotes
Financial & competing interests disclosure
PR Taub is supported by grant P30DK079337 from the NIDDK O’Brien Center. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
For reprint orders, please contact reprints@expert-reviews.com
References
- 1.Ronco C, Haapio M, House AAN, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(19):1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 2.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34(7):1913–1917. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 3.Cruz DN, Bolgan I, Perazella MA, et al. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI) Investigators. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE criteria. Clin J Am Soc Nephrol. 2007;2(3):418–425. doi: 10.2215/CJN.03361006. [DOI] [PubMed] [Google Scholar]
- 4.Aghel A, Shrestha K, Mullens W, Borowski A, Tang WH. Serum neutrophil gelatinase-associated lipocalin (NGAL) in predicting worsening renal function in acute decompensated heart failure. J Card Fail. 2010;16(1):49–54. doi: 10.1016/j.cardfail.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 7.Bagshaw SM, George C, Bellomo R ANZICS Database Management Committe. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23(5):1569–1574. doi: 10.1093/ndt/gfn009. [DOI] [PubMed] [Google Scholar]
- 8.Taub PR, Gabbai-Saldate P, Maisel A. Biomarkers of heart failure. Congest Heart Fail. 2010;16(Suppl 1):S19–S24. doi: 10.1111/j.1751-7133.2010.00168.x. [DOI] [PubMed] [Google Scholar]
- 9.Uttenthal O. NGAL: a marker molecule for the distressed kidney? Clin Lab Int. 2005;29:39–41. [Google Scholar]
- 10.Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63(5):1714–1724. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 11.Kieran NE, Doran PP, Connolly SB, et al. Modification of the transcriptomic response to renal ischemia/reperfusion injury by lipoxin analog. Kidney Int. 2003;64(2):480–492. doi: 10.1046/j.1523-1755.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 12.Yuen PS, Jo SK, Holly MK, Hu X, Star RA. Ischemic and nephrotoxic acute renal failure are distinguished by their broad transcriptomic responses. Physiol Genomics. 2006;25(3):375–386. doi: 10.1152/physiolgenomics.00223.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolignano D, Basile G, Parisi P, Coppolino G, Nicocia G, Buemi M. Increased plasma neutrophil gelatinase-associated lipocalin levels predict mortality in elderly patients with chronic heart failure. Rejuvenation Res. 2009;12(1):7–14. doi: 10.1089/rej.2008.0803. [DOI] [PubMed] [Google Scholar]
- 14.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A NGAL Meta-analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57(17):1752–1761. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yndestad A, Landrø L, Ueland T, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J. 2009;30(10):1229–1236. doi: 10.1093/eurheartj/ehp088. [DOI] [PubMed] [Google Scholar]
- 17.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 18.Herget-Rosenthal S, Marggraf G, Hüsing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66(3):1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 19.Lassus J, Harjola VP. Cystatin C: a step forward in assessing kidney function and cardiovascular risk. Heart Fail Rev. 2012;17(2):251–261. doi: 10.1007/s10741-011-9242-6. [DOI] [PubMed] [Google Scholar]
- 20.Lassus J, Harjola VP, Sund R, et al. for the FINN-AKVA Study group. Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur Heart J. 2007;28(15):1841–1847. doi: 10.1093/eurheartj/ehl507. [DOI] [PubMed] [Google Scholar]
- 21.Lassus JP, Nieminen MS, Peuhkurinen K, et al. FINN-AKVA study group. Markers of renal function and acute kidney injury in acute heart failure: definitions and impact on outcomes of the cardiorenal syndrome. Eur Heart J. 2010;31(22):2791–2798. doi: 10.1093/eurheartj/ehq293. [DOI] [PubMed] [Google Scholar]
- 22.Eriksen BO, Mathisen UD, Melsom T, et al. Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int. 2010;78(12):1305–1311. doi: 10.1038/ki.2010.321. [DOI] [PubMed] [Google Scholar]
- 23.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290(2):F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 24.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 25.Han WK, Waikar SS, Johnson A, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73(7):863–869. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liangos O, Tighiouart H, Perianayagam MC, et al. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers. 2009;14(6):423–431. doi: 10.1080/13547500903067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damman K, Ng Kam Chuen MJ, MacFadyen RJ, et al. Volume status and diuretic therapy in systolic heart failure and the detection of early abnormalities in renal and tubular function. J Am Coll Cardiol. 2011;57(22):2233–2241. doi: 10.1016/j.jacc.2010.10.065. [DOI] [PubMed] [Google Scholar]
- 28.Tschoeke SK, Oberholzer A, Moldawer LL. Interleukin-18: a novel prognostic cytokine in bacteria-induced sepsis. Crit Care Med. 2006;34(4):1225–1233. doi: 10.1097/01.CCM.0000208356.05575.16. [DOI] [PubMed] [Google Scholar]
- 29.Melnikov VY, Faubel S, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest. 2002;110(8):1083–1091. doi: 10.1172/JCI15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melnikov VY, Ecder T, Fantuzzi G, et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107(9):1145–1152. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miaolin C, Bo X, Song X, et al. Clinical usefulness of novel biomarkers for the detection of acute kidney injury following elective cardiac surgery. Nephron Clin Prac. 2010;115:66–72. doi: 10.1159/000286352. [DOI] [PubMed] [Google Scholar]
- 32.Parikh CR, Mishra J, Thiessen-Philbrook H, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70(1):199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 33.Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008;73(9):1008–1016. doi: 10.1038/sj.ki.5002729. [DOI] [PubMed] [Google Scholar]
- 34.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16(10):3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 35.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50(25):2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 36.de Cal M, Haapio M, Cruz DN, et al. B-type natriuretic peptide in the critically ill with acute kidney injury. Int J Nephrol. 2011;2011:951629. doi: 10.4061/2011/951629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maisel AS, Katz N, Hillege HL, et al. Acute Dialysis Quality Initiative Consensus Group. Biomarkers in kidney and heart disease. Nephrol Dial Transplant. 2011;26(1):62–74. doi: 10.1093/ndt/gfq647. [DOI] [PubMed] [Google Scholar]
- 38.Xie Y, Sakatsume M, Nishi S, Narita I, Arakawa M, Gejyo F. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int. 2001;60(5):1645–1657. doi: 10.1046/j.1523-1755.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 39.Lorenzen JM, Hafer C, Faulhaber-Walter R, et al. Osteopontin predicts survival in critically ill patients with acute kidney injury. Nephrol Dial Transplant. 2011;26(2):531–537. doi: 10.1093/ndt/gfq498. [DOI] [PubMed] [Google Scholar]
- 40.Damman K, Masson S, Hillege HL, et al. Clinical outcome of renal tubular damage in chronic heart failure. Eur Heart J. 2011;32(21):2705–2712. doi: 10.1093/eurheartj/ehr190. [DOI] [PubMed] [Google Scholar]
- 41.Tögel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67(5):1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 42.Van Balkom BWM, Pisitjun T, Verhaar MC, Knepper MA. Exosomes and the kidney: prospects for diagnosis and therapy of renal diseases. Kidney Int. 2011;80:1138–1145. doi: 10.1038/ki.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knepper MA, Pisitkun T. Exosomes in urine: who would have thought? Kidney Int. 2007;72(9):1043–1045. doi: 10.1038/sj.ki.5002510. [DOI] [PubMed] [Google Scholar]
- 44.Zhou H, Cheruvanky A, Hu X, et al. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. 2008;74(5):613–621. doi: 10.1038/ki.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hata N, Yokoyama S, Shinada T, et al. Acute kidney injury and outcomes in acute decompensated heart failure: evaluation of the RIFLE criteria in an acutely ill heart failure population. Eur J Heart Fail. 2010;12(1):32–37. doi: 10.1093/eurjhf/hfp169. [DOI] [PubMed] [Google Scholar]
- 46.Maisel AS, Mueller C, Fitzgerald R, et al. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: the NGAL EvaLuation Along with B-type NaTriuretic Peptide in acutely decompensated heart failure (GALLANT) trial. Eur J Heart Fail. 2011;13(8):846–851. doi: 10.1093/eurjhf/hfr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Damman K, Van Veldhuisen DJ, Navis G, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart. 2010;96(16):1297–1302. doi: 10.1136/hrt.2010.194878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haase M, Bellomo R, Haase-Fielitz A. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J Am Coll Cardiol. 2010;55(19):2024–2033. doi: 10.1016/j.jacc.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 49.Bellomo R, Auriemma S, Fabbri A, et al. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI) Int J Artif Organs. 2008;31(2):166–178. doi: 10.1177/039139880803100210. [DOI] [PubMed] [Google Scholar]
- 50.Vercaemst L. Hemolysis in cardiac surgery patients undergoing cardiopulmonary bypass: a review in search of a treatment algorithm. J Extra Corpor Technol. 2008;40(4):257–267. [PMC free article] [PubMed] [Google Scholar]
- 51.Vermeulen Windsant IC, Snoeijs MG, Hanssen SJ, et al. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int. 2010;77(10):913–920. doi: 10.1038/ki.2010.24. [DOI] [PubMed] [Google Scholar]
- 52.Ho J, Lucy M, Krokhin O, et al. Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: a nested case–control study. Am J Kidney Dis. 2009;53(4):584–595. doi: 10.1053/j.ajkd.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 53.Kemna E, Pickkers P, Nemeth E, van der Hoeven H, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106(5):1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 54.Bernard AM, Vyskocil AA, Mahieu P, Lauwerys RR. Assessment of urinary retinol-binding protein as an index of proximal tubular injury. Clin Chem. 1987;33(6):775–779. [PubMed] [Google Scholar]
- 55.Hofstra JM, Deegens JK, Steenbergen EJ, Wetzels JF. Urinary excretion of fatty acid-binding proteins in idiopathic membranous nephropathy. Nephrol Dial Transplant. 2008;23(10):3160–3165. doi: 10.1093/ndt/gfn190. [DOI] [PubMed] [Google Scholar]
- 56.Portilla D, Dent C, Sugaya T, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73(4):465–472. doi: 10.1038/sj.ki.5002721. [DOI] [PubMed] [Google Scholar]
- 57.Negishi K, Noiri E, Doi K, et al. Monitoring of urinary L-type fatty acid-binding protein predicts histological severity of acute kidney injury. Am J Pathol. 2009;174(4):1154–1159. doi: 10.2353/ajpath.2009.080644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ronco C, McCullough P, Anker SD. Cardio-renal syndromes: report from the consensus conference of the Acute Dialysis Quality Initiative. Eur Heart J. 2010;31(6):703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murray PT, Devarajan P, Levey AS, et al. A framework and key research questions in AKI diagnosis and staging in different environments. Clin J Am Soc Nephrol. 2008;3(3):864–868. doi: 10.2215/CJN.04851107. [DOI] [PubMed] [Google Scholar]
- 60.Ahlström A, Tallgren M, Peltonen S, Pettilä V. Evolution and predictive power of serum cystatin C in acute renal failure. Clin Nephrol. 2004;62(5):344–350. doi: 10.5414/cnp62344. [DOI] [PubMed] [Google Scholar]
- 61.McIlroy DR, Wagener G, Lee HT. Biomarkers of acute kidney injury: an evolving domain. Anesthesiology. 2010;112(4):998–1004. doi: 10.1097/ALN.0b013e3181cded3f. [DOI] [PubMed] [Google Scholar]
- 62.Maisel AS, Krishnaswamy P, Nowak RM, et al. Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]