Abstract
A major obstacle to efficacious T cell-based cancer immunotherapy is the tolerizing tumor microenvironment that rapidly inactivates tumor-infiltrating lymphocytes. In an autochthonous model of prostate cancer, we have previously shown that intratumoral injection of antigen loaded dendritic cells (DCs) delays T cell tolerance induction as well as refunctionalizes already tolerized T cells in the tumor tissue. In this study, we have defined molecular interactions that mediate DCs’ effects. We show that pretreating antigen-loaded DCs with anti-CD70 antibody abolishes DCs’ ability to delay tumor-mediated T cell tolerance induction, whereas interfering with 4-1BBL, CD80, CD86 or both CD80 and CD86 had no significant effect. In contrast, CD80−/− or CD80−/−CD86−/− DCs failed to reactivate already-tolerized T cells in the tumor tissue, whereas interfering with CD70 and 4-1BBL had no effect. Furthermore, despite a high level of PD-1 expression by tumor infiltrating T cells and PD-L1 expression in the prostate, disrupting PD-1/PD-L1 interaction did not enhance T cell function in this model. These findings reveal dynamic requirements for costimulatory signals to overcome tumor induced tolerance and have significant implications for developing more effective cancer immunotherapies.
Introduction
A major focus of cancer immunotherapy has been stimulating patients’ CD8+ cytolytic T cells to kill tumor cells. In one treatment modality, tumor-infiltrating leukocytes (TILs) are isolated from the patient, activated ex vivo and infused back into the same patient. Such adoptive cell therapy (ACT) has shown clinical benefit in treating melanoma (1). In another treatment modality, DC based vaccines are used to stimulate the patients’ endogenous anti-tumor immune response, and recently has been approved for treating prostate cancer (2). Despite these successes, a major hurdle to widespread use of these and other treatments utilizing CD8+ T cells is the tolerizing environment within the tumor tissue (1), which rapidly inactivates TILs and render the therapies ineffective.
T cell activation and function is regulated by both costimulatory and inhibitory signals. In concert with peptide MHC (pMHC) and T cell receptor (TCR) signaling, additional receptors on T cells promote or negate expansion, differentiation, and survival (3). Programmed death-1 (PD-1) expressed on activated T cells inhibits T cell function upon engagement with its ligand, PD-Ligand 1 (PD-L1). PD-L1 is expressed on tumor and/or tumor associated stroma, and sites of immune privilege, and is considered a promising candidate for checkpoint blockade in tumor immunotherapy (4). Indeed, blockade of PD-L1 along with adoptive transfer of tumor specific T cells, delays tumor growth in preclinical melanoma models (5). Among costimulatory molecules, engagement of CD28 on T cells with CD80 and CD86 on antigen presenting cells (APCs) promotes activation of both naïve and memory T cells (3). Specific to anti-tumor responses, enforced expression of CD80 and/or CD86 on tumor cells stimulates their destruction by the immune system (6), a strategy of cancer immunotherapy that has been tested in clinical trials (7). The TNF family contains a diverse array of molecules critical for positively regulating T cell function, including the CD27/CD70 and 4-1BB/4-1BBL receptor ligand pairs, expressed on T cells/APCs, respectively (8). Overexpression of CD70 in transgenic mice enhances priming of T cells, leading to rejection of EL-4 thymomas that express the nucleoprotein (NP) model antigen (9). Similarly, in vivo stimulation of clonotypic T cells with an anti-4-1BB antibody promotes T cell rejection of established murine plasmacytoma tumors (10).
In our study of CD8+ T cell-tumor cell interaction, we have developed an autochthonous TRP-SIY prostate cancer model, based on TRAMP mice, where tumor cells express a nominal MHC class I epitope (SIYRYYGL or SIY) recognized by the 2C clonotypic TCR (11). Adoptive transfer of naïve CD8+ 2C T cells into TRP-SIY mice followed by infection with influenza virus expressing the SIY epitope leads to activation and differentiation of transferred T cells into potent effector cells. As in human patients, effector T cells infiltrate into the prostate tumor tissue and rapidly become inactivated (tolerized). The tolerized 2C T cells persist in the prostate tumor tissue (12) expressing high levels of PD-1, analogous to TILs in patients. Importantly, we have found that antigen-loaded bone marrow-derived DCs (BMDCs), when injected intraprostatically, delay the rapid tolerance induction of effector 2C T cells as they initially infiltrate the tumor tissue (13). In addition, when antigen-loaded BMDCs are injected after initial tolerance induction, they refunctionalize the persisting tolerized 2C T cells in the tumor tissue. These previous studies set the stage to define molecular interactions that are required for prostate tumor-mediated T cell tolerance induction and DC-mediated delay and reactivation of tolerized T cells in the prostate tumor microenvironment.
In this study, we have evaluated the role of PD-1/PD-L1 interaction in T cell tolerance induction in the prostate tumor tissue and the role of CD80, CD86, 4-1BBL, and CD70 in DC-mediated delay of T cell tolerance induction and refunctionalization of tolerized T cells in the prostate tumor tissue. Our results show that despite the high levels of PD-1 expression by prostate-infiltrating T cells and PD-L1 expression in the prostate, blocking PD-1/PD-L1 interaction has no effect on T cell tolerance induction in the prostate tumor tissue. While CD70 is required for DC-mediated delay of T cell tolerance induction, CD80 and CD86 are required for refunctionalizing the tolerized T cells in the prostate tumor tissue. These findings show that different costimulatory signals are required to overcome tumor tolerizing signals during the initial tolerance induction and reactivation of previously tolerized T cells. They also suggest approaches to overcome the tolerizing tumor environment to achieve more efficacious cancer immunotherapy by ACT and DC vaccination.
Materials and Methods
Mice, Adoptive Transfer and Influenza Infection
TRP-SIY mice were generated as previously described (11). 2C TCR transgenic mice were maintained on C57BL/6 and RAG1−/− backgrounds (2C/RAG mice). CD86, and CD80/CD86 knockout mice on the C57BL/6 background were from Jackson Laboratories (Bar Harbor, ME). CD80, PD-L1 knockout and TRAMP mice were bred in house. Where indicated, mice were retroorbitally injected with 1.5×106 naïve 2C cells from 2C/RAG mice and were intranasally infected with 100 pfu WSN-SIY influenza A virus. All experiments were approved by the Committee on Animal Care (CAC) at Massachusetts Institute of Technology.
Generation of Bone Marrow-Derived DCs (BMDCs) and Intraprostatic Injection
Bone marrow was collected from the femurs of C57BL/6 mice, or indicated knockout mice. Cells were resuspended at 2×105 cells/mL in RPMI plus 10%FCS, 50µM 2-mercaptoethanol, 4mM L-glutamine and 100U/mL-100ug/mL penicillin-streptomycin supplemented 1:30 with the supernatant from J5 cells secreting GM-CSF. Cells were washed and media changed on day 2 and 5, 1µg/mL LPS was added on day 6, and cells were harvested on day 7. Where indicated, BMDCs were loaded with 1µg/mL SIY peptide for 1hr at 37°C and/or were labeled for 10 min at 37°C with 5µM CFSE (Invitrogen) in PBS plus 0.1% BSA on the day of harvest. For blocking experiments, 1×106 cell/mL were incubated in 10 µg/mL of F(ab’)2 of indicated antibodies for 2.5 hours on ice, as previously described (14). BMDCs were rinsed and resuspended at 1×106 cells / 40μL PBS for intraprostatic injection (1×106 cells per mouse).
Cells and Flow Cytometry
Single cell suspensions were prepared from lymphoid tissues by grinding them between frosted glass slides and filtering the suspensions through 70µm nylon mesh. Prostate tissues were digested with 2µg/mL collagenase A (Roche) and 170U/mL DNase I (Sigma-Aldrich) in RPMI 1640 plus 10% FCS for 1hr at 37°C. Digested samples were then ground between frosted glass slides and filtered through 70µm nylon mesh. Intracellular IFN-γ staining was performed with a BD Cytofix/Cytoperm Kit (BD Biosciences). Recovered cells were restimulated with 1µg/mL SIY peptide for 4hr at 37°C in the presence of BD GolgiPlug containing brefeldin A. Surface antigens were stained, dead cells were identified using a LIVE/DEAD Fixable Red Dead Cell Stain Kit (Invitrogen), and all cells were fixed and permeabilized. Samples were then stained with a PE-conjugated anti-IFN-γ antibody (BD Pharmingen). Gates were set using a panel of fluorescent minus one controls. Statistical analysis was conducted using a Student’s t test and data considered significant if p<0.05.
Immunohistochemistry
Prostate glands were excised from mice, flash frozen in OCT compound (Tissue-Tek, Sakura, Torrance, CA) and cyrosectioned at 10 μM by the Koch Institute Histology Core Facility. Sections were fixed with acetone and stained with Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA). Visualization of PD-L1 staining was carried out with 3,3’-diaminobenzidine (DAB) substrate (Vector Laboratories) and counterstained with eosin (Sigma-Aldrich, St. Louis, MO). Images were acquisitioned using a Zeiss Axioplan II microscope, using either Zeiss Plan-Neofluar 20× (0.8) lenses and a QImaging MicroPublisher 5.0 color camera. Images were captured using Openlab 5.5 (Improvision, PerkinElmer), and analyzed using ImageJ (US National Institutes of Health).
Antibodies and F(ab’)2 Production
Fluorescently labeled Anti-Thy1.1 (Clone OX-7), CD27 (LG.3A10), CD11c (N418), CD28 (37.51), PD1 (RMP1-30), 41BB (17B5), OX40 (OX-86), CD30 (CD30.1), LAG-3 (C9B7W) were from BioLegend, anti-CD86 (P03.1), CD70 (FR70), 4-1BB-L (TKS-1), PD-L1 (MIH5), Tim3 (8B.2C12) from eBioscience, anti-CD80 (16–10A1), CD44 (IM7) and rat IgG2bκ isotype (clone A95-1) from BD Pharmingen, and biotinylated anti-rat IgG (BA-4001) from Vector Laboratories. 2C TCR was stained using a biotin-conjugated clonotypic 1B2 antibody detected with streptavidin-APC (BioLegend). Stained cells were analyzed using a FACSCalibur or LSR II (BD Biosciences) instrument, and the data was processed using FlowJo software (Tree Star).
F(ab’)2 fragments were generated from purified anti-CD70 (FR70, eBioscience), anti-4-1BBL (TKS-1, eBioscience), anti-FcR (CD16/CD32) (2.4G2-BD Pharmingen) (PMID: 6736648) using the Pierce F(ab’)2 Preparation Kit (Thermo Scientific) and concentration determined by absorbance on 280nm, as recommended. For F(ab’)2 competition experiments, 1×106 cell/mL were incubated in 10 µg/mL of F(ab’)2 fragment and full length fluorescently labeled antibody, followed by staining with CD11c and analysis by flow cytometry.
In Vivo Cytotoxicity Assay
In vivo cytotoxicity was performed as previously described (13). Briefly, C57BL/6 (Thy1.1+) splenocytes were activated on anti-CD3 coated plates in the presence of 50 U/mL IL-2 for 3 days. Half of the activated splenocytes were then labeled with 20 μM CFSE (CFSEHi) and pulsed with SIY peptide, and half labeled with 1 μM (CFSELo). CFSEHi and CFSELo cells were mixed 1:1 and retroorbitally injected into mice (20×106 cells/mouse). Approximately 24 hrs later, the ratio of CFSEHi to CFSELo cells in various tissues was evaluated by flow cytometry.
Results
PD-1 is expressed on prostate resident T cells and PD-L1 on the prostate stroma
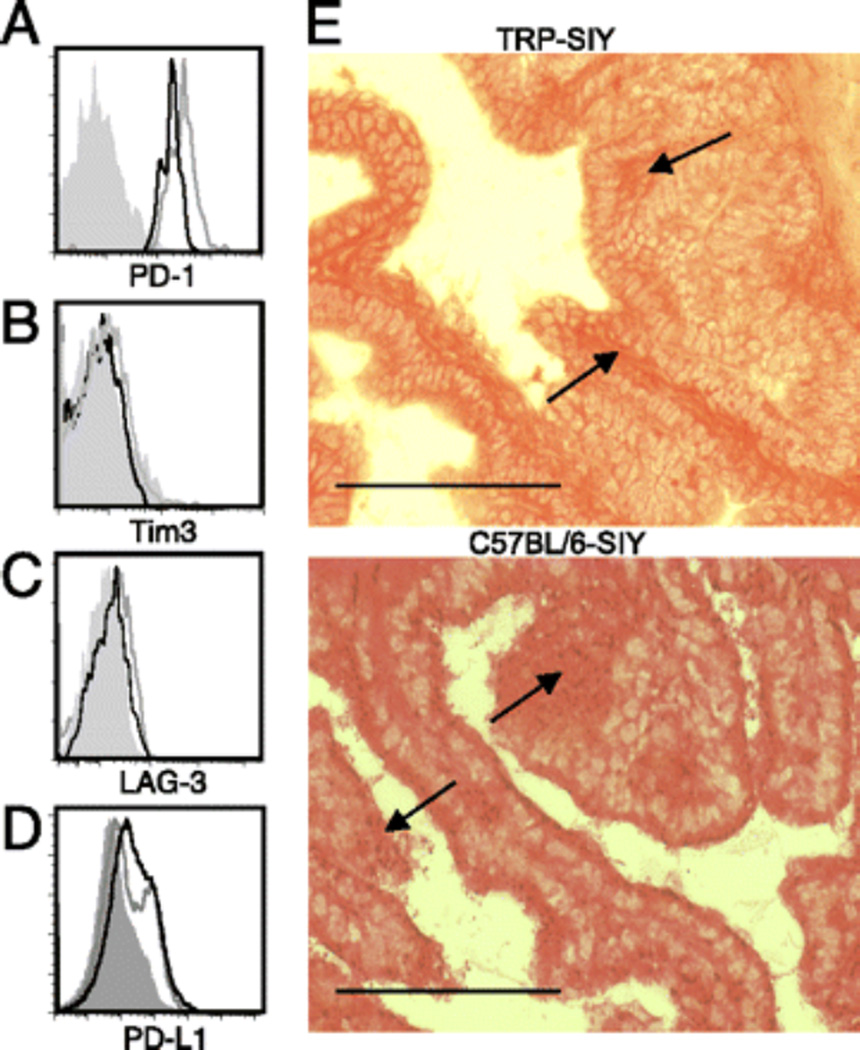
To define the pathways that regulate tolerance of 2C T cells in the TRP-SIY system we assessed the expression of inhibitory receptors on the surface of 2C T cells. PD-1, Tim3, and LAG-3, individually or in combination, negatively regulate T cell responses in tumor tissues (15, 16). T cells recovered thirteen days post transfer, (or approximately 6 days after initial infiltration into the prostate) expressed high levels of PD-1 (Fig. 1A). Expression of PD-1 was maintained on prostate resident 2C T cells after 36 day post transfer into TRP-SIY mice (Fig. 1A). In contrast to PD-1, prostate resident 2C T cells did not express Tim3 or LAG-3 either 13 or 36 days post transfer (Fig. 1B, 1C). PD-L1 expression was detected on both C57BL/6-SIY and TRP-SIY prostate cells by flow cytometry (Fig. 1D) and confirmed by histological staining of prostate tissue sections (Fig. 1E).
FIGURE 1.
PD-1 is expressed on prostate resident T cells and PD-L1 on the prostate stroma. Naive 2C T cells were transferred into TRP-SIY mice, followed by intranasal infection with WSN-SIY virus. Prostates were harvested day 13 and day 36 post transfer, and Thy1.1+2C TCR+ cells were analyzed for PD-1 (A), Tim3 (B), or LAG-3 (C) expression. Histograms show relative levels of cell surface expression on 2C T cells at day 13 (gray) or day 36 (black), compared with isotype control (filled gray line). (D) Prostates from TRP-SIY (black) and C57BL/6-SIY (gray) mice were harvested and digested into single-cell suspension and stained with PD-L1 or isotype (filled gray line) and analyzed by flow cytometry. Histograms show relative levels of PD-L1 expression on prostate cells. (E) Sectioned prostates of TRP-SIY and C57BL/6-SIY mice were stained for PD-L1, visualized with peroxidase staining (brown), and counterstained with eosin (red). Arrows indicate areas of positive staining. Scale bars, 100 µm.
Modulating the PD-1/PD-L1 axis does not improve T cell responses in TRP-SIY mice
The expression of PD-1 on 2C T cells and PD-L1 on prostate cells provides the basis for a possible role of PD-1/PD-L1 interaction in tolerance induction in the TRP-SIY system. To test this notion, we generated TRP-SIY mice on a PD-L1−/− background. Following adoptive transfer of 2C T cells and intranasal infection with WSN-SIY influenza virus, effector 2C T cells infiltrated the prostate tumor tissue of TRP-SIY PD-L1−/− mice but rapidly lost IFN-γ expression, comparable to 2C T cells in prostates of TRP-SIY mice heterozygous for PD-L1 (Fig. 2A). Thus, PD-L1 is not required for T cell tolerance induction in the prostate tumor tissue of TRP-SIY mice.
FIGURE 2.
Modulation of the PD-1/PD-L1 interaction between 2C T cells and prostate or BMDCs does not affect T cell activity. (A) Naive 2C T cells were transferred into PD-L1+/− or PD-L1−/− TRP-SIY mice, along with intranasal infection with WSN-SIY virus. On day 14, 2C T cells were harvested from prostate tissue, stimulated with SIY peptide, and analyzed for IFN-γ expression. CD8 versus Thy1.1 plots were gated on all live cells from prostate. IFN-γ versus Thy1.1 plots were gated on CD8+ Thy1.1+ cells. Representative FACS plots from three experiments are shown. (B) Experimental scheme for analyzing BMDC-mediated delay of tolerance induction and refunctionalization of tolerized 2C cells in the prostate of TRP-SIY mice. On day 0, naive 2C T cells were adoptively transferred into TRP-SIY mice and given intranasal infection with WSN-SIY virus. On day 7 or 30, mice were injected intraprostatically with PBS or 1 × 106 ex vivo matured SIY-loaded wild-type (WT) or PD-L1−/− BMDCs. At 6–7 d later, 2C T cells were harvested from prostate tissue, stimulated with SIY peptide and analyzed for IFN-γ expression. (C) Representative plots of flow cytometry analyses of cells from prostate tissues of mice that were injected with PBS, wild type (WT), or PD-L1−/− BMDCs on either day 7 or day 30 after initial 2C cell transfer. 2C TCR versus Thy1.1 plots were gated on all live cells from prostate. IFN-γ versus Thy1.1 staining was gated on 2C TCR+ Thy1.1+ cells. The numbers indicate percentage of IFNγ+ cells. (D and E) Percentages (mean ± SD) of IFN-γ+ 2C cells from three independent experiments normalized to PBS control. Number of mice for each treatment are indicated. *p < 0.05 comparing DC versus PBS injection. (F and G) Number of 2C TCR+Thy1.1+ cells from prostates injected with PBS, WT, or PD-L1−/− BMDCs either 7 d (F) or 30 d (G) after T cell transfer and analyzed after 6 d (F) or 7 d (G) later. Graphs are from three independent experiments, with at least four mice per group. NS, Compared with PBS control.
While PD-L1 expression was not directly responsible for tolerance induction, the expression of PD-1 on 2C T cells could affect their subsequent function. Therefore, we tested whether the PD-1/PD-L1 interaction between 2C T cells and BMDCs affects the BMDC mediated activation of 2C T cells. 2C T cells were transferred into TRP-SIY mice and activated by intranasal WSN-SIY infection. Seven days later, as newly induced effector 2C T cells entered the prostate, SIY-loaded BMDCs from wild type or PD-L1−/− mice were injected directly into the prostate tumor tissue to test if they delay the tolerance induction (Fig. 2B). 2C T cells were recovered from the prostate another 6 days later and analyzed for their ability to express IFN-γ. Intraprostatic injection of SIY-loaded wild type or PD-L1−/− BMDCs stimulated similar percentages of 2C T cells to express IFN-γ when compared to PBS control (Fig. 2C, 2D). Furthermore, 30 days after initial T cell transfer and infection, when infiltrating 2C T cells were already tolerized, SIY-loaded BMDCs from wild type or PD-L1−/− mice were injected intraprostatically to assess whether they reactivate the tolerized T cell in situ to a similar extent (Fig. 2B). Intraprostatic injection of SIY-loaded wild type or PD-L1−/− BMDCs reactivated similar percentages of 2C T cells to express IFN-γ as compared to PBS control (Fig. 2C and 2E). These results were further confirmed by using blocking antibodies to PD-L1 (Supplemental Fig. 1). To determine if PD-L1−/− BMDCs affect the number of 2C T cells within the prostate, we analyzed the numbers of 2C T cells recovered from the prostates after BMDC injection 7 or 30 days post transfer (Fig. 2F, 2G,). Injection of PD-L1−/− BMDCs did not increase the number of 2C T cells as compared to PBS or wild-type BMDCs at either time point. Taken together, these data suggests that despite expression of PD-1 on 2C T cells and PD-L1 in the prostate tissue, disruption of PD-1/PD-L1 interaction does not enhance DC-mediated delay of tolerance induction or refunctionalization of already tolerized T cells in the prostate tumor tissue.
BMDCs act directly on 2C T cells in the TRP-SIY prostate
We assessed the mechanisms by which BMDCs activate 2C T cells within the prostate tissue. To exclude the possibility that the injected BMDCs non-specifically activate 2C T cells in the prostate, we measured IFN-γ expression by endogenous SIY-non-specific CD8+ T cells. Injection of BMDCs either 7 or 30 days after Thy1.1+ 2C T cell transfer did not stimulate IFN-γ production by endogenous Thy1.1−CD8+ T cells (Fig. 3A). To determine if injection of activated BMDCs affects the endogenous prostate-resident DCs, we assessed the costimulatory receptor expression by the endogenous CD11c+ population following injection of either PBS or BMDCs. As compared to PBS injection, the injection of activated BMDCs into the prostate of TRP-SIY did not alter the expression of CD80, CD86, CD70, or 4-1BBL on endogenous CD11c+ cells from the prostate (Fig. 3B). Furthermore, injection of SIY-loaded BMDCs did not stimulate IL-2 production by 2C T cells either 7 or 30 days after 2C T cell transfer as compared to PBS (Fig. 3C).
FIGURE 3.
BMDCs act directly on 2C T cells in the prostate tissue. (A) TRP-SIY mice were injected intraprostatically with PBS-or SIY-loaded wild-type (WT) BMDCs on either day 7 or day 30 after initial 2C cell transfer and infection. At 6–7 d later, Thy1.1−CD8+ T cells were harvested from prostate tissue and analyzed for IFN-γ expression. IFN-γ versus CD8 plots were gated on live CD8+ Thy1.1− cells. The numbers indicate percentage of IFN-γ+ cells. (B) TRP-SIY mice were injected with PBS or WT-DCs in the prostate. At 6 d later, prostate tissues were dissociated, and single-cell suspensions were stained with CD11c and CD80, CD86, CD70, 4-1BBL (black line), or isotype control (filled gray line). Histograms are gated on live CD11c+ cells. (C) TRP-SIY mice were injected with PBS-or SIY-loaded WT BMDCs on either day 7 or day 30 after initial 2C cell transfer and infection. At 6–7 d later, Thy1.1+ 2C T cells were harvested from prostate tissue, stimulated with SIY peptide, and analyzed for IL-2 expression. IFN-γ versus Thy1.1 flow cytometry plots were gated on live 2C TCR+ Thy1.1+ cells. The numbers indicate percentage of IL-2+ cells. (D) 2C T cells recovered from the prostates of TRP-SIY were assayed for CD44 expression 13 d post transfer and infection with WSN-SIY virus. Histograms are gated on Thy1.1+2C TCR+ T cells with either CD44 (black line) or isotype (filled gray line) and representative of three independent experiments. (E) TRP-SIY mice were injected with PBS, SIY peptide, or LPS-activated wild type (WT) BMDCs not pulsed with SIY peptide 7 d after initial 2C cell transfer and infection. At 6 d later, Thy1.1+ 2C T cells were harvested from prostate tissue, stimulated with SIY peptide, and analyzed for IFN-γ expression. IFN-γ versus Thy1.1 flow cytometry plots were gated on live 2C TCR+ Thy1.1+ cells. The numbers indicate percentage of IFNγ+ cells.
In our experimental system, 2C T cells are activated in the periphery by WSN-SIY infection and CD44+ effector 2C T cells traffic to the prostate (Fig. 3D). We therefore confirmed the activity of injected BMDCs is dependent on the direct presentation of the SIY peptide to the 2C T cells. We compared the effect of injection of PBS, SIY peptide alone, or activated BMDCs that had not been pulsed with the SIY peptide on IFN-γ expression by 2C T cells in the prostate. Injection of SIY peptide alone did not significantly stimulate IFN-γ expression by 2C T cells above control PBS injection (Fig. 3E), suggesting that presentation of SIY by the endogenous DCs is not a major factor in reactivation of 2C T cells. Injection of activated BMDCs without being loaded with the SIY peptide in vitro stimulated a higher fraction of 2C T cells to produce IFN-γ (Fig. 3E) than PBS or SIY peptide alone. However, the effect was lesser than SIY-loaded BMDCs (compare to Fig. 2). The observed effect is unlikely due to non-specific effect of injected BMDCs but likely due to uptake and presentation of SIY peptide in the prostate of TRP-SIY mice where the SIY transgene is robustly expressed (11). Taken together, these results suggest that BMDCs activate 2C T cells in the prostate tumor partly by directly engaging 2C T cells through pMHC/TCR interaction.
CD80 and CD86 are required to reactivate tolerized T cells in the prostate tumor tissue
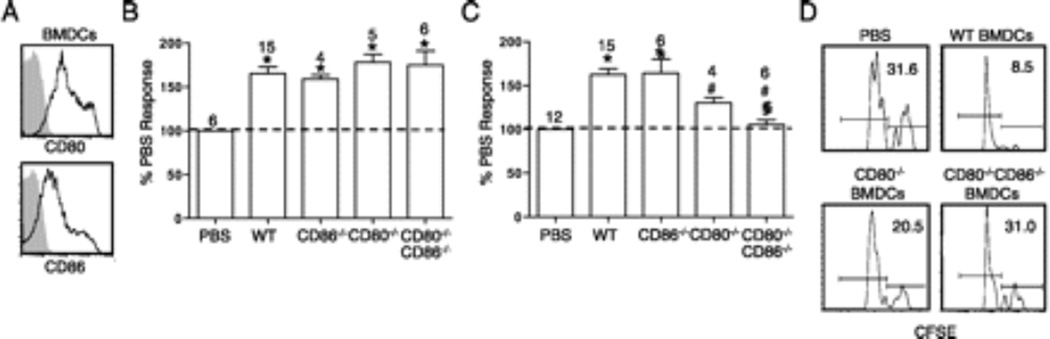
Next, we determined cell surface molecules on BMDCs that are required for reactivating 2C T cells in addition to SIY/MHC. Upregulated upon maturation of DCs, CD80 and CD86 provide an important stimulus in the context of the TCR/peptide MHC-1 engagement (3). Specific to prostate cancer, provision of antagonistic CD80 or CD86 antibodies abrogates the immunosuppressive environment and reduces tumor growth (17). BMDCs exhibit robust expression of CD80 and CD86 before intraprostate injection (Fig. 4A). Therefore, we compared the effect of intraprostatic injection of SIY-loaded wild type DC and DCs deficient in CD80, CD86, or both CD80 and CD86 in TRP-SIY mice. Experiments were carried out as in Fig. 2B, except SIY-loaded BMDCs from CD80−/−, CD86−/−, or CD80−/−CD86−/− mice were injected 7 days post initial T cell transfer and WSN-SIY infection. Compared to PBS injected mice, injection of SIY-loaded wild type BMDCs stimulated 2C T cells to express IFN-γ (Fig. 4B). Surprisingly, intraprostatic injection of BMDCs from CD80−/−, CD86−/−, or CD80−/−CD86−/− mice prolonged IFN-γ expression of infiltrating 2C T cells to a comparable extent, suggesting that neither CD80 nor CD86 is required for DC-mediated delay of tolerance induction in prostate tumor tissue.
FIGURE 4.
CD80 and CD86 expression on BMDCs is necessary for 2C T cell reactivation, but not the delay of 2C T cell tolerance. (A) Day 7 LPS-activated BMDCs were stained with anti-CD11c, -CD80, or -CD86 Abs (black line) or isotype control (filled gray line). CD80 and CD86 expression histograms are gated on CD11c+ cells. (B and C) Mice were treated and cells analyzed as in Fig. 2B, with prostate tissues injected with PBS, wild-type (WT), CD80−/−, CD86−/−, or CD80−/−CD86−/− BMDCs on either (B) day 7 or (C) day 30 after initial 2C cell transfer and infection. Percentages (mean ± SD) of IFN-γ+ 2C cells from at least three independent experiments are normalized to PBS control. Number of mice for each treatment is indicated. *p < 0.05, comparing DC with PBS injection, #p < 0.05 comparing WT with knockout DCs, §p < 0.05 comparing CD80−/−CD86−/− DCs with CD80−/− DCs. (D) At 30 d post 2C cell transfer and infection, TRP-SIY mice were injected intraprostatically with PBS, or SIY-loaded WT, CD80−/−, CD80−/−/CD86−/− BMDCs. On day 36, mice were injected retro-orbitally with a 1:1 mixture of SIY-pulsed (CFSEHi) and unpulsed (CFSELo) activated T cells (Thy1.1+). The following day, the proportions of CFSEHi versus CFSELo target cells were determined by flow cytometry. CFSE histograms are shown for live Thy1.1+ cells. Numbers indicate percentage of CFSE+ cells. Representative data from one of the two experiments are shown.
In addition to their importance in T cell primary responses, CD80 and CD86 are important for simulating productive secondary responses (18, 19). To determine the requirement for CD80 and/or CD86 in DC-mediated refunctionalization of persisting tolerized T cells in the prostate tumor tissue, we injected SIY-loaded DCs from wild type, CD80−/−, CD86−/−, or CD80−/−CD86−/− mice 30 days post initial 2C cell transfer and WSN-SIY infection. Intraprostatic injection of SIY-loaded wild type BMDCs reactivated tolerized T cells to express IFN-γ as compared to PBS control (Fig. 4C). While injection of SIY-loaded CD86−/− BMDCs stimulated similar percentage of 2C cells to express IFN-γ in the prostate tumor tissue, the percentage of 2C cells that was induced to express IFN-γ was significantly reduced following injection of SIY-loaded CD80−/− BMDCs. Most dramatically, the ability of SIY-loaded BMDCs to induce 2C cell expression of IFN-γ was completely abrogated by deficiency of CD80 and CD86. These results suggest that although deficiency of CD86 can be compensated by the presence of CD80, CD80 deficiency cannot be completely compensated by the presence of CD86. Thus, CD80 is critically required for DC-mediated refunctionalization of tolerized T cells in the prostate tumor tissue.
The requirement of CD80 and CD86 in reactivating tolerized T cells in the tumor tissue was further supported by an in vivo cytotoxicity assay. Thirty days post initial 2C T cell transfer into, and infection of, TRP-SIY mice and 6 days after injection of PBS or BMDCs, a 1:1 ratio of CFSEHi SIY pulsed target cells and CFSELo control target cells were transferred into the mice. Approximately 24 hours later, the ratio of CFSEHi to CFSELo cells in the prostate was determined by flow cytometry to assess the level of antigen-specific target cell lysis. When wild type BMDCs were injected, only 8% of CFSEHi target cells remained compared to 30% in PBS injected prostates (Fig. 4D). When CD80−/− or CD80−/−CD86−/− BMDCs were injected, 21% and 30% of CFSEHi target cells remained, respectively. These results suggest that deficiency of CD80 and CD86 impairs the ability of antigen-loaded DCs to reactivate tolerized 2C cells in the prostate tumor tissue.
CD70 but not 4-1BBL is required for BMDC mediated delay in T cell tolerance
CD86 and CD80 do not affect BMDCs’ ability to delay 2C T cell tolerance, indicating other costimulatory molecules may potentiate these effects. TNF costimulatory molecules, such as 4-1BBL and CD70, promote T cell activation during initial priming, and may be important for 2C T cell function during initial infiltration within the prostate tissue (8). For example, engagement of 4-1BB on CD8+ T cells by 4-1BBL enhances T cell cytotoxic function (20, 21). Similarly, CD70 functions to maintain T cell survival and proliferation in the periphery (22, 23). Activated BMDCs express higher levels of CD70 and 4-1BBL relative to the expression on endogenous prostate DCs (Fig. 5A, compared to Fig. 3B). We used anti-CD70 and anti-4-1BBL antibodies previously shown to block CD70/CD27 and 4-1BBL/4-1BB interactions in vivo, to define the contribution of CD70 and 4-1BB in DC-mediated delay of tolerance induction (14, 24). To exclude possible Fc receptor (FcR)-mediated effect on the DCs, we generated F(ab’)2 fragment of each antibody lacking the Fc portion of the molecule but still retaining binding activity as assessed by the ability to compete fluorescently labeled full length antibody for cell surface binding (Fig. 5B). As a control, we used F(ab’)2 of the antibody cocktail against FcRs CD16 and CD32 (25). SIY-loaded BMDCs were incubated with each antibody fragment and then injected into prostate tumor tissue of TRP-SIY mice 7 days after 2C T cell transfer and WSN-SIY infection. FcR-blocking antibodies did not diminish the ability of BMDCs to stimulate prolonged IFN-γ expression by infiltrating 2C T cells (Fig. 5C). Blocking the interaction between 4-1BB on 2C T cells and 4-1BBL on BMDCs did not impair DCs’ ability to extend the IFN-γ expression by infiltrating 2C T cells. However, blockade of CD70 on BMDCs significantly reduced their ability to stimulate prolonged IFN-γ expression by infiltrating 2C T cells in the prostate (Fig. 5C). The observed difference was not due to differential retention of anti-CD70 treated BMDCs within the prostate tissue as compared to control treated BMDCs (Fig. 5E), consistent with previous experiments with untreated BMDCs (13). These results show that CD70/CD27 interaction is required for DC-mediated delay of 2C T cell tolerance induction in the prostate tumor tissue.
FIGURE 5.
CD70 is required for DC-mediated delay in 2C T cell tolerance. (A) Day 7 LPS-activated BMDCs were stained for CD11c plus CD70, 4-1BBL (black line), or isotype control (filled gray line). Histograms are gated on CD11c+ cells. (B) F(ab'′)2 fragments are confirmed to maintain their Ag-binding capacity using a competition assay. BMDCs were incubated with 10 µg/ml of fluorescently labeled full-length Ab in the presence of the same amount of either F(ab'′)2 of interest or control anti-FcR F(ab '′)2 fragment. The cells were then stained with anti-CD11c and analyzed by flow cytometry. Center histogram shows binding of fluorescently labeled anti-CD70 to BMDCs in the presence of either anti-CD70 F(ab'′ )2 (gray) or control anti-FcR F(ab'′)2(black). Left histogram shows binding of fluorescently labeled anti-CD16/CD32 to BMDCs in the presence of either anti-FcR F(ab'′)2 (gray) or control anti-CD70 F(ab'′ )2(black). Right histogram shows binding of fluorescently labeled anti– 4-1BBL to BMDCs in the presence of either anti–4-1BBL F(ab'′)2 (gray) or control anti-FcR F(ab'′)2 (black). Histograms are gated on CD11c+ cells. (C) Experiments were conducted as in Fig. 2B with SIY-pulsed BMDCs incubated with anti-FcR, CD70, or 4-1BBL F(ab'′)2 fragments. Shown are percentages (mean ± SD) of IFN-γ+ 2C cells from three independent experiments normalized to PBS control. Number of mice for each treatment is indicated. (D) As in (C), except BMDCs were injected on day 30 post T cell transfer, and analysis was carried out on day 37. Shown are percentages (mean ± SD) of IFN-γ+ 2C cells from three independent experiments normalized to PBS control. Number of mice for each treatment is indicated. *p < 0.05 comparing DC with PBS injection, #p < 0.05 comparing BMDCs treated with anti-CD70 F(ab'′)2 and those treated with anti-FcR F(ab'′)2. (E) Day 7 LPS-matured BMDCs were labeled with CFSE and incubated with F(ab'′)2 fragments specific for FcR or CD70. DCs (10 × 103 per mouse) were surgically injected into one of the dorsal prostate lobes. At 3 and 5 d later, mice were sacrificed, and CSFE+ DCs in each of the prostate tissues were assessed by flow cytometry. Shown are the average numbers of DCs (±SD) from three prostates per group, per time point.
As 4-1BBL and CD70 have been shown to enhance recall responses and reactivate previously tolerized T cells (26, 27), we examined the effect of 4-1BBL and CD70 on reactivation of already tolerized 2C T cells. We injected antibody-treated, SIY-loaded BMDCs into the prostate of TRP-SIY mice 30 days after 2C T cell transfer and WSN-SIY infection. Blockade of either CD70 or 4-1BBL did not significantly reduce the fractions of persisting 2C T cells that were induced to express IFN-γ (Fig. 5D), indicating that CD70 and 4-1BBL are not required for reactivation of tolerized 2C T cells in the prostate tumor tissue.
CD27 is downregulated on prostate resident T cells
The costimulatory molecules necessary to delay tolerance induction and reactivate already tolerized 2C T cells are distinct. BMDCs expressed similar levels of costimulatory molecules whether they were used to delay tolerance induction or reactivate already tolerized T cells. The functional differences between these two time points may be a result of phenotypic changes on prostate resident 2C T cells. Thus, we assessed 2C T cell expression of CD28, CD27, and 4-1BB before transfer and 13, 23, and 36 days following transfer and infection in TRP-SIY mice. CD28 expression on 2C T cells from prostate remained similar at the three different time points and was similar to that of naïve 2C T cells (Fig. 6A-6C). The expression of 4-1BB was low on 2C T cells recovered from TRP-SIY prostate throughout the 36 days following transfer (Fig. 6B, 6C). CD27 was highly expressed on naïve 2C T cells (Fig. 6A). In contrast to CD28 and 4-1BB, expression of CD27 was progressively lost from 2C T cells following infiltration into TRP-SIY prostate tissue (Fig. 6B, 6C). To determine if CD27 downregulation is specific to the TRP-SIY prostate, we transferred 2C T cells into TRAMP and C57BL/6 mice. As shown in Figure 6D, CD27 expression on 2C T cells were also downregulated on the prostate of TRAMP and C57BL/6 mice. The observed downregulation is specific to the prostate, as the level of CD27 on 2C T cells from spleen of the same mice remained stable (Fig. 6E). These results show that the lack of effect of anti-CD70 treatment during reactivation is correlated with the downregulation of CD27 on persisting tolerized 2C T in the prostate.
FIGURE 6.
CD27 expression is progressively lost on TRP-SY prostate resident 2C T cells. (A) Naive 2C T cells pooled from spleens and lymph nodes were assayed for expression of CD28, 4-1BB, and CD27. Histograms are gated on Thy1.1+ 2C TCR+ cells stained with isotype Ab (shaded gray) or specific Ab (black line). (B) Naive Thy1.1+ 2C T cells were retro-orbitally injected into TRP-SIY mice, along with intranasal infection with WSN-SIY virus. Mice were sacrificed 13 (bold black line), 23 (gray line), and 36 (thin black line) d post transfer, and CD28, 4-1BB, and CD27 expression on prostate resident 2C T cells was assayed by flow cytometry. Histograms are gated on live 2C TCR+ Thy1.1+ cells with isotype Ab (shaded gray) or specific Ab (black line). (C) Mean fluorescence intensity of CD28, CD27, or 4-1BB staining from Thy1.1+2C TCR+T cells from naive mice or recovered from prostates at indicated times post transfer and WSN-SIY infection. Data shown are from at least two independent experiments (mean ± SD). (D) Time course of CD27 expression on 2C T cells recovered from TRAMP and C57BL/6 (B6) prostates 13 (bold black line), 23 (gray line), and 36 (thin black line) d post transfer. (E) As above, with 2C transfer/WSN-SIY infection and analysis of CD27 on 2C TCR+Thy1.1+ 2C T cells 13 (bold black line), 23 (gray line), and 36 (thin black line) d post transfer in spleens from TRP-SIY, TRAMP, and B6 mice.
Discussion
Molecular interactions that underlie DCs’ ability to overcome tumor-induced T cell tolerance are largely unknown. In an autochthonous model of prostate cancer, we have previously defined two stages when activated DCs can overcome tumor microenvironment: delaying tolerance induction of tumor infiltrating T cells and reactivating already tolerized T cells in the tumor tissue. In this study, we have now identified molecules necessary for DCs’ effects. Our data show that CD70-CD27 interaction between T cells and BMDCs is required for DC mediated delay in 2C T cell tolerance. Our study further refines the role of CD70 by demonstrating that CD70-CD27 interaction can sustain intra-tumoral T cell activity in an otherwise tolerizing environment. Previous reports of CD70 function in tumor models have been restricted to transplantable cell lines. Over-expression of CD70 by endogenous antigen presenting cells enhances priming of T cells against the EL4-NP and B6F10 tumor cell lines (9, 28). Furthermore, activation of clonotypic T cells with an anti-CD27 antibody enhances the rejection of another transplantable melanoma cell line (29). These studies focus on priming of anti-tumor T cells in transplantable tumor models. In contrast we analyze the requirement of costimulatory molecules to overcome tolerization in an autochthonous prostate model. Studies have shown that provision of antigen specific CD4 T cells to TRAMP mice sustains SV40 specific CD8 T cell function through CD40L activation of endogenous DCs (30). As CD40L stimulation promotes CD70 expression on DCs (31), CD70 dependent signals from DCs may be a common pathway to activate prostate tumor reactive T cells. Notably, CD80, CD86, and 4-1BBL do not play a direct role in delaying prostate tumor-induced T cell tolerance, perhaps because they do not influence CD70 expression.
The CD80 and CD86 costimulatory molecules are best known for their role in naïve T cell activation. Our results presented in this study demonstrate that CD80 is also required to reactivate tolerized T cells in the prostate tumor tissue. It is notable that CD86−/− DCs are as potent as wild type DCs in reactivating tolerized T cells. CD80−/− DCs exhibit a significantly reduced ability to reactivate tolerized T cells, and this function is completely abolished when combined with CD86 deficiency. These results reveal distinct functions for CD80 and CD86 in DC-mediated reactivation of tolerized T cells. In contrast, blocking CD70 or 4-1BBL does not affect DC’s ability to reactivate tolerized 2C T cells. This is perhaps explained by the lack of CD27 and low 4-1BB expression on persisting T cells in the TRP-SIY prostate. These findings highlight the spatiotemporal specificity of costimulatory molecules in augmenting tumor specific T cell responses.
Our studies demonstrate that the ability of BMDCs to overcome T cell tolerance is based on a combination of Ag specificity and costimulation. Despite Ag expression within the TRP-SIY prostate (11), and a population of tissue resident DCs (13), 2C T cells are tolerized within the prostate. Indeed, we show that endogenous DCs within the prostate express levels of costimulatory molecules barely above background (Fig. 3B). Previous work has shown that depletion of prostate DCs reverses 2C T cell tolerance (13). Considering that tolerance can result from pMHC presentation by DCs lacking costimulatory signals (32), these data suggest that steady-state prostate resident DCs promote T cell tolerance, perhaps through pMHC/TCR engagement without costimulation. However, when peptide-pulsed, LPS-activated, BMDCs are injected into the prostate, 2C T cells are reactivated in a CD70 or CD80/CD86-dependent manner. The injection of activated BMDCs may provide a costimulatory signal that is normally lacking in the TRP-SIY prostate. This notion is supported by three lines of evidence. First, we have demonstrated that 2C T cell reactivation is dependent on the interaction between costimulatory receptors and ligands (Figs. 4, 5). Second, LPS-activated BMDCs without SIY peptide activate a greater percentage of 2C T cells, compared with PBS injection, but less than SIY pulsed LPS activated DCs. This observation suggests that enough residual SIY peptide is present in the prostate to be acquired and presented by activated BMDCs, and further highlights the importance of the pMHC/TCR interaction. Third, the window in which 2C T cells remain activated in this model directly depends on the presence of BMDCs within the prostate (13), reinforcing the importance of the presence of costimulatory molecules.
In addition to stimulatory molecules, 2C T cells within the prostate express high levels of the inhibitory receptor PD-1. In human patients and mouse models of cancer, T cells express PD-1, Tim3, and LAG3 individually or in combination depending on tumor type (16, 33, 34). We find tumor-infiltrating 2C T cells express, and maintain, uniformly high levels of PD-1 but do not express Tim3 or LAG3. Despite the expression of PD-L1 within the tumor islets of the TRP-SIY prostate, genetic deletion of PD-L1 on the TRP-SIY background did not inhibit the induction of T cell tolerance in the prostate tumor tissue. This indicates that PD-1 is not critical for tolerance induction, but perhaps a marker of exhausted T cells, similar to dysfunctional T cells in chronic viral infections (35). Furthermore, PD-L1−/− BMDCs were as active as wild-type DCs in reactivating tolerzied 2C T cells in the prostate tumor tissue. This unexpected finding is seemingly at odds with the literature outlining the increase in tumor associated T cell function after blockade of PD-1 (5, 36). The specifics of our model system likely account for these differences. We note that in many systems restoration of tumor tolerized T cell function require blockade of multiple inhibitory pathways to regain full function (16, 33, 37). As we found that 2C T cells upregulate PD-1 and not LAG-3 or Tim3, there may be additional, unidentified coinhibitory receptors functioning in this system. Further, inefficacy of PD-L1 blockade in the context of BMDC injection may be a consequence of the powerful stimulatory capacity of DCs. BMDCs may provide the maximal stimulation of tolerzied T cells regardless of PD-1/PD-L1 engagement. This highlights the tissue (TRP-SIY prostates) and context (DC mediated reactivation) specificity in the utility of checkpoint blockades in tumor resident T cell activity.
In summary, the differential requirement for CD70 and CD80/CD86 in T cell function within the tumor environment suggests approaches to enhance ACT and DC-based vaccines for cancer immunotherapy. For example, enhancing CD27-CD70 interaction may delay tolerization of adoptively transferred T cells in the tumor microenvironment and enhancing CD80 expression by DCs could stimulate anti-tumor response by endogenous tolerzied CD8+ T cells. Defining the molecular interactions necessary to overcome tumor-induced tolerance is critical, as provision of costimulatory Fc fusion proteins, transduction of whole cell tumor vaccines with costimulatory molecules, and viral vectors expressing costimulatory molecules are under development for the treatment of tumors (2). Our findings provide a basis for the rational design of ACT and DC vaccines targeting tumor specific immune responses.
Supplementary Material
Acknowledgements
We thank Camille Jusino, Carol McKinley, Marisha Mikell and the Swanson Biotechnology Core Facility at the Koch Institute for their technical support, and members of the Chen Lab for their helpful discussions. We thank Dr. Arlene Sharpe (Harvard Medical School, Boston, MA) and Dr Leiping Chen (Yale University, New Haven, CT) for CD80 and PD-L1 knockouts, respectively.
This work is supported in part by a Postdoctoral Fellowship (12109-PF-11-025-01-LIB) from the American Cancer Society (S.P.B.), the Margaret A. Cunningham Immune Mechanisms in Cancer Research Fellowship (S.P.B.) from the John D. Proctor Foundation, and a Prostate Cancer Research Program grant from USAMRMC (to J.C.).
Abbreviations used in this paper
- TRAMP
TRansgenic Adenocarcinoma of the Mouse Prostate
- TRP-SIY
TRAMP mice expressing SIY
- WSN-SIY
WSN influenza A virus strain expressing SIY
- DCs
dendritic cells
- BMDCs
bone marrow-derived DCs
- TILs
tumor-infiltrating lymphocytes
- APCs
Antigen presenting cells
References
- 1.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gulley JL, Drake CG. Immunotherapy for prostate cancer: recent advances, lessons learned, and areas for further research. Clin Cancer Res. 2011;17:3884–3891. doi: 10.1158/1078-0432.CCR-10-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 5.Pilon-Thomas S, Mackay A, Vohra N, Mule JJ. Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J Immunol. 2010;184:3442–3449. doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, McGowan P, Ashe S, Johnston JV, Hellstrom I, Hellstrom KE. B7-1/CD80-transduced tumor cells elicit better systemic immunity than wild-type tumor cells admixed with Corynebacterium parvum. Cancer Res. 1994;54:5420–5423. [PubMed] [Google Scholar]
- 7.Antonia SJ, Seigne J, Diaz J, Muro-Cacho C, Extermann M, Farmelo MJ, Friberg M, Alsarraj M, Mahany JJ, Pow-Sang J, Cantor A, Janssen W. Phase I trial of a B7-1 (CD80) gene modified autologous tumor cell vaccine in combination with systemic interleukin-2 in patients with metastatic renal cell carcinoma. J Urol. 2002;167:1995–2000. [PubMed] [Google Scholar]
- 8.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 9.Arens R, Schepers K, Nolte MA, van Oosterwijk MF, van Lier RA, Schumacher TN, van Oers MH. Tumor rejection induced by CD70-mediated quantitative and qualitative effects on effector CD8+ T cell formation. J Exp Med. 2004;199:1595–1605. doi: 10.1084/jem.20031111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.May KF, Jr, Chen L, Zheng P, Liu Y. Anti-4-1BB monoclonal antibody enhances rejection of large tumor burden by promoting survival but not clonal expansion of tumor-specific CD8+ T cells. Cancer Res. 2002;62:3459–3465. [PubMed] [Google Scholar]
- 11.Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc Natl Acad Sci U S A. 2008;105:13003–13008. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olurinde MO, Shen CH, Drake A, Bai A, Chen J. Persistence of tumor-infiltrating CD8 T cells is tumor-dependent but antigen-independent. Cell Mol Immunol. 2011 doi: 10.1038/cmi.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higham EM, Shen CH, Wittrup KD, Chen J. Cutting edge: delay and reversal of T cell tolerance by intratumoral injection of antigen-loaded dendritic cells in an autochthonous tumor model. J Immunol. 2010;184:5954–5958. doi: 10.4049/jimmunol.1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Deusen KE, Rajapakse R, Bullock TN. CD70 expression by dendritic cells plays a critical role in the immunogenicity of CD40-independent, CD4+ T cell-dependent, licensed CD8+ T cell responses. J Leukoc Biol. 2009;87:477–485. doi: 10.1189/jlb.0809535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakuishi K, Jayaraman P, Behar SM, Anderson AC, Kuchroo VK. Emerging Tim-3 functions in antimicrobial and tumor immunity. Trends Immunol. 2011;32:345–349. doi: 10.1016/j.it.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DA. Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-cell Function to Promote Tumoral Immune Escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou P, Zheng X, Zhang H, Liu Y, Zheng P. B7 blockade alters the balance between regulatory T cells and tumor-reactive T cells for immunotherapy of cancer. Clin Cancer Res. 2009;15:960–970. doi: 10.1158/1078-0432.CCR-08-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuse S, Zhang W, Usherwood EJ. Control of memory CD8+ T cell differentiation by CD80/CD86-CD28 costimulation and restoration by IL-2 during the recall response. J Immunol. 2008;180:1148–1157. doi: 10.4049/jimmunol.180.2.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habib-Agahi M, Phan TT, Searle PF. Co-stimulation with 4-1BB ligand allows extended T-cell proliferation, synergizes with CD80/CD86 and can reactivate anergic T cells. Int Immunol. 2007;19:1383–1394. doi: 10.1093/intimm/dxm106. [DOI] [PubMed] [Google Scholar]
- 20.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP, Pearson TC, Ledbetter JA, Aruffo A, Mittler RS. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169:4882–4888. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 22.Schildknecht A, Miescher I, Yagita H, van den Broek M. Priming of CD8+ T cell responses by pathogens typically depends on CD70-mediated interactions with dendritic cells. Eur J Immunol. 2007;37:716–728. doi: 10.1002/eji.200636824. [DOI] [PubMed] [Google Scholar]
- 23.Tesselaar K, Xiao Y, Arens R, van Schijndel GM, Schuurhuis DH, Mebius RE, Borst J, van Lier RA. Expression of the murine CD27 ligand CD70 in vitro and in vivo. J Immunol. 2003;170:33–40. doi: 10.4049/jimmunol.170.1.33. [DOI] [PubMed] [Google Scholar]
- 24.Seko Y, Takahashi N, Oshima H, Shimozato O, Akiba H, Takeda K, Kobata T, Yagita H, Okumura K, Azuma M, Nagai R. Expression of tumour necrosis factor (TNF) ligand superfamily co-stimulatory molecules CD30L, CD27L, OX40L, and 4-1BBL in murine hearts with acute myocarditis caused by Coxsackievirus B3. J Pathol. 2001;195:593–603. doi: 10.1002/path.986. [DOI] [PubMed] [Google Scholar]
- 25.Kurlander RJ, Ellison DM, Hall J. The blockade of Fc receptor-mediated clearance of immune complexes in vivo by a monoclonal antibody (2.4G2) directed against Fc receptors on murine leukocytes. J Immunol. 1984;133:855–862. [PubMed] [Google Scholar]
- 26.Keller AM, Schildknecht A, Xiao Y, van den Broek M, Borst J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity. 2008;29:934–946. doi: 10.1016/j.immuni.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Bertram EM, Lau P, Watts TH. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J Immunol. 2002;168:3777–3785. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- 28.Keller AM, Xiao Y, Peperzak V, Naik SH, Borst J. Costimulatory ligand CD70 allows induction of CD8+ T-cell immunity by immature dendritic cells in a vaccination setting. Blood. 2009;113:5167–5175. doi: 10.1182/blood-2008-03-148007. [DOI] [PubMed] [Google Scholar]
- 29.Roberts DJ, Franklin NA, Kingeter LM, Yagita H, Tutt AL, Glennie MJ, Bullock TN. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J Immunother. 2010;33:769–779. doi: 10.1097/CJI.0b013e3181ee238f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shafer-Weaver KA, Watkins SK, Anderson MJ, Draper LJ, Malyguine A, Alvord WG, Greenberg NM, Hurwitz AA. Immunity to murine prostatic tumors: continuous provision of T-cell help prevents CD8 T-cell tolerance and activates tumor-infiltrating dendritic cells. Cancer Res. 2009;69:6256–6264. doi: 10.1158/0008-5472.CAN-08-4516. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Bullock TN, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J Immunol. 2005;174:710–717. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- 32.Hochweller K, Anderton SM. Kinetics of costimulatory molecule expression by T cells and dendritic cells during the induction of tolerance versus immunity in vivo. Eur J Immunol. 2005;35:1086–1096. doi: 10.1002/eji.200425891. [DOI] [PubMed] [Google Scholar]
- 33.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blackburn SD, Crawford A, Shin H, Polley A, Freeman GJ, Wherry EJ. Tissue-specific differences in PD-1 and PD-L1 expression during chronic viral infection: implications for CD8 T-cell exhaustion. J Virol. 2010;84:2078–2089. doi: 10.1128/JVI.01579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenblatt J, Glotzbecker B, Mills H, Vasir B, Tzachanis D, Levine JD, Joyce RM, Wellenstein K, Keefe W, Schickler M, Rotem-Yehudar R, Kufe D, Avigan D. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother. 2011;34:409–418. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V, Zarour HM. CD8+ T Cells Specific for Tumor Antigens Can Be Rendered Dysfunctional by the Tumor Microenvironment through Upregulation of the Inhibitory Receptors BTLA and PD-1. Cancer Res. 2012;72:887–896. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.