Abstract
Significance: Targeted irradiation is an effective cancer therapy but damage inflicted to normal tissues surrounding the tumor may cause severe complications. While certain pharmacologic strategies can temper the adverse effects of irradiation, stem cell therapies provide unique opportunities for restoring functionality to the irradiated tissue bed. Recent Advances: Preclinical studies presented in this review provide encouraging proof of concept regarding the therapeutic potential of stem cells for treating the adverse side effects associated with radiotherapy in different organs. Early-stage clinical data for radiation-induced lung, bone, and skin complications are promising and highlight the importance of selecting the appropriate stem cell type to stimulate tissue regeneration. Critical Issues: While therapeutic efficacy has been demonstrated in a variety of animal models and human trials, a range of additional concerns regarding stem cell transplantation for ameliorating radiation-induced normal tissue sequelae remain. Safety issues regarding teratoma formation, disease progression, and genomic stability along with technical issues impacting disease targeting, immunorejection, and clinical scale-up are factors bearing on the eventual translation of stem cell therapies into routine clinical practice. Future Directions: Follow-up studies will need to identify the best possible stem cell types for the treatment of early and late radiation-induced normal tissue injury. Additional work should seek to optimize cellular dosing regimes, identify the best routes of administration, elucidate optimal transplantation windows for introducing cells into more receptive host tissues, and improve immune tolerance for longer-term engrafted cell survival into the irradiated microenvironment. Antioxid. Redox Signal. 21: 338–355.
Introduction
Targeted radiotherapy is an effective treatment for cancer, but the amount of curative radiation that can be delivered to the tumor is limited by the sensitivity of normal tissues surrounding the lesion. While cancer therapies increasingly achieve cure and extend survival, they often result in chronic side effects in patients (29, 99, 142, 148, 149). Paradoxically, modern therapies threaten to increase the burden of chronic toxicity, not reduce it (6). Acute reactions impacting normal tissue injury early after treatment are related to oxidative stress and inflammation that alter the microenvironment which includes sensitive stem cell niches (78). These changes prime the irradiated tissue bed for a wide-range of multifaceted late effects.
Recent research has identified a wealth of pharmacologic strategies to temper the adverse effects of radiation exposure (23, 163). Although pharmacologic interventions have only limited ability to mitigate severe side effects of radiotherapy, some studies have shown the potential of these treatments to inhibit the onset of tissue damage (163). While certain pharmacologic approaches are beneficial, none offer the potential of stem cell-based strategies, which afford the opportunity to functionally replace cells lost or damaged during irradiation. The use of stem cell therapy to promote recovery of normal tissues exposed to ionizing radiation aims at ameliorating the unintended side effects due to normal tissue damage. With the exception of bone marrow transplants, used for many years to reconstitute the hematopoietic compartment after ablative irradiation (83), the application of stem cell therapies for reducing other normal tissue sequelae remains a new but burgeoning area of research.
The potential benefits of stem cell therapy are certainly not limited to cell replacement, as significant evidence exists, showing that engrafted cells provide trophic support to the surrounding host tissue (123, 172, 196, 198). Regardless of the mechanism, protecting and/or restoring endogenous cell function will reduce normal tissue injury and hasten the recovery of patients who are subjected to irradiation. Mesenchymal stem cells (MSCs) were originally proposed for therapeutic purposes in regenerative medicine, based on their multipotent, proliferative, and anti-inflammatory properties. However, in recent years, therapeutic strategies are now evolving in which other stem/progenitor cell types are used alone or in co-transplantation with endothelial or epithelial progenitors to hasten the recovery and reconstitution of normal tissue.
The identification of stem cell populations and the capability to isolate and expand them for therapeutic uses has stimulated a wealth of research into regenerating injured tissue. Despite this promise, the past decade has also shown that translating the potential of stem cell therapy into actual practice is not easy, and many barriers, including immunorejection (22, 129), teratogenesis (106), regulatory (107) and ethical issues (46), still need to be overcome before such strategies become commonplace in the clinic. Thus, discussions of therapeutic efficacy to restore functionality to irradiated tissues are provided along with the caveats associated with such treatments. The present review will highlight recent advances in the application of various stem cell-based strategies to ameliorate radiation-induced normal tissue damage occurring within selected target organ sites.
Stem Cell Therapy to Ameliorate Radiation-Induced Cognitive Impairment
Worldwide, ∼240,000 patients are diagnosed with brain tumors per year. Delayed adverse cognitive effects can occur in approximately 50% of irradiated patients, depending on radiation dose, fractionation schedule, irradiated volume, and location (29, 59, 82). Brain necrosis developing >6 months after radiotherapy is uncommon when total doses of 50 Gray (Gy) are delivered in fractions of 2 Gy or less; a tolerance dose of 57 Gy has been suggested. However, children who receive 30–35 Gy of whole brain irradiation frequently develop intellectual deficiencies over the next few years (84, 156, 180, 189).
Acute and early delayed effects are associated with short-term memory loss, fatigue, and somnolence (197). Acute responses involving oxidative stress and inflammation can elicit apoptosis and signaling changes that contribute to disruption of the blood–brain barrier, hypoxia, transient demyelination, and more persistent injury (208). Late effects typified by demyelination, vascular breakdown leading to edema, and radionecrosis of the white matter are progressive and generally irreversible (181–183, 201). Current concepts of radiation injury now recognize that alterations to the microenvironment involving oxidative stress, inflammation, vascular dysfunction, and impaired neurogenesis contribute to central nervous system (CNS) pathology and disrupted cognitive processing (68, 69, 197, 208).
Some of the more promising interventions for ameliorating radiation-induced neurocognitive sequelae are agents that inhibit inflammatory processes (169, 170, 217). Agonists targeting the peroxisomal proliferator-activated receptor family can attenuate neuroinflammation, resulting in preserved hippocampal neurogenesis and cognitive ability after irradiation (169, 170, 216). Inhibiting the renin–angiotensin system also has shown promise at ameliorating late effects in the CNS after irradiation (41, 176).
One well-defined effect of cranial irradiation is its adverse effect on endogenous neurogenesis. Generation of CNS progenitor cells occurs in the subventricular (SVZ) and dentate subgranular zones (SGZ) (74, 202). Each of these processes contributes new glia and neurons to the brain. SVZ-derived neuroblasts migrate along the rostral migratory stream to repopulate the olfactory bulb, while SGZ-derived neural precursors repopulate the granule cell layer of the hippocampus (74). Irradiation depletes radiosensitive populations of multipotent precursors in the hippocampus, leading to an inhibition of neurogenesis and cognitive impairment (144, 167, 177). The growing evidence linking neural stem and precursor cell depletion to neurocognitive sequelae has stimulated efforts that are aimed at cell replacement strategies in the CNS.
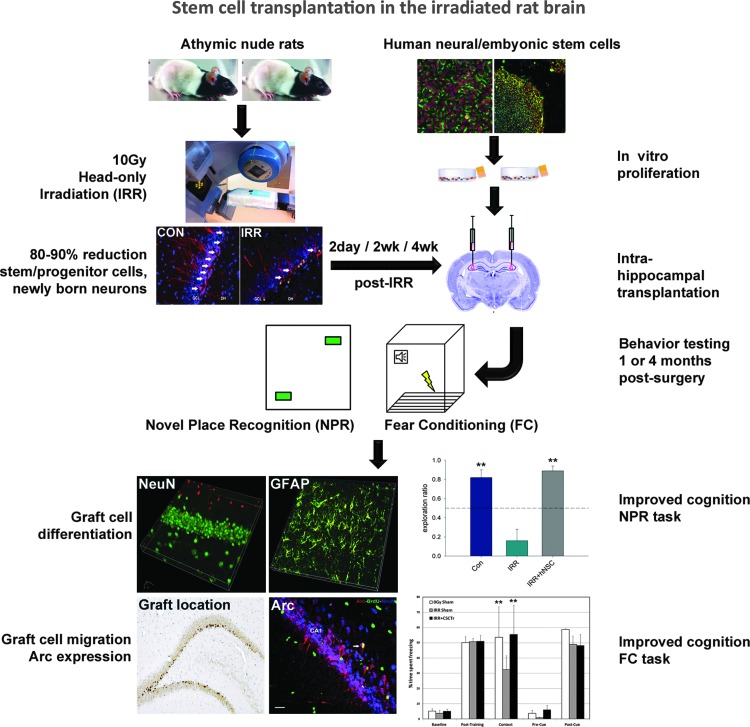
Regenerative medicine targeted to the CNS took shape soon after the first isolation of neural stem cells from the rodent brain (173, 190). Application of stem cell therapies to ameliorate radiation-induced normal tissue injury soon followed, as neural stem cell transplants to the spinal cord were found to ameliorate radiation-induced myelopathy (174). While radionecrosis remains a complicating factor for spinal cord treatments (34), neurologic dysfunction is now considered the major dose-limiting factor for radiotherapy of the brain (56). The sensitivity of adult neurogenesis to irradiation has stimulated efforts to explore the use of stem cell transplantation for reversing cognitive dysfunction after cranial radiotherapy. Recent work has demonstrated the benefits of intrahippocampal transplantation of either human embryonic or human neural stem cells (hNSCs) to ameliorate radiation-induced impairment of cognition (1–3). In these studies, rats were cranially irradiated 2 days before bilateral intrahippocampal transplantation of human stem cells. At 1 and 4 months after irradiation and transplantation, significant survival of the transplanted cells was demonstrated. Engrafted cells migrated extensively throughout the septo-temporal axis of the hippocampus and differentiated along neuronal and astroglial lineages. Importantly, rats receiving stem cell transplants showed improved cognitive performance compared with irradiated rats receiving sham surgery (1–3). Key features of the foregoing experimental approach are depicted in Figure 1.
FIG. 1.
Stem cell transplantation for ameliorating radiation-induced cognitive dysfunction. Immunocompromised athymic rats are subjected to cranial irradiation, while human stem cells are grown in parallel. At specified times (2 days, 2 or 4 weeks) after irradiation, animals are subjected to hippocampal transplantation with fixed numbers of human stem cells. At specific times (1 or 4 months) after irradiation, animals are then evaluated for cognitive performance using NPR and FC tasks. Irradiated animals transplanted with stem cells (IRR+hNSC) exhibit higher exploration ratios in NPR and increased time spent freezing in the context phase of FC, demonstrating improved hippocampal-dependent performance. After completion of cognitive testing, the brains of animals are analyzed for engrafted cell survival, migration, differentiated fate (NeuN-neuronal fate, GFAP-astroglial fate), and functional integration by the expression of the Arc. Asterisks represent significance (p<0.05) compared to irradiated groups. Arc, activity-regulated cytoskeleton-associated protein; FC, fear conditioning; hNSC, human neural stem cell; NPR, novel place recognition.
The mechanisms of stem cell-based cognitive rescue after irradiation are definitely complex. The beneficial effects are likely to involve both the replacement of lost or damaged cells and increased trophic support to the brain by transplanted cells or endogenous stem cells homing to the site of radiation injury. Research by the group of Limoli has shown that transplanted cells express activity-regulated cytoskeleton-associated protein, suggesting their capability to functionally integrate into the hippocampus (2). Moreover, trophic factors such as glial cell line-derived neurotrophic factor and basic fibroblast growth factor (FGF) released from engrafted astro-glial cells may support the functions of mature host neurons or foster neurogenesis and the maturing neuroblasts (35, 80, 101, 192). The release of brain-derived neurotrophic factor from transplanted hNSCs has been shown to provide some cognitive benefits in a mouse model of Alzheimer Disease (18). Moreover, bone marrow-derived stem cells may provide vascular support and help maintain the specialized vascular niche for stem cell maintenance (54, 91, 150, 195, 220). They can be recruited directly to the site of radiation injury in the brain, where they appear to provide support to the microvasculature (28).
While regenerative medicine holds considerable promise, inherent limitations to the repair of neural tissue present practical limitations. The migration of engrafted cells toward damaged tissue is a critical step in stem cell therapy, and further work is required to elucidate the chemokine signaling networks that regulate this process (16, 31, 95). The risk of immunorejection also weighs heavily on any transplantation study (9). These issues impact the assessment of stem cell safety and efficacy in efforts to translate animal studies to the clinic (9). Clearly, there is much to learn, but despite these inherent shortcomings, the treatment of neurocognitive sequelae through stem cell therapy is a worthy pursuit, especially in the absence of any satisfactory, long-term interventions for this serious complication of cranial radiotherapy.
Stem Cell Therapy to Ameliorate Radiation-Induced Xerostomia
Worldwide, about 500,000 patients are diagnosed with head and neck cancer (HNC) per year. Of these, 70% are treated with radiotherapy alone or in combination with surgery and/or chemotherapy (94). Radiotherapy of HNC often involves co-irradiation of the salivary glands that may induce hyposalivation, an important factor in the development of xerostomia (58, 204). To avoid a long-term loss of salivary function, at least one parotid gland should be spared to a mean dose of less than 20 Gy to preserve function if the other is targeted with a higher dose. Alternatively, when both glands are irradiated, total doses need to be less than 25 Gy, whereas the submandibular gland needs to be spared to doses less than 35 Gy (25, 50, 116).
Current strategies that prevent radiation-induced salivary gland dysfunction include the use of protective medication, such as amifostine or pilocarpine (24, 27, 120), surgical relocation of the submandibular gland (215), and minimization of the radiation dose administered to the major salivary glands (43, 62, 153, 203, 206). Although intensity-modulated radiotherapy has provided a significant reduction of xerostomia, 40% of patients still develop life-long complaints (62). Radiation-induced damage to salivary glands mainly results from the inability of stem cells to produce a sufficient number of mature functional cells (110). Increasing the regenerative potential of salivary glands by stem cell therapy should help restore tissue homeostasis, and adult tissue stem cells have shown great clinical potential (43, 44, 55).
Interestingly, the non-functioning ducts mostly remain intact after irradiation (43), offering the opportunity for transplanted stem cells to engraft to a proper niche and to regenerate the salivary gland. Indeed, the protective effect of prophylactic pilocarpine (26) and keratinocyte growth factor (KGF) (126) seems to be due to stimulated proliferation of ductal stem/progenitor cells. Ductal stem/progenitor cells can also be stimulated by factors which are secreted by bone marrow-derived cells (BMDCs) that home to damaged salivary glands after mobilization (125). Some studies controversially claim that salivary glands which are damaged by irradiation can be rescued by bone marrow stem cells that transdifferentiate or have adopted an acinar-like phenotype (121, 199). Similar observations have been made for MSCs (137). However, characterization of the most potent salivary gland stem cell is still ongoing.
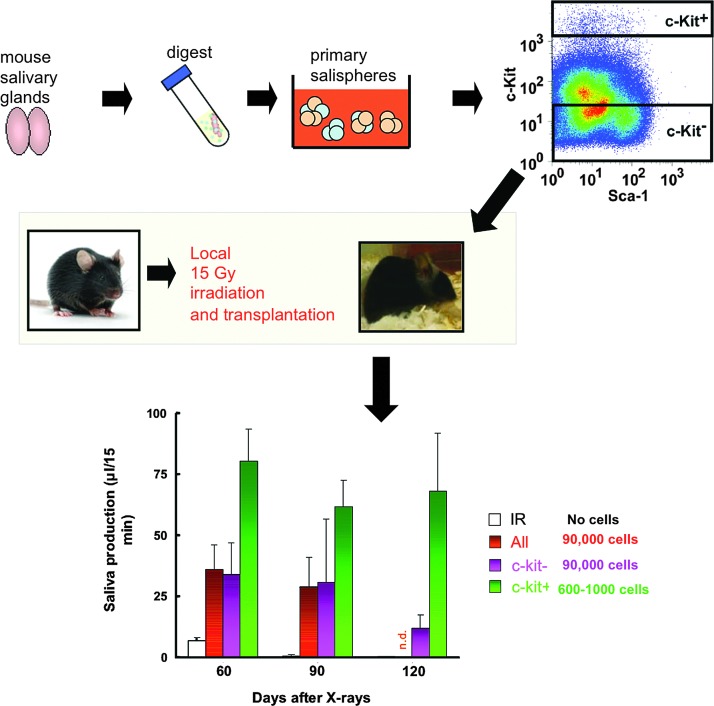
Current research is largely focused on the characterization, isolation, and transplantation of tissue-derived stem cells. Both mouse (164) and human (67) salivary gland stem cells can be isolated from cultured salispheres. While identification of the bonafide salivary stem cell remains elusive, c-Kit+ cells exhibited a remarkable capability to ameliorate radiation damage, as shown in Figure 2 (124). Furthermore, CD24/CD29, CD49f, and CD133 expressing cells were able to differentiate into all salivary gland lineages (67, 124, 151, 164) and were able to regenerate the irradiated salivary gland (124, 151).
FIG. 2.
Transplantation of salivary gland stem cells rescues mice from radiation-induced hyposalivation. Salivary gland tissue is dispersed to a cell solution and allowed to form salispheres in culture. From these salispheres, c-Kit expressing stem cells are selected and injected into locally irradiated glands to improve saliva secretion [adapted from Gao et al. (75)]. n.d., not determined.
Despite these advances, several issues need to be addressed before these laboratory-based findings can be translated to the clinic (165). Evidence substantiating that functional recovery can be achieved through the preferential administration of transplanted cells via retrograde ductal injection needs to be obtained. Moreover, while several candidate salivary gland stem/progenitor cells have been suggested for human use (13, 42, 108, 127, 136, 157), safety issues need to be addressed experimentally before their clinical application (79).
Stem Cell Therapy to Ameliorate Osteoradionecrosis
Irradiation of bone may result in four major complications: osteoradionecrosis (ORN) (139), insufficiency fractures (40), severe alterations of bone growth, and radiation-induced neoplasms. Septic ORN typically occurs in HNC patients, combining superficial epithelial radionecrotic ulceration and deep exposed mandible, whereas aseptic ORN may affect the hip in pelvic cancer or the ribs in breast cancer. The estimated tolerance doses for mandibular bone are 60 and 72 Gy for 5% and 50% incidences of necrosis (63, 93).
ORN is a non-healing wound resulting from hypoxia, hypovascularization, and tissue hypocellularity followed by tissue breakdown (139). Early stages of bone atrophy are linked to the imbalance between bone formation and bone resorption (103). Irradiation leads to the inhibition of MSCs and osteogenic cell proliferation (179) and may promote the terminal differentiation of osteoblasts (48). During progression of the lesion, irradiated bone shows signs of osteoporosis characterized by atrophy and a disorganized trabecular structure, resulting from an imbalance in osteogenesis/osteolysis, defects in neovascularization, and fibrosis. After irradiation, the myofibroblasts appear at the initial inflammatory phase and persist during the constitutive fibrotic phase (14). Transforming growth factor-β1 (TGF-β1) is considered the main cytokine involved in this process, resulting in increased extracellular matrix secretion and decreased metalloproteinase excretion (51). TGF-β1 plays an important role in establishing and maintaining vessel integrity by ameliorating damage to the arteries and veins surrounding the bone after irradiation (161). Vascular damage and ischemia appear before osteoblast alteration (60, 152).
Treatment of squamous cell carcinoma of the upper aero-digestive tract often requires large surgical bone removal and reconstruction with anastomosed free flaps (90). Irradiation reduces healing capacities and is associated with a higher risk of vascularized flap failure. The use of calcium phosphate biomaterials has been considered an alternative to autogenous bone grafts. However, calcium phosphate alone is not sufficient for bone substitution in irradiated areas (119), and it needs to be used in conjunction with total bone marrow (TBM) for more optimal stimulation of bone healing (Fig. 3). Biphasic calcium phosphate (BCP) associated with TBM provides better bone reconstruction after radiotherapy (131), whereas association of MSCs with calcium phosphate leads to better bone reconstruction in non-irradiated areas (12, 134, 211, 221). Studies in an irradiated mouse model also showed that TBM was superior to MSC for association with BCP for bone reconstruction in irradiated areas (64). These results highlight the potential benefits of stem cells used in conjunction with biomaterials for combined regenerative strategies proposed in clinical applications (64). A current phase I clinical trial for mandible ORN treatment (ClinicalTrials.gov, identifier: NCT01147315) will provide some of the first results in humans.
FIG. 3.
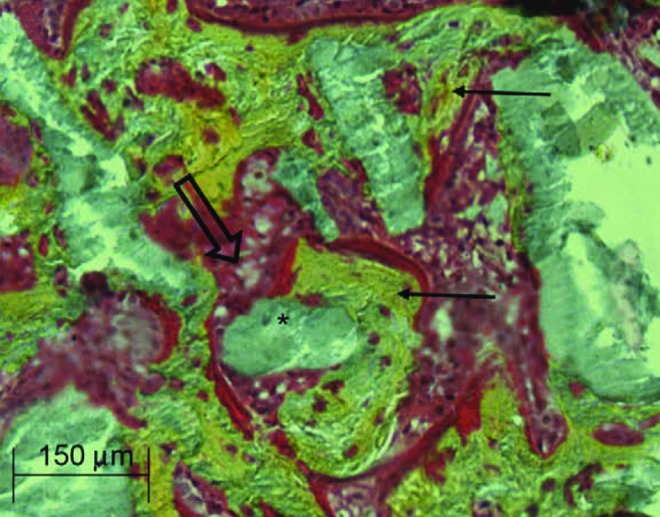
Bone marrow grafts treated with BCP. Histologic section reveals healing of defective bone, shown as the BCP substitute associated with the bone marrow graft in the irradiated bone area. New bone formation (yellow, ←) in contact with the BCP (gray, *). Rich bone marrow (red, ⇒) in contact with the BCP. BCP, biphasic calcium phosphate.
Stem Cell Therapy to Ameliorate Radiation-Induced Skin Fibro-Necrosis
Acute skin reactions that range from a mild rash to severe ulceration are one of the most common side effects of external radiotherapy. This pathological condition leads to pain, delays in treatment, and an overall decrease in quality of life (130, 141). Radiation dermatitis may occur after a total dose of 55 Gy and usually manifests within 1–4 weeks. At later times, subcutaneous fibrosis and occasionally radionecrosis may develop (20). Acute reactions result from the loss of rapidly proliferating cells, combined with vascular and inflammatory responses. Late radiation effects manifest in the slowly proliferating cell populations, resulting in progressive TGF-β1-mediated fibrosis and tissue hypoxia. Radiation-induced fibrosis and necrosis are usually considered intractable, manifesting as impaired healing and prone to recurrence even after minor trauma.
A current management strategy for skin fibrosis includes anti-inflammatory treatment with corticosteroids or interferon gamma, vascular therapy with pentoxifylline or hyperbaric oxygen, and antioxidant treatment with superoxide dismutase or tocopherol (52). Under certain circumstances, the radiolesion becomes refractory to treatment, leaving surgery as a final option, with increased risk of disability and morbidity. Such complications suggest the need to develop new therapeutic approaches using adult stem cells for the repair of radiation-induced tissue injury. Preclinical studies have identified MSCs as promising cell-based agents for the treatment of tissue necrosis. For clinical applications, MSCs can be expanded to mass culture, and they can be cryopreserved without loss of viability or stem cell potency (5, 118). Recent work has successfully demonstrated the preclinical benefit of systemic MSC injections for ulcerated skin and muscle restoration after high-dose irradiation (17). The beneficial effects rely on paracrine mechanisms, by which cytokines and growth factors that are released from engrafted cells favorably influence wound healing (73).
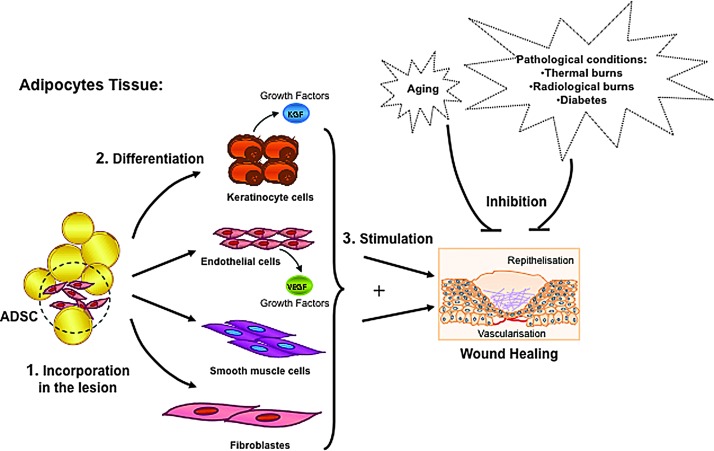
Recent studies have demonstrated a marked plasticity of adipose-derived stem cells (ADSC), mainly comprising mesenchymal cells. The harvest of adipose tissue is easy, generally involving minimally invasive lipoaspiration procedures. Topical administration of ADSC into full-thickness wounds normalizes defective wound healing in irradiated mice (61). Interestingly, ADSC can differentiate into keratinocytes, and ADSC-derived KGF released at the site of injury may regulate the healing of the cutaneous wound. ADSC have also been shown to incorporate into vessels and differentiate into endothelial cells, leading to improved perfusion and vessel density (61). A schematic demonstrating the plasticity and capability of ADSC to facilitate wound healing is shown in Figure 4. Endothelial progenitor cells (EPC) similarly incorporated into vessels, differentiated into CD31-expressing endothelial cells, and increased perfusion and vessel density (102). An injection of EPC increased vascular permeability, suggesting that progenitor cell-induced nitric oxide-dependent vasodilatation and hyperpermeability were required for their proangiogenic effects (210, 212).
FIG. 4.
ADSC for the resolution of skin injury. ADSC exhibit marked plasticity toward keratinocyte and endothelial cells. Stem cells of the adipose lineage have the ability to differentiate into epithelial cells, acquire a functional keratinocyte phenotype, and differentiate into vascular cells. These cells can be used to facilitate the repair of radiation-induced skin injury as well as other pathological conditions of the skin. ADSC, adipose-derived stem cells.
The group of Rigotti (175) demonstrated that lipoaspirate transplantation was clinically effective in the treatment of the side effects of skin radiation. Autologous fat grafts showed a generalized improvement of clinical symptoms in all 20 treated patients (104). Clinically purified autologous lipoaspirates were also proposed by the group of Akita (7) for the treatment of radiation-induced late skin complications. ADSC showed the capability to promote the growth of a microvascular bed that facilitated the subsequent correction of chronic ischemic damage. The group of Klinger also recently demonstrated (32) the efficacy of autologous fat grafts in the post-mastectomy pain syndrome (PMPS). The cohort was composed of 113 patients who were affected by PMPS and who underwent mastectomy with axillary dissection and radiotherapy. The reported pain control with a mean follow up of 13 months could be linked to inflammation reduction along with an improvement in tissue quality and scar softness with beneficial effects on nerve entrapment (115). Finally, adequate debridement combined with skin autograft and an injection of MSC showed significant therapeutic benefit for approximately 7 years in the medical management of severe skin and underlying muscle necrosis in eight patients (17).
The foregoing pre-clinical and clinical studies have now demonstrated proof of concept for the use of stem cells in the therapeutic management of radiation-induced skin damage. Further innovations are now required to efficiently translate these findings into clinical protocols for minimizing the side effects associated with radiotherapy.
Stem Cell Therapy to Ameliorate Radiation-Induced Liver Disease
Whole liver cannot tolerate more than 35 Gy of radiation therapy (RT) in standard fractionation because of the induction of potentially lethal radiation-induced liver disease (RILD). RILD was recently analyzed in the Quantitative Assessment of Normal Tissue Effects in the Clinic project (63, 96, 158). Patients with classic RILD present with symptoms of fatigue, rapid weight gain, enlarged liver, ascites, and an isolated elevation in alkaline phosphatase, ∼2 weeks to 4 months after liver RT (117). All other liver functions, including serum bilirubin and ammonia levels, are usually normal, unless the patients have radiation-induced reactivation of hepatitis B virus replication. Histopathologically, RILD is characterized as a hepatic central veno-occlusive disease with occlusion of the central vein lumina and hepatic sinusoids by reticulin and collagen fibers, resulting in vascular congestion and atrophy of centrilobular hepatocytes (66, 154, 171). Since radiation induces apoptosis of the liver sinusoidal endothelial cells (LSECs) in a dose-dependent manner, the clinical syndrome has also been named sinusoidal obstructive syndrome (53). Besides endothelial injury, regenerating hepatocytes are sensitive to radiation-induced mitotic catastrophe, as noted in patients with cirrhotic livers, who have elevated serum transaminases and ascites, indicating radiation-induced hepatocellular death (36).
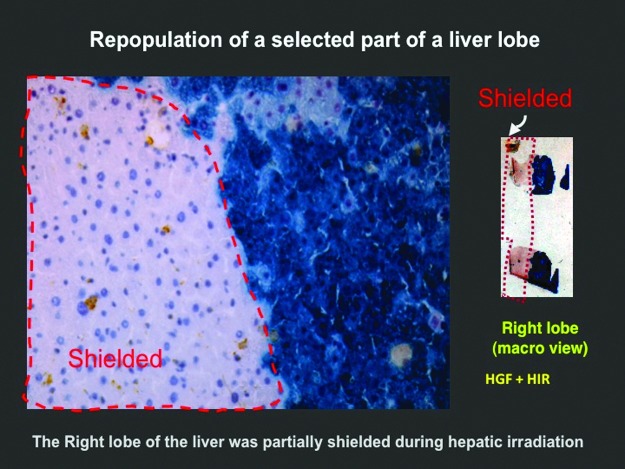
Currently, there is no effective treatment for RILD (85). Since irradiation inhibits hepatocellular regeneration, it was hypothesized that intra-portal or intra-splenic transplantation of unirradiated hepatocytes would enable donor cells to efficiently engraft in irradiated liver lobes, and selectively proliferate in response to mitogenic growth factors as the irradiated host hepatocytes undergo cell cycle arrest and/or mitotic death. A model of RILD was developed in F344 rats after treatment with partial hepatectomy and hepatic irradiation (HIR) (88). Within 6 weeks after HIR, acute effects included perivenous steatosis and hepatocellular atrophy, while late effects manifested as severe bile duct proliferation and periportal fibrosis by 12 weeks. Mortality from RILD was 60–75%. Intrasplenic transplantation of 5×106 congenic hepatocytes ameliorated the acute and late effects of RILD and significantly improved survival (88) with normal liver function. Immunohistochemical staining for donor hepatocytes revealed progressive proliferation with near-total replacement of the irradiated host hepatocytes by donor cells over 3 months. Such results are shown in Figure 5, where donor-derived repopulation of a liver lobe subjected to partial irradiation was demonstrated after hepatocyte transplantation (HT). Strikingly, proliferation of the donor hepatocytes occurred primarily in the irradiated liver lobes, evident in the lower power histomicrographs that highlight the efficiency of the preparative regime for replacing hepatocytes depleted during irradiation (Fig. 5).
FIG. 5.
Preparative liver irradiation for hepatocyte transplantation. The anterior half of the right lobe of liver was irradiated with a single fraction of 50 Gray in C57Bl/6 mice, followed by intrasplenic transplantation of 1×106 beta-galactosidase-proficient hepatocytes isolated from congeneic Rosa mouse, 1 day after irradiation. Hepatic mitogenic signal was provided by intravenous administration of an adenovirus expressing recombinant human HGF within 1 h after liver irradiation. Animals were sacrificed 16 weeks after hepatocyte transplantation. Data show beta-galactosidase staining of a fresh frozen section of the right lobe of the liver 16 weeks after hepatocyte transplantation. Note preferential repopulation of the donor beta-galactosidase-positive hepatocytes (blue) in the irradiated liver lobe. HGF, hepatocyte growth factor.
Since classic RILD manifests as sinusoidal obstructive syndrome, it was reasoned that acute loss of LSEC in irradiated livers would require rapid restoration of the sinusoidal endothelium. Therefore, LSECs were transplanted via an intra-portal or intra-splenic injection after HIR and a recombinant adenovirus-expressing hepatocyte growth factor (HGF) was administered to provide mitogenic signals for donor cells. LSEC transplantation ameliorated radiation-induced sinusoidal obstructive syndrome with donor cells selectively engrafting and repopulating the irradiated hepatic sinusoidal endothelium by 8 weeks (100).
Orthotopic liver transplantation (OLT) is the only curative therapy for many patients with terminal liver diseases. However, due to shortage of donor livers, there is a long waiting list for OLT. HT is being explored as a therapeutic alternative. However, HT is limited because the number of cells that can be safely transplanted into the liver is inadequate to compensate for liver function. Encouraged by the massive repopulation of donor hepatocytes after HT for ameliorating RILD in rodents, focal liver irradiation was examined as a preparative regimen for HT in the treatment of metabolic liver diseases (85–87). Several investigators have demonstrated that preparative HIR in combination with hepatic mitogenic signals can enable selective engraftment (209) and proliferation of donor hepatocytes in irradiated host livers (37, 111, 132). Thus, preparative HIR has been used to correct liver function in murine and rodent models of inherited metabolic diseases, such as primary hyperoxaluria (89, 97), Wilson's disease (133), and hypercholesterolemia (218).
A phase I clinical trial (NCT01345578) of preparative HIR to facilitate HT has been initiated in patients with metabolic liver diseases (70). Partial liver irradiation could be safely administered using stereotactic body RT via three dimensional conformal or intensity modulated radiotherapy followed by HT for the treatment of inherited liver diseases, liver failure, hepatic gene therapy, RILD, and for generating human-mouse liver chimera animal models.
Cardiac Damage and Stem Cell Replacement
Thoracic irradiation significantly increases the risk of cardiovascular disease in cancer survivors. Long-term survivors of Hodgkin's lymphoma and childhood cancers have 2 to >7-fold increased risks of cardiac death for total doses of 30–40 Gy (200). Increased cardiac morbidity and mortality also occur after irradiation for breast cancer, despite having a relatively small part of the heart included in the irradiation field. The Early Breast Cancer Trialists' Collaborative Group evaluated data from >30,000 women in randomized trials of radiotherapy versus no radiotherapy and showed a 27% excess of heart disease in the irradiated group (38, 140). The risk of cardiac death was related to the estimated mean cardiac dose, increasing by 3% per Gy (140).
Animal studies show that radiation-induced damage to the myocardium is primarily caused by damage to the microvasculature, leading to inflammatory and thrombotic changes and vascular leakage (184, 185). Progressive reduction in the number of capillaries led to reduced perfusion of the cardiac muscle, ischemia, myocardial cell death, and fibrosis (65, 138). Animal data are supported by clinical studies that demonstrate regional perfusion defects in non-symptomatic breast cancer patients at 6 months to 5 years after radiotherapy (4, 47). Radiation also predisposes to the formation of inflammatory, unstable atherosclerotic lesions, which are prone to rupture and may cause a fatal heart attack (155, 191).
There is currently no specific pharmacological treatment for cancer therapy-related cardiomyopathy, although symptomatic patients may receive standard treatments for congestive heart failure, including angiotensin-converting-enzyme inhibitors. Animal studies have demonstrated some benefit from the radioprotector amifostine when administered before cardiac irradiation (112), or pentoxifylline, in combination with the anti-oxidant vitamin E (19, 122).
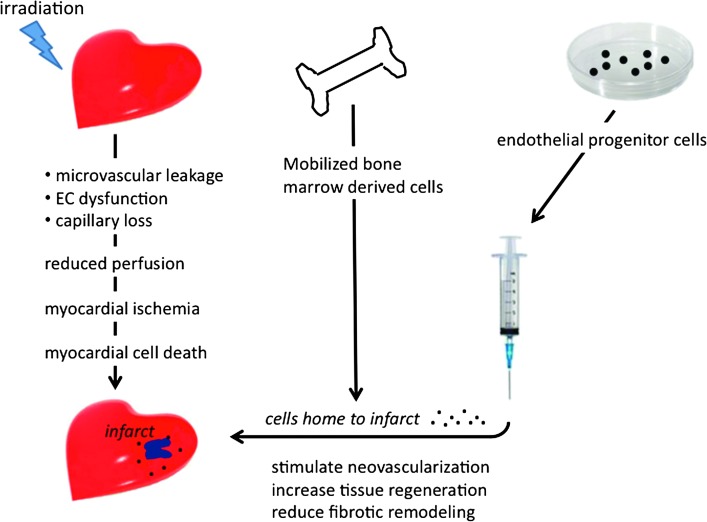
Recent studies have identified stem and progenitor cells that can generate myocytes, smooth muscle cells, and endothelial cells and participate in regeneration of the adult heart (11, 15, 30). Circulating BMDCs also migrate to sites of ischemic damage in the heart and can contribute to new vessel formation by fusion with resident cardiac cells and by secretion of pro-angiogenic and survival factors. Preclinical studies show that both mobilization and transplantation of BMDC improve the microvascularity and cardiac function after acute myocardial ischemia and in models of chronic cardiac fibrosis (39, 45, 49, 147). Since radiation is known to cause progressive microvascular damage and capillary loss (65, 138, 184, 185), this technology may also have potential for treating radiation-induced cardiac injury (Fig. 6), although this has not yet been tested.
FIG. 6.
Radiation-induced myocardial damage and stem cell treatments. Irradiation causes microvascular damage and capillary loss, which leads to inadequate perfusion of the tissue and may result in myocardial infarct. Bone marrow progenitor cells can either be mobilized to the blood using growth factors, or extracted and cultured in vitro to enrich populations of endothelial progenitor cells. Mobilized or injected progenitor cells home to the infarct area and stimulate surviving cells to proliferate and regenerate damaged tissue.
Meta-analyses of randomized clinical trials demonstrate small, but significant, improvements in short-term cardiac function after BMDC therapy, with reduced benefit at later times (207, 214, 219). There was a positive correlation between the number of cells injected and an improvement in cardiac function, but there were no significant improvements in long-term survival (207). Preclinical studies indicate that only 1–3% of injected BMDC are found at infarcted areas of the heart, although ex-vivo enrichment of specific cell populations, for example, CD34+ cells, can considerably increase this engraftment. Cardiomyocyte progenitor cells (CPC) and embryonic stem cells (ESC) have greater capacity for stable engraftment and proliferation, and they can differentiate into cardiomyocytes. There are several ongoing phase 1 trials using CPC isolated from a biopsy of the patient's own heart and expanded ex-vivo (92, 166). Preliminary results seem promising, but the numbers of patients included are very small (21). Challenges for the future in applying stem cell therapy for radiation injury to the heart include the identification and expansion of optimal cell types, the number of cells, the route of administration, and the timing of therapy.
Stem Cell Therapy to Ameliorate Radiation-Induced Proctitis
Approximately 500,000 patients per year undergo abdominal or pelvic radiotherapy worldwide. Of these, 5–10% will develop pelvic radiation disease (PRD) within 10 years (10). Acute radiation responses, including abdominal pain, diarrhea/constipation, and malabsorption, may interrupt or delay the radiotherapy protocol. Late radiation responses, including fibro-necrosis, fistulae, hemorrhage, and occlusion, can result in significant morbidity and mortality.
Acute epithelial ulceration, mucosal and submucosal inflammation, and chronic radiation enteropathy are characterized by excessive extracellular matrix deposition, vascular sclerosis, and muscular dystrophy. The cellular and molecular mechanisms of PRD include oxidative stress, stem cell death with compromised epithelial renewal (162), microvascular damage with endothelial cell death, and pro-inflammatory and pro-thrombotic changes (77, 143, 159). A current management strategy for PRD includes anti-inflammatory treatment with sulfasalazine, vascular therapy with hyperbaric oxygen or argon plasma coagulation, and pharmacological modulation of fibrosis. The management of severe radio-necrosis requires surgical intervention.
Stem cell therapy may offer a novel strategy to treat PRD. In abdominally irradiated mice, MSCs injected via the tail vein engraft to the enteric mucosa (213), enhance structural recovery, and delay death (186). The implantation of MSCs into the wall of the irradiated intestine facilitates repair of radiation-induced damage by inhibiting ulceration (113). Lorenzi et al. (128) reported that MSCs improved muscle regeneration and increased contractile function of anal sphincters after injury. Engrafted MSCs might regulate epithelial stem cells niches that provide and maintain an optimal microenvironment for stem cell function by releasing cytokines and growth factors such as interleukin-11, HGF, FGF-2, and insulin growth factor-I (187). Interestingly, repeated infusion of MSC-derived bioactive components after abdominal irradiation increased survival, decreased diarrhea, and improved the small intestinal structural integrity of irradiated mice (75). Mitigation of lethal radiation-induced intestinal injury can also be achieved by the transplantation of bone marrow-derived adipose stromal cells (BMASCs). Transplantation of BMASC increased blood levels of intestinal growth factors and induced the regeneration of intestinal villi, thereby accelerating functional recovery of the intestine (178). A summary of pre-clinical data regarding the treatment of radiation pelvic disease with MSCs is provided in Table 1.
Table 1.
Pre-Clinical Model of Mesenchymal Stem Cell to Treat Pelvic Radiation Disease
| Main results | ||
|---|---|---|
| Configuration | Non human primate | References |
| Total body irradiation | Engraft in gut | 72 |
| Mice | ||
| Abdominal | MSC when infused to mice that received either extended or localized irradiation, migrate to almost all tissues (including gut) where they engraft transiently usually at very low levels of detection | 72, 145 |
| Engraft into enteric mucosa | 113 | |
| Repair radiation-induced intestinal damage by inhibiting ulceration | 186 | |
| Favor reestablishment of cellular homeostasis by both increasing endogenous proliferation processes and inhibiting apoptosis of radiation induced of small epithelial stem cells | 187 | |
| MSC release cytokine and growth factors such as IL-11, hepatocyte growth factor, fibroblast growth factor-2, and insulin growth factor-1 that prevent intestinal radiation injury. Repeated infusion of MSC-derived bioactive components increased survival rate, decreased diarrhea occurrence, and improved small intestine structural integrity | 75 | |
| Improved muscle regeneration, increased contractile function of anal sphincters after injury (without radiation) | 128 | |
| Mitigation of radiation-induced lethal intestinal injury can similarly be achieved by transplantation of BMASC. BMASC increases blood levels of intestinal growth factors and induces regeneration of intestinal villi, thereby accelerating functional recovery of the intestine | 178 | |
| Rescue epithelial integrity. Limit radiation effect on the small intestine in an interleukine-6-dependent manner by reducing the levels of pro-inflammatory cytokines, while inducing anti-inflammatory cytokines, MSC dampen the systemic inflammatory response in radiation-induced syndrome |
71 | |
| Improve liver function and modulate hepatocellular death. Regenerate the small intestine epithelium, which, in turn, restored the enterohepatic recirculation pathway initially damaged by irradiation. The consequence was a distant hepatic protection without engraftment of MSC in the liver |
146 |
BMASC, bone marrow-derived adipose stromal cell; IL-11, interleukin-11; MSCs, mesenchymal stem cells.
In 2006, the radiation oncology accident at the public hospital in Épinal-France affected 425 patients who were under treatment for prostate cancer. Patients were overdosed by approximately 10–20%. Clinical consequences were severe, and sequelae classified from grade 2 to 5 on the common terminology criteria for adverse events 3.0 scale were noted (135, 160). Three patients received intravenous infusions of MSCs (5×106/kg) from allogeneic bone marrow derived from family donors for the treatment of hemorrhagic refractory radiation-induced fistulizing colitis. Clinical parameters, including pain, hemorrhage, and fistulization, were evaluated (magnetic resonance imaging, colonoscopy) before and after (1 and 6 months) MSC therapy. Two patients showed a substantial clinical response for pain and hemorrhage after MSC therapy with no adverse events. One patient experienced a relapse in pain after 6 months and was responsive to a second infusion of MSCs. In another patient, early fistulization was stopped by MSC treatment, with stable remission since then observed over a 3-year follow up. These positive clinical responses suggest that MSC therapy may represent a safe and effective intervention for patients developing rectitis and/or cystitis of varying severity (205).
Conclusions
Allogeneic hematopoietic stem cell transplantation has been successfully used over the past 4 decades for patients with hematologic diseases (81). Conventional myeloablative transplantation includes conditioning with high-dose radiotherapy and chemotherapy to eradicate residual disease and recipient (host) immunity in preparation for healthy donor-derived hematopoietic stem cells (graft). This has been the first and most robust demonstration of the efficacy of stem cell therapies to completely reconstitute a sterilized tissue after high-dose radiotherapy, thereby opening new avenues for the treatment of other radiation-induced normal tissue sequelae.
After more than a decade of intense activity, the science of stem cells seems to be catching up with its promise. Clinical trials have established techniques for cell delivery and protocols for establishing feasibility, safety, and early-stage efficacy in humans. The first clinical trials for pathologies, including diabetes (98), cardiac infarct (57), Crohn's disease (76), or Graft versus Host disease (118), have demonstrated safety with trends of efficacy.
Preclinical studies presented in this review provide encouraging proof of concept regarding the therapeutic potential of stem cells for treating the side effects associated with radiotherapy at different organ sites, including brain, salivary glands, bone, skin, heart, liver, and gut. Initial clinical data for radiation-induced lung (114), bone (ClinicalTrials.gov, identifier: NCT01147315), and skin (17, 32) injury indicate that certain complications may be amenable to stem cell-based approaches. However, successful translation to the clinic still faces many hurdles. Subsequent work will be critical in determining if/how and when to incorporate stem cell interventions into clinical trials. By gaining a further understanding of the molecular interactions between donor stem cells and host tissue, and how such interactions are modulated by host immunity (109), we could discover ways to expedite the use of stem cells in the clinic. While data demonstrating the efficacy of stem cells in the clinic remain sparse, additional evidence demonstrating long-term safety is also needed; transplanted stem cells should not form teratomas or undergo transformation and they should not promote tumor recurrence, even after complete tumor sterilization. Many of these issues are likely to be resolved by the appropriate selection of stem cell types for transplantation, but requires further preclinical testing.
Lessons from clinical trials should also be taken into account; since the first reported trial in 1995, cultured MSCs have been used in 125 registered clinical trials (registered at http://clinicaltrial.gov/) without any reported confounding side effects related to the cell therapy. The resistance to transformation of MSCs produced in four clinical-grade cell therapy facilities was investigated, and data demonstrated that MSCs with or without chromosomal alterations showed progressive growth arrest and entered senescence without evidence of transformation either in vitro or in vivo (194). The long-term genomic stability of cultured adult stem cells may minimize certain concerns regarding their oncogenic potential (188).
While current clinical data support the long-term safety of MSCs, concerns do persist, and some are related to the potential of transplanted MSCs to adversely impact tumor growth and metastasis after radiotherapy. A variety of experimental tumor models involving the exogenous addition of MSCs have led to conflicting findings regarding their capability to promote or suppress tumor growth (105). Depending on the model, mechanisms put forth have included the inhibition or promotion of apoptosis, angiogenesis, or immunity and related interactions within the tumor microenvironment. Understanding the mechanisms in which adult somatic stem cells modulate tumor growth will be essential for the safe and efficient advancement of stem cell therapies that counteract radiation injury. A summary of data regarding stem cell therapies that are specific to the organ site from selected clinical trials and pre-clinical studies are provided in Table 2.
Table 2.
Clinical Trials and Pre-Clinical Studies to Treat the Adverse Consequences of Radiotherapy
| Organ system | Radiation dose (Gy) | Normal tissue endpoint | Paradigm | Stem cell type administration (preclinical studies) | Stem cell type administration (clinical trial) | Follow up time |
|---|---|---|---|---|---|---|
| Bone marrow | 12 | Bone marrow aplasia | Hematopoietic stem cell/progenitor depletion and stem cell “niche” destruction | BM, HSC, MSC | BM (81) | 30 years |
| Brain | >57 | Brain radionecrosis, cognitive dysfunction | Inflammation, vascular breakdown, disruption of BBB, CNS progenitor depletion, stem cell “niche” destruction (74, 144, 167, 177, 202), hypoxia, demyelination, necrosis (197, 208) | hESC (1, 3), hNSC (2, 3) | No | — |
| Salivary glands | >35 | Xerostomia (58, 62, 204), salivary flow | Stem cell/progenitor depletion (110) | BM (121, 199), MSC (37), salivary gland stem cell (13, 42, 67, 124, 127, 151, 165) | No | — |
| Bone | >60 | Bone growth alteration, bone weakening, and osteo radionecrosis (139) | Hypocellularity (48, 179), hypovascularization, hypoxia (139), and fibro-necrosis (14, 51, 60, 152, 161) | BM, MSC (64) | BM associated to biomaterial (Phase I) (64, 131) | Few months |
| Skin | >50 | Skin radionecrosis (20), pain (130, 141) | Chronic inflammation, damage to the microvasculature, epidermis stem cell/progenitor depletion, ischemia, fibroblast death, and fibro-necrosis | MSC (17, 73), ADSC (61), EPC (102) | MSC (local injection, 2×106/kg, repetitive injections, curative startegy) (compationnal treatment) (17) and lipoaspirate (Phase I) (7, 32, 104, 115, 175) | 8 years and 13 months |
| Liver | >35 | Radiation-induced liver disease (96, 158), sinusoidal obstructive syndrome (36, 53, 66, 154, 171) | Vascular (sinusoidal) breakdown, hepatocyte cell death, and inhibition of hepatocellular regeneration | Hepatocyte | Hepatocyte (intraspelnic transplantation, 6.106) (Phase I) (70) | — |
| Heart | >30–40 | Atherosclerosis (155, 191), cardiac attack (38, 191) | Inflammation, damage to the microvasculature (184, 185), ischemia, myocardial cell death, and fibro-necrosis (4, 47, 65, 138) | — | No | — |
| Colon-rectum | >35 Gy | Pelvic radiation disease (10), colo-rectal ulceration, rectitis, cystitis, and fistulae | Chronic inflammation, damage to the microvasculature (77, 143, 159), epithelial stem cell/progenitor depletion (162), ischemia, myofibroblast death, and fibro-necrosis | MSC (33, 75, 113, 128, 186, 187, 213) | MSC (i.v. injection, 2×106/kg, repetitive injections) (compassional treatment) | 4 years |
ADSC, adipose-derived stem cell; BBB, blood brain barrier; BM, bone marrow; CNS, central nervous system; EPC, endothelial progenitor cells; GFAP, glial fibrillary acidic protein; hESC, human embryonic stem cell; HSC, human stem cell; hNSC, human neural stem cell; IRR, irradiation.
Pluripotent stem cells represent attractive sources of starting material for cell-based therapies, largely due to their self-renewing properties and their capability to differentiate into tissue from all three germ layers (8). The use of pluripotent ESC is still fraught with ethical controversy and concerns of genetic instability and immune tolerance (46, 106, 129). The group of Yamanaka (193) has recently developed a technique for in-vitro reprogramming of terminally differentiated cells, such as skin fibroblast, into pluripotent cells that closely resemble ESC. These induced pluripotent stem cells (iPS) could be used for transplantation, with theoretical reductions in the risk of immune rejection. Such a technology also provides the capability to generate cell banks for future uses. Despite these potential benefits, several issues with iPS technology still need to be resolved before clinical use is possible. Viral integration strategies used for cellular reprogramming add an element of oncogenic risk, and reprogrammed somatic cells may harbor underlying mutations of unknown consequence (168).
Regenerative therapy for radiation injury is at an important juncture. Breaking down traditional barriers between specialized fields will be challenging, but necessary, if we are to move stem cell biology beyond basic science and toward the development of truly beneficial regenerative therapies. To foster this sort of multidisciplinary collaboration, a parallel effort needs to bring academic science in closer contact with clinicians. The fate of future stem cell physician–scientists and patients suffering from the adverse side effects of radiotherapy will certainly depend on the outcome.
Abbreviations Used
- ADSC
adipose derived stem cells
- Arc
activity-regulated cytoskeleton-associated protein
- BCP
biphasic calcium phosphate
- BMASCs
bone marrow-derived adipose stromal cells
- BMDC
bone marrow-derived cell
- CNS
central nervous system
- CPC
cardiomyocyte progenitor cells
- EPC
endothelial progenitor cells
- ESC
embryonic stem cells
- FC
fear conditioning
- FGF-2
fibroblast growth factor-2
- Gy
Gray
- hESC
human embryonic stem cell
- HGF
hepatocyte growth factor
- HIR
hepatic irradiation
- HNC
head and neck cancer
- hNSC
human neural stem cell
- HT
hepatocyte transplantation
- IL-11
interleukin-11
- iPS
induced pluripotent stem cell
- KGF
keratinocyte growth factor
- LSECs
liver sinusoidal endothelial cells
- MSCs
mesenchymal stem cells
- NPR
novel place recognition
- OLT
orthotopic liver transplantation
- ORN
osteoradionecrosis
- PMPS
post mastectomy pain syndrome
- PRD
pelvic radiation disease
- RILD
radiation-induced liver disease
- RT
radiation therapy
- SGZ
subgranular zone
- SVZ
subventricular zone
- TBM
total bone marrow
- TGF-β1
transforming growth factor-β1
Acknowledgments
The topics selected were derived from a regenerative medicine symposium that focused on the treatment of radiation-induced side effects in normal tissues, presented at the most recent International Congress of Radiation Research convened in Warsaw Poland 2012. The speakers at that symposium have contributed to this review. A part of this work was supported by the National Institutes of Health NINDS Grant R01 NS074388 (C.L.L.).
References
- 1.Acharya MM, Christie LA, Lan ML, Donovan PJ, Cotman CW, et al. . Rescue of radiation-induced cognitive impairment through cranial transplantation of human embryonic stem cells. Proc Natl Acad Sci U S A 106: 19150–19155, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acharya MM, Christie LA, Lan ML, Giedzinski E, Fike JR, et al. . Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res 71: 4834–4845, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acharya MM, Christie LA, Lan ML, and Limoli CL. Comparing the functional consequences of human stem cell transplantation in the irradiated rat brain. Cell Transplant 22: 55–64, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams MJ, Lipshultz SE, Schwartz C, Fajardo LF, Coen V, et al. . Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol 13: 346–356, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal S. and Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105: 1815–1822, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Ahmad SS, Duke S, Jena R, Williams MV, and Burnet NG. Advances in radiotherapy. BMJ 345: e7765, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Akita S, Akino K, Hirano A, Ohtsuru A, and Yamashita S. Noncultured autologous adipose-derived stem cells therapy for chronic radiation injury. Stem Cells Int 2010: 532704, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, et al. . Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol 227: 271–278, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Anderson AJ, Haus DL, Hooshmand MJ, Perez H, Sontag CJ, et al. . Achieving stable human stem cell engraftment and survival in the CNS: is the future of regenerative medicine immunodeficient? Regen Med 6: 367–406, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreyev HJ, Wotherspoon A, Denham JW, and Hauer-Jensen M. Defining pelvic-radiation disease for the survivorship era. Lancet Oncol 11: 310–312, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Anversa P, Leri A, Rota M, Hosoda T, Bearzi C, et al. . Concise review: stem cells, myocardial regeneration, and methodological artifacts. Stem Cells 25: 589–601, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Arinzeh TL, Peter SJ, Archambault MP, van den Bos C, Gordon S, et al. . Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am 85-A: 1927–1935, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Banh A, Xiao N, Cao H, Chen CH, Kuo P, et al. . A novel aldehyde dehydrogenase-3 activator leads to adult salivary stem cell enrichment in vivo. Clin Cancer Res 17: 7265–7272, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barcellos-Hoff MH. How do tissues respond to damage at the cellular level? The role of cytokines in irradiated tissues. Radiat Res 150: S109–S120, 1998 [PubMed] [Google Scholar]
- 15.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, et al. . Human cardiac stem cells. Proc Natl Acad Sci U S A 104: 14068–14073, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belmadani A, Tran PB, Ren D, and Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci 26: 3182–3191, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benderitter M, Gourmelon P, Bey E, Chapel A, Clairand I, et al. . New emerging concepts in the medical management of local radiation injury. Health Phys 98: 851–857, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, et al. . Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A 106: 13594–13599, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boerma M, Roberto KA, and Hauer-Jensen M. Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and alpha-tocopherol. Int J Radiat Oncol Biol Phys 72: 170–177, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolderston A, Lloyd NS, Wong RK, Holden L, and Robb-Blenderman L. The prevention and management of acute skin reactions related to radiation therapy: a systematic review and practice guideline. Support Care Cancer 14: 802–817, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, et al. . Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378: 1847–1857, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Boyd AS. and Fairchild PJ. Approaches for immunological tolerance induction to stem cell-derived cell replacement therapies. Expert Rev Clin Immunol 6: 435–448, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Brizel DM. Pharmacologic approaches to radiation protection. J Clin Oncol 25: 4084–4089, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, et al. . Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol 18: 3339–3345, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Brockstein BE. and Vokes EE. Head and neck cancer in 2010: maximizing survival and minimizing toxicity. Nat Rev Clin Oncol 8: 72–74, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Burlage FR, Faber H, Kampinga HH, Langendijk JA, Vissink A, et al. . Enhanced proliferation of acinar and progenitor cells by prophylactic pilocarpine treatment underlies the observed amelioration of radiation injury to parotid glands. Radiother Oncol 90: 253–256, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Burlage FR, Roesink JM, Kampinga HH, Coppes RP, Terhaard C, et al. . Protection of salivary function by concomitant pilocarpine during radiotherapy: a double-blind, randomized, placebo-controlled study. Int J Radiat Oncol Biol Phys 70: 14–22, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Burrell K, Hill RP, and Zadeh G. High-resolution in-vivo analysis of normal brain response to cranial irradiation. PLoS One 7: e38366, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butler JM, Rapp SR, and Shaw EG. Managing the cognitive effects of brain tumor radiation therapy. Curr Treat Options Oncol 7: 517–523, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Caplice NM. and Doyle B. Vascular progenitor cells: origin and mechanisms of mobilization, differentiation, integration, and vasculogenesis. Stem Cells Dev 14: 122–139, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Carbajal KS, Schaumburg C, Strieter R, Kane J, and Lane TE. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc Natl Acad Sci U S A 107: 11068–11073, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caviggioli F, Maione L, Forcellini D, Klinger F, and Klinger M. Autologous fat graft in postmastectomy pain syndrome. Plast Reconstr Surg 128: 349–352, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, et al. . Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med 5: 1028–1038, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Chari DM, Gilson JM, Franklin RJ, and Blakemore WF. Oligodendrocyte progenitor cell (OPC) transplantation is unlikely to offer a means of preventing X-irradiation induced damage in the CNS. Exp Neurol 198: 145–153, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Chen B, Gao XQ, Yang CX, Tan SK, Sun ZL, et al. . Neuroprotective effect of grafting GDNF gene-modified neural stem cells on cerebral ischemia in rats. Brain Res 1284: 1–11, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Cheng JC, Wu JK, Lee PC, Liu HS, Jian JJ, et al. . Biologic susceptibility of hepatocellular carcinoma patients treated with radiotherapy to radiation-induced liver disease. Int J Radiat Oncol Biol Phys 60: 1502–1509, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Christiansen H, Koenig S, Krause P, Hermann RM, Rave-Frank M, et al. . External-beam radiotherapy as preparative regimen for hepatocyte transplantation after partial hepatectomy. Int J Radiat Oncol Biol Phys 65: 509–516, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, et al. . Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366: 2087–2106, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, et al. . Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev 2: CD006536, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Conill C, Tomas X, Combalia-Aleu A, Palacin A, Planas I, et al. . Pathological femur fracture secondary to radiation therapy for soft tissue sarcoma. Clin Transl Oncol 9: 537–539, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Conner KR, Payne VS, Forbes ME, Robbins ME, and Riddle DR. Effects of the AT1 receptor antagonist L-158,809 on microglia and neurogenesis after fractionated whole-brain irradiation. Radiat Res 173: 49–61, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coppes RP. and Stokman MA. Stem cells and the repair of radiation-induced salivary gland damage. Oral Dis 17: 143–153, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Coppes RP, van der Goot A, and Lombaert IM. Stem cell therapy to reduce radiation-induced normal tissue damage. Semin Radiat Oncol 19: 112–121, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Cotroneo E, Proctor GB, and Carpenter GH. Regeneration of acinar cells following ligation of rat submandibular gland retraces the embryonic-perinatal pathway of cytodifferentiation. Differentiation 79: 120–130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai W. and Kloner RA. Bone marrow-derived cell transplantation therapy for myocardial infarction: lessons learned and future questions. Am J Transplant 11: 2297–2301, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Daley GQ, Ahrlund Richter L, Auerbach JM, Benvenisty N, Charo RA, et al. . Ethics. The ISSCR guidelines for human embryonic stem cell research. Science 315: 603–604, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, et al. . Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys 76: 656–665, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dare A, Hachisu R, Yamaguchi A, Yokose S, Yoshiki S, et al. . Effects of ionizing radiation on proliferation and differentiation of osteoblast-like cells. J Dent Res 76: 658–664, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Davani S, Deschaseaux F, Chalmers D, Tiberghien P, and Kantelip JP. Can stem cells mend a broken heart? Cardiovasc Res 65: 305–316, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, et al. . Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys 76: S58–S63, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delanian S. and Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol 73: 119–131, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Delanian S. and Lefaix JL. Current management for late normal tissue injury: radiation-induced fibrosis and necrosis. Semin Radiat Oncol 17: 99–107, 2007 [DOI] [PubMed] [Google Scholar]
- 53.DeLeve LD, Shulman HM, and McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis 22: 27–42, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Deng J, Zou ZM, Zhou TL, Su YP, Ai GP, et al. . Bone marrow mesenchymal stem cells can be mobilized into peripheral blood by G-CSF in vivo and integrate into traumatically injured cerebral tissue. Neurol Sci 32: 641–651, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Denny PC, Liu P, and Denny PA. Evidence of a phenotypically determined ductal cell lineage in mouse salivary glands. Anat Rec 256: 84–90, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Dietrich J, Monje M, Wefel J, and Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist 13: 1285–1295, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Dill T, Schachinger V, Rolf A, Mollmann S, Thiele H, et al. . Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. Am Heart J 157: 541–547, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Dirix P. and Nuyts S. Evidence-based organ-sparing radiotherapy in head and neck cancer. Lancet Oncol 11: 85–91, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Douw L, Klein M, Fagel SS, van den Heuvel J, Taphoorn MJ, et al. . Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol 8: 810–818, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Dudziak ME, Saadeh PB, Mehrara BJ, Steinbrech DS, Greenwald JA, et al. . The effects of ionizing radiation on osteoblast-like cells in vitro. Plast Reconstr Surg 106: 1049–1061, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Ebrahimian TG, Pouzoulet F, Squiban C, Buard V, Andre M, et al. . Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol 29: 503–510, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Eisbruch A. Radiotherapy: IMRT reduces xerostomia and potentially improves QoL. Nat Rev Clin Oncol 6: 567–568, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Emami B, Lyman J, Brown A, Coia L, Goitein M, et al. . Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21: 109–122, 1991 [DOI] [PubMed] [Google Scholar]
- 64.Espitalier F, Vinatier C, Lerouxel E, Guicheux J, Pilet P, et al. . A comparison between bone reconstruction following the use of mesenchymal stem cells and total bone marrow in association with calcium phosphate scaffold in irradiated bone. Biomaterials 30: 763–769, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Fajardo LF, Berthrong M, and Anderson RE. Testis. In Radiation Pathology, New York: Oxford University Press, 2001:312–318 [Google Scholar]
- 66.Fajardo LF. and Colby TV. Pathogenesis of veno-occlusive liver disease after radiation. Arch Pathol Lab Med 104: 584–588, 1980 [PubMed] [Google Scholar]
- 67.Feng J, van der Zwaag M, Stokman MA, van Os R, and Coppes RP. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother Oncol 92: 466–471, 2009 [DOI] [PubMed] [Google Scholar]
- 68.Fike JR, Rola R, and Limoli CL. Radiation response of neural precursor cells. Neurosurg Clin N Am 18: 115–127, x, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Fike JR, Rosi S, and Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin Radiat Oncol 19: 122–132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fox IJ. Hepatocyte Transplantation for Liver Based Metabolic Diosorders. NCT01345578, 2011
- 71.Francois M, Birman E, Forner KA, Gaboury L, and Galipeau J. Adoptive transfer of mesenchymal stromal cells accelerates intestinal epithelium recovery of irradiated mice in an interleukin-6-dependent manner. Cytotherapy 14: 1164–1170, 2012 [DOI] [PubMed] [Google Scholar]
- 72.Francois S, Bensidhoum M, Mouiseddine M, Mazurier C, Allenet B, et al. . Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells 24: 1020–1029, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Francois S, Mouiseddine M, Mathieu N, Semont A, Monti P, et al. . Human mesenchymal stem cells favour healing of the cutaneous radiation syndrome in a xenogenic transplant model. Ann Hematol 86: 1–8, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Gage FH. Mammalian neural stem cells. Science 287: 1433–1438, 2000 [DOI] [PubMed] [Google Scholar]
- 75.Gao Z, Zhang Q, Han Y, Cheng X, Lu Y, et al. . Mesenchymal stromal cell-conditioned medium prevents radiation-induced small intestine injury in mice. Cytotherapy 14: 267–273, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Olmo D, Garcia-Arranz M, Herreros D, Pascual I, Peiro C, et al. . A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum 48: 1416–1423, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Gaugler MH, Vereycken-Holler V, Squiban C, Vandamme M, Vozenin-Brotons MC, et al. . Pravastatin limits endothelial activation after irradiation and decreases the resulting inflammatory and thrombotic responses. Radiat Res 163: 479–487, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Globus RK, Caiozzo VJ, Acharya MM, Fike JR, and Limoli CL. Redox regulation of stem cell compartments: the convergence of radiation-induced normal tissue damage and oxidative stress. In: Oxidative Stress in Applied Basic Research and Clinical Practice: Cancer, edited by Spitz DR, Dornfeld KJ, Gius D, and Krishnan K. Totowa, NJ: Humana Press, Inc., 2012, pp. 169–192 [Google Scholar]
- 79.Goldring CE, Duffy PA, Benvenisty N, Andrews PW, Ben-David U, et al. . Assessing the safety of stem cell therapeutics. Cell Stem Cell 8: 618–628, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Gomez-Pinilla F, So V, and Kesslak JP. Spatial learning and physical activity contribute to the induction of fibroblast growth factor: neural substrates for increased cognition associated with exercise. Neuroscience 85: 53–61, 1998 [DOI] [PubMed] [Google Scholar]
- 81.Gorin NC. [Bone marrow autograft. Current status and perspectives]. Nouv Rev Fr Hematol 25: 391–399, 1983. [Article in French] [PubMed] [Google Scholar]
- 82.Gorlia T, Stupp R, Brandes AA, Rampling RR, Fumoleau P, et al. . New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: a pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer 48: 1176–1184, 2012 [DOI] [PubMed] [Google Scholar]
- 83.Greenberger JS. and Epperly M. Bone marrow-derived stem cells and radiation response. Semin Radiat Oncol 19: 133–139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grill J, Renaux VK, Bulteau C, Viguier D, Levy-Piebois C, et al. . Long-term intellectual outcome in children with posterior fossa tumors according to radiation doses and volumes. Int J Radiat Oncol Biol Phys 45: 137–145, 1999 [DOI] [PubMed] [Google Scholar]
- 85.Guha C. and Kavanagh BD. Hepatic radiation toxicity: avoidance and amelioration. Semin Radiat Oncol 21: 256–263, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guha C, Parashar B, Deb NJ, Garg M, Gorla GR, et al. . Normal hepatocytes correct serum bilirubin after repopulation of Gunn rat liver subjected to irradiation/partial resection. Hepatology 36: 354–362, 2002 [DOI] [PubMed] [Google Scholar]
- 87.Guha C, Parashar B, Deb NJ, Sharma A, Gorla GR, et al. . Liver irradiation: a potential preparative regimen for hepatocyte transplantation. Int J Radiat Oncol Biol Phys 49: 451–457, 2001 [DOI] [PubMed] [Google Scholar]
- 88.Guha C, Sharma A, Gupta S, Alfieri A, Gorla GR, et al. . Amelioration of radiation-induced liver damage in partially hepatectomized rats by hepatocyte transplantation. Cancer Res 59: 5871–5874, 1999 [PubMed] [Google Scholar]
- 89.Guha C, Yamanouchi K, Jiang J, Wang X, Roy Chowdhury N, et al. . Feasibility of hepatocyte transplantation-based therapies for primary hyperoxalurias. Am J Nephrol 25: 161–170, 2005 [DOI] [PubMed] [Google Scholar]
- 90.Head C, Alam D, Sercarz JA, Lee JT, Rawnsley JD, et al. . Microvascular flap reconstruction of the mandible: a comparison of bone grafts and bridging plates for restoration of mandibular continuity. Otolaryngol Head Neck Surg 129: 48–54, 2003 [DOI] [PubMed] [Google Scholar]
- 91.Hess DC, Hill WD, Martin-Studdard A, Carroll J, Brailer J, et al. . Bone marrow as a source of endothelial cells and NeuN-expressing cells After stroke. Stroke 33: 1362–1368, 2002 [DOI] [PubMed] [Google Scholar]
- 92.Hoover-Plow J. and Gong Y. Challenges for heart disease stem cell therapy. Vasc Health Risk Manag 8: 99–113, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hubner W, Blume A, Pushnjakova R, Dekhtyar Y, and Hein HJ. The influence of X-ray radiation on the mineral/organic matrix interaction of bone tissue: an FT-IR microscopic investigation. Int J Artif Organs 28: 66–73, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Hunter KD, Parkinson EK, and Harrison PR. Profiling early head and neck cancer. Nat Rev Cancer 5: 127–135, 2005 [DOI] [PubMed] [Google Scholar]
- 95.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, et al. . Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A 101: 18117–18122, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ingold JA, Reed GB, Kaplan HS, and Bagshaw MA. Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med 93: 200–208, 1965 [PubMed] [Google Scholar]
- 97.Jiang J, Salido EC, Guha C, Wang X, Moitra R, et al. . Correction of hyperoxaluria by liver repopulation with hepatocytes in a mouse model of primary hyperoxaluria type-1. Transplantation 85: 1253–1260, 2008 [DOI] [PubMed] [Google Scholar]
- 98.Jiang R, Han Z, Zhuo G, Qu X, Li X, et al. . Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Front Med 5: 94–100, 2011 [DOI] [PubMed] [Google Scholar]
- 99.Joly F, Rigal O, Noal S, and Giffard B. Cognitive dysfunction and cancer: which consequences in terms of disease management? Psychooncology 20: 1251–1258, 2011 [DOI] [PubMed] [Google Scholar]
- 100.Kabarriti R, Zhou JV, and Vainshtein S. Transplantation of liver sinusoidal endothelial cells repairs HIR induced hepatic endothelial cell damage. Int J Radiat Biol 78: S41, 2010 [Google Scholar]
- 101.Kameda M, Shingo T, Takahashi K, Muraoka K, Kurozumi K, et al. . Adult neural stem and progenitor cells modified to secrete GDNF can protect, migrate and integrate after intracerebral transplantation in rats with transient forebrain ischemia. Eur J Neurosci 26: 1462–1478, 2007 [DOI] [PubMed] [Google Scholar]
- 102.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, et al. . Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 107: 461–468, 2003 [DOI] [PubMed] [Google Scholar]
- 103.King MA, Casarett GW, and Weber DA. A study of irradiated bone: I. histopathologic and physiologic changes. J Nucl Med 20: 1142–1149, 1979 [PubMed] [Google Scholar]
- 104.Klinger M, Caviggioli F, Vinci V, Salval A, and Villani F. Treatment of chronic posttraumatic ulcers using autologous fat graft. Plast Reconstr Surg 126: 154e–155e, 2010 [DOI] [PubMed] [Google Scholar]
- 105.Klopp AH, Gupta A, Spaeth E, Andreeff M, and Marini F, 3rd. Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells 29: 11–19, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells 27: 1050–1056, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Knoepfler PS. Key anticipated regulatory issues for clinical use of human induced pluripotent stem cells. Regen Med 7: 713–720, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Knox SM, Lombaert IM, Reed X, Vitale-Cross L, Gutkind JS, et al. . Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 329: 1645–1647, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kokaia Z, Martino G, Schwartz M, and Lindvall O. Cross-talk between neural stem cells and immune cells: the key to better brain repair? Nat Neurosci 15: 1078–1087, 2012 [DOI] [PubMed] [Google Scholar]
- 110.Konings AW, Coppes RP, and Vissink A. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys 62: 1187–1194, 2005 [DOI] [PubMed] [Google Scholar]
- 111.Krause P, Wolff HA, Rave-Frank M, Schmidberger H, Becker H, et al. . Fractionated external beam radiotherapy as a suitable preparative regimen for hepatocyte transplantation after partial hepatectomy. Int J Radiat Oncol Biol Phys 80: 1214–1219, 2011 [DOI] [PubMed] [Google Scholar]
- 112.Kruse JJ, Strootman EG, and Wondergem J. Effects of amifostine on radiation-induced cardiac damage. Acta Oncol 42: 4–9, 2003 [DOI] [PubMed] [Google Scholar]
- 113.Kudo K, Liu Y, Takahashi K, Tarusawa K, Osanai M, et al. . Transplantation of mesenchymal stem cells to prevent radiation-induced intestinal injury in mice. J Radiat Res 51: 73–79, 2010 [DOI] [PubMed] [Google Scholar]
- 114.Kursova LV, Konoplyannikov AG, Pasov VV, Ivanova IN, Poluektova MV, et al. . Possibilities for the use of autologous mesenchymal stem cells in the therapy of radiation-induced lung injuries. Bull Exp Biol Med 147: 542–546, 2009 [DOI] [PubMed] [Google Scholar]
- 115.Kwekkeboom K. Postmastectomy pain syndromes. Cancer Nurs 19: 37–43, 1996 [DOI] [PubMed] [Google Scholar]
- 116.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, et al. . Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol 26: 3770–3776, 2008 [DOI] [PubMed] [Google Scholar]
- 117.Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, et al. . Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys 31: 1237–1248, 1995 [DOI] [PubMed] [Google Scholar]
- 118.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, et al. . Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371: 1579–1586, 2008 [DOI] [PubMed] [Google Scholar]
- 119.LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res: 81–98, 2002 [DOI] [PubMed] [Google Scholar]
- 120.LeVeque FG, Montgomery M, Potter D, Zimmer MB, Rieke JW, et al. . A multicenter, randomized, double-blind, placebo-controlled, dose-titration study of oral pilocarpine for treatment of radiation-induced xerostomia in head and neck cancer patients. J Clin Oncol 11: 1124–1131, 1993 [DOI] [PubMed] [Google Scholar]
- 121.Lin CY, Chang FH, Chen CY, Huang CY, Hu FC, et al. . Cell therapy for salivary gland regeneration. J Dent Res 90: 341–346, 2011 [DOI] [PubMed] [Google Scholar]
- 122.Liu H, Xiong M, Xia YF, Cui NJ, Lu RB, et al. . Studies on pentoxifylline and tocopherol combination for radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys 73: 1552–1559, 2009 [DOI] [PubMed] [Google Scholar]
- 123.Llado J, Haenggeli C, Maragakis NJ, Snyder EY, and Rothstein JD. Neural stem cells protect against glutamate-induced excitotoxicity and promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol Cell Neurosci 27: 322–331, 2004 [DOI] [PubMed] [Google Scholar]
- 124.Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, et al. . Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One 3: e2063, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lombaert IM, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G, et al. . Cytokine treatment improves parenchymal and vascular damage of salivary glands after irradiation. Clin Cancer Res 14: 7741–7750, 2008 [DOI] [PubMed] [Google Scholar]
- 126.Lombaert IM, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G, et al. . Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells 26: 2595–2601, 2008 [DOI] [PubMed] [Google Scholar]
- 127.Lombaert IM, Knox SM, and Hoffman MP. Salivary gland progenitor cell biology provides a rationale for therapeutic salivary gland regeneration. Oral Dis 17: 445–449, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lorenzi B, Pessina F, Lorenzoni P, Urbani S, Vernillo R, et al. . Treatment of experimental injury of anal sphincters with primary surgical repair and injection of bone marrow-derived mesenchymal stem cells. Dis Colon Rectum 51: 411–420, 2008 [DOI] [PubMed] [Google Scholar]
- 129.Lui KO, Waldmann H, and Fairchild PJ. Embryonic stem cells: overcoming the immunological barriers to cell replacement therapy. Curr Stem Cell Res Ther 4: 70–80, 2009 [DOI] [PubMed] [Google Scholar]
- 130.Maddocks-Jennings W, Wilkinson JM, and Shillington D. Novel approaches to radiotherapy-induced skin reactions: a literature review. Complement Ther Clin Pract 11: 224–231, 2005 [DOI] [PubMed] [Google Scholar]
- 131.Malard O. Curative Treatment of Osteoradionecrosis (ORN) with Hybrid Bone Substitution. Clinicaltrialsgov NCT01147315, 2013
- 132.Malhi H, Gorla GR, Irani AN, Annamaneni P, and Gupta S. Cell transplantation after oxidative hepatic preconditioning with radiation and ischemia-reperfusion leads to extensive liver repopulation. Proc Natl Acad Sci U S A 99: 13114–13119, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Malhi H, Joseph B, Schilsky ML, and Gupta S. Development of cell therapy strategies to overcome copper toxicity in the LEC rat model of Wilson disease. Regen Med 3: 165–173, 2008 [DOI] [PubMed] [Google Scholar]
- 134.Maniatopoulos C, Sodek J, and Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res 254: 317–330, 1988 [DOI] [PubMed] [Google Scholar]
- 135.Marchesi V, Aigle D, Peiffert D, Noel A, and Simon JM. [Securitization of the bi-site radiotherapy activity as part of the resumption of treatments in the Hospital of Epinal by the team of Alexis Vautrin Nancy Cancer Center]. Cancer Radiother 13: 740–743, 2009. [Article in French] [DOI] [PubMed] [Google Scholar]
- 136.Maria OM, Maria AM, Cai Y, and Tran SD. Cell surface markers CD44 and CD166 localized specific populations of salivary acinar cells. Oral Dis 18: 162–168, 2012 [DOI] [PubMed] [Google Scholar]
- 137.Maria OM. and Tran SD. Human mesenchymal stem cells cultured with salivary gland biopsies adopt an epithelial phenotype. Stem Cells Dev 20: 959–967, 2011 [DOI] [PubMed] [Google Scholar]
- 138.Marks LB, Yu X, Prosnitz RG, Zhou SM, Hardenbergh PH, et al. . The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys 63: 214–223, 2005 [DOI] [PubMed] [Google Scholar]
- 139.Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg 41: 283–288, 1983 [DOI] [PubMed] [Google Scholar]
- 140.McGale P, Darby SC, Hall P, Adolfsson J, Bengtsson NO, et al. . Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol 100: 167–175, 2011 [DOI] [PubMed] [Google Scholar]
- 141.McQuestion M. Evidence-based skin care management in radiation therapy. Semin Oncol Nurs 22: 163–173, 2006 [DOI] [PubMed] [Google Scholar]
- 142.Meyers CA. Neurocognitive dysfunction in cancer patients. Oncology (Williston Park) 14: 75–79; discussion 79, 81–72, 85, 2000 [PubMed] [Google Scholar]
- 143.Milliat F, Francois A, Isoir M, Deutsch E, Tamarat R, et al. . Influence of endothelial cells on vascular smooth muscle cells phenotype after irradiation: implication in radiation-induced vascular damages. Am J Pathol 169: 1484–1495, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mizumatsu S, Monje M, Morhardt D, Rola R, Palmer T, et al. . Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res 63: 4021–4027, 2003 [PubMed] [Google Scholar]
- 145.Mouiseddine M, Francois S, Semont A, Sache A, Allenet B, et al. . Human mesenchymal stem cells home specifically to radiation-injured tissues in a non-obese diabetes/severe combined immunodeficiency mouse model. Br J Radiol 80 Spec No 1: S49–S55, 2007 [DOI] [PubMed] [Google Scholar]
- 146.Mouiseddine M, Francois S, Souidi M, and Chapel A. Intravenous human mesenchymal stem cells transplantation in NOD/SCID mice preserve liver integrity of irradiation damage. Methods Mol Biol 826: 179–188, 2012 [DOI] [PubMed] [Google Scholar]
- 147.Mummery CL, Davis RP, and Krieger JE. Challenges in using stem cells for cardiac repair. Sci Transl Med 2: 27ps17, 2010 [DOI] [PubMed] [Google Scholar]
- 148.Myers JS. Chemotherapy-related cognitive impairment. Clin J Oncol Nurs 13: 413–421, 2009 [DOI] [PubMed] [Google Scholar]
- 149.Myers JS. Neuropsychologic testing for chemotherapy-related cognitive impairment. Adv Exp Med Biol 678: 55–69, 2010 [DOI] [PubMed] [Google Scholar]
- 150.Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, et al. . Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110: 429–441, 2002 [DOI] [PubMed] [Google Scholar]
- 151.Nanduri LS, Maimets M, Pringle SA, van der Zwaag M, van Os RP, et al. . Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol 99: 367–372, 2011 [DOI] [PubMed] [Google Scholar]
- 152.Niehoff P, Springer IN, Acil Y, Lange A, Marget M, et al. . HDR brachytherapy irradiation of the jaw—as a new experimental model of radiogenic bone damage. J Craniomaxillofac Surg 36: 203–209, 2008 [DOI] [PubMed] [Google Scholar]
- 153.Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, et al. . Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 12: 127–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ogata K, Hizawa K, Yoshida M, Kitamuro T, Akagi G, et al. . Hepatic injury following irradiation—a morphologic study. Tokushima J Exp Med 43: 240–251, 1963 [PubMed] [Google Scholar]
- 155.Pakala R, Leborgne L, Cheneau E, Chan RC, Yazdi H, et al. . Radiation-induced atherosclerotic plaque progression in a hypercholesterolemic rabbit: a prospective vulnerable plaque model? Cardiovasc Radiat Med 4: 146–151, 2003 [DOI] [PubMed] [Google Scholar]
- 156.Palmer SL, Reddick WE, and Gajjar A. Understanding the cognitive impact on children who are treated for medulloblastoma. J Pediatr Psychol 32: 1040–1049, 2007 [DOI] [PubMed] [Google Scholar]
- 157.Palmon A, David R, Neumann Y, Stiubea-Cohen R, Krief G, et al. . High-efficiency immunomagnetic isolation of solid tissue-originated integrin-expressing adult stem cells. Methods 56: 305–309, 2012 [DOI] [PubMed] [Google Scholar]
- 158.Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, et al. . Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 76: S94–S100, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, et al. . Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293: 293–297, 2001 [DOI] [PubMed] [Google Scholar]
- 160.Peiffert D, Simon JM, and Eschwege F. [Epinal radiotherapy accident: passed, present, future]. Cancer Radiother 11: 309–312, 2007. [Article in French] [DOI] [PubMed] [Google Scholar]
- 161.Pepper MS. Transforming growth factor-beta: vasculogenesis, angiogenesis, and vessel wall integrity. Cytokine Growth Factor Rev 8: 21–43, 1997 [DOI] [PubMed] [Google Scholar]
- 162.Potten CS, Owen G, and Roberts SA. The temporal and spatial changes in cell proliferation within the irradiated crypts of the murine small intestine. Int J Radiat Biol 57: 185–199, 1990 [DOI] [PubMed] [Google Scholar]
- 163.Prasanna PG, Stone HB, Wong RS, Capala J, Bernhard EJ, et al. . Normal tissue protection for improving radiotherapy: where are the Gaps? Transl Cancer Res 1: 35–48, 2012 [PMC free article] [PubMed] [Google Scholar]
- 164.Pringle S, Nanduri LS, van der Zwaag M, van Os R, and Coppes RP. Isolation of mouse salivary gland stem cells. J Vis Exp pii: 2484, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pringle S, van Os R, and Coppes RP. Adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells 31: 613–619, 2013 [DOI] [PubMed] [Google Scholar]
- 166.Ptaszek LM, Mansour M, Ruskin JN, and Chien KR. Towards regenerative therapy for cardiac disease. Lancet 379: 933–942, 2012 [DOI] [PubMed] [Google Scholar]
- 167.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, et al. . Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res 162: 39–47, 2004 [DOI] [PubMed] [Google Scholar]
- 168.Rajasingh J. Reprogramming of somatic cells. Prog Mol Biol Transl Sci 111: 51–82, 2012 [DOI] [PubMed] [Google Scholar]
- 169.Ramanan S, Kooshki M, Zhao W, Hsu FC, Riddle DR, et al. . The PPARalpha agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int J Radiat Oncol Biol Phys 75: 870–877, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ramanan S, Zhao W, Riddle DR, and Robbins ME. Role of PPARs in radiation-induced brain injury. PPAR Res 2010: 234975, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Reed GB, Jr., and Cox AJ, Jr., The human liver after radiation injury. A form of veno-occlusive disease. Am J Pathol 48: 597–611, 1966 [PMC free article] [PubMed] [Google Scholar]
- 172.Ren G, Chen X, Dong F, Li W, Ren X, et al. . Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Transl Med 1: 51–58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reynolds BA. and Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255: 1707–1710, 1992 [DOI] [PubMed] [Google Scholar]
- 174.Rezvani M, Birds DA, Hodges H, Hopewell JW, Milledew K, et al. . Modification of radiation myelopathy by the transplantation of neural stem cells in the rat. Radiat Res 156: 408–412, 2001 [DOI] [PubMed] [Google Scholar]
- 175.Rigotti G, Marchi A, Galie M, Baroni G, Benati D, et al. . Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 119: 1409–1422; discussion 1423–1404, 2007 [DOI] [PubMed] [Google Scholar]
- 176.Robbins ME, Payne V, Tommasi E, Diz DI, Hsu FC, et al. . The AT1 receptor antagonist, L-158,809, prevents or ameliorates fractionated whole-brain irradiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys 73: 499–505, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, et al. . Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol 188: 316–330, 2004 [DOI] [PubMed] [Google Scholar]
- 178.Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, et al. . Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One 6: e24072, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sakurai T, Sawada Y, Yoshimoto M, Kawai M, and Miyakoshi J. Radiation-induced reduction of osteoblast differentiation in C2C12 cells. J Radiat Res 48: 515–521, 2007 [DOI] [PubMed] [Google Scholar]
- 180.Saury JM. and Emanuelson I. Cognitive consequences of the treatment of medulloblastoma among children. Pediatr Neurol 44: 21–30, 2011 [DOI] [PubMed] [Google Scholar]
- 181.Schultheiss TE, Kun LE, Ang KK, and Stephens LC. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys 31: 1093–1112, 1995 [DOI] [PubMed] [Google Scholar]
- 182.Schultheiss TE. and Stephens LC. Invited review: permanent radiation myelopathy. Br J Radiol 65: 737–753, 1992 [DOI] [PubMed] [Google Scholar]
- 183.Schultheiss TE, Stephens LC, and Maor MH. Analysis of the histopathology of radiation myelopathy. Int J Radiat Oncol Biol Phys 14: 27–32, 1988 [DOI] [PubMed] [Google Scholar]
- 184.Schultz-Hector S. Radiation-induced heart disease: review of experimental data on dose response and pathogenesis. Int J Radiat Biol 61: 149–160, 1992 [DOI] [PubMed] [Google Scholar]
- 185.Schultz-Hector S. and Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys 67: 10–18, 2007 [DOI] [PubMed] [Google Scholar]
- 186.Semont A, Francois S, Mouiseddine M, Francois A, Sache A, et al. . Mesenchymal stem cells increase self-renewal of small intestinal epithelium and accelerate structural recovery after radiation injury. Adv Exp Med Biol 585: 19–30, 2006 [DOI] [PubMed] [Google Scholar]
- 187.Semont A, Mouiseddine M, Francois A, Demarquay C, Mathieu N, et al. . Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ 17: 952–961, 2010 [DOI] [PubMed] [Google Scholar]
- 188.Sensebe L, Tarte K, Galipeau J, Krampera M, Martin I, et al. . Limited acquisition of chromosomal aberrations in human adult mesenchymal stromal cells. Cell Stem Cell 10: 9–10; author reply 10–11, 2012 [DOI] [PubMed] [Google Scholar]
- 189.Smee RI, Williams JR, De-Loyde KJ, Meagher NS, and Cohn R. Medulloblastoma: progress over time. J Med Imaging Radiat Oncol 56: 227–234, 2012 [DOI] [PubMed] [Google Scholar]
- 190.Snyder EY, Deitcher DL, Walsh C, Arnold-Aldea S, Hartwieg EA, et al. . Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell 68: 33–51, 1992 [DOI] [PubMed] [Google Scholar]
- 191.Stewart FA, Heeneman S, Te Poele J, Kruse J, Russell NS, et al. . Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE−/− mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol 168: 649–658, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sun D, Bullock MR, McGinn MJ, Zhou Z, Altememi N, et al. . Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp Neurol 216: 56–65, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. . Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872, 2007 [DOI] [PubMed] [Google Scholar]
- 194.Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, et al. . Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 115: 1549–1553, 2010 [DOI] [PubMed] [Google Scholar]
- 195.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, et al. . A specialized vascular niche for adult neural stem cells. Cell Stem Cell 3: 279–288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Teng YD, Benn SC, Kalkanis SN, Shefner JM, Onario RC, et al. . Multimodal actions of neural stem cells in a mouse model of ALS: a meta-analysis. Sci Transl Med 4: 165ra164, 2012 [DOI] [PubMed] [Google Scholar]
- 197.Tofilon PJ. and Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res 153: 357–370, 2000 [DOI] [PubMed] [Google Scholar]
- 198.Tolar J, Le Blanc K, Keating A, and Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells 28: 1446–1455, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tran SD, Sumita Y, and Khalili S. Bone marrow-derived cells: a potential approach for the treatment of xerostomia. Int J Biochem Cell Biol 43: 5–9, 2011 [DOI] [PubMed] [Google Scholar]
- 200.Tukenova M, Guibout C, Oberlin O, Doyon F, Mousannif A, et al. . Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol 28: 1308–1315, 2010 [DOI] [PubMed] [Google Scholar]
- 201.van der Kogel AJ. Radiation-induced damage in the central nervous system: an interpretation of target cell responses. Br J Cancer Suppl 7: 207–217, 1986 [PMC free article] [PubMed] [Google Scholar]
- 202.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, et al. . Functional neurogenesis in the adult hippocampus. Nature 415: 1030–1034, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vergeer MR, Doornaert PA, Rietveld DH, Leemans CR, Slotman BJ, et al. . Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a nonrandomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys 74: 1–8, 2009 [DOI] [PubMed] [Google Scholar]
- 204.Vissink A, Jansma J, Spijkervet FK, Burlage FR, and Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med 14: 199–212, 2003 [DOI] [PubMed] [Google Scholar]
- 205.Voswinkel J, Francois S, Simon JM, Benderitter M, Gorin NC, et al. . Use of mesenchymal stem cells (MSC) in chronic inflammatory fistulizing and fibrotic diseases: a comprehensive review. Clin Rev Allergy Immunol 45: 180–192, 2013 [DOI] [PubMed] [Google Scholar]
- 206.Wang ZH, Yan C, Zhang ZY, Zhang CP, Hu HS, et al. . Impact of salivary gland dosimetry on post-IMRT recovery of saliva output and xerostomia grade for head-and-neck cancer patients treated with or without contralateral submandibular gland sparing: a longitudinal study. Int J Radiat Oncol Biol Phys 81: 1479–1487, 2011 [DOI] [PubMed] [Google Scholar]
- 207.Wen Y, Meng L, Xie J, and Ouyang J. Direct autologous bone marrow-derived stem cell transplantation for ischemic heart disease: a meta-analysis. Expert Opin Biol Ther 11: 559–567, 2011 [DOI] [PubMed] [Google Scholar]
- 208.Wong CS. and Van der Kogel AJ. Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol Interv 4: 273–284, 2004 [DOI] [PubMed] [Google Scholar]
- 209.Yamanouchi K, Zhou H, Roy-Chowdhury N, Macaluso F, Liu L, et al. . Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology 49: 258–267, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.You D, Waeckel L, Ebrahimian TG, Blanc-Brude O, Foubert P, et al. . Increase in vascular permeability and vasodilation are critical for proangiogenic effects of stem cell therapy. Circulation 114: 328–338, 2006 [DOI] [PubMed] [Google Scholar]
- 211.Yuan J, Cui L, Zhang WJ, Liu W, and Cao Y. Repair of canine mandibular bone defects with bone marrow stromal cells and porous beta-tricalcium phosphate. Biomaterials 28: 1005–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 212.Zampetaki A, Kirton JP, and Xu Q. Vascular repair by endothelial progenitor cells. Cardiovasc Res 78: 413–421, 2008 [DOI] [PubMed] [Google Scholar]
- 213.Zhang J, Gong JF, Zhang W, Zhu WM, and Li JS. Effects of transplanted bone marrow mesenchymal stem cells on the irradiated intestine of mice. J Biomed Sci 15: 585–594, 2008 [DOI] [PubMed] [Google Scholar]
- 214.Zhang S, Sun A, Xu D, Yao K, Huang Z, et al. . Impact of timing on efficacy and safetyof intracoronary autologous bone marrow stem cells transplantation in acute myocardial infarction: a pooled subgroup analysis of randomized controlled trials. Clin Cardiol 32: 458–466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang Y, Guo CB, Zhang L, Wang Y, Peng X, et al. . Prevention of radiation-induced xerostomia by submandibular gland transfer. Head Neck 34: 937–942, 2012 [DOI] [PubMed] [Google Scholar]
- 216.Zhao W, Payne V, Tommasi E, Diz DI, Hsu FC, et al. . Administration of the peroxisomal proliferator-activated receptor gamma agonist pioglitazone during fractionated brain irradiation prevents radiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys 67: 6–9, 2007 [DOI] [PubMed] [Google Scholar]
- 217.Zhao W. and Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem 16: 130–143, 2009 [DOI] [PubMed] [Google Scholar]
- 218.Zhou H, Dong X, Roy-Chowdhury J, and Guha C. Preparatory liver irradiation followed by hepatocyte transplantation and engraftment corrects serum cholesterol in a murine hypercholesterolemia model. Int J Radiat Biol 72: S710, 2008 [Google Scholar]
- 219.Zimmet H, Porapakkham P, Sata Y, Haas SJ, Itescu S, et al. . Short- and long-term outcomes of intracoronary and endogenously mobilized bone marrow stem cells in the treatment of ST-segment elevation myocardial infarction: a meta-analysis of randomized control trials. Eur J Heart Fail 14: 91–105, 2012 [DOI] [PubMed] [Google Scholar]
- 220.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57: 178–201, 2008 [DOI] [PubMed] [Google Scholar]
- 221.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, et al. . Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211–228, 2001 [DOI] [PubMed] [Google Scholar]