Abstract
The spontaneous formation of closed bilayer structures from prebiotically plausible amphiphiles is an essential requirement for the emergence of early cells on prebiotic Earth. The sources of amphiphiles could have been both endo- and exogenous (accretion of meteorite carbonaceous material or interstellar dust particles). Among all prebiotic possible amphiphile candidates, those containing phosphate are the least investigated species because their self-assembly occurs in a seemingly too narrow range of conditions. The self-assembly of simple phosphate amphiphiles should, however, be of great interest, as contemporary membranes predominantly contain phospholipids. In contrast to common expectations, we show that these amphiphiles can be easily synthesized under prebiotically plausible environmental conditions and can efficiently form bilayer structures in the presence of various co-surfactants across a large range of pH values. Vesiculation was even observed in crude reaction mixtures that contained 1-decanol as the amphiphile precursor. The two best co-surfactants promoted vesicle formation over the entire pH range in aqueous solutions. Expanding the pH range where bilayer membranes self-assemble and remain intact is a prerequisite for the emergence of early cell-like compartments and their preservation under fluctuating environmental conditions. These mixed bilayers also retained small charged solutes, such as dyes. These results demonstrate that alkyl phosphate amphiphiles might have played a significant role as early compartment building blocks. Key Words: Vesicles—Alkyl phosphate—Prebiotic synthesis—Amphiphile mixtures. Astrobiology 14, 462–472.
1. Introduction
Contemporary cell metabolism is dependent on compartmentalization. The compartment boundaries are mainly composed of self-assembled lipid bilayers or membranes. Their functions are not limited to the spatial definition of the cell as a whole (identity definition and preservation) (Szostak et al., 2001) but are essential for the spatial separation of the metabolic subsystems and play a regulating role in exchanges with the environment (up-concentrating valuable molecules, uptake of nutrients, and energy and waste management) (Deamer, 1997). The boundaries of early cells and cell-like systems that preceded them (Walde et al., 1994; Monnard and Deamer, 2002; Luisi et al., 2006; Walde, 2010; Patil et al., 2012) must have fulfilled at least the same functions with comparable building blocks. However, early membranes might have also played a crucial role in the development and selection of precellular systems by imparting them with evolutionary advantages (Budin and Szostak, 2011; Adamala and Szostak, 2013).
The composition of these early cellular membranes is still an open question. Among all possible membrane-like-structure–forming molecules, amphiphiles have been extensively studied (Morigaki and Walde, 2007; Chen and Walde, 2010), although alternative boundary compositions have been proposed (Oparin et al., 1976; Russell et al., 1994; Zhang et al., 2008; Williams et al., 2012). It seems likely that the early amphiphiles were simpler than modern phospholipids, especially because of the complexity of their non-enzymatic synthesis (Epps et al., 1978). Investigation of the organic contents of carbonaceous chondrite meteorites (Mautner et al., 1995), as well as laboratory synthetic models (McCollom et al., 1999; Rushdi and Simoneit, 2001), has established that amphiphilic molecules that are composed of a single hydrocarbon chain with a polar headgroup, mostly an acid function, were very likely available on early Earth, with the abundance of the species increasing with decreasing hydrocarbon chain length. However, such amphiphiles were probably minor components of rather complex mixtures. The presence of other amphiphiles with functional headgroups, such as phosphate, phosphonate, and amine/ammonium, can be also inferred; traces of short alkyl chains with a functional group containing phosphorus or nitrogen have been found in meteorites even though these particular molecules cannot self-assemble (Cooper et al., 1992; Degraaf et al., 1995; Pizzarello et al., 2011).
The investigation of the self-assembly of single-hydrocarbon chain amphiphiles has thus focused on fatty acids and derivatives because of their documented availability and the fact that phospholipids contain fatty acid as their main hydrophobic moiety. Bilayer formation by fatty acids was found to be sensitive to pH (Apel et al., 2002), ionic strength (Monnard et al., 2002; Namani and Deamer, 2008), and temperature of the medium (Mansy and Szostak, 2008). In addition, the minimal amount of fatty acid to form membranes was shown to be dependent on the number of methylene groups in the hydrocarbon chain. The shortest self-assembling species, octanoic acid, forms membranes at concentrations 150 mM and above (Apel et al., 2002; Maurer et al., 2009). This relatively high critical vesicle concentration, in conjunction with the likely absence of synthetic pathways delivering a single product, makes early membranes made of a single compound appear unlikely. Recent research has suggested that mixed membranes would have had properties that remedy in part the shortcomings of their single-component counterparts, and could even represent a form of evolutionary advantage. Indeed, mixed membranes have been shown to tolerate higher ionic strengths (Monnard et al., 2002; Chen et al., 2005), higher temperatures (Mansy and Szostak, 2008; Maurer et al., 2009) while forming at lower amphiphile concentrations (Cape et al., 2011), and being stable within a larger pH range (Apel et al., 2002). Primitive membranes composed of mixed amphiphiles could also have imparted an evolutionary edge to an early cell by increasing its ability to take up amphiphiles from the environment (Budin and Szostak, 2011) or to scavenge amphiphiles from nonmixed competitors. Hence, early cells with mixed membranes would have been better equipped to grow and multiply.
In the present study, we decided to reinvestigate the self-assembly of phosphate amphiphiles mixed with co-surfactants, in particular alkanols. Indeed, an endogenic synthesis of phosphate-containing amphiphiles has been recently carried out (Powner and Sutherland, 2011) by using alkanols as precursors, ammonium hydrogen phosphate as reactant, and urea as a catalyst under dehydrating conditions (100°C). Under such conditions, syntheses starting from alkanol mixtures (decanol, hexanol, and ethanol as representative medium and short chain alkanols) led to the accumulation of decyl- and hexyl-phosphate products, even with the shorter chain alkanol in large molar excess. Thus, a spontaneous fractionation of the amphiphile precursor could have taken place (likely based on their volatility) and would have led to the accumulation of medium hydrocarbon chain amphiphiles. Such a result is significant, as short hydrocarbon chain alcohols seem to be the predominant products in prebiotic syntheses (McCollom et al., 1999), and the corresponding alkyl phosphates do not self-assemble. A source for phosphate, as a soluble anion, could have been schreibersite (Pasek and Lauretta, 2005; Bryant and Kee, 2006), which could have formed ferrous and nickel phosphates under anoxic corrosion and further redox transformation. The subsequent precipitation of the two metals with sulfide or complexation with cyanide could then release phosphate into solution. The availability of phosphate amphiphiles would then depend on that of schreibersite on early Earth. In a recent report, Pasek and Lauretta (2008) reinvestigated the influx of phosphorus, in the form of phosphides in iron meteorites, and estimated that phosphorus would have been present in concentrations that could sustain prebiotic chemistry in at least some locations.
A second reason for this reinvestigation of phosphate amphiphile self-assembly is related to the absence of studies dealing with their mixtures with co-surfactants. Indeed, whereas investigations of fatty acid structure formation have been carried out in mixtures, the few studies dealing with single-chain phosphate amphiphiles, which established their ability to form bilayers in a narrow acidic pH range (around pH 2) (Walde et al., 1997; Sakai et al., 2012), have only pertained to pure phosphate amphiphile membranes.
The present study shows that mixed bilayer vesicles composed of decyl phosphate and small amounts of prebiotically plausible co-surfactant can form over a wider range of pH values and are able to encapsulate and retain small molecules more efficiently than comparable mixed fatty acid membranes.
2. Experimental
All chemicals were from Sigma-Aldrich and of the highest purity available, except decyl amine at 95% purity and 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (pyranine) at≥97% purity. Milli-Q grade water was used throughout the experiments.
2.1. Synthesis
The prebiotic simulation reaction was performed in a 50 mL round-bottom flask with 1 mmol of NH4H2PO4, 10 mmol of urea, and 1 mmol of 1-decanol dissolved in 10 mL of Milli-Q water and 5 mL of ethanol. The open flask was heated at 30°C for 48 h, after which the temperature was increased to 100°C for 24 h, which resulted in a white crystalline solid. Conventional synthesis of decyl phosphate was done according to the procedure described in the literature (Virag et al., 2003).
2.2. Vesicle formation
Decyl phosphate, usually 10 mg/mL (35.4 μmol), was dissolved in Milli-Q water by application of vortex mixing. The pH of the resulting suspension was then adjusted to the desired value with either 2 M NaOH or 1 M HCl. The addition of co-surfactant to the samples occurred after the pH adjustment. The samples were then sonicated on a bath sonicator for 10 min and allowed to equilibrate for 30 min before use.
To investigate self-assembly, the crude reaction product was dissolved in 1.5 mL of Milli-Q water, and the resultant solution was adjusted to pH 7 with either 2 M NaOH or 1 M HCl. Finally, the sample volume was increased to 2.0 mL with Milli-Q water before investigation by microscopy.
2.3. Microscopy
Epifluorescence microscopy was carried out on a Nikon TE2000-S microscope (Japan), connected to a BD CARV II (New Jersey, USA), with a Photometrics Cascade II 512 camera (Arizona, USA). The samples were illuminated with a Nikon Intensilight C-HGFI (Japan) fluorescent lamp or a Zeiss Axioplan (Carl Zeiss, Göttingen, Germany), with an integrated FluoArc, a Photometrics Coolsnap HQ2 camera (Arizona, USA), and a Lambda 10-2 shutter (Shutter Instrument, California, USA).
To visualize the vesicles on the microscope, the samples were stained with a 1 mM solution of Nile red in ethanol (volume ratio of sample to Nile red solution 10:1), unless otherwise noted.
2.4. Determination of the critical vesicle concentration (CVC)
The minimal amount of decyl phosphate and precursor necessary to form bilayer structures in aqueous solution was determined by using solvatochromic probes; merocyanine 540 and pyrene were used for the low (2 and 7) and high (12) pH values, respectively. The probes were added to a serial dilution of the samples, and dilution was performed with Milli-Q water adjusted to the pH of the amphiphile stock solution. The diluted samples were sonicated for 10 min and left to equilibrate for half an hour before the measurements.
For merocyanine 540, the solvatochromic shift was observed as a change in absorbance between 530 and 570 nm. It was added as an ethanol solution immediately before the measurements to obtain a 10 μM final concentration. For pyrene, a change in fluorescence signal between 365 and 405 nm was recorded, which corresponds to the formation/dissolution of excimers. In this case, the dye was added as a tetrahydrofuran solution to obtain a 1.0 μM final concentration. In both cases, the samples were left to rest for at least 30 min before measurement.
2.5. Encapsulation
Encapsulation of the solutes, either 0.1 mM pyranine or 0.4 mM carboxyfluorescein, was done by pH titration with a final pH of 7. The vesicle suspension was passed through a size exclusion column, GE Lifescience PD miditrap G-25 column, to remove non-encapsulated dye molecules before microscopic examination.
Alternatively, samples containing 35 mM of decyl phosphate, 4%mol decylamine or 1-decanol, and 0.5 mM 5(6)-carboxyfluorescein in Milli-Q water at pH 7.5 were prepared for photobleaching experiments. Such a surfactant concentration ensured the formation of a large number of vesicles. The mixtures were sonicated for 10 min and left to equilibrate for 30 min. Immediately prior to investigation, the mixtures were diluted with one volume equivalent of pH-adjusted Milli-Q water. The presence of vesicles was first confirmed by microscopy (i.e., a suspension aliquot was stained with Nile red) before carrying out the photobleaching experiments on the microscope (the 455–490 nm excitation filter and the 510–590 nm emission filter).
3. Results
To evaluate the capacity of decyl phosphate to self-assemble into vesicles, a series of different systems were prepared: a pure decyl phosphate system along with four other mixtures containing each one co-surfactant, 1-decanol, decylamine, decyltrimethylammonium bromide (DTAB), and 1-decanoyl-rac-glycerol (GMD). The goal was to optimize the structure formation at the lowest possible concentration of co-surfactant and thereby ensure that the primary component in the membrane was the decyl phosphate.
3.1. Vesicle formation in mixtures as function of pH
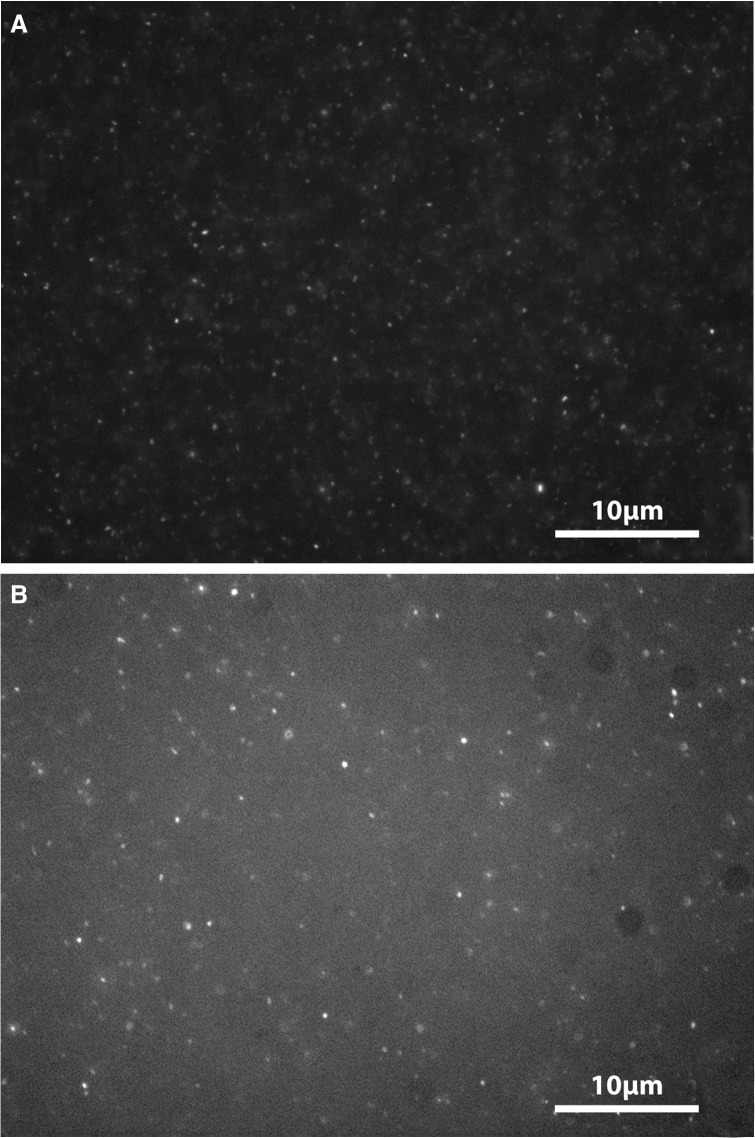
The pH range conducive to the self-assembly of the various amphiphile systems was examined by microscopy at pH 2, 7, and 12. Pure decyl phosphate formed structures at pH 2 only (see Fig. 1). At higher pH, no membranous aggregates were observed, but solid precipitates were present.
FIG. 1.
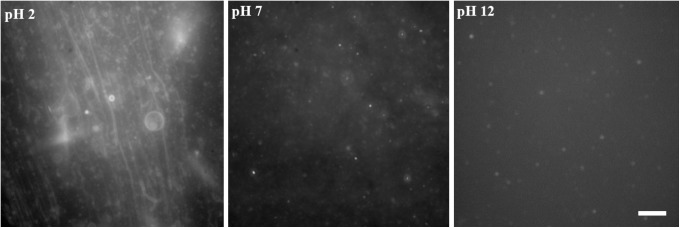
Formation of structures by pure sodium decyl phosphate 10 mg/mL (35.4 mM) at pH 2, 7, and 12, respectively. Nile red at a final concentration of approximately 0.1 mM was used to visualize any structure present in the sample. The scale bar is 10 μm and applies to all micrographs.
The most effective concentration of co-surfactant to induce vesicle formation at higher pH was first determined by using 1-decanol; 2%mol 1-decanol already led to the formation of vesicles, and 4%mol yielded more structures as determined by microscopic observations. At 6%mol, the appearance of oil droplets was recorded, which indicated that the alcohol was in excess and did not completely insert into the bilayers. As the concentration of 1-decanol was further increased (up to 20%mol), a clear trend emerged; oil droplets became the predominant aggregate type at the cost of the membranous structures. This result was comparable to those obtained in alcohol/fatty acid systems (Apel et al., 2002) where a 1:9 (alcohol:fatty acid) ratio was determined to be optimal, higher alcohol contents leading to oil droplet formation.
The presence of a co-surfactant at 4%mol led to two different outcomes in samples containing 10 mg/mL phosphate amphiphiles. In the case of decanol and decylamine, vesicles and tubes were detected at all pH values investigated (see Fig. 2). Both GMD and DTAB co-surfactant systems did not form vesicles at pH 7 and 12. But structures could be observed with 33%mol GMD as co-surfactant at all pH values. It should be noted here that the same molar decanoic acid–to–GMD ratio of 2:1 leads to an optimized formation of mixed vesicles (Maurer et al., 2009).
FIG. 2.
A collage of the vesicles produced at different pH values and with different system compositions. All solutions contained 10 mg/mL (35.4 mM) of sodium decyl phosphate and 4%mol of the co-surfactant given at the top left corner of each micrograph. Note that both GMD samples contained 33%mol GMD. The samples were stained with 0.1 mM Nile red. The scale bar applies to all micrographs and represents 10 μm.
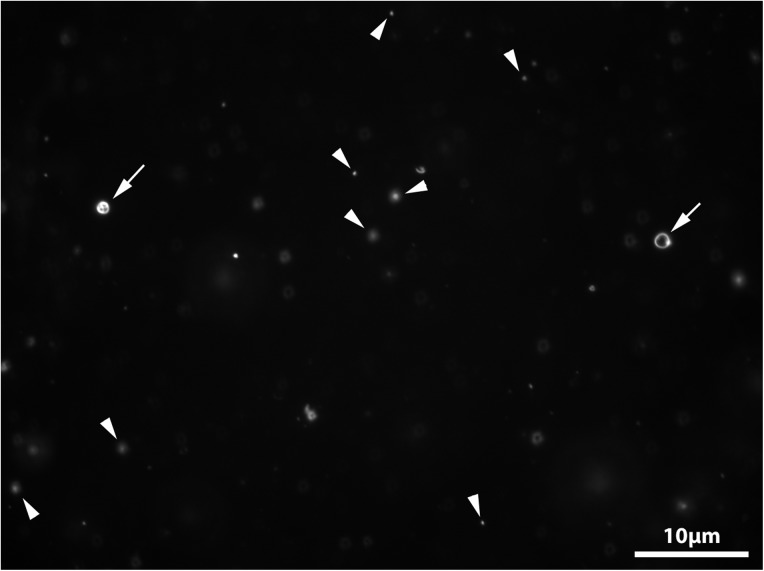
Since a mixture of 1-decanol and decyl phosphate produced vesicles, vesicle formation in the crude reaction mixture used to synthesize decyl phosphate (Powner and Sutherland, 2011) was investigated. The crude reaction solid was re-suspended in Milli-Q water, and the pH of the resulting solution was adjusted to 7.0. Numerous small vesicles and a few larger ones could be seen in the samples (see Fig. 3). This result was consistent with the continued presence of 1-decanol, the amphiphile precursor, among the products in the reaction mixture.
FIG. 3.
Vesicles formed by the reaction mixture used to synthesize decyl phosphate (pH 7). 40 μM Nile red was used to visualize the sample on the microscope. Scale bar 10 μm. The semicrystalline crude reaction product was resuspended in Milli-Q water and the pH adjusted to pH 7 yielding a final volume of 2 mL. The arrows point to vesicles, and the arrowheads point to oil droplets in the frame.
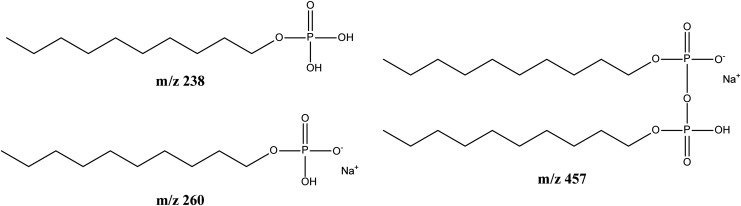
Electrospray mass spectroscopy was carried out to qualitatively determine the composition of the crude reaction mixture. Three main products were detected whose masses corresponded to hydrogen monodecyl phosphate, sodium monodecyl phosphate, and didecyl pyrophosphate (see Fig. 4). The amount of didecyl pyrophosphate was unfortunately too low for this product to be purified; hence its self-assembly properties will be tested in future experiments by using material prepared by conventional synthesis. 1-Decanol could not be detected by electrospray mass spectroscopy, but its presence as unreacted starting material can be inferred from the observed vesicle formation as well as the presence of oil droplets (see Fig. 3).
FIG. 4.
Products of the prebiotically plausible decyl phosphate synthesis and their masses.
3.2. Determination of the CVC
Initially, the CVCs, at the three different pH values, were estimated by using serial dilution and microscopy. During this preliminary work, the observed trends for the CVCs indirectly explained the self-assembly behavior observed earlier; the CVC of decyl phosphate/DTAB was approximately 10 times higher than it was for the other systems above pH 2.
Because of the potential significance of 1-decanol in the prebiotic synthesis of decyl phosphate, the mixture of 4%mol 1-decanol and decyl phosphate was further investigated to determine the mixture's CVC at the three pH values. These CVCs were then measured by using merocyanine 540 (Cape et al., 2011) at pH 2 and 7 or pyrene (Delample et al., 2011), as the solvatochromic probe, at pH 12 (see Table 1).
Table 1.
Critical Vesicle Concentration Constants for Sodium Decyl Phosphate and Mixed Samples Containing 4%mol 1-Decanol
| System | pH | CVC (mM) |
|---|---|---|
| Decyl phosphate | 2±0.5 | 0.67±0.12 |
| Decyl phosphate 4%mol 1-decanol | 2±0.5 | 0.81±0.12 |
| Decyl phosphate 4%mol 1-decanol | 7.0±0.2 | 2.69±0.91 |
| Decyl phosphate 4%mol 1-decanol | 12±1.5 | 6.38±0.40 |
The concentration refers to the decyl phosphate alone to allow for easy comparison.
The addition of 1-decanol at pH 2 did not significantly influence the CVC. The other CVCs established an increase of the minimal concentration for vesicle formation as the pH increased, attaining a maximum value 10-fold larger than the pure decyl phosphate at pH 2.
3.3. Encapsulation and permeability
The encapsulation of 0.1 mM pyranine and 0.4 mM carboxyfluorescein was investigated at pH 7 by using vesicles consisting of decyl phosphate and 4%mol 1-decanol. After removal of the non-encapsulated solutes, the samples were investigated by microscopy. Micrographs of the suspension displayed the usual characteristics of dye encapsulation (see Fig. 5). Thus, small fluorescent points corresponding to small vesicles with fluorescent dyes in their aqueous lumen were visible.
FIG. 5.
Micrographs of decyl phosphate vesicles encapsulating 0.1 mM pyranine (A) and 0.4 mM carboxyfluorescein (B). The vesicles displayed in both micrographs were formed in mixtures of decyl phosphate 10 mg/mL (35.4 mM) and 1-decanol in the ratio 25:1. The micrographs were obtained by using the fluorescent signal of pyranine (A) and carboxyfluorescein (B).
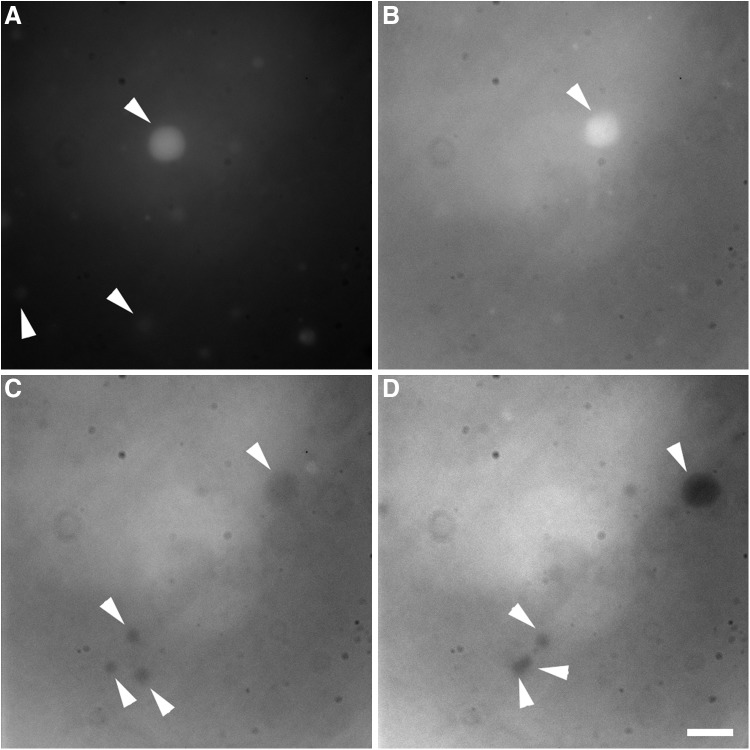
The assessment of the permeability of these vesicles was then performed by using bleaching of encapsulated dyes as an indicator. In the case of leaky membranous structures (Maurer et al., 2009), the bleaching effect rapidly disappears as the equilibration of dyes between the internal lumen and the external medium takes place. A solution containing vesicles as well as 0.5 mM carboxyfluorescein was prepared as usual with mixtures containing 4%mol 1-decanol or decylamine. Just before the bleaching experiment, the suspensions were diluted with one volume equivalent of Milli-Q water resulting in a 2-fold concentration gradient for the dye across the bilayers. The samples were then irradiated with a mercury lamp while being observed by microscopy. The series of pictures is displayed in Fig. 6. At first, the higher internal concentration of dye can be observed. As the exposure proceeded, the bleaching effect on the limited supply of dye in the internal vesicle lumen became apparent (darkening of the lumen), indicating that the dye equilibration across the bilayers did not occur over the time span of the experiments (a few minutes). Similar results were obtained in the case of 1-decanol mixed vesicles.
FIG. 6.
Photobleaching of carboxyfluorescein encapsulated inside sodium decyl phosphate vesicles. The sample was diluted with one volume equivalent of pH-adjusted Milli-Q water immediately before the micrographs were obtained. The vesicles were visualized by using the fluorescence signal of 0.25 mM carboxyfluorescein. The vesicles are composed of 17.7 mM decyl phosphate with 4%mol decylamine. Arrowheads highlight the vesicles containing the dye before (A) and after (D) bleaching. The scale bar is 10 μm and applies to all micrographs.
4. Discussion
Phosphates are today present ubiquitously in the cell as components of nucleic acid backbones, lipids, and other metabolites (Westheimer, 1987), as well as in energy currency molecules. Interestingly, phosphate amphiphiles have not yet attracted much attention from researchers who investigate protocell or precellular compartments. This lack of interest is likely due to the very narrow pH range in which stable vesicles can be formed by pure phosphate amphiphiles (Walde et al., 1997).
The stabilization of amphiphile structures, which reflects the propensity for an amphiphile molecule to self-assemble, is governed by three main types of interactions: (i) hydrophobic effect due to the negative interactions between hydrocarbon chains and the water molecules and van der Waals forces between the chains themselves, which both are proportional to the length of the hydrocarbon chain, (ii) headgroup to headgroup interactions (either through H bonds or electrostatic pairing), and (iii) the interactions between the amphiphile headgroups and solutes present in the aqueous environment or/and the water molecules themselves. The balance between these three interactions or their actual contribution to the structure stabilization is a complex issue, as can be seen in previous investigations of mixed fatty acid membranes with GMD or DTAB (Maurer et al., 2009; Caschera et al., 2011).
In this report, the self-assembly of simple phosphate amphiphiles was investigated in mixtures with several co-surfactants; in particular, decyl phosphate and its mixtures with 1-decanol, 1-decanoyl-rac-glycerol, decylamine, and decyltrimethylammonium bromide were used. These co-surfactants were chosen because of their likely availability on primitive Earth. Indeed, the presence of alkyl alcohols has been documented in Fischer-Tropsch–type reactions (McCollom et al., 1999); monoglycerides can be easily prepared from their constituents glycerol and fatty acids (Apel and Deamer, 2005). Alkyl amines and tetraalkylammoniums have been detected in the carbon content of nitrogen-rich meteorites (Pizzarello and Holmes, 2009). However, none of them are reported to form bilayer structures alone, which is in clear contrast to their capacity to form micelles or distinct phases in aqueous media.
Moreover, these co-surfactants have all the potential to stabilize phosphate amphiphile structures by either H-bond formation or electrostatic interactions.
4.1. Pure decyl phosphate vesicles
Phosphate amphiphiles are protonatable like fatty acids. However, they have two exchangeable protons. As the propensity of protonatable amphiphiles to form vesicles at a pH around their pKa is well established experimentally for fatty acids (Hargreaves and Deamer, 1978; Cistola et al., 1988) and theoretically predicted for phosphate amphiphiles (Schuler et al., 2001), the investigation of phosphate amphiphile self-assembly was pursued at pH 2, 7, and 12. The two first pH values correspond to the reported pKa values of 2.2 and 7.4 (White, 1950). The last pH value allowed for the full coverage of the pH range in aqueous solution. The length of the hydrophobic hydrocarbon chain was chosen to be comparable to that of decanoic acid, a fatty acid that is known to form a bilayer structure around the second pKa of phosphate, at relatively low total amphiphile concentration and at room temperature (Kanicky et al., 2000; Morigaki et al., 2003; Maurer et al., 2009).
Pure decyl phosphate vesicles were observed at pH 2 (see Table 2), close to the reported first pKa of the headgroup. At high pH values, no vesicles of pure sodium decyl phosphate were observed even at pH 7, which is close to the second pKa of this amphiphile. However, a striking difference exists between decanoic acid and decyl phosphate. Whereas the fatty acid molecules were only partially carrying a single negative charge, which could be easily stabilized by an H bond, all phosphate headgroups were anionic, with approximately 50% of them being in principle dianionic, a state that certainly induces an increase in the electrostatic repulsion between the amphiphiles. As a result, half the molecules should be considered to have a wedge shape, which is known to promote the formation of micelles instead of bilayers (Walde et al., 1997; Schuler et al., 2001). Indeed, the higher curvature of micellar structures allows the reduction of the repulsive interactions between the charged headgroups. The self-assembly behavior of pure phosphate amphiphiles confirmed previous observations reported in the literature (Walde et al., 1997).
Table 2.
Summary of the pH Range for Vesicle Formation Depending on the Amphiphile Mixture (Determined by Epifluorescence Microscopy)
| Vesicle formation | ||||
|---|---|---|---|---|
| Co-surfactant | %mol | pH 2 | pH 7 | pH 12 |
| None | — | Yes | No | No |
| 1-Decanol | 4 | Yes | Yes | Yes |
| Decylamine | 4 | Yes | Yes | Yes |
| Decyltrimethylammonium bromide | 4 | Yes | No | No |
| 1-Decanoyl-rac-glycerol | 4 | Yes | No | No |
| 1-Decanoyl-rac-glycerol | 33 | Yes | Yes | Yes |
Phosphate amphiphile concentration was 10 mg/mL in all samples.
4.2. Mixed vesicles
The set of co-surfactants was chosen in this study to permit the exploration of two possible routes toward the stabilization of phosphate amphiphile bilayers: the formation of H bonds between headgroups and the electrostatic stabilization by formation of ionic amphiphile pairs. Note that the vesiculation process was not optimized to maximize the total amount of vesicles and membranous structures.
4.3. Stabilization of phosphate amphiphile membranous structures by H-bonding
An alcohol and a polyol, 1-decanol and GMD, were investigated at various molar ratios to determine their promotion of self-assembly while keeping their concentrations at a minimum. The expected effect of the co-surfactant was observed, as they both supported vesiculation at high pH values (7 and 12) and did not disrupt it at pH 2. Thus, they permitted the observation of membranous structures at pH values never reported before. As is the case for fatty acids, the stabilization of membranous structures at high pH is most likely attributable to the non-protonatable character of the alcohol group, which allows for the formation of H bonds with the phosphate group at any pH between 2 and 12 in aqueous solutions.
A significant difference in suspension composition leading to self-assembly was observed; GMD systems needed a significantly higher concentration of the co-surfactant (approximately 8 fold), a fact that suggests that its stabilization of the mixed bilayer was less effective than in the case of 1-decanol. The reason for the different behavior between the two co-surfactant headgroups was likely less than optimal H-bonding. The sterically larger glycerol headgroup could have induced packing defects in the phosphate bilayers.
These results correspond well with the behavior of both co-surfactants in mixed fatty acid membranes (Apel et al., 2002; Monnard et al., 2002). However, whereas the presence of alkyl alcohol or GMD [for every medium hydrocarbon chain length (Maurer et al., 2009)] decreases the CVC of mixed fatty acid vesicles, indicating a strong bilayer stabilization, it resulted here in a slight increase of this parameter (Table 2) at pH 2.
4.4. Stabilization of phosphate amphiphile membranous structures by electrostatic interactions
The formation of vesicles at pH values above 2 was facilitated by the addition of decylamine but not by DTAB. Both these co-surfactants are positively charged at pH 2 and 7; hence vesiculation in the presence of amine can be attributed to charge pairs forming between amphiphiles in the mixed bilayers. At pH 12, the amine is neutral; thus the observed promotion of self-assembly could be attributed to H-bonding.
Interestingly, the preliminary investigations of the CVC for decyl phosphate/DTAB at pH 2 also established that it was 10-fold higher than those of all other mixed systems. Thus, this different behavior hinted at a steric hindrance that would prevent a favorable packing of the amphiphiles in a bilayer configuration, although this repulsive interaction between the primary surfactant and the co-surfactant did not hinder the formation of vesicles at pH 2, whose mixed character has yet to be confirmed.
This DTAB behavior in mixed membranes is not surprising, as even in membranes that require its presence to form, for example, hexadecanedioic acid/DTAB (Caschera et al., 2011), DTAB did not present a strong co-surfactant character, as it tended to self-assemble preferentially in micelles. Alternatively, the lack of dissociation of DTAB into its ion constituents (DTA+ and Br−), which may be as low as 26% (Bales and Zana, 2002), could have significantly reduced the amount of positive charge available in the bilayer, thereby preventing the stabilization of the phosphate amphiphile in a bilayer structure. These results led to the conclusion that DTAB did not induce vesicle formation in the system, and it was not investigated further.
In comparison with decanoic acid alone, or its mixtures with amine amphiphiles, decyl phosphate/decylamine formed vesicles at concentrations below the CVC of the pure fatty acid, in a much larger pH range than similar mixtures reported by Namani and Deamer (2008). In their case, decanoic acid/decylamine formed vesicles at high pH (≥8) and low pH (≤4) but failed to maintain the vesicular structures at intermediate pH values (4–8). The authors surmised from the expected protonation states of the amphiphiles that mixed vesicles were stabilized by charge pairs between them, although the stabilization at pH 2 might have been related to more complex electrostatic interactions involving the buffer (borate).
A possible explanation for the difference in behavior between large headgroup (glycerol and trimethylammonium in GMD and DTAB, respectively) and small headgroup co-surfactants (alcohol and amine) can be proposed on the basis of general amphiphile self-assembly behavior. In the case of GMD, the impact of the headgroup-to-headgroup stabilization decreased with increasing hydrocarbon chain length (Maurer et al., 2009). That is, the contribution of this interaction to the structure stabilization was significantly higher in the case of short or medium alkyl chain length amphiphiles used in this study. The headgroup interactions alone cannot ensure the stable bilayer formation, as the CVC increase with decreasing alkyl chain length proves it. The “bulky” character of ionized phosphate headgroups (which leads to the preferential formation of micelles at high pH values) should require an extensive stabilization based on both the headgroup interaction and a dense packing of the hydrophobic moieties to form bilayers. Thus, the two large bulky molecules that likely need a large surface area to properly interact with the phosphate headgroups may well destabilize the bilayer by decreasing the packing density of the hydrophobic core, which would result in the loss of van der Waals interactions, ultimately preventing the formation of bilayers.
4.5. Encapsulation and permeability
Encapsulation of hydrophilic molecules within the aqueous lumen and their retention is considered to be one of the important parameters to judge whether amphiphile systems could model precellular membranes. Indeed, the retention of large charged molecules is of paramount importance for the development of metabolic networks and information systems as postulated, for example, by researchers in the protocell community (Szostak, et al., 2001).
1-Decanol/decyl phosphate mixed vesicles were shown not to be significantly permeable to pyranine at pH 7 and could withstand the osmotic stress of an encapsulated solute. These vesicles were also capable of retaining carboxyfluorescein along with the product of the photobleaching process. By contrast, encapsulation of carboxyfluorescein and pyranine was not achieved in GMD/decanoic acid (Maurer et.al., 2009) and decylamine/decanoic acid mixed vesicles (Namani and Deamer, 2008), respectively, as these molecules readily crossed the mixed membranes. That is, provided that small solutes were produced inside the phosphate amphiphile mixed vesicles, they could be available to perform or participate in reactions for longer periods of time, a clear evolutionary advantage.
By analogy with phospholipids/fatty acid membranes (Budin and Szostak, 2011), another possible evolutionary advantage of mixed membranes containing phosphate amphiphile could lie in the enhancement in amphiphile uptake from the environment, if the improved stability of mixed structures containing a large amount of phosphate amphiphiles does apply to similar mixed systems predominantly composed of fatty acid. Such a result will strengthen the prebiotic significance of simple phosphate amphiphiles.
5. Conclusion
We have shown that decyl phosphate mixtures with co-surfactants are capable of forming vesicles over a wide range of pH values and that the vesicles can retain small charged molecules and, by inference, larger charged molecules such as RNA. Moreover, vesicles can form directly from crude alkanol phosphorylation reaction products upon resuspension in unbuffered water. All these properties make decyl phosphate an interesting candidate as a component of primitive bilayers.
The reported results are interesting in terms of evolutionary pathways toward contemporary cells. Indeed, the ubiquity of phosphate groups in biochemistry points to early incorporation of phosphates in its various functions. At first, the incorporation would likely have had to rely on the spontaneous formation of phosphate-containing molecules, the phosphate source of which would have been soluble inorganic phosphates. Such a process can be proposed for both RNA and amphiphiles. The fact that both explored synthetic pathways are chemically compatible allows us to envision a synthetic emergence of precellular systems with a concomitant evolution toward the chemically more complex biomolecules life uses today.
Acknowledgments
We wish to thank the members of FLinT for fruitful scientific discussions. This work was supported for J.D.S. and C.D.D. by the Medical Research Council (project number MC_UP_A024_1009) and an award from the Origin of Life Challenge—we thank Harry Lonsdale for the latter. For P.-A.M. and A.N.A., we thank the Danish National Research Foundation, which supported the Center for Fundamental Living Technologies (FLinT, headed by center leader Prof. Steen Rasmussen) and the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 249032 (MATCHIT).
Author Disclosure Statement
No competing financial interests exist.
Abbreviations
CVC, critical vesicle concentration; DTAB, decyltrimethylammonium bromide; GMD, 1-decanoyl-rac-glycerol.
References
- Adamala K. and Szostak J.W. (2013) Competition between model protocells driven by an encapsulated catalyst. Nat Chem 5:495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel C.L. and Deamer D.W. (2005) The formation of glycerol monodecanoate by a dehydration/condensation reaction: increasing the chemical complexity of amphiphiles on the early Earth. Orig Life Evol Biosph 35:323–332 [DOI] [PubMed] [Google Scholar]
- Apel C.L., Deamer D.W., and Mautner M.N. (2002) Self-assembled vesicles of monocarboxylic acids and alcohols: conditions for stability and for the encapsulation of biopolymers. Biochim Biophys Acta Biomembranes 1559:1–9 [DOI] [PubMed] [Google Scholar]
- Bales B.L. and Zana R. (2002) Characterization of micelles of quaternary ammonium surfactants, as reaction media I: dodecyltrimethylammonium bromide and chloride. J Phys Chem B 106:1926–1939 [Google Scholar]
- Bryant D.E. and Kee T.P. (2006) Direct evidence for the availability of reactive, water soluble phosphorus on the early Earth. H-Phosphinic acid from the Nantan meteorite. Chem Commun (Camb) 14:2344–2346 [DOI] [PubMed] [Google Scholar]
- Budin I. and Szostak J.W. (2011) Physical effects underlying the transition from primitive to modern cell membranes. Proc Natl Acad Sci USA 108:5249–5254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cape J.L., Monnard P.-A., and Boncella J.M. (2011) Prebiotically relevant mixed fatty acid vesicles support anionic solute encapsulation and photochemically catalyzed trans-membrane charge transport. Chem Sci 2:661–671 [Google Scholar]
- Caschera F., Bernardino de la Serna J., Loffler P.M.G., Rasmussen T.E., Hanczyc M.M., Bagatolli L.A., and Monnard P.-A. (2011) Stable vesicles composed of monocarboxylic or dicarboxylic fatty acids and trimethylammonium amphiphiles. Langmuir 27:14078–14090 [DOI] [PubMed] [Google Scholar]
- Chen I.A. and Walde P. (2010) From self-assembled vesicles to protocells. Cold Spring Harb Perspect Biol 2, doi: 10.1101/cshperspect.a002170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I.A., Salehi-Ashtiani K., and Szostak J.W. (2005) RNA catalysis in model protocell vesicles. J Am Chem Soc 127:13213–13219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cistola D.P., Hamilton J.A., Jackson D., and Small D.M. (1988) Ionization and phase-behavior of fatty-acids in water—application of the Gibbs phase rule. Biochemistry 27:1881–1888 [DOI] [PubMed] [Google Scholar]
- Cooper G.W., Onwo W.M., and Cronin J.R. (1992) Alkyl phosphonic acids and sulfonic acids in the Murchison meteorite. Geochim Cosmochim Acta 56:4109–4115 [DOI] [PubMed] [Google Scholar]
- Deamer D.W. (1997) The first living systems: a bioenergetic perspective. Microbiol Mol Biol Rev 61:239–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degraaf R.M., Visscher J., and Schwartz A.W. (1995) A plausibly prebiotic synthesis of phosphonic-acids. Nature 378:474–477 [DOI] [PubMed] [Google Scholar]
- Delample M., Jerome F., Barrault J., and Douliez J.-P. (2011) Self-assembly and emulsions of oleic acid-oleate mixtures in glycerol. Green Chem 13:64–68 [Google Scholar]
- Epps D.E., Sherwood E., Eichberg J., and Oro J. (1978) Cyanamide mediated syntheses under plausible primitive Earth conditions. 5. Synthesis of phosphatidic acids. J Mol Evol 11:279–292 [DOI] [PubMed] [Google Scholar]
- Hargreaves W.R. and Deamer D.W. (1978) Liposomes from ionic, single-chain amphiphiles. Biochemistry 17:3759–3768 [DOI] [PubMed] [Google Scholar]
- Kanicky J.R., Poniatowski A.F., Mehta N.R., and Shah D.O. (2000) Cooperativity among molecules at interfaces in relation to various technological processes: effect of chain length on the pKa of fatty acid salt solutions. Langmuir 16:172–177 [Google Scholar]
- Luisi P.L., Ferri F., and Stano P. (2006) Approaches to semi-synthetic minimal cells: a review. Naturwissenschaften 93:1–13 [DOI] [PubMed] [Google Scholar]
- Mansy S.S. and Szostak J.W. (2008) Thermostability of model protocell membranes. Proc Natl Acad Sci USA 105:13351–13355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer S.E., Deamer D.W., Boncella J.M., and Monnard P.-A. (2009) Chemical evolution of amphiphiles: glycerol monoacyl derivatives stabilize plausible prebiotic membranes. Astrobiology 9:979–987 [DOI] [PubMed] [Google Scholar]
- Mautner M.N., Leonard R.L., and Deamer D.W. (1995) Meteorite organics in planetary environments—hydrothermal release, surface-activity, and microbial utilization. Planet Space Sci 43:139–147 [DOI] [PubMed] [Google Scholar]
- McCollom T.M., Ritter G., and Simoneit B.R.T. (1999) Lipid synthesis under hydrothermal conditions by Fischer-Tropsch–type reactions. Orig Life Evol Biosph 29:153–166 [DOI] [PubMed] [Google Scholar]
- Monnard P.-A. and Deamer D.W. (2002) Membrane self-assembly processes: steps toward the first cellular life. Anat Rec 268:196–207 [DOI] [PubMed] [Google Scholar]
- Monnard P.-A., Apel C.L., Kanavarioti A., and Deamer D.W. (2002) Influence of ionic inorganic solutes on self-assembly and polymerization processes related to early forms of life: implications for a prebiotic aqueous medium. Astrobiology 2:139–152 [DOI] [PubMed] [Google Scholar]
- Morigaki K. and Walde P. (2007) Fatty acid vesicles. Curr Opin Colloid Interface Sci 12:75–80 [Google Scholar]
- Morigaki K., Walde P., Misran M., and Robinson B.H. (2003) Thermodynamic and kinetic stability. Properties of micelles and vesicles formed by the decanoic acid/decanoate system. Colloids Surf A Physicochem Eng Asp 213:37–44 [Google Scholar]
- Namani T. and Deamer D.W. (2008) Stability of model membranes in extreme environments. Orig Life Evol Biosph 38:329–341 [DOI] [PubMed] [Google Scholar]
- Oparin A.I., Orlovskii A.F., Bukhlaeva V.Y., and Gladilin K.L. (1976) Effect of polyadenylic acid enzymatic-synthesis on coacervate system. Doklady Akademii Nauk SSSR 226:972–974 [PubMed] [Google Scholar]
- Pasek M.A. and Lauretta D.S. (2005) Aqueous corrosion of phosphide minerals from iron meteorites: a highly reactive source of prebiotic phosphorus on the surface of the early Earth. Astrobiology 5:515–535 [DOI] [PubMed] [Google Scholar]
- Pasek M.A. and Lauretta D.S. (2008) Extraterrestrial flux of potentially prebiotic C, N, and P to the early Earth. Orig Life Evol Biosph 38:5–21 [DOI] [PubMed] [Google Scholar]
- Patil A.J., Lee Y.-C., Yang J.-W., and Mann S. (2012) Mesoscale integration in titania/J-aggregate hybrid nanofibers. Angew Chem Int Ed Engl 51:733–737 [DOI] [PubMed] [Google Scholar]
- Pizzarello S. and Holmes W. (2009) Nitrogen-containing compounds in two CR2 meteorites: N-15 composition, molecular distribution and precursor molecules. Geochim Cosmochim Acta 73:2150–2162 [Google Scholar]
- Pizzarello S., Williams L.B., Lehman J., Holland G.P., and Yarger J.L. (2011) Abundant ammonia in primitive asteroids and the case for a possible exobiology. Proc Natl Acad Sci USA 108:4303–4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powner M.W. and Sutherland J.D. (2011) Prebiotic chemistry: a new modus operandi. Philos Trans R Soc Lond B Biol Sci 366:2870–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushdi A.I. and Simoneit B.R.T. (2001) Lipid formation by aqueous Fischer-Tropsch–type synthesis over a temperature range of 100 to 400 degrees C. Orig Life Evol Biosph 31:103–118 [DOI] [PubMed] [Google Scholar]
- Russell M.J., Daniel R.M., Hall A.J., and Sherringham J.A. (1994) A hydrothermally precipitated catalytic iron sulfide membrane as a first step toward life. J Mol Evol 39:231–243 [Google Scholar]
- Sakai T., Miyaki M., Tajima H., and Shimizu M. (2012) Precipitate deposition around CMC and vesicle-to-micelle transition of monopotassium monododecyl phosphate in water. J Phys Chem B 116:11225–11233 [DOI] [PubMed] [Google Scholar]
- Schuler L.D., Walde P., Luisi P.L., and van Gunsteren W.F. (2001) Molecular dynamics simulation of n-dodecyl phosphate aggregate structures. Eur Biophys J 30:330–343 [DOI] [PubMed] [Google Scholar]
- Szostak J.W., Bartel D.P., and Luisi P.L. (2001) Synthesizing life. Nature 409:387–390 [DOI] [PubMed] [Google Scholar]
- Virag T., Elrod D.B., Liliom K., Sardar V.M., Parrill A.L., Yokoyama K., Durgam G., Deng W.L., Miller D.D., and Tigyi G. (2003) Fatty alcohol phosphates are subtype-selective agonists and antagonists of lysophosphatidic acid receptors. Mol Pharmacol 63:1032–1042 [DOI] [PubMed] [Google Scholar]
- Walde P. (2010) Building artificial cells and protocell models: experimental approaches with lipid vesicles. Bioessays 32:296–303 [DOI] [PubMed] [Google Scholar]
- Walde P., Goto A., Monnard P.-A., Wessicken M., and Luisi P.L. (1994) Oparin's reactions revisited—enzymatic-synthesis of poly(adenylic acid) in micelles and self-reproducing vesicles. J Am Chem Soc 116:7541–7547 [Google Scholar]
- Walde P., Wessicken M., Rädler U., Berclaz N., Conde-Frieboes K., and Luisi P.L. (1997) Preparation and characterization of vesicles from mono-n-alkyl phosphates and phosphonates. J Phys Chem B 101:7390–7397 [Google Scholar]
- Westheimer F. (1987) Why nature chose phosphates. Science 235:1173–1178 [DOI] [PubMed] [Google Scholar]
- White J.R. (1950) Dissociation constants of higher alkyl phosphate esters, phosphonic acids, phosphonous acids, phosphinic acids and carboxylic acids. J Am Chem Soc 72:1859–1860 [Google Scholar]
- Williams D.S., Koga S., Hak C.R.C., Majrekar A., Patil A.J., Perriman A.W., and Mann S. (2012) Polymer/nucleotide droplets as bio-inspired functional micro-compartments. Soft Matter 8:6004–6014 [Google Scholar]
- Zhang J., Song Y.-F., Cronin L., and Liu T. (2008) Self-assembly of organic–inorganic hybrid amphiphilic surfactants with large polyoxometalates as polar head groups. J Am Chem Soc 130:14408–14409 [DOI] [PubMed] [Google Scholar]