Abstract
The aim of this study was to assess the antinociceptive activity of ginseng total saponins (GTS) on hyperalgesia induced by repeated intramuscular injections of acidic saline in rats and to examine the mechanisms involved. Rats were injected intraperitoneally with a 0.9% saline vehicle or various doses of GTS after the development of hyperalgesia. Rats were then injected with N-methyl-D-aspartate (NMDA) or naloxone 10 min before GTS injection. The mechanical withdrawal threshold (MWT) was assessed with von Frey filaments. The MWT was significantly increased after intraperitoneal injection of 100 mg/kg and 200 mg/kg of GTS when compared with the MWT after the development of hyperalgesia. Injection of GTS with NMDA showed a significant decrease in the MWT when compared with GTS injection. GTS showed an antinociceptive activity against chronic muscle-induced pain, and the effect of GTS may be mediated by NMDA.
Key Words: : acid, chronic, hyperalgesia, muscle, Panax ginseng
Introduction
Chronic musculoskeletal pain syndromes such as fibromyalgia and myofascial pain syndrome are major but poorly understood clinical syndromes. Therefore, an animal model of chronic muscle-induced pain has been developed to improve understanding of the diseases. Specifically, two injections of acidic saline into one gastrocnemius muscle 2 or 5 days apart produce long-lasting, bilateral hyperalgesia without associated tissue injury and without continued primary afferent input.1,2
Ginseng, the root of Panax ginseng Meyer, is a popular herbal medicine that has been shown to exhibit a variety of medicinal effects. In particular, ginseng has been used to improve various types of pain, including toothache, abdominal pain, chest pain, and neuralgia in traditional folk medicine. Ginseng saponins, or ginsenosides, have a four-ring, steroid-like structure with sugar moieties attached. They are the main bioactive molecules responsible for the actions of ginseng,3 and show properties similar to acetylcholine, adrenaline, histamine, and opioids.4
A few experimental studies have suggested that ginseng saponins have antinociceptive activity,5,6 but the production of antinociception in chronic muscle-induced pain has not been characterized until now. Some mechanisms of antinociception have been suggested but are not yet established. We hypothesized that ginseng total saponins (GTS) have antinociceptive activity on hyperalgesia induced by repeated intramuscular injection of acidic saline, and the antinociceptive effect is antagonized by N-methyl-D-aspartate (NMDA) and naloxone. The goal of this research was first to assess the antinociceptive activity of GTS in chronic muscle-induced pain, and second to examine the mechanisms involved.
Materials and Methods
These experiments were reviewed and approved by the Institutional Animal Care and Use Committee at Chung-ang University (2012-0003). The animals were treated in accordance with the National Institute of Health and the International Association for the Study of Pain policies on the use of laboratory animals.
Animals and drugs
Adult male Sprague–Dawley rats (weight: 250–300 g) from Coretec Laboratories (Seoul, Korea) were used for all experiments. The animals were housed two per cage in a room maintained at 22±0.5°C with an alternating 12 hour light–dark cycle. Food and water were unrestricted. GTS and ginseng saponin Rb1 were obtained from Ambo Institute (Daejon, Korea), and GTS was a mixture of various types of individual ginseng saponins, the ratios of which were as follows: Rb1 (18.26%), Rb2 (9.07%), Rc (9.65%), Rd (8.24%), Re (9.28%), Rf (3.48%), Rg1 (6.42%), Rg2 (3.63%), Rg3 (4.70%), Ro (3.82%), Ra (2.91%), and other minor ginseng saponins. All chemicals except GTS and ginseng saponin Rb1 were purchased from Sigma-Aldrich (St. Louis, MO, USA). All drugs used for injection were dissolved in sterile saline (0.9% NaCl solution).
Muscle-induced hyperalgesia
Animals were anesthetized briefly with isoflurane (1–4%) and injected with pH 4 preservative-free sterile saline (100 μL) into one lateral gastrocnemius muscle on day 0 and again on day 2.
Drug administration
To assess the antinociceptive activity of GTS on chronic muscle-induced pain, conscious rats were injected intraperitoneally with a 0.9% saline vehicle or various doses (25, 50, 100, 200 mg/kg) of GTS dissolved in the vehicle after the development of hyperalgesia (24 h after second injection). In the same way, rats were injected with an 18 mg/kg of ginseng saponin Rb1 to compare the antinociceptive effect of GTS (100 mg/kg) with single ginseng saponin because the ratio of Rb1 is 18.26% of GTS. The vehicle, ginseng saponin Rb1, and GTS solution were in a volume of 10 mL/kg.
To examine whether the observed effects of GTS against mechanical hyperalgesia induced by repeated intramuscular injections of acidic saline were mediated by NMDA or opioid receptors, rats were injected with NMDA (30 μg/kg) or naloxone (5 mg/kg) 10 min before GTS (200 mg/kg) injection.
Behavioral measurements
Animals were tested for their withdrawal threshold to mechanical stimuli (von Frey filaments) applied to the plantar aspect of the hindpaw (Fig. 1). Von Frey filaments, with bending forces at 4, 9, 20, 59, 78, 98, 147, and 254 mN, were applied in a progressively increasing manner until the hindpaw was withdrawn or 254 mN was reached. Each filament was applied three times. The filament with the lowest bending force from which the animal withdrew was considered the mechanical withdrawal threshold (MWT) of the hindpaw. After a response, the filaments above and below were tested to confirm the MWT. The test–retest reliability of this method was previously established (r2=0.7; P=.007).7
FIG. 1.

Test for withdrawal threshold to mechanical stimuli applied to the plantar aspect of the hindpaw using von Frey filaments. Mechanical withdrawal threshold of the hindpaw was the filament with the lowest bending force from which the animal withdrew among three applications.
In the antinociceptive activity test of GTS and ginseng saponin Rb1, the MWT was assessed using the following schedule: before the first injection (BI); 24 h after the second injection (AI); and 30 min, 60 min, 120 min, 1 day, and 2 days after the injection of vehicle, ginseng saponin Rb1, or various doses of GTS.
In the experiment to investigate whether NMDA or opioid receptors are involved in the antinociceptive effect of GTS, the MWT was tested every 10 min after GTS injection for 60 min.
To rule out the possibility of nonspecific motor or sedative effects of GTS, rats were tested for their ability to perform a rotorod treadmill test 30 min after the administration of 200 mg/kg of GTS and compared to vehicle controls.1
Statistical analysis
To estimate the group size for a study assessing the antinociceptive activity of ginseng saponin Rb1 and GTS, a pilot study was conducted for measuring withdrawal thresholds to mechanical stimuli in eight hyperalgesia-induced rats (control group). The average and standard deviation of the withdrawal threshold were 55.1 mN and 19.2 mN respectively. Autocorrelation between adjacent measurements on the same subject was 0.7. For our power calculation, we assumed that first-order autocorrelation adequately represented the autocorrelation pattern. We wanted to detect a 30% difference in the MWT of different groups and a 10% difference at different time points. Therefore, with an alpha of 0.05 and a power of 80%, we needed eight rats per group.
In the study examining the mechanism of GTS, sample size estimation was performed using the results of the study assessing the antinociceptive effect of GTS. The average and standard deviation of the MWT in the 200 mg/kg of GTS were 115.2 mN and 42.3 mN respectively. The autocorrelation between adjacent measurements on the same subject was 0.6. For our power calculation, we assumed that first-order autocorrelation adequately represented the autocorrelation pattern. We wanted to detect a 30% difference in the MWT of different groups and a 20% difference at different time points. Therefore, with an alpha of 0.05 and a power of 80%, we needed 12 rats per group. The PASS 11™ software (NCSS, Kaysville, UT, USA) was used to calculate the sample size.
Individual measurements were expressed as the mean±standard error and analyzed with SPSS v20.0 (IBM Corp., Armonk, NY, USA). A P-value≤.05 was considered statistically significant. The Shapiro–Wilk test was used to test for normality of variables. Since MWT were not distributed normally (P<.05), Friedman's repeated-measures analysis of variance was used to evaluate their differences, followed by a Tukey test for multiple pairwise comparisons.
Results
Mechanical hyperalgesia induced by repeated intramuscular injection of acidic saline
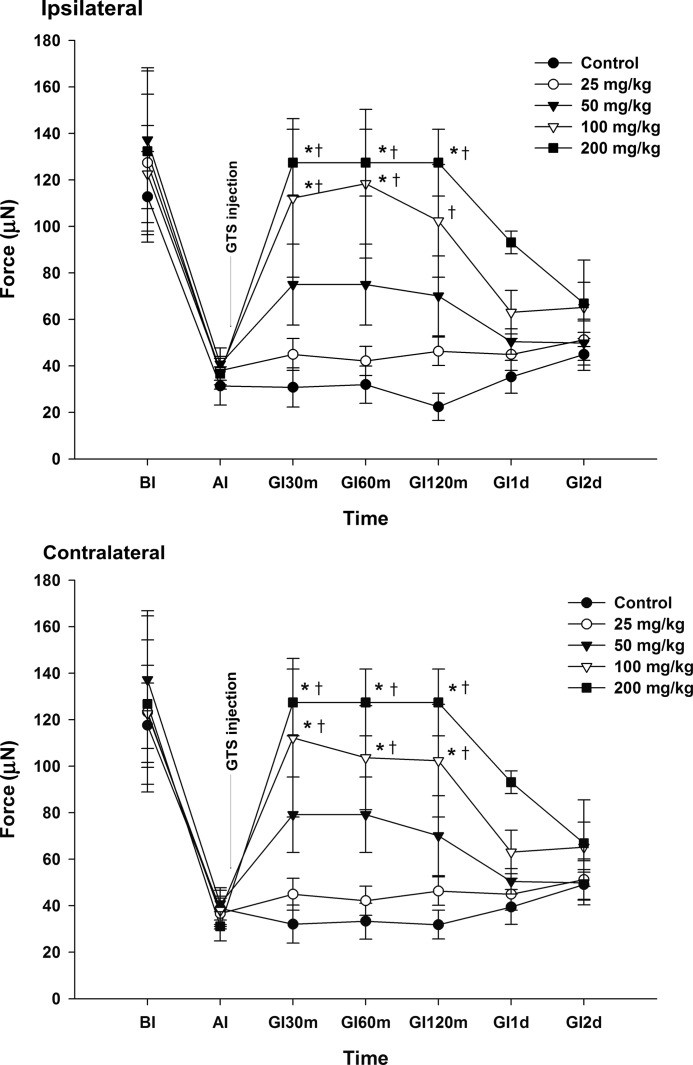
Two injections of pH 4.0 saline into one gastrocnemius muscle 2 days apart resulted in a bilateral decrease in the MWT during the measurement (Fig. 2).
FIG. 2.
Effect of ginseng total saponins (GTS) on hyperalgesia. Rats were injected intraperitoneally with experimental medication after the development of hyperalgesia. The mechanical withdrawal threshold (MWT) was significantly increased after the injection of 100 mg/kg and 200 mg/kg of GTS when compared with the MWT at AI. The MWT was significantly increased bilaterally after intraperitoneal injection of 100 mg/kg and 200 mg/kg of GTS when compared with the vehicle controls. *P<.05 compared with AI. †P<.05 compared with vehicle control. BI, before the first injection of acidic saline; AI, 24 h after the second injection of acidic saline; GI30m, GI60m, GI120m, GI1d, and GI2d: 30 min, 60 min, 120 min, 1 day, and 2 days after the injection of vehicle or various doses of GTS, respectively.
Effects of GTS on hyperalgesia
An intraperitoneal injection of 200 mg/kg of GTS resulted in a significant increase in the MWT within 30 min, which remained elevated for 120 min bilaterally when compared with the MWT at AI. The MWT was significantly increased 30, 60, and 120 min on the contralateral side, and 30 and 60 min on the ipsilateral side after intraperitoneal injection of 100 mg/kg of GTS when compared with the MWT at AI.
When compared with the vehicle controls, the MWT was significantly increased bilaterally 30, 60, and 120 min after intraperitoneal injection of 100 mg/kg and 200 mg/kg of GTS (Fig. 2).
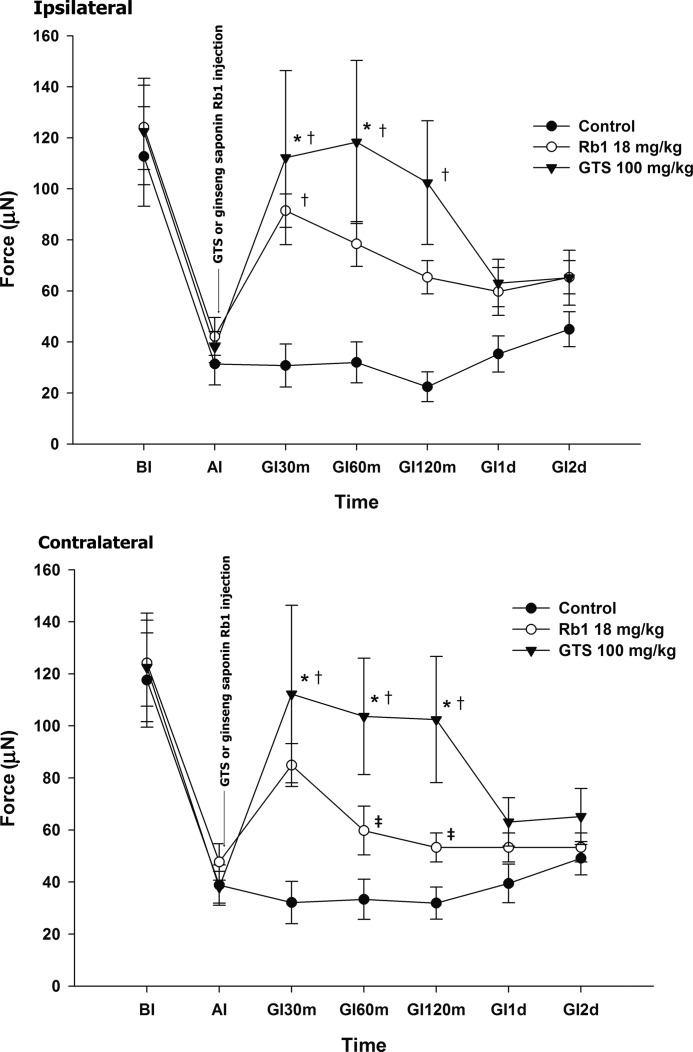
Comparison of the effect on hyperalgesia between ginseng saponin Rb1 and GTS
Intraperitoneal injection of 18 mg/kg of ginseng saponin Rb1 showed a significant increase in the MWT at 30 min on the ipsilateral side when compared with the vehicle controls. Compared with the 100 mg/kg of GTS, ginseng saponin Rb1 exhibited a significant decrease in the MWT at 60 and 120 min on the contralateral side (Fig. 3).
FIG. 3.
Comparison of the effect on hyperalgesia between ginseng saponin Rb1 and GTS. GI values were measured at the indicated interval after the injection of vehicle, ginseng saponin Rb1, or 100 mg/kg of GTS. Rats were injected intraperitoneally with experimental medication after the development of hyperalgesia. An intraperitoneal injection of 18 mg/kg of ginseng saponin Rb1 showed a significant increase in the mechanical withdrawal threshold (MWT) at 30 min on the ipsilateral side when compared with the vehicle control. Compared with the 100 mg/kg of GTS, ginseng saponin Rb1 showed a significant decrease of the MWT at 60 and 120 min on the contralateral side. *P<.05 compared with AI. †P<.05 compared with vehicle control. ‡P<.05 compared between ginseng saponin Rb1 and 100 mg/kg of GTS.
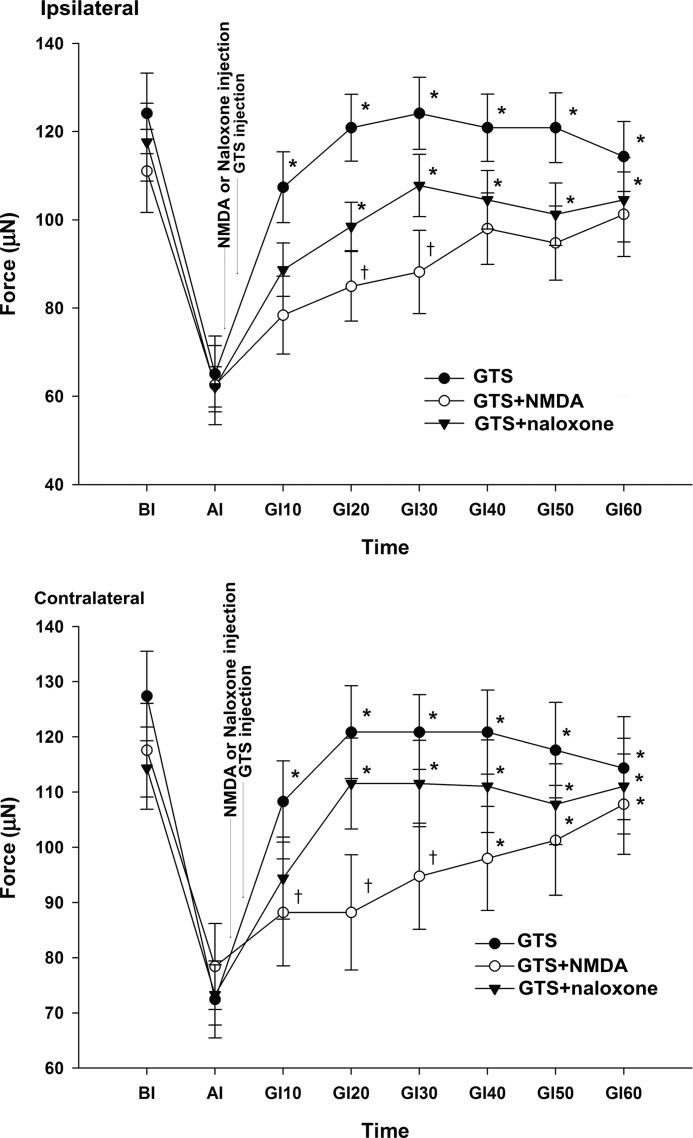
Inhibition of GTS-induced analgesia by NMDA or naloxone
Among the GTS groups, the MWT was significantly increased bilaterally during all measurements when compared with the MWT at AI. Within the GTS with naloxone, the MWT was significantly increased bilaterally at 20, 30, 40, 50, and 60 min after GTS injection compared to the MWT at AI. In rats administered GTS with NMDA, the MWT on the contralateral side was significantly increased at 40, 50, and 60 min after GTS injection, but the ipsilateral side was not significantly increased when compared with the MWT at AI.
Compared with the GTS, the GTS with NMDA showed a significant decrease of the MWT at 20 and 30 min on the ipsilateral side, and at 10, 20, and 30 min on the contralateral side. The GTS with naloxone did not show a significant decrease in the MWT on either side during all measurements (Fig. 4).
FIG. 4.
Effect of N-methyl-D-aspartate (NMDA) or naloxone on the reversal of the antinociception of GTS. GI values were measured at the indicated interval after the injection of experimental drugs. NMDA or naloxone was given as a pretreatment 10 min before GTS (200 mg/kg) treatment. Injection of GTS with NMDA showed a significant decrease of the mechanical withdrawal threshold when compared with GTS injection. *P<.05 compared with AI. †P<.05 compared with GTS.
Discussion
Our findings show that GTS has an antinociceptive activity against hyperalgesia induced by repeated intramuscular injections of acidic saline, the analgesic effects are proportional to the dosage, and GTS is more effective than single ginseng saponin (Rb1) alone. The antinociception of GTS was significantly reserved by NMDA. This suggests GTS have an antinociceptive effect in a chronic muscle-induced pain model and may be associated with the NMDA receptor.
Several studies have demonstrated the effects of ginsenosides on analgesia using a variety of animal models. GTS and the ginseng saponins Rc, Rd, and Re have exhibited analgesic effects in the writhing test and the second phase of pain in the formalin test.5,8 The ginseng saponins Rf had dose-dependent antinociception in the acetic acid abdominal constriction test and in the tonic phase of the biphasic formalin test.9 Ginseng saponins were effective for alleviating postoperative pain evoked by paw incision.10
Ginseng saponins produce antinociceptive effects at the spinal or supraspinal sites but not at nociceptors in the periphery. Intraperitoneally, intrathecally, or intracerebroventricularly injected ginseng saponins suppressed pain-related behavior produced by capsaicin injection into the plantar surface of the hindpaw, but subcutaneously injected ginseng saponins did not.6 Some studies have shown that ginseng saponins may exert their analgesic effects by acting on the presynaptic site. Ginseng saponins inhibit voltage-dependent Ca2+ channels in sensory neurons at the cellular level.11 Voltage dependent Ca2+ channels in sensory neurons play an important role in the release of pain transmitters from afferent presynaptic nerve terminals into the dorsal horn of the spinal cord after peripheral stimulation, such as with formalin treatment.12
Skyba et al. demonstrated that the production of long-lasting mechanical hyperalgesia following the repeated application of low pH saline involved the NMDA receptor.13 Many studies have suggested that NMDA receptors play an important role in the development and maintenance of chronic pain, where noxious inputs are tonically active and generate hyperexcitability in the pain-transmitting neurons of the spinal cord dorsal horn.14,15 This could induce central sensitization, wind-up phenomena, and pain memory. The blockade of spinal NMDA receptors prevents “windup” of both dorsal horn neurons and alpha motor neurons resulting from C-fiber strength conditioning stimuli.16
Ginseng saponins modulate the expression and function of NMDA receptors.17 Pretreatment with ginseng saponins via intrathecal administration attenuated NMDA-induced nociceptive behavior. Nah et al. investigated excitatory amino acid-induced nociceptive responses in mice and found that coadministration of ginseng saponins with NMDA via an intrathecal route inhibited NMDA-induced pain behaviors.18,19 This is consistent with our results.
It has been reported that ginseng-induced analgesia was not blocked by naltrexone in rats.20 Studies have also shown that ginseng saponins mimic the actions of opioids without activating opioid receptors during electrically evoked contractions in the ilea of guinea pigs and vasa deferentia of mice.21 In addition, ginseng extract was reported to inhibit Ca2+ channels in rat sensory neurons, an effect that was not blocked by naloxone, which indicated the involvement of nonopioid mediated effects.11 Our results showed that naloxone tended to inhibit the analgesic effects of GTS, but the effect was not significant. Thus, the opioid receptor seems less relevant to the antinociceptive effect of GTS when compared with the NMDA receptor.
We aimed to determine whether the antinociceptive effect of GTS can be applied to patients with chronic musculoskeletal pain syndrome. Thus, we injected GTS via an intraperitoneal route, as systemic absorption may be more clinically reasonable with this method. When delivered intraperitoneally, the dose of drug required to exert an effect is much higher than that for an intrathecal route, which raises concerns about toxicity. Ginseng is generally considered safe and has not shown to have dangerous interactions with other drugs.22,23 In addition, the administration of ginseng saponins at high doses has not been shown to induce nonspecific motor or sedative effects based on rotorod performance.5
We performed this study to investigate the properties of GTS in chronic muscle-induced pain to explore the potential of ginseng for future clinical applications. In conclusion, GTS showed antinociceptive activity against chronic muscle-induced pain, and the effect was superior to a single ginseng saponin. In addition, the antinociceptive effects of GTS on mechanical hyperalgesia may be mediated by NMDA. This suggests that ginseng can be useful in the treatment of chronic musculoskeletal pain syndrome. However, further studies will be needed for assessing the antinociceptive effects of each single saponin.
Acknowledgment
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2012R1A1A1003700).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Sluka KA, Kalra A, Moore SA: Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 2001;24:37–46 [DOI] [PubMed] [Google Scholar]
- 2.Kang H, Woo YC, Baek CW, et al. : The effect of minocycline on allodynia produced by repeated injection of low pH saline in rats. Korean J Anesthesiol 2007;53:97–103 [Google Scholar]
- 3.Nah SY: Ginseng: recent advances and Trends. J Ginseng Res 1997;21:1–12 [Google Scholar]
- 4.Kaku T, Miyata T, Uruno T, Sako I, Kinoshita A: Chemico-pharmacological studies on saponins of Panax ginseng C. A. Meyer. II. Pharmacological part. Arzneimittelforschung 1975;25:539–547 [PubMed] [Google Scholar]
- 5.Shin YH, Jung OM, Nah JJ, et al. : Ginsenosides that produce differential antinociception in mice. Gen Pharmacol 1999;32:653–659 [DOI] [PubMed] [Google Scholar]
- 6.Nah JJ, Hahn JH, Chung S, et al. : Effect of ginsenosides, active components of ginseng, on capsaicin-induced pain-related behavior. Neuropharmacology 2000;39:2180–2184 [DOI] [PubMed] [Google Scholar]
- 7.Gopalkrishnan P, Sluka KA: Effect of varying frequency, intensity and pulse duration of TENS on primary hyperalgesia in inflamed rats. Arch Phys Med Rehabil 2000;81:984–990 [DOI] [PubMed] [Google Scholar]
- 8.Shin YH, Kim SC, Han JW, et al. : Study on ginseng rotopanaxadiol and protopanaxatriol saponins-induced antinociception. Korean J Physiol Pharmacol 1997;1:143–149 [Google Scholar]
- 9.Mogil JS, Shin YH, McCleskey EW, Kim SC, Nah SY: Ginsenoside Rf, a trace component of ginseng root, produces antinociception in mice. Brain Res 1998;792:218–228 [DOI] [PubMed] [Google Scholar]
- 10.Shin DJ, Yoon MH, Lee HG, et al. : The Effect of treatment with intrathecal ginsenosides in a rat model of postoperative pain. Korean J Pain 2007;20:100–105 [Google Scholar]
- 11.Nah SY, McCleskey EW: Ginseng root extract inhibits calcium channels in rat sensory neurons through a similar path, but different receptor, as μ-type opioids. J Ethnopharmacol 1994;42:45–51 [DOI] [PubMed] [Google Scholar]
- 12.Go VL, Yaksh TL: Release of substance P from the cat spinal cord. J Physiol 1987;391:141–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skyba DA, King EW, Sluka KA: Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain 2002;98:69–78 [DOI] [PubMed] [Google Scholar]
- 14.Chizh BA, Headley PM: NMDA antagonists and neuropathic pain—multiple drug targets and multiple uses. Curr Pharm Des 2005;11:2977–2994 [DOI] [PubMed] [Google Scholar]
- 15.Haley JE, Sullivan AF, Dickenson AH: Evidence for spinal N-methyl-D-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res 1990;518:218–226 [DOI] [PubMed] [Google Scholar]
- 16.Davies SN, Lodge D: Evidence for involvement of N-methyl-D-aspartate receptors in “windup” of class 2 neurones in the dorsal horn of the rat. Brain Res 1987;424:402–406 [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Hwang SL, Oh S: Ginsenoside Rc and Rg1 differentially modulate NMDA receptor subunit mRNA levels after intracerebroventricular infusion in rats. Neurochem Res 2000;25:1149–1154 [DOI] [PubMed] [Google Scholar]
- 18.Nah JJ, Choi S, Kim YH, et al. : Effect of spinally administered ginseng total saponin on capsaicin-induced pain and excitatory amino acid-induced nociceptive responses. J Ginseng Res 1999;23:38–43 [Google Scholar]
- 19.Yoon SR, Nah JJ, Shin YH, et al. : Ginsenosides induce differential antinociception and inhibit substance P induced-nociceptive response in mice. Life Sci 1998;62:319–325 [DOI] [PubMed] [Google Scholar]
- 20.Bhargava HN, Ramarao P: The effect of Panax ginseng on the development of tolerance to the pharmacological actions of morphine in the rat. Gen Pharmacol 1991;22:429–434 [DOI] [PubMed] [Google Scholar]
- 21.Watanabe J, Oh KW, Kim HS, Takahashi MT, Kaneto H: A non-opioid mechanism in the inhibitory effect of ginseng saponins on electrically evoked contractions of guinea pig ileum and mouse vas deferens. J Pharmacobiodyn 1988;11:453–458 [DOI] [PubMed] [Google Scholar]
- 22.Coon JT, Ernst E: Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf 2002;25:323–344 [DOI] [PubMed] [Google Scholar]
- 23.Shin TJ, Hwang SH, Choi SH, et al. : Effects of protopanaxatriol-ginsenoside metabolites on rat N-methyl-D-aspartic Acid receptor-mediated ion currents. Korean J Physiol Pharmacol 2012;16:113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]