Abstract
Liver steatosis is characterized by lipid dysregulation and fat accumulation in the liver and can lead to oxidative stress in liver. Since proanthocyanidins are present in plant-based foods and have powerful antioxidant properties, we investigated whether proanthocyanidins can prevent oxidative stress and subsequent liver injury. Carbon tetrachloride (CCl4) treatment can cause steatosis in rats that models both alcoholic and non-alcoholic fatty liver disease in humans. We pre-treated rats by oral administration of proanthocyanidins extracted from grape seeds 7 days prior to intragastrically administering CCl4. Proanthocyanidin treatment continued for an additional 2 weeks, after which time liver and serum were harvested, and mediators of liver injury, oxidative stress, and histological features were evaluated. CCl4-treated rats exhibited significant increases in the following parameters as compared to non-treated rats: fat droplets in the liver, liver injury (ALT, AST), and DNA damage (8-OHdG). Additionally, CCl4 treatment decreased antioxidant enzymes SOD, GSH, GPX, and CAT in the liver due to their rapid depletion after battling against oxidative stress. Compared to CCl4-treated rats, treatment with proanthocyanidins effectively suppressed lipid accumulation, liver injury, DNA damage, as well as restored antioxidant enzyme levels. Further investigation revealed that proanthocyanidins treatment also inhibited expression of CYP2E1 in liver, which prevented the initial step of generating free radicals from CCl4. The data presented here show that treatment with orally administered proanthocyanidins prevented liver injury in the CCl4-induced steatosis model, likely through exerting antioxidant actions to suppress oxidative stress and inhibiting the free radical–generating CYP2E1 enzyme.
Key Words: : CYP2E1, fatty liver, grape seed extracts, hepatotoxicity, oxidative stress
Introduction
Liver steatosis, a condition in which lipid metabolism is disrupted and lipids accumulate in fat droplets within liver cells, is a major complication of obesity, hyperlipidemia, insulin resistance, and alcoholic/nonalcoholic fatty liver disease.1 If untreated, benign steatosis can gradually advance to steatohepatitis, fibrosis, cirrhosis, even liver cancer.2,3 Therefore, there is a need to explore ways to reduce the development of steatosis, including the study of naturally-occurring compounds.
Several animal models are used to study the mechanisms underlying steatosis and to test the efficacy of potential treatments, including the carbon tetrachloride (CCl4)-mediated rat model of steatosis.4 CCl4 is a well-known hepatotoxin that is mainly metabolized in the liver. One distinctive feature of CCl4 toxicity is rapid triglyceride (TG) accumulation in the liver, similar to observations in steatotic liver tissue from human patients.5,6 Liver injury induced by CCl4 is mediated by the formation of reactive intermediates that are generated by cytochrome P450-mediated CCl4 metabolism in the liver microsome, including trichloromethyl radical (CCl3•) and its derivative trichloromethylperoxy radical (CCl3OO•).
Specifically, the CYP2E1 enzyme catalyzes this reaction.7,8 The free radicals generated by this process are thought to react with membrane lipids causing lipid peroxidation, leading to injury of cellular membranes, and consequently altering liver cell structure and function. Therefore, inhibition and/or removal of intracellular reactive oxygen species (ROS) would play an important role in preventing and treating liver disease.9,10
Proanthocyanidins are natural compounds found in plant-based foods, including grape seed extracts. Proanthocyanidins are one of the most well-known and powerful antioxidants in the plant world due to their ability to absorb oxygen radicals and have been shown to mediate anti-inflammatory as well as anti-cancer effects.11 Since ROS can cause oxidative stress in liver steatosis,12,13 we hypothesized that the antioxidant properties of proanthocyanidins would relieve the oxidative stress brought on by the CCl4-induced free radicals and subsequently prevent liver injury. Therefore, we investigated the effect of proanthocyanidin treatment on CCl4-induced hepatic steatosis and liver injury in rats. Our data reveal that treatment with proanthocyanidins inhibits CCl4-induced hepatic steatosis and liver injury via decreasing oxidative stress and inhibiting cytochrome CYP2E1.
Materials and Methods
Animals and treatment schedules
Male Wistar rats, weighing 200–225 g, were provided by the Laboratory Animal Center at Dalian Medical University. Rats were housed individually. A standard laboratory diet and water were available ad libitum. The animal room was maintained at constant temperature of 23±1°C and 50% relative humidity with a 12 h (7:00 a.m.–7:00 p.m.) light/dark cycle. Food was removed the night before the experiment. All experimental procedures were conducted in conformity with institutional guidelines for the care and use of laboratory animals in Dalian Medical University and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication no. 85-23, revised 1996). All animal work was performed according to the NIH guidelines and protocols were approved by Institutional Animal Care and Use Committee of Dalian Medical University. After 1 week on the basal diet, 30 animals were randomly divided into 3 groups of 10 rats each. Rats in the proanthocyanidin group were pre-treated with proanthocyanidins (400 mg/kg body weight) by intragastric (i.g.) gavage for 7 days (proanthocyanidins extracted from grape seeds [purity>95] were provided by NC (Pittsburgh, PA, USA). The extract contained, oligomeric proanthocyanidins 85.52%, catechin 9.32%, L-epicatechin 4.79%, and were diluted in distilled water before use). Control rats (group I) received i.g. the same volume of liquid paraffin oil as Group II twice a week for 2 weeks. Rats in the CCl4 group (group II) received i.g. administration of CCl4 in 50% liquid paraffin oil (0.5 mL/kg body weight) twice a week for 2 weeks. Rats pre-treated with proanthocyanidins (group III) received i.g. administration of CCl4 in 50% liquid paraffin oil (0.5 mL/kg body weight) twice a week and also continued to receive i.g. proanthocyanidins administration (400 mg/kg body weight) every day until the end of the experiment. After 2 weeks, all rats were anesthetized at 24 h after the last treatment. Blood was collected by cervical decapitation and centrifuged at 1500 g for 20 min at 4°C to obtain serum. The study design is illustrated in Figure 1.
FIG. 1.
Study design.
Evaluation of liver pathology
A histological study was performed following a midline laparotomy to remove the liver. Livers were harvested at the end of the experiment, weighed, immediately placed in 10% buffered formalin, and embedded in paraffin. Liver sections were stained with hematoxylin and eosin using standard techniques. Investigators were blinded to the group identity of each section, and biopsies were classified into four categories depending on fat accumulation using a previously established method14 as follows: Grade 0, no fat observed in the liver; Grade 1, <33% of hepatocytes contained fat vacuoles; Grade 2, 33–66% of hepatocytes contained fat vacuoles; Grade 3, 66% of hepatocytes contained fat vacuoles.
Hepatocellular injury assay
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum were used as indicators of hepatocyte function and injury. ALT and AST levels were measured by a Bayer 1650 automatic biochemical analyzer.
Triglyceride detection and antioxidative enzyme activity in hepatocytes
Liver tissue was homogenized in ice-cold PBS buffer and centrifuged at 1800 g for 10 min at 4°C to precipitate the insoluble material, and the supernatant was used in the following assays. Triglycerides (TG), malondialdehyde (MDA), and glutathione (GSH) levels as well as the enzymatic activities of superoxide dismutase (SOD), glutathione peroxidase (GPX), and catalase (CAT) were measured using commercial testing kits (Nanjing Jiancheng Bioengineering Institute, Nanjing City, China) according to the manufacturer's instructions. MDA and GSH levels are expressed as nmol/mg protein and mg/g protein, respectively. The enzymatic activity of the antioxidant enzymes SOD, GPX, and CAT are expressed as U/mg protein.
8-OHdG levels in liver DNA
Liver tissue DNA was extracted from frozen tissues using a DNeasy tissue kit according to the manufacturer's instructions. The 8-OHdG levels in liver DNA were determined using an ELISA kit (R&D Systems, Minneapolis, MN, USA). The anti–8-OHdG antibody and liver DNA samples were added to an ELISA plate pre-coated with 8-OHdG, and the 8-OHdG in the sample competed with 8-OHdG bound on the plate for 8-OHdG antibody binding sites. The average 8-OHdG concentration per microgram of protein for each experimental group was calculated. DNA samples were assayed in duplicate.
Immunohistochemical staining
Immunohistochemistry was performed on 4-μm-thick sections cut from formalin-fixed, paraffin-embedded tissue. Briefly, the sections were deparaffinized and stained with anti-CYP2E1 antibody (Santa Cruz Biotechnology, Dallas, TX, USA) followed by treatment with the streptavidin-peroxidase (SP) Kit (Fuzhou Maixin Biotechnology Co., Fujian, China), and DAB was used as the chromogen. No staining was observed in the absence of the primary antibody anti-CYP2E1 IgG used, as a staining control. Immunohistochemical staining of CYP2E1 was digitally quantified from the acquired images using IPP5.1 software (IOD=integrated optical density).
Statistical analyses
Differences among groups were examined by one-way ANOVA followed by Tukey-Kramer multiple comparison tests. Values are expressed as the mean±SEM. A value of P<.05 was considered statistically significant.
Results
Treatment with proanthocyanidins prevents CCl4-induced liver steatosis
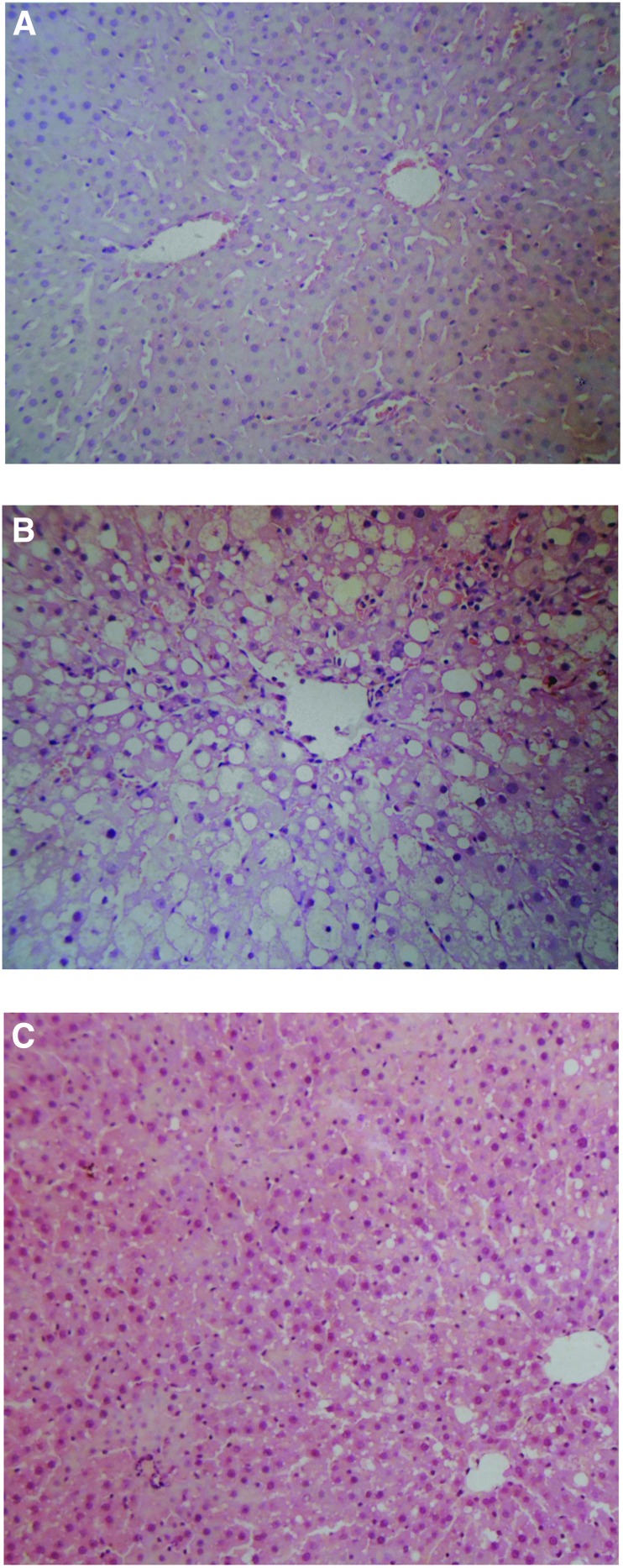
Whereas fatty infiltration was induced in CCl4 treated rats as compared to control rats, proanthocyanidins were able to prevent this CCl4-induced fatty infiltration as assessed by H&E-stained liver sections (Fig. 2). Quantifying the fatty infiltration by a previously published scoring system,14 we observed that the mean fatty infiltration in the steatosis group was 2, as the fat deposits in the steatosis group were classified as mixed (Fig. 2). In contrast, the mean fatty infiltration in the proanthocyanidins-treated group was quantified as 0, as we only observed the occasional fat droplet scattered in the histology field, which was similar to what was observed in the livers from the control group not treated with CCl4 (Fig. 2). This liver histology result suggested that proanthocyanidin treatment was able to suppress the CCl4-induced fatty infiltration.
FIG. 2.
Proanthocyanidins improved histological features of carbon tetrachloride (CCl4)-induced steatosis and liver injury. Rats were treated by intragastric (i.g.) administration of proanthocyanidins each day for 7 days before inducing liver injury with i.g. administration of CCl4 every other day along with continued proanthocyanidin treatment every day for 2 weeks. Liver was harvested at this time point, and liver sections from (A) control, (B) CCl4 control, and (C) CCl4+proanthocyanidins rats were stained with H&E and evaluated for fatty infiltration. Original magnification: 150×. Color images available online at www.liebertpub.com/jmf
We assessed CCl4-induced liver injury by measuring AST and ALT liver enzyme levels in serum harvested from rats after 2 weeks of CCl4 treatment with or without treatment with proanthocyanidins. Serum ALT and AST levels were both elevated in the steatosis group compared with those in control group (Fig. 3), which indicated that liver damage occurred in response to CCl4 treatment. Upon proanthocyanidin treatment, the adverse effects of CCl4-induced steatosis were completely prevented, as AST and ALT levels were not significantly different from baseline control levels in rats that were not treated with CCl4 (Fig. 3). Liver function related to lipid metabolism was also disrupted by CCl4 treatment as measured by the liver TG levels. While CCl4-treated rats produced a significantly increased hepatic TG content in liver homogenate compared with the controls (P<.01), proanthocyanidins effectively decreased the CCl4-induced TG content in the liver (Fig. 4). Taken together, these data suggest that proanthocyanidin treatment can prevent liver damage associated with lipid dysregulation.
FIG. 3.
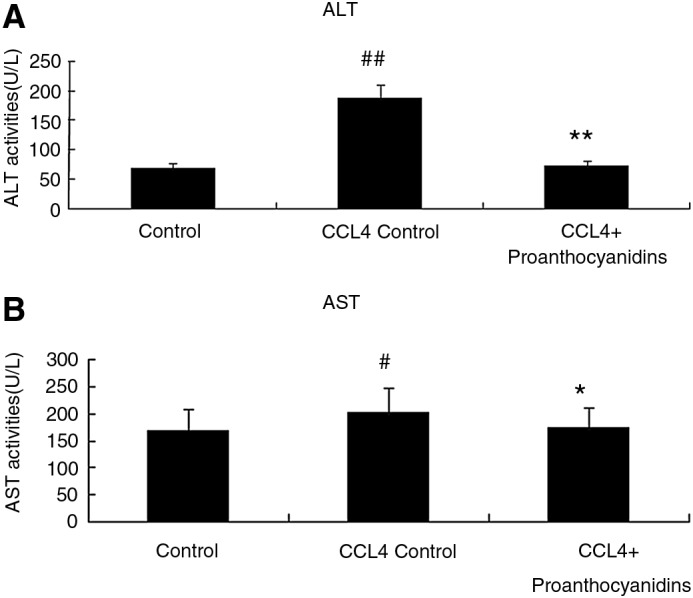
Proanthocyanidins decreased the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). At the completion of the experiment described in Figure 1, blood was drawn, and serum concentrations of ALT (A) and AST (B) in control, CCl4 control, and CCl4+proanthocyanidins rats were evaluated. (n=10, mean±SEM). #P<.05, ##P<.01 compared to control; *P<.05, **P<.01 compared to CCl4 control.
FIG. 4.
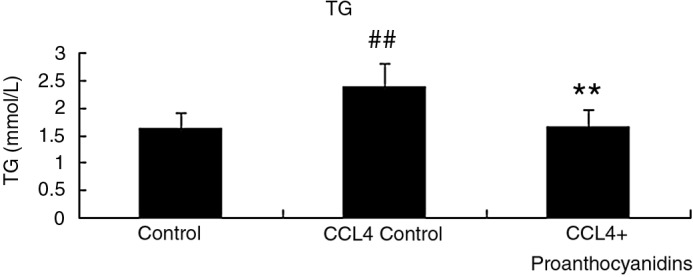
Proanthocyanidins decreased the levels of triglyceride (TG) in liver. At the completion of the experiment described in Figure 1, liver was harvested, and TG levels in control, CCl4 control, and CCl4+proanthocyanidins rats were evaluated from homogeneates. (n=10, mean±SEM). ##P<.01 compared to control; **P<.01 compared to CCl4 control.
Proanthocyanidins suppress liver injury–induced oxidative stress mediators
Considering the key role of ROS in the development of liver injury and steatosis in the rat model of CCl4-mediated liver injury, assessing whether proanthocyanidins could prevent CCl4-mediated changes to the hepatic antioxidant system was crucial. Characteristically, CCl4 downregulates antioxidative stress mediators because cellular stores are depleted after combatting oxidative stress to stabilize the liver.15 Indeed, CCl4 treatment significantly increased MDA, a reactive species generated by lipid peroxidation, and significantly decreased the antioxidation enzymes SOD, GSH, GPX, and CAT in the liver as compared to the control (Table 1). Compared with the corresponding levels in CCl4-treated rats experiencing steatosis, proanthocyanidin treatment significantly decreased MDA and increased SOD, GSH, GPX, and CAT levels in CCl4-treated rats. These data indicate that proanthocyanidin treatment is able to prevent the peroxidation cascade and maintain high levels of antioxidative enzymes in rat liver tissue after CCl4 treatment.
Table 1.
Changes in Lipid Peroxidation and Antioxidant Status in Liver Homogenates
| Groups | MDA (nmol/mg prot) | SOD (U/mg prot) | GSH (mg/g prot) | GPX (U/mg prot) | CAT (U/mg prot) |
|---|---|---|---|---|---|
| Control | 0.71±0.25 | 102.51±3.58 | 14.25±2.75 | 435.71±47.21 | 32.51±5.21 |
| CCl4 control | 1.72±0.24## | 45.42±9.21## | 7.58±1.38## | 305.33±52.75## | 24.53±3.26## |
| CCl4+proanthocyanidins | 0.77±0.19** | 99.21±3.5** | 12.51±1.52** | 380.42±72.51* | 28.38±3.45* |
n=10, mean±SEM.
P<.05, ##P<.01 compared to control; *P<.05, **P<.01, compared to CCl4 control.
MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione; GPX, glutathione peroxidase; CAT, catalase; CCl4, carbon tetrachloride.
Proanthocyanidins inhibits oxidative stress–induced DNA damage upon liver injury in steatosis
To determine whether proanthocyanidins could also suppress oxidative-stress–mediated DNA damage, we evaluated the levels of 8-hydroxy-2′-deoxyguanosine (8-OHdG), a marker of oxidative stress in DNA. As shown in Figure 5, 8-OHdG levels significantly increased in liver tissue under CCl4-induced steatosis compared with the control group. However, this CCl4-induced elevation of 8-OHdG was significantly inhibited in rats pre-treated with proanthocyanidins, suggesting that proanthocyanidis can prevent oxidative-stress–mediated intracellular damage to DNA and further suggesting that proanthocyanidins can also prevent apoptosis, necrosis, or carcinogenesis of cells within liver tissue.
FIG. 5.
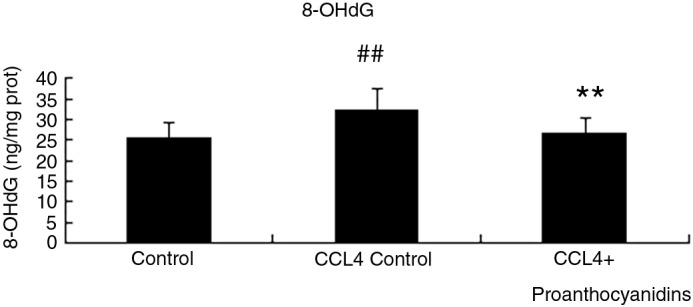
Proanthocyanidins decreased the levels of 8-OHdG in liver. At the completion of the experiment described in Figure 1, liver was harvested and 8-OHdG levels in control, CCl4 control and CCl4+ proanthocyanidins rats were evaluated in liver tissue DNA by ELISA. (n=10, mean±SEM). ##P<.01 compared to control; **P<.01 compared to CCl4 control.
Proanthocyanidins suppress CYP2E1 expression in the liver to inhibit free-radical generation by CCl4
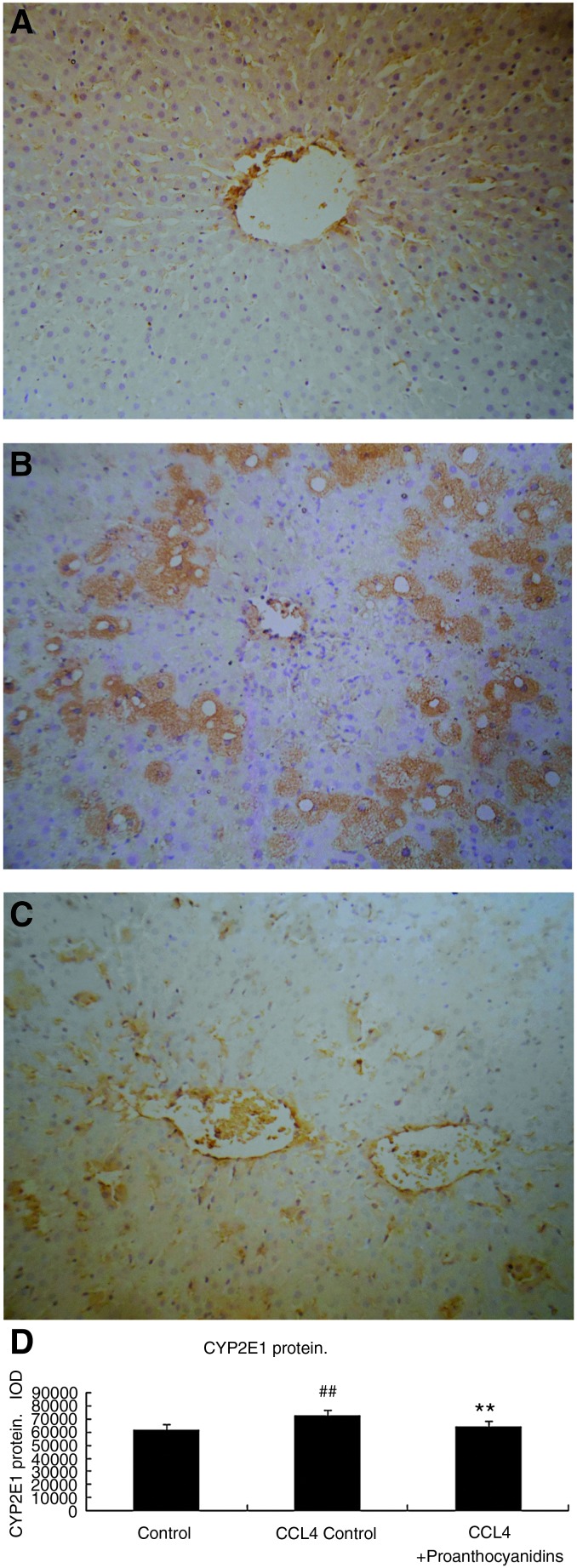
Since free-radical generation by CCl4 metabolism is catalyzed by CYP2E1 to initiate CCl4-induced liver dysfunction, proanthocyanidins could possibly interrupt this initial step in the signaling pathway, since we observed that all of the downstream effects, including oxidative stress and fatty infiltration, were inhibited by this treatment. We therefore tested whether proanthocyanidins could alter the increased CYP2E1 expression that occurs after CCl4 treatment. In normal rat livers, CYP2E1 expression was not detected (Fig. 6A). After CCl4-induced steatosis, rat livers became positive for CYP2E1 expression (Fig. 6B), where positive staining in steatotic livers was localized in the cytoplasm, but not in the nuclei, and corresponded with distribution of the fat droplets. In the proanthocyanidin-treated group, the cytosolic expression of CYP2E1 expression observed in the steatotic condition was greatly decreased to levels similar to control rats (Fig. 6C), suggesting that treatment with proanthocyanidins effectively inhibited CCl4-induced CYP2E1 expression (IOD data; Fig. 6D). Thus, proanthocyanidins could inhibit the CYP2E1 enzyme from generating free radicals from CCl4, and thereby prevent the cascade that causes oxidative stress and liver injury in steatosis.
FIG. 6.
Effects of proanthocyanidin administration on the expression of CYP2E1 in liver by immunohitochemical staining. At the completion of the experiment described in Figure 1, liver was harvested, and paraffin-embedded liver tissue from (A) control, (B) CCl4 control, and (C) CCl4+proanthocyanidins rats were stained with anti-CYP2E1 antibody and evaluated for CYP2E1 expression. Original magnification: 200×. (D) Changes in the cumulative value of optical density in immunohistochemical staining of CYP2E1. ##P<.01 compared to control; **P<.01, compared with CCl4 control. Color images available online at www.liebertpub.com/jmf
Discussion
In this study, our data reveal that treatment with proanthocyanidins extracted from grape seeds can inhibit the oxidative stress brought on by the free radicals generated CCl4-induced steatosis in a rat, likely due to the antioxidative properties of proanthocyanidins. Proanthocyanidin treatment can suppress liver injury, as assessed by AST and ALT levels, and fat accumulation associated with CCl4 treatment (Figs. 2–4).
Antioxidants can prevent insulin resistance and ameliorate hepatic steatosis formation.16 Furthermore, proanthocyanidin treatment decreased the oxidative stress within the liver tissue by lowering the levels of oxidative products and oxidative-stress-induced DNA damage due to CCl4 (Table 1 and Fig. 5), and restoring the antioxidant enzyme levels (Table 1) that were depleted after battling against CCl4-mediated oxidative stress. Our data further reveal that proanthocyanidins can also exert their antioxidant effect upstream of the CYP2E1 enzyme that is required for catalyzing the reaction of CCl4 into free radicals, as CYP2E1 protein levels were reduced to near baseline levels in rats pre-treated with proanthocyanidins before CCl4 treatment (Fig. 6).
CYP2E1 is the most important hepatic cytochrome P450 isoform for metabolizing CCl4 and creates free radicals in phase I enzymes.17 Our study is one of only a few reports to reveal that proanthocyanidins can prevent the upregulation of the cytochrome P450 enzyme CYP2E1, suggesting that the mechanism of action for proanthocyanidins acts early in the process of oxidative stress and can therefore prevent the entire cascade leading to liver injury and inflammation. This has also been observed in a mouse model of acetaminophen (APAP)-induced liver injury, where proanthocyanidins were shown to prevent the CYP2E1-dependent aniline hydroxylation both in vivo and in vitro on liver microsomes.18 Further studies are necessary to determine the precise cellular and molecular targets of proanthocyanidins in our model that protects from liver injury upstream of suppressing CYP2E1. It is also important to mediate free-radical generation in other mechanisms of steatosis, including non-alcoholic fatty liver and alcohol-induced steatosis.17,19 Since our data show that proanthocyanidins can suppress the function of this cytochrome P450 enzyme, proanthocyanidin treatment can also be broadly applied to suppression of free-radical generation brought on by a variety of mechanisms.
Interestingly, one study investigated whether CYP2E1 was regulated by proanthocyanidins in a cancer model of azoxymethane (AOM)-induced colonic aberrant crypt foci (ACF) formation in rats, and found that orally adminstered proanthocyanidins did not affect levels of CYP2E1 in the gut.20 Although this is seemingly contradictory to our data, it is possible that the antioxidant effects in the liver are different than in the gut. It is also possible that differences in the rat models and/or the molecules targeted by the different treatments may also affect whether proanthocyanidins affect CYP2E1 levels.
Our observation that proanthocyanidins can suppress oxidative stress in the liver is consistent with previous work showing that proanthocyanidins inhibit oxidative stress and liver injury in other animal models, such as alcohol-induced oxidative stress, lipid peroxidation by 12-O-tetradecanoylphorbol-13-acetate (TPA), liver injury by dimethylnitrosamine (DMN), oxidative liver injury by obstructing bile ducts, and liver injury associated with chemotherapeutic cisplatin treatment.20–24 These previous studies confirmed and supported the data shown in the present study, and demonstrate that proanthocyanidins can suppress liver injury in response to all of the various methods used to perturb liver redox homeostasis. In phase II enzymes, glutathione-S-transferases (GSTs) are a class of important detoxification enzymes in the antioxidant system. The presence of proanthocyanidins can normalize the levels of GST and other GSH-dependent antioxidant enzymes to nearly normal values of control.24,25 Indeed, we observed here that proanthocyanidins can restore the levels of antioxidant mediators.
By decreasing oxidative stress at an upstream step, proanthocyanidins effectively prevented the DNA-damage–associated molecule, 8-OHdG, and would also likely suppress induction of apoptosis, necrosis, or carcinogenesis that follow DNA damage.26,27 This would further prevent the possibility of incurring irreparable damage and injury to the liver and liver function.28,29
There has been some suggestion that high doses of proanthocyanidins can actually cause celluar toxicity.30 However, these studies have so far been limited to showing these toxic effects directly on cultured cells, and evidence for in vivo effects of ingested proanthocyanidins from foods remains to be determined. One study showed that high doses of guarana extract, which contains proanthocyanidins along with several other mediators, showed some in vivo toxicity in terms of weight loss and elevated liver enzymes when using doses similar to what was used here (<300 mg/kg).31 However, because the guarana extract was not pure proanthocyanidins, this effect may be mediated by other components present in the extract. In the present study, we observed that the levels of oxidative stress and liver injury mediators returned to control levels, we therefore conclude that proanthocyanidin treatment at the doses we used here are protective and not toxic to the rat.
Conclusions
In summary, the data presented in the present study demonstrate that treatment with orally administered proanthocyanidins, which are naturally occurring compounds present in many plant-based foods, prevent liver injury in the rat model of CCl4-induced steatosis, likely through exerting antioxidant function to suppress oxidative stress as well as inhibiting the production of the free-radical–generating CYP2E1 enzyme. These results suggest that ingesting proanthocyanidin-containing foods or proanthocyanidin supplements can help to treat both alcoholic and non-alcoholic fatty liver disease by suppressing the damaging liver peroxidation mechanisms associated with steatosis, and therefore delay or prevent the development of pathology that can lead to steatohepatitis, fibrosis, and cirrhosis, as well as the development of liver cancer.
Author Disclosure Statement
The authors have no conflicts of interest to declare in conducting this research.
References
- 1.Bradbury MW: Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am J Physiol Gastrointest Liver Physiol 2006;290:G194–198 [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto E, Yatsuji S, Tobari M, et al. : Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol 2009;44:589–595 [DOI] [PubMed] [Google Scholar]
- 3.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN: The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010;51:1972–1978 [DOI] [PubMed] [Google Scholar]
- 4.Tunon MJ, Alvarez M, Culebras JM, Gonzalez-Gallego J: An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol 2009;15:3086–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan X, Hussain FN, Iqbal J, Feuerman MH, Hussain MM: Inhibiting proteasomal degradation of microsomal triglyceride transfer protein prevents CCl4-induced steatosis. J Biol Chem 2007;282:17078–17089 [DOI] [PubMed] [Google Scholar]
- 6.Lee GH, Bhandary B, Lee EM, et al. : The roles of ER stress and P450 2E1 in CCl4-induced steatosis. Int J Biochem Cell Biol 2011;43:1469–1482 [DOI] [PubMed] [Google Scholar]
- 7.Sun F, Hamagawa E, Tsutsui C, Ono Y, Ogiri Y, Kojo S: Evaluation of oxidative stress during apoptosis and necrosis caused by carbon tetrachloride in rat liver. Biochim Biophys Acta 2001;1535:186–191 [DOI] [PubMed] [Google Scholar]
- 8.Lewis MD, Roberts BJ: Role of CYP2E1 activity in endoplasmic reticulum ubiquitination, proteasome association, and the unfolded protein response. Arch Biochem Biophys 2005;15:237–245 [DOI] [PubMed] [Google Scholar]
- 9.Ohyama T, Sato K, Kishimoto K, et al. : Azelnidipine is a calcium blocker that attenuates liver fibrosis and may increase antioxidant defence. Br J Pharmacol 2012;165:1173–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chacko BK, Srivastava A, Johnson MS, et al. : Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology 2011;54:153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouedraogo M, Charles C, Ouedraogo M, Guissou IP, Stevigny C, Duez P: An overview of cancer chemopreventive potential and safety of proanthocyanidins. Nutr Cancer 2011;63:1163–1173 [DOI] [PubMed] [Google Scholar]
- 12.Zhu W, Jia Q, Wang Y, Zhang Y, Xia M: The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: involvement of a cAMP-PKA-dependent signaling pathway. Free Radic Biol Med 2012;15:314–327 [DOI] [PubMed] [Google Scholar]
- 13.Garcia MC, Amankwa-Sakyi M, Flynn TJ: Cellular glutathione in fatty liver in vitro models. Toxicol In Vitro 2011;25:1501–1506 [DOI] [PubMed] [Google Scholar]
- 14.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR: Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467–2474 [DOI] [PubMed] [Google Scholar]
- 15.Cemek M, Yılmaz F, Büyükokuroğlu ME, Büyükben A, Aymelek F, Ayaz A: Serum and liver tissue bio-element levels, and antioxidant enzyme activities in carbon tetrachloride-induced hepatotoxicity: protective effects of royal jelly. J Med Food 2012;15:747–752 [DOI] [PubMed] [Google Scholar]
- 16.Hsieh FC, Lee CL, Chai CY, Chen WT, Lu YC, Wu CS: Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr Metab (London) 2013;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kathirvel E, Chen P, Morgan K, French SW, Morgan TR: Oxidative stress and regulation of anti-oxidant enzymes in cytochrome P4502E1 transgenic mouse model of non-alcoholic fatty liver. J Gastroenterol Hepatol 2010;25:1136–1143 [DOI] [PubMed] [Google Scholar]
- 18.Ray SD, Parikh H, Hickey E, Bagchi M, Bagchi D: Differential effects of IH636 grape seed proanthocyanidin extract and a DNA repair modulator 4-aminobenzamide on liver microsomal cytochrome 4502E1-dependent aniline hydroxylation. Mol Cell Biochem 2001;218:27–33 [DOI] [PubMed] [Google Scholar]
- 19.Wu D, Wang X, Zhou R, Yang L, Cederbaum AI: Alcohol steatosis and cytotoxicity: the role of cytochrome P4502E1 and autophagy. Free Radic Biol Med 2012;53:1346–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singletary KW, Meline B: Effect of grape seed proanthocyanidins on colon aberrant crypts and breast tumors in a rat dual-organ tumor model. Nutr Cancer 2001;39:252–258 [DOI] [PubMed] [Google Scholar]
- 21.Bagchi D, Garg A, Krohn RL, et al. : Protective effects of grape seed proanthocyanidins and selected antioxidants against TPA-induced hepatic and brain lipid peroxidation and DNA fragmentation, and peritoneal macrophage activation in mice. Gen Pharmacol 1998;30:771–776 [DOI] [PubMed] [Google Scholar]
- 22.Shin MO, Yoon S, Moon JO: The proanthocyanidins inhibit dimethylnitrosamine-induced liver damage in rats. Arch Pharm Res 2010;33:167–173 [DOI] [PubMed] [Google Scholar]
- 23.Dulundu E, Ozel Y, Topaloglu U, et al. : Grape seed extract reduces oxidative stress and fibrosis in experimental biliary obstruction. J Gastroenterol Hepatol 2007;22:885–892 [DOI] [PubMed] [Google Scholar]
- 24.Yousef MI, Saad AA, El-Shennawy LK: Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem Toxicol 2009;47:1176–1183 [DOI] [PubMed] [Google Scholar]
- 25.Puiggros F, Llópiz N, Ardévol A, Bladé C, Arola A, Salvadoa MJ: Grape seed procyanidins prevent oxidative injury by modulating the expression of antioxidant enzyme systems. J Agric Food Chem 2005;53,6080–6086 [DOI] [PubMed] [Google Scholar]
- 26.Valavanidis A, Vlachogianni T, Fiotakis C: 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2009;27:120–139 [DOI] [PubMed] [Google Scholar]
- 27.Romilda C, Marika P, Alessandro S, et al. : Oxidative DNA damage correlates with cell immortalization and mir-92 expression in hepatocellular carcinoma. BMC Cancer 2012;12:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irie M, Sohda T, Iwata K, et al. : Levels of the oxidative stress marker γ-glutamyltranspeptidase at different stages of nonalcoholic fatty liver disease. J Int Med Res 2012;40:924–933 [DOI] [PubMed] [Google Scholar]
- 29.Ishii Y, Okamura T, Inoue T, Tasaki M, Umemura T, Nishikawa A: Dietary catechol causes increased oxidative DNA damage in the livers of mice treated with acetaminophen. Toxicology 2009;19;263:93–99 [DOI] [PubMed] [Google Scholar]
- 30.Shao ZH, Hsu CW, Chang WT, et al. : Cytotoxicity induced by grape seed proanthocyanidins: role of nitric oxide. Cell Biol Toxicol 2006;22:149–158 [DOI] [PubMed] [Google Scholar]
- 31.Antonelli-Ushirobira TM, Kaneshima EN, Gabriel M, Audi EA, Marques LC, Mello JC: Acute and subchronic toxicological evaluation of the semipurified extract of seeds of guarana (Paullinia cupana) in rodents. Food Chem Toxicol 2010;48:1817–1820 [DOI] [PubMed] [Google Scholar]