Abstract
Genome-wide association studies have provided strong evidence for association of the SNP rs1344706 in the ZNF804A gene with schizophrenia and bipolar disorder. Neuroimaging studies have suggested that variation at rs1344706 may be associated with neural endophenotypes such as white matter volumes and densities. However, analyses of white matter microstructure using diffusion tensor imaging (DTI) have produced conflicting results. We examined the association between rs1344706 and white matter microstructure in 107 healthy individuals using Tract-Based Spatial Statistics (TBSS). TBSS analysis showed significant association between the risk allele and lower fractional anisotropy in the corpus callosum, left forceps minor, and right parietal white matter (p < .05; FWE corrected). Post-hoc analyses indicated that this association was largely driven by alterations in radial diffusivity, consistent with an effect of genotype on myelination. In light of the strong DTI evidence for white matter microstructural abnormalities in schizophrenia, the current results implicate a potential mechanism for schizophrenia risk formation by ZNF804A rs1344706 genotype.
Keywords: schizophrenia, genetics, endophenotype, ZNF804A, diffusion tensor imaging, white matter
Introduction
The ZNF804A single nucleotide polymorphism (SNP) rs1344706 has been associated with schizophrenia, and to a lesser degree, bipolar disorder, across multiple studies (O'Donovan et al., 2008; Riley et al., 2010; Schwab et al., 2013; Williams et al., 2010). However, the biological functions of ZNF804A and the mechanisms through which it conveys the illness risk remain to be elucidated.
White matter abnormalities have also been strongly linked with schizophrenia (Davis et al., 2003). For example, an early post-mortem study found smaller corpus callosum fiber density in women with schizophrenia (Highley et al., 1999). Within the last decade, diffusion tensor imaging (DTI) studies have consistently reported lower white matter integrity as measured by fractional anisotropy (FA) in patients with schizophrenia compared to healthy cohorts (Buchsbaum et al., 2006; Kubicki et al., 2007; Lee et al., 2013). FA deficits have been reported in the first episode of schizophrenia, prior to treatment with antipsychotic medication, as well as in unaffected first-degree relatives of patients with schizophrenia, consistent with a genetic underpinning for this abnormality (Karlsgodt et al., 2012; Peters et al., 2010). Moreover, FA deficits have been correlated with symptom severity, underscoring the potential clinical relevance of understanding this mechanism (Cheung et al., 2011; Whitford et al., 2010).
Prior studies have shown that rs1344706 is associated with brain white matter volume (Lencz et al., 2010; Wassink et al., 2012) and density (Wei et al., 2012). Findings from DTI studies of white matter microstructure, however, are inconsistent. One study, which utilized a 3T MRI scanner, showed association between rs1344706 genotype and white matter microstructure (Kuswanto et al., 2012), while three studies using 1.5T MRI have reported negative results (Sprooten et al., 2012; Voineskos et al., 2011; Wei et al., 2013). This inconsistency in DTI findings may be related to differences in methodology between studies (Table 1), including magnet strength and resultant image resolution and signal-to-noise. Moreover, analytic approaches have varied as two studies employed Tract-Based Spatial Statistics (TBSS) (Sprooten et al., 2012; Wei et al., 2013), with one study using tractography (Voineskos et al., 2011), and another using an approach based on large regions of interest (ROI) (Kuswanto et al., 2012). A number of tracts has been examined in Voineskos et al. (2011) to examine association between risk allele and white matter FA using deterministic tractography, as well as cortical gray matter thickness measures from T1 structural brain images. The risk variant showed negative association with thickness in the superior temporal and cingulate gyri, while it failed to show association with white matter FA. In Kuswanto et al. (2012), FA in frontal, temporal and parietal lobules and cingulate gyrus were examined between risk allele homozygotes vs. others in patients of schizophrenia and healthy controls. Risk homozygotes showed higher FA than others in right temporal lobe among healthy controls, and showed lower FA in left and right parietal lobe and left cingulate gyrus among schizophrenia patients. Sample sizes were moderate in these studies (healthy controls n=50-69), and appeared not related to the variability in finding s between these studies. In summary, previous 1.5T studies showed no association between rs1344706 and DTI measurements (Sprooten et al., 2012; Voineskos et al., 2011; Wei et al., 2013), and one 3T study showed association between rs1344706 and lobular averages of FA (Kuswanto et al., 2012). The current study is, to our knowledge, the first 3T TBSS study to test the association between rs1344706 and voxelwise FA, in the largest sample to date (n = 107). Whereas tractography and ROI approaches can have greater sensitivity to differences across an entire tract or region, TBSS is a voxel-wise approach that is therefore more sensitive to differences in smaller sub-regions, as well as areas that are not demarcated a priori.
Table 1. Previous DTI studies on ZNF804A rs1344706 genotype and white matter microstructure.
| First Author | Year | MRI | N | Age | Method | Result |
|---|---|---|---|---|---|---|
| Voineskos (Voineskos et al., 2011) | 2011 | 1.5T | 62 HC | 37.4±12.7 | Tractography | Negative |
| Kuswanto (Kuswanto et al., 2012) | 2011 | 3T | 64 HC89 SZ | 31.9±10.1 34.3±9.2 | ROI | Positive |
| Sprooten (Sprooten et al., 2012) | 2012 | 1.5T | 50 HC83 HC84 sib-BP84 sib-BP | 22.7±1.7 21.1±2.4 21.4±2.8 | TBSS | Negative |
| Wei (Wei et al., 2013) | 2013 | 1.5T | 69 HC100 SZ | 25.4±5.7 26.5±6.9 | TBSS | Negative |
Kuswanto et al. (Kuswanto et al., 2012): Patients with AA genotype (homozygous for risk allele) showed lower FA in parietal lobes than patients with AC or CC genotype. HC with AA genotype showed higher FA in the right temporal lobe than HC with AC or CC genotype.
DTI = diffusion tensor imaging; FA = fractional anisotropy; HC = healthy controls; SZ = schizophrenia; Sib = sibling; BP = bipolar disorder; ROI = region of interest; TBSS = tract-based spatial statistics.
The original GWAS studies reporting the association of ZNF804A with schizophrenia utilized an additive model (O'Donovan et al., 2008). However, each of the previous DTI studies have used dominant models, in which heterozygous and homozygous risk allele carriers were grouped together and compared to non-risk allele homozygotes, potentially reducing sensitivity. Moreover, it has been shown that age and white matter microstructure show a non-linear relationship across the lifespan in most of the major white matter tracts (Kochunov et al., 2012; Lebel et al., 2012; Peters et al., 2013; Peters et al., 2012; Salat et al., 2005). In samples with wide age ranges, genetic effects on DTI white matter microstructure may be masked by considerable variance derived from age effects.
In the present study, we employed 3T MRI and TBSS to examine associations between rs1344706 genotype and brain white matter microstructure using an additive model, while accounting for the non-linear effects of age,. We hypothesized that the ZNF804A risk allele (A allele of SNP rs1344706) would be associated with lower FA in psychiatrically healthy individuals.
Methods
Participants
One-hundred seven healthy Caucasians (52% male) between the ages of 8.8 and 68.1 years (mean 31.8 ±16.0; median 26.2) were recruited through local advertisements and by word of mouth. Written informed consent was obtained from participants or if the participant was a minor, from a parent or guardian; all minors provided assent. Participants had no history of a DSM-IV axis I major mood or psychotic disorder as assessed by structured diagnostic interview (Schedule for Affective Disorders and Schizophrenia for School-Age Children -Present and Lifetime Version (Kaufman et al., 1997) or Structured Clinical Interview for DSM-IV disorders Non-Patient Edition (First et al., 2001)). Other exclusion criteria included: (i) intellectual or learning disability; (ii) medications with known adverse cognitive effects; (iii) MRI contraindications; (iv) pregnancy; (v) significant medical illness that could affect brain structure. Mean IQ as estimated from the Wide Range Achievement Test (Reading subtest) was 105.9 ±9.6 (data missing for 14 subjects). Handedness was determined using the Edinburgh Handedness Inventory; median laterality quotient was 0.9 (range -1 to 1). This study was approved by the Institutional Review Board of the North Shore - Long Island Jewish Health System.
Genotyping
Genotyping was performed for ∼ 770K genome wide SNPs using Illumina Omni Express arrays according to manufacturers’ specifications. SNPs were filtered based on call rate < 98 %, minor allele frequency < 0.025 and Hardy-Weinberg exact test P < 0.000001. Samples were filtered based on genotype quality control filtration (sample call rate < 97 %, gender mismatch). Principal component analysis was performed with 98,629 LD pruned (r2 > 0.2) genome wide SNPs to identify the non-Caucasian outliers using ethnicity information. A total 107 individuals passed QC and were identified as Caucasian and considered for further analysis.
DTI Acquisition and Preprocessing
All subjects received a DTI exam at the North Shore University Medical Center, Manhasset, NY, on a GE Signa HDx 3.0 T system (General Electric, Milwaukee, WI). The sequence included volumes with diffusion gradients applied along 31 non-parallel directions (b = 1000 s/mm2) and 5 volumes without diffusion weighting (TR = 14 s, TE = min., matrix = 128 × 128, FOV = 240 mm). Each volume consisted of 51 contiguous 2.5-mm axial slices acquired parallel to the anterior-posterior commissural line using a ramp sampled, double spin-echo, single shot echo-planar imaging method.
All scans were reviewed by a radiologist and all images were visually inspected to ensure that no gross abnormalities were evident. Image processing was conducted using the Functional Magnetic Resonance Imaging of the Brain Software Library (FSL; Oxford, United Kingdom; http://fsl.fmrib.ox.ac.uk/fsl/). Eddy-current distortions and head-motion displacements were corrected through affine registration of the 31 diffusion volumes to the first b0 volume using FSL's Linear Registration Tool (FLIRT) (Jenkinson and Smith, 2001). The b-vector table (i.e. gradient directions) for each participant was then adjusted according to the rotation parameters of this linear correction. Non-brain tissue was removed using FSL's Brain Extraction Tool. Fractional anisotropy (FA) and diffusivity parameters, putative measures of white matter integrity (Beaulieu, 2002), were then calculated at each voxel of the brain by fitting a diffusion tensor model to the raw diffusion data using weighted least squares in FSL's Diffusion Toolbox.
Tract Based Spatial Statistics
Voxel-wise statistical analysis of the FA data was carried out using TBSS (Tract-Based Spatial Statistics) in FSL (Smith et al., 2006). All subjects' FA data were aligned to the FMRIB58 FA standard brain using the nonlinear registration tool FNIRT (Smith et al., 2006). Next, the mean FA image was created and thinned to create a mean FA skeleton which represents the centers of all tracts common to the group. The FA threshold for the mean FA skeleton was set at 0.2. Each subject's aligned FA data was then projected onto this skeleton.
Prior to statistical testing, studentized residuals were calculated from FA regressions on age and sex using a Poisson model (c + a*Age*exp(-b*Age2) for each voxel (Lebel et al., 2012), and the resulting data fed into voxel-wise cross-subject statistics. Studentized age and sex residuals were also calculated for each voxel in the axial and radial diffusivity maps for post-hoc analysis.
To test for local associations between genotype and FA, linear regressions of A allele ‘dosage’ on studentized FA residuals were performed using permutation-based testing. An additive model was tested where A allele dosage was numbered as 0, 1, or 2 alleles and then mean centered. Inference on the statistic maps was carried out using threshold-free cluster enhancement (Smith and Nichols, 2009). The null distribution was built up over 5000 random permutations across the image. The clusters were then thresholded at a level of P<0.05, which is corrected for multiple comparisons (i.e. family-wise error). Anatomic location of significant FA clusters was determined with the probabilistic cortical, subcortical and white matter tractography atlases provided in FSL, and an MRI atlas of human white matter anatomy (Mori et al., 2006). For each of the significant FA clusters, the mean studentized residual values for FA, Axial Diffusivity (AD) and Radial Diffusivity (RD) within the clusters were extracted and examined with post-hoc stepwise linear regressions to determine the relative contributions of AD and RD.
Results
Genotyping
Allele frequencies of rs1344706 almost precisely matched those in the original GWAS sample (O'Donovan et al.,). Specifically, overall A-allele frequency was 59.81%. Subjects were grouped according to rs1344706 SNP genotype, yielding 43 AA homozygotes (8 to 60 years old, 34% of whom were younger than 18 years old), 42 AC heterozygotes (14 to 66 years old, 17% < 18 years old) and 22 CC homozygotes (9 to 68 years old, 22% < 18 years old).
TBSS
The TBSS voxelwise analysis revealed that higher A allele dosage significantly predicted lower FA in three regions: right parietal white matter, left forceps minor, and the anterior body/genu of the corpus callosum (Table 2 and Figure 1). There were no regions in which higher A allele dosage predicted higher FA values. Within each of the three clusters, A allele dosage significantly predicted the mean FA, AD and RD studentized residual values, with the exception of AD in the corpus callosum (Figure 1).
Table 2. Higher dosage of ZNF804A rs1344706 risk allele (A) predicted lower white matter FA in tract-based spatial statistics analysis (p<0.05, corrected).
| Tract | Size (voxels) | Peak p-value (corrected) | Peak Coordinates | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Right Parietal White Matter | 822 | 0.033 | 22 | -44 | 39 |
| Left Forceps Minor | 346 | 0.036 | -19 | 45 | 5 |
| Corpus Genu and Body | 186 | 0.041 | -3 | 9 | 23 |
FA = fractional anisotropy
Figure 1. Three white matter regions in which lower fractional anisotropy (FA) was associated with ZNF804A rs1344706 risk allele (A) dosage.
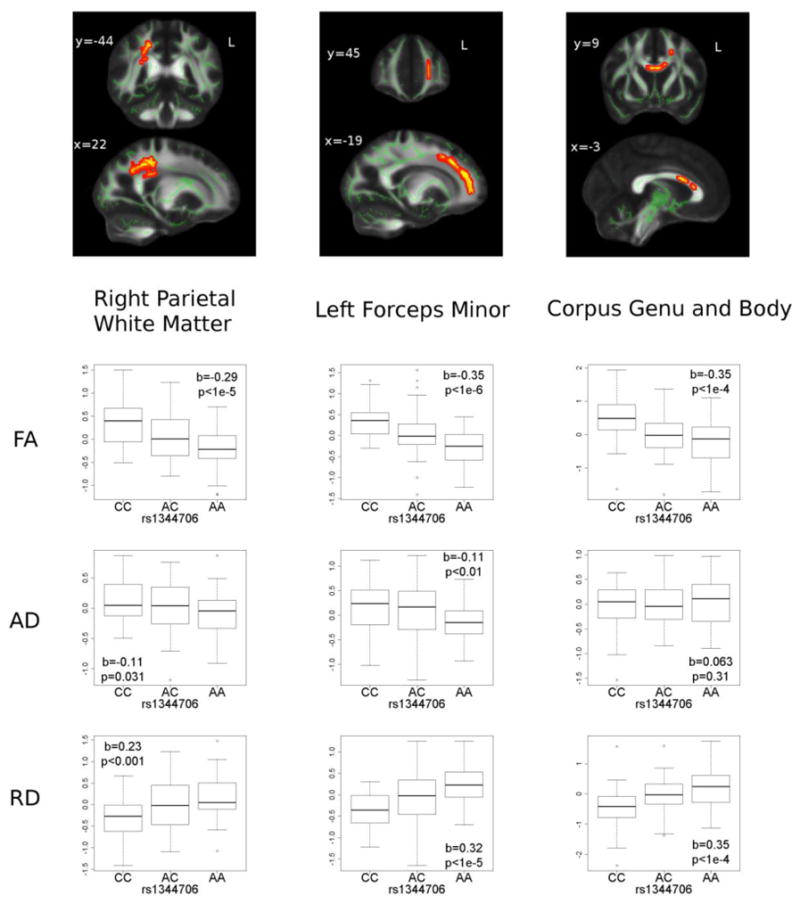
The significant clusters (red-yellow) in the FA images in the top row are ‘thickened’ into the local tracts by the tbss-fill tool for display purposes, and overlaid on the TBSS (tract-based spatial statistics analysis) skeleton in green. The mean studentized residuals from regressions on age and sex were determined for FA, Axial Diffusivity (AD) and Radial Diffusivity (RD) for each of the three clusters. The beta coefficients (b) of linear regressions with A allele dosage are shown in each boxplot.
In all three clusters, A allele dosage had significant goodness of fit (R-squared) to RD for all three ROIs, while goodness of fit to AD was only significant for the left forceps minor (Table 3, columns 2 and 3), with values for RD approaching those for FA (Table 3, column 1). In order to further test the associations of A allele dosage to AD and to RD, two two-stage regressions were performed for each of the clusters assessing the improvement of R-squared when RD was included in addition to AD in the model to predict A allele dosage compared to when RD was not included, as well as the improvement of R-squared when AD was included in the model in addition to RD compared to when AD was not included. Regressions between AD and A allele dosage was improved by having RD in the model, whereas regressions between RD and A allele was only minimally improved by having AD, further indicating very limited associations between AD and A allele dosage (Table 3, columns 4 and 5).
Table 3. Comparisons of linear regressions on A allele dosage.
| Adjusted R-squared (p-value) | |||||
|---|---|---|---|---|---|
| FA∼A | RD∼A | AD∼A | (A∼AD+RD) - (A∼AD) | (A∼RD+AD) - (A∼RD) | |
| Right Parietal White Matter | 0.18 (<0.01) | 0.09 (<0.01) | 0.03 (0.03) | 0.12 | 0.07 |
| Left Forceps Minor | 0.22 (<0.01) | 0.17 (<0.01) | 0.06 (<0.01) | 0.16 | 0.04 |
| Corpus Genu and Body | 0.13 (<0.01) | 0.13 (<0.01) | 0.00 (0.32) | 0.13 | 0.00 |
FA∼A = Linear FA regression on A allele dosage;
AD∼A = Linear AD regression on A allele dosage;
RD∼A = Linear RD regression on A allele dosage;
A∼AD+RD = Linear A allele dosage regression on AD and RD
A∼AD = Linear A allele dosage regression on AD
(A∼AD+RD) - (A∼AD) = Improvement of the model by including RD in the model in addition to AD to predict A allele dosage
A∼RD+AD = Linear A allele dosage regression on RD and AD
A∼RD = Linear A allele dosage regression on RD
(A∼RD+AD) - (A∼RD) = Improvement of the model by including AD in the model in addition to RD to predict A allele dosage
FA = fractional anisotropy
AD = Axial Diffusivity
RD = Radial Diffusivity.
Bold Face = significant association or improvement of the model
Discussion
This is the first study that shows statistically significant voxel-wise associations between ZNF804A rs1344706 genotype and white matter microstructure, using a stringent TBSS analysis of high-resolution 3T DTI data. Specifically, higher dosage of the schizophrenia risk allele (A) was associated with lower white matter FA and higher RD in the corpus callosum, left forceps minor and right parietal lobe.
The finding in the right parietal lobe is consistent with DTI findings in schizophrenia, which have demonstrated delayed growth trajectories in parietal white matter of patients with childhood-onset schizophrenia (Gogtay et al., 2008) and their siblings (Gogtay et al., 2012), as well as lower parietal white matter FA in adult schizophrenia patients (Ardekani et al., 2003), including never medicated patients with a first psychotic episode (Cheung et al., 2011) and patients with deficit schizophrenia (Rowland et al., 2008). Although the current sample consisted of participants without schizophrenia or other psychotic disorders, the lower FA by increased risk allele dosage is consistent with the observed associations between compromised parietal white matter (micro)structure and schizophrenia, and ZNF804A being a risk gene for schizophrenia. Taken together, the current result suggests that ZNF804A may exert its effect on risk for schizophrenia via effects on white matter microstructure in the right parietal lobe.
TBSS analysis also revealed significant regions in the corpus callosum and the forceps minor (Figure 1), showing an association between higher risk allele dosage and lower FA. Lower FA values in the genu and its extensions (i.e. forceps minor) have also been implicated in schizophrenia (Buchsbaum et al., 2006; Kanaan et al., 2006; Lee et al., 2013; Price et al., 2007; Whitford et al., 2010), and found to correlate with degree of reality distortion in patients (Whitford et al., 2010). Our finding also corresponds to the reduced interhemispheric functional connectivity between dorsolatetal prefrontal cortexes (DLPFC) by ZNF804A risk genotype carriage during a working memory task (Esslinger et al., 2009). Lower FA by risk allele dosage in the corpus callosum and forceps minor can be interpreted as the structural homologue to this functional connectivity finding, since these structures contain connections between the bilateral DLPFCs.
Post-hoc analysis of axial and radial diffusivities revealed that the associations between A allele dosage and RD were greater than the associations between A allele dosage and AD. It has been shown that dysmyelination results in increased RD (Song et al., 2003; Song et al., 2002). The association between RD and A allele dosage may therefore suggest that ZNF804A rs1344706 schizophrenia risk genotype contributes to poorer myelination in these white matter regions (Davis et al., 2003). However, this interpretation should be considered tentative since we do not have direct evidence in humans that increased RD solely reflects dysmyelination. Although the biological functions of ZNF804A remain largely unknown, functional brain connectivity studies have shown associations with rs1344706 risk genotype (Esslinger et al., 2011; Esslinger et al., 2009; Paulus et al., 2013; Rasetti et al., 2011; Walter et al., 2011). In these studies, prefrontal and prefrontal-temporal connectivity have been consistently reported to be lower in rs1344706 risk allele carriers, which at least partly corresponds to our findings in the corpus callosum and forceps minor. Functional connectivity between DLPFC and parietal regions has also been found to be reduced in risk genotype carriers (Esslinger et al., 2011), which may be interpreted as a functional counterpart to our structural finding in the right parietal white matter, corpus callosum and forceps minor altogether.
In sum, the present study indicates that the ZNF804A schizophrenia risk gene may affect regional white matter microstructure, including white matter regions that have been found compromised in schizophrenia patients. Our results may provide insight into the relationship between ZNF804A rs1344706 and pathophysiology of schizophrenia. Further studies are needed to shed light on the causative mechanisms by which the ZNF804A gene affects white matter microstructure in healthy individuals and patients with schizophrenia.
Acknowledgments
We thank Michelle Bergman, Jamie Wagner and Kimberly Cameron for their coordination of the study.
Funding Sources: This work was supported in part by grants from NARSAD and the National Institute of Mental Health to Dr. Szeszko (R01 MH076995), Dr. Malhotra (MH79800), the NSLIJ Research Institute General Clinical Research Center (M01 RR018535), an Advanced Center for Intervention and Services Research (P30 MH090590) and a Center for Intervention Development and Applied Research (P50 MH080173).
Footnotes
Financial Disclosures: Dr. Ikuta, Dr. Peters, Dr. Guha, Dr. John, Dr. Karlsgodt, Dr. Lencz and Dr. Szeszko reported no biomedical financial interests or potential conflicts of interest. Dr Malhotra has received compensation from Eli Lilly, Schering-Plough/Merck, Sunovion Pharmaceuticals, Genomind, Shire, and Abbott.
Contributors: Dr. Ikuta designed the study, conducted the literature search and imaging analyses, and wrote the first draft of the manuscript. Drs. Szeszko, Lencz and Malhotra wrote the study protocol and edited the manuscript. Drs. Peters and Karlsgodt recruited participants and edited the manuscript. Drs. Guha and John conducted the genetic and statistical analyses. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO. MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport. 2003;14(16):2025–2029. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR in Biomedicine. 2002;15(7-8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Friedman J, Buchsbaum BR, Chu KW, Hazlett EA, Newmark R, Schneiderman JS, Torosjan Y, Tang C, Hof PR, Stewart D, Davis KL, Gorman J. Diffusion Tensor Imaging in Schizophrenia. Biological Psychiatry. 2006;60(11):1181–1187. doi: 10.1016/j.biopsych.2005.11.028. [DOI] [PubMed] [Google Scholar]
- Cheung V, Chiu CPY, Law CW, Cheung C, Hui CLM, Chan KKS, Sham PC, Deng MY, Tai KS, Khong PL, McAlonan GM, Chua SE, Chen E. Positive symptoms and white matter microstructure in never-medicated first episode schizophrenia. Psychological Medicine. 2011;41(08):1709–1719. doi: 10.1017/S003329171000156X. [DOI] [PubMed] [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: Evidence for myelin-related dysfunction. Archives of General Psychiatry. 2003;60(5):443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- Esslinger C, Kirsch P, Haddad L, Mier D, Sauer C, Erk S, Schnell K, Arnold C, Witt SH, Rietschel M, Cichon S, Walter H, Meyer-Lindenberg A. Cognitive state and connectivity effects of the genome-wide significant psychosis variant in ZNF804A. NeuroImage. 2011;54(3):2514–2523. doi: 10.1016/j.neuroimage.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, Haddad L, Mier D, Opitz von Boberfeld C, Raab K, Witt SH, Rietschel M, Cichon S, Meyer-Lindenberg A. Neural Mechanisms of a Genome-Wide Supported Psychosis Variant. Science. 2009;324(5927):605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Biometrics Research. New York State Psychiatric Institute; New York: 2001. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Non-patient Edition. (SCID-I/NP) [Google Scholar]
- Gogtay N, Hua X, Stidd R, Boyle CP, Lee S, Weisinger B, Chavez A, Giedd JN, Clasen L, Toga AW, Rapoport JL, Thompson PM. Delayed white matter growth trajectory in young nonpsychotic siblings of patients with childhood-onset schizophrenia. Archives of General Psychiatry. 2012;69(9):875–884. doi: 10.1001/archgenpsychiatry.2011.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Lu A, Leow AD, Klunder AD, Lee AD, Chavez A, Greenstein D, Giedd JN, Toga AW, Rapoport JL, Thompson PM. Three-dimensional brain growth abnormalities in childhood-onset schizophrenia visualized by using tensor-based morphometry. Proceedings of the National Academy of Sciences. 2008;105(41):15979–15984. doi: 10.1073/pnas.0806485105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highley JR, Esiri MM, McDonald B, Cortina-Borja M, Herron BM, Crow TJ. The size and fibre composition of the corpus callosum with respect to gender and schizophrenia: a post-mortem study. Brain. 1999;122(1):99–110. doi: 10.1093/brain/122.1.99. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kanaan RA, Shergill SS, Barker GJ, Catani M, Ng VW, Howard R, McGuire PK, Jones DK. Tract-specific anisotropy measurements in diffusion tensor imaging. Psychiatry Research: Neuroimaging. 2006;146(1):73–82. doi: 10.1016/j.pscychresns.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Jacobson S, Seal M, Fusar-Poli P. The relationship of developmental changes in white matter to the onset of psychosis. Curr Pharm Des. 2012;18(4):422–433. doi: 10.2174/138161212799316073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Williamson DE, Lancaster J, Fox P, Cornell J, Blangero J, Glahn DC. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiology of Aging. 2012;33(1):9–20. doi: 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. Journal of Psychiatric Research. 2007;41(1-2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuswanto CN, Woon PS, Zheng XB, Qiu A, Sitoh YY, Chan YH, Liu J, Williams H, Ong WY, Sim K. Genome-wide supported psychosis risk variant in ZNF804A gene and impact on cortico–limbic WM integrity in schizophrenia. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2012;159B(3):255–262. doi: 10.1002/ajmg.b.32032. [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kubicki M, Asami T, Seidman LJ, Goldstein JM, Mesholam-Gately RI, McCarley RW, Shenton ME. Extensive white matter abnormalities in patients with first-episode schizophrenia: A diffusion tensor imaging (DTI) study. Schizophrenia Research. 2013;143:231–238. doi: 10.1016/j.schres.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Szeszko PR, DeRosse P, Burdick KE, Bromet EJ, Bilder RM, Malhotra AK. A Schizophrenia Risk Gene, ZNF804A, Influences Neuroanatomical and Neurocognitive Phenotypes. Neuropsychopharmacology. 2010;35(11):2284–2291. doi: 10.1038/npp.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae-Poetscher LM, Zijl PCMv. MRI Atlas of Human White Matter. Elsevier; Amsterdam: 2006. [Google Scholar]
- O'Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V, Nikolov I, Hamshere M, Carroll L, Georgieva L, Dwyer S, Holmans P, Marchini JL, Spencer CCA, Howie B, Leung HT, Hartmann AM, Moller HJ, Morris DW, Shi Y, Feng G, Hoffmann P, Propping P, Vasilescu C, Maier W, Rietschel M, Zammit S, Schumacher J, Quinn EM, Schulze TG, Williams NM, Giegling I, Iwata N, Ikeda M, Darvasi A, Shifman S, He L, Duan J, Sanders AR, Levinson DF, Gejman PV, Cichon S, Nothen MM, Gill M, Corvin A, Rujescu D, Kirov G, Owen MJ. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nature Genetics. 2008;40(9):1053–1055. doi: 10.1038/ng.201. [DOI] [PubMed] [Google Scholar]
- Paulus FM, Krach S, Bedenbender J, Pyka M, Sommer J, Krug A, Knake S, Nöthen MM, Witt SH, Rietschel M, Kircher T, Jansen A. Partial support for ZNF804A genotype-dependent alterations in prefrontal connectivity. Human Brain Mapping. 2013;34(2):304–313. doi: 10.1002/hbm.21434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Blaas J, de Haan L. Diffusion tensor imaging in the early phase of schizophrenia: What have we learned? Journal of Psychiatric Research. 2010;44(15):993–1004. doi: 10.1016/j.jpsychires.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Peters BD, Ikuta T, DeRosse P, John M, Burdick KE, Gruner P, Prendergast DM, Szeszko PR, Malhotra AK. Age-Related Increases in White Matter Tract Integrity Predict Increases in Cognitive Performance from Childhood into Adulthood. Biological Psychiatry in press. 2013 doi: 10.1016/j.biopsych.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P, Zhang JP, Giorgio A, Qiu D, Tapert SF, Brauer J, Asato MR, Khong PL, James AC, Gallego JA, Malhotra AK. White Matter Development in Adolescence: Diffusion Tensor Imaging and Meta-Analytic Results. Schizophrenia Bulletin. 2012;38(6):1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G, Cercignani M, Parker GJM, Altmann DR, Barnes TRE, Barker GJ, Joyce EM, Ron MA. Abnormal brain connectivity in first-episode psychosis: A diffusion MRI tractography study of the corpus callosum. NeuroImage. 2007;35(2):458–466. doi: 10.1016/j.neuroimage.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasetti R, Sambataro F, Chen Q, Callicott JH, Mattay VS, Weinberger DR. Altered cortical network dynamics: A potential intermediate phenotype for schizophrenia and association with ZNF804A. Archives of General Psychiatry. 2011;68(12):1207–1217. doi: 10.1001/archgenpsychiatry.2011.103. [DOI] [PubMed] [Google Scholar]
- Riley B, Thiselton D, Maher BS, Bigdeli T, Wormley B, McMichael GO, Fanous AH, Vladimirov V, O'Neill FA, Walsh D, Kendler KS. Replication of association between schizophrenia and ZNF804A in the Irish Case-Control Study of Schizophrenia sample. Molecular Psychiatry. 2010;15(1):29–37. doi: 10.1038/mp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LM, Spieker EA, Francis A, Barker PB, Carpenter WT, Buchanan RW. White Matter Alterations in Deficit Schizophrenia. Neuropsychopharmacology. 2008;34(6):1514–1522. doi: 10.1038/npp.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM. Age-Related Changes in Prefrontal White Matter Measured by Diffusion Tensor Imaging. Annals of the New York Academy of Sciences. 2005;1064(1):37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Schwab SG, Kusumawardhani AAAA, Dai N, Qin W, Wildenauer MDB, Agiananda F, Amir N, Antoni R, Arsianti T, Asmarahadi A, Diatri H, Djatmiko P, Irmansyah I, Khalimah S, Kusumadewi I, Kusumaningrum P, Lukman PR, Mustar L, Nasrun MW, Naswati S, Prasetiyawan P, Semen GM, Siste K, Tobing H, Widiasih N, Wiguna T, Wulandari WD, Benyamin B, Wildenauer DB. Association of rs1344706 in the ZNF804A gene with schizophrenia in a case/control sample from Indonesia. Schizophrenia Research. 2013;147(1):46–52. doi: 10.1016/j.schres.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination Revealed through MRI as Increased Radial (but Unchanged Axial) Diffusion of Water. NeuroImage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sprooten E, McIntosh AM, Lawrie SM, Hall J, Sussmann JE, Dahmen N, Konrad A, Bastin ME, Winterer G. An investigation of a genomewide supported psychosis variant in ZNF804A and white matter integrity in the human brain. Magnetic Resonance Imaging. 2012;30(10):1373–1380. doi: 10.1016/j.mri.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Lerch JP, Felsky D, Tiwari A, Rajji TK, Miranda D, Lobaugh NJ, Pollock BG, Mulsant BH, Kennedy JL. The ZNF804A Gene: Characterization of a Novel Neural Risk Mechanism for the Major Psychoses. Neuropsychopharmacology. 2011;36(9):1871–1878. doi: 10.1038/npp.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Schnell K, Erk S, Arnold C, Kirsch P, Esslinger C, Mier D, Schmitgen MM, Rietschel M, Witt SH, Nothen MM, Cichon S, Meyer-Lindenberg A. Effects of a genome-wide supported psychosis risk variant on neural activation during a theory-of-mind task. Molecular Psychiatry. 2011;16(4):462–470. doi: 10.1038/mp.2010.18. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Epping EA, Rudd D, Axelsen M, Ziebell S, Fleming FW, Monson E, Ho BC, Andreasen NC. Influence of ZNF804A on brain structure volumes and symptom severity in individuals with schizophrenia. Archives of General Psychiatry. 2012;69(9):885–892. doi: 10.1001/archgenpsychiatry.2011.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Kang Z, Diao F, Guidon A, Wu X, Zheng L, Li L, Guo X, Hu M, Zhang J, Liu C, Zhao J. No association of ZNF804A rs1344706 with white matter integrity in schizophrenia: A tract-based spatial statistics study. Neuroscience Letters. 2013;532(0):64–69. doi: 10.1016/j.neulet.2012.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Kang Z, Diao F, Shan B, Li L, Zheng L, Guo X, Liu C, Zhang J, Zhao J. Association of the ZNF804A gene polymorphism rs1344706 with white matter density changes in Chinese schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;36(1):122–127. doi: 10.1016/j.pnpbp.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Schneiderman JS, O'Donnell LJ, King R, Alvarado JL, Khan U, Markant D, Nestor PG, Niznikiewicz M, McCarley RW, Westin CF, Shenton ME. Corpus Callosum Abnormalities and Their Association with Psychotic Symptoms in Patients with Schizophrenia. Biological Psychiatry. 2010;68(1):70–77. doi: 10.1016/j.biopsych.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams HJ, Norton N, Dwyer S, Moskvina V, Nikolov I, Carroll L, Georgieva L, Williams NM, Morris DW, Quinn EM, Giegling I, Ikeda M, Wood J, Lencz T, Hultman C, Lichtenstein P, Thiselton D, Maher BS, Malhotra AK, Riley B, Kendler KS, Gill M, Sullivan P, Sklar P, Purcell S, Nimgaonkar VL, Kirov G, Holmans P, Corvin A, Rujescu D, Craddock N, Owen MJ, O'Donovan MC. Fine mapping of ZNF804A and genome-wide significant evidence for its involvement in schizophrenia and bipolar disorder. Molecular Psychiatry. 2010;16(4):429–441. doi: 10.1038/mp.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]


