Abstract
OBJECTIVE
To determine the clinical relevance, if any, of traumatic intracranial findings on early head CT and brain MRI to 3-month outcome in mild traumatic brain injury (MTBI)
METHODS
135 MTBI patients evaluated for acute head injury in emergency departments of three Level I Trauma Centers were enrolled prospectively. In addition to admission head CT, early brain MRI was performed 12+/-3.9 days after injury. Univariate and multivariate logistic regression were used to assess for demographic, clinical, socioeconomic, CT, and MRI features that were predictive of Extended Glasgow Outcome Scale (GOS-E) at 3 months post-injury.
RESULTS
Twenty-seven percent of MTBI patients with normal admission head CT had abnormal early brain MRI. CT evidence of subarachnoid hemorrhage was associated with a multivariate odds ratio of 3.5 (p=0.01) for poorer 3-month outcome, after adjusting for demographic, clinical, and socioeconomic factors. One or more brain contusions on MRI, and ≥4 foci of hemorrhagic axonal injury on MRI, were each independently associated with poorer 3-month outcome, with multivariate odds ratios of 4.5 (p=0.01) and 3.2 (p=0.03), respectively, after adjusting for head CT findings and demographic, clinical, and socioeconomic factors.
INTERPRETATION
In this prospective multicenter observational study, the clinical relevance of abnormal findings on early brain imaging after MTBI is demonstrated. The addition of early CT and MRI markers to a prognostic model based on previously known demographic, clinical, and socioeconomic predictors resulted in a greater than two-fold increase in the explained variance in 3-month GOS-E.
INTRODUCTION
Mild traumatic brain injury (MTBI) comprises 75% of the 1.7 million patients who seek medical attention annually in the U.S. for acute head injury. The most widely accepted definitions of MTBI1-3 consist of 1) non-penetrating head trauma resulting in one or more of the following: confusion/disorientation, loss of consciousness (LOC) <30 minutes and/or post-traumatic amnesia (PTA) <24 hours in duration, or transient focal neurological signs/seizure; and 2) Glasgow Coma Scale (GCS) 13-15 upon acute medical evaluation.
As a group, MTBI patients have generally been ascribed a good prognosis. However, there is convincing evidence that, within MTBI, there is a subset of patients who develop persistent dysfunction.4-9 To date, there remains a dearth of effective clinical, laboratory and imaging markers in MTBI. The WHO Collaborating Centre Task Force on MTBI has endorsed an urgent need for well-designed studies that determine risk factors for persistent impairment after MTBI, as a prerequisite for better triage to therapeutic interventions.4 Such treatments include early educational intervention, structured cognitive-behavioral therapy, and early mild physical activity, which result in fewer symptoms, lower mean severity of symptoms, less social disability, and fewer days off work.10, 11
MRI is a standard imaging technique for the assessment of many brain disorders. Many recent studies, however, have reported that the acute focal traumatic lesions detected on early MRI in MTBI patients are not correlated with clinical outcome.6, 12-15 The implication of such studies is that the exquisite sensitivity of MRI, particularly at 3 Tesla, reveals numerous small lesions, such as axonal injury and small cortical contusions, that are clinically irrelevant. As a result, no consensus exists regarding the significance of such lesions, even among clinicians who routinely care for TBI patients. In many hospitals, in the absence of a lesion requiring surgical intervention, patients are discharged without follow-up care. In a geographically diverse study of 878 Emergency Department (ED) visits for MTBI in the U.S.,16 9% of patients received no discharge recommendations; 28% were instructed to return to ED only as needed, without other follow-up; 19% were referred to primary care; and 42% were referred to another, unspecified physician.
In contrast to CT and MRI, certain clinical, demographic, and socioeconomic characteristics, including age, prior head injury, educational background, and employment status have been widely accepted as factors in poorer outcome after MTBI.17, 18 In this study, we sought to assess the clinical relevance of early CT and MRI to 3-month outcome after controlling for such factors. Progress beyond mere definition of MTBI, toward evidence-based diagnosis, is essential for clinical trials that evaluate treatments and, ultimately, more effective triage to follow-up care.4, 19-22
PATIENTS AND METHODS
Study population
MTBI patients were enrolled at three Level I trauma centers as part of the prospective multicenter TRACK-TBI (Transforming Research and Clinical Knowledge in Traumatic Brain Injury) study. Institutional review boards of participating centers (San Francisco General Hospital; University of Pittsburgh; University Medical Center Brackenridge) approved all study protocols, and all patients or their legal representatives gave written informed consent. Inclusion criteria were GCS 13-15 upon ED arrival and triage to head CT to assess for traumatic intracranial injury using the American College of Emergency Physicians/Centers for Disease Control (ACEP/CDC) evidence-based joint practice guideline (Supplementary Table 1).23 To maximize the generalizability of study conclusions, the limited exclusion criteria included age <15 years, LOC ≥30 minutes, PTA ≥24 hours, and contraindication to MRI.
A total of 1023 patients with GCS 13-15 upon ED arrival, and who underwent head CT for the indication of acute head injury, were screened to obtain the final study population of 135 study participants. Independent-samples t-test showed that the 135 study participants were younger (mean 40 years, median 38 years, S.D. 17 years, range 15-86 years) than the 888 non-participants (mean 49 years, median 47 years, S.D. 21 years, range 15-100 years), (p=2×10-6, two-tailed), likely due in part to greater difficulty we experienced in coordinating outpatient MRIs for elderly patients, who related more difficulties in traveling to the outpatient imaging facility, and more frequently had problems with mobility at baseline. Mann-Whitney U test showed the 135 participants had higher GCS scores (mean GCS 15, median GCS 15) than the 888 non-participants (mean GCS 15, median GCS 15) (U=53618, p=0.01). A χ2 test with Yates’ continuity correction showed gender did not differ significantly between participants (72%) and non-participants (67%) (χ2 = 0.85, p=0.36). LOC and PTA duration information were missing for many non-participants, so that some of the 888 non-participants may have sustained “moderate” rather than “mild” TBI on the basis of LOC > 30 minutes and/or PTA > 24 hours; therefore, the reported group difference in GCS may be exaggerated by the presence of moderate TBI patients in the non-participant group. Non-participants’ socioeconomic data were not collected.
Table 1 and Supplementary Figures 1 and 2 summarize clinical, demographic and socioeconomic characteristics of participants and screened non-participant patients.
Table 1.
Clinical, demographic and socioeconomic characteristics of study participants (N=135)
| Age | Mean +/- S.D. = 40 +/- 17 years Range 15 to 86 years |
|
|---|---|---|
| Gender | Male | 97 (72%) |
| Female | 38 (28%) | |
| Race | White | 102 (76%) |
| Black | 13 (10%) | |
| Asian | 9 (7%) | |
| Hawaiian/Pacific Islander | 7 (5%) | |
| American Indian/Alaska Native | 2 (1%) | |
| Unknown | 2 (1%) | |
| Ethnicity | Hispanic | 23 (17%) |
| Non-Hispanic | 111 (82%) | |
| Unknown | 1 (1%) | |
| GCS | 15 | 106 (79%) |
| 14 | 26 (19%) | |
| 13 | 3 (2%) | |
| LOC OR PTA | Yes | 108 (80%) |
| No | 26 (19%) | |
| Unknown | 1 (1%) | |
| Prior TBI resulting in acute medical evaluation |
Yes | 45 (33%) |
| No | 89 (66%) | |
| Unknown | 1 (1%) | |
| Educational background | Full-time student | 8 (6%) |
| Adult ≥19 years old with less than | 12 (9%) | |
| High school diploma/G.E.D. only | 43 (32%) | |
| College student, bachelor’s or | 70 (52%) | |
| Unknown | 2 (1%) | |
| Employment status at time of TBI |
Unemployed | 24 (18%) |
| Part-time or full-time employed; | 110 (78%) | |
| Unknown | 1 (1%) | |
| GOS-E at 3 months post-injury |
8 | 52 (39%) |
| 7 | 37 (27%) | |
| 6 | 24 (18%) | |
| 5 | 19 (14%) | |
| 4 | 2 (1%) | |
| 3 | 0 | |
| 2 | 0 | |
| 1 | 1 (1%) | |
S.D. – Standard deviation GCS – Glasgow Coma Scale LOC – Loss of consciousness PTA – Posttraumatic amnesia G.E.D. – Graduate Equivalency Degree GOS-E – Extended Glasgow Outcome Scale
Evaluation of CT and MRI studies according to TBI Common Data Elements
Each patient’s head CT upon ED presentation and early brain MRI (mean 12+/-3.9 days post-injury) were characterized using the TBI Common Data Elements (TBI-CDEs). The TBI-CDEs are consensus-based recommendations for data collection, data definitions, and best practices in TBI research established jointly by the National Institute of Neurological Disorders and Stroke, Defense Centers of Excellence, National Institute on Disability and Rehabilitation Research, and Veterans Administration.19, 20, 22 Each CT and MRI was reviewed by a board-certified neuroradiologist blinded to demographic and clinical data except gender and age; and without concurrent access to patients’ other imaging studies. CT and MR imaging parameters are in Supplementary Tables 2 and 3.
Outcome measure
The primary outcome measure was the 8-point Extended Glasgow Outcome Scale (GOS-E) at 3 months post-injury, obtained through structured interview with each participant by research assistants trained to uniformly assess the GOS-E. The GOS-E is a well-validated, widely employed summary assessment of global function after MTBI suitable for clinical trials. Prospective studies have shown that outcomes as determined by the GOS-E are strongly, consistently associated with outcome category on numerous alternative functional scales.24-28
Statistical analysis
We performed univariate and multivariate ordinal logistic regression of 3-month GOS-E upon clinical, demographic, socioeconomic, and imaging features using SPSS Statistics 19 (IBM, Chicago, IL). We used ordinal logistic regression, an extension of binary logistic regression to the case of an ordinal outcome variable,29 because it would not require arbitrary dichotomization of the ordinal 8-point GOS-E outcome measure. Such dichotomization of ordinal outcome variables discards valuable information and reduces statistical power to detect relationships between outcome and predictor variables, in some cases equivalent to discarding one-third of the data.30-32 All ordinal regression analyses employed a standard logit link function. All multivariate models satisfied standard tests for parallel lines, confirming the proportional odds assumption. To provide sensitivity and specificity measures for the multivariate models, we dichotomized the 3-month GOS-E into scores of 8 versus 7 and below, and calculated the receiver operating characteristics (ROC) for binary logistic regression analogs of the multivariate ordinal logistic regression models. We tested for statistically significant differences in area under the curve (AUC) for the different models using the method of DeLong et al.33
RESULTS
Categorization of CT and MRI studies: MRI demonstrates more traumatic intracranial lesions than CT
TBI-CDE-defined pathoanatomic features observed on initial head CT and early brain MRI consisted of: skull fracture, epidural hematoma, subdural hematoma, subarachnoid hemorrhage, brain contusion, traumatic axonal injury (TAI), and diffuse axonal injury (DAI). TAI and DAI are defined in the TBI-CDEs as 1 to 3 foci (TAI) and ≥4 foci (DAI) of axonal injury, respectively. TBI-CDE features expected to be more characteristic of moderate-to-severe TBI, including midline shift≥5 mm and partial/complete basal cistern effacement, were not observed in any patient in this MTBI population.
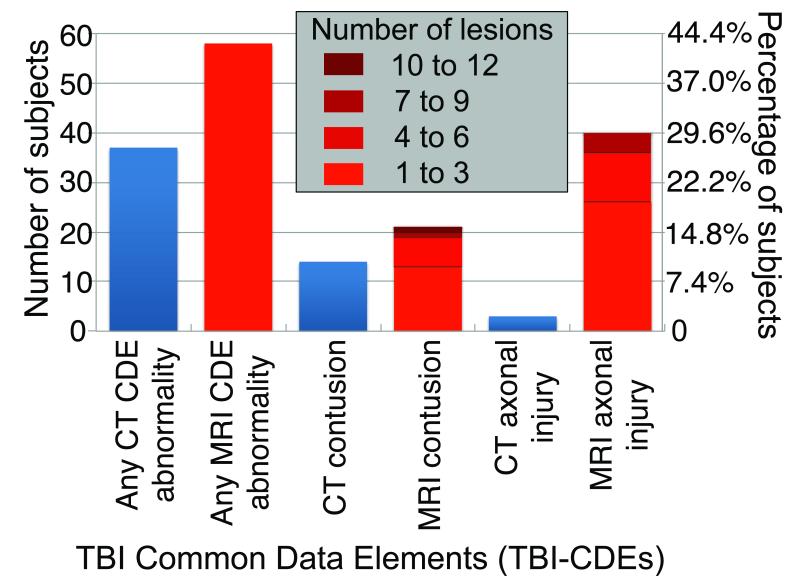
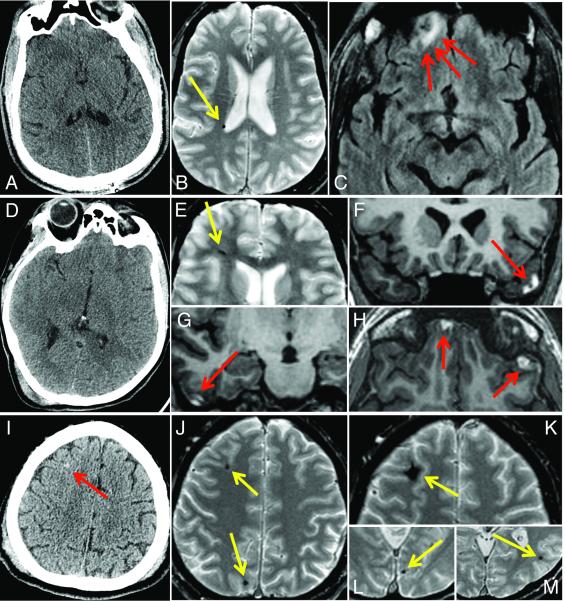
Figure 1 shows MRI identified many more acute traumatic intracranial lesions than CT. Of 135 study participants, 27% had abnormal head CT (31 with acute intracranial injury and 6 with isolated skull fracture). Of 98 patients without CT evidence of skull fracture or acute intracranial injury, 27 of 98 patients (28%) had abnormal MRI, including 23 patients with hemorrhagic axonal injury, 3 patients with brain contusions, and 4 patients with extraaxial hematomas. Figure 2 shows the more extensive pathology demonstrated by MRI compared to CT in three representative cases.
Figure 1.
Figure 2.
Univariate ordinal logistic regression: 3-month GOS-E versus age, gender, GCS, LOC/PTA, prior TBI, educational, employment status, CT and MRI TBI-CDEs
First, we performed univariate ordinal logistic regression to assess for associations between the 3-month GOS-E and 7 clinical and demographic/socioeconomic characteristics previously shown to be correlated with outcome in TBI. Participants with prior TBI resulting in acute medical evaluation, adults lacking high school diploma or equivalent, and unemployed adults had significantly worse outcomes at p≤0.05 (Table 2). In contrast, age, gender, GCS upon ED arrival, and LOC/PTA were not statistically significant univariate predictors at p≤0.05.
Table 2.
Univariate ordinal logistic regression of 3-month GOS-E upon clinical, demographic, socioeconomic, CT, and MRI predictors
| CLINICAL, DEMOGRAPHIC, AND SOCIOECONOMIC |
ADMISSION HEAD CT | EARLY MRI (12+/-3.9 days post-injury) |
||||||
|---|---|---|---|---|---|---|---|---|
| Predictor | Category (number of patients) |
Univariate odds ratio per unit decrease in GOS-E (95% CI, p-value) |
Predictor | Category | Univariate odds ratio per unit decrease in GOS-E (95% CI, p-value) |
Predictor | Category | Univariate odds ratio per unit decrease in GOS-E (95% CI, p-value) |
| Age | Years | 1.01 (0.99- 1.02 per year, p = 0.55) |
Skull fracture | Yes (15) | 1.3 (0.5-3.3, p = 0.64) |
Brain contusion |
One or more (21) |
3.5** (1.5-8.1, p = 0.004) |
| Gender | Female(38) | 0.9 (0.4-1.7, p = 0.66) |
Epidural hematoma |
Yes (3) | 1.6 (0.2-12.6, p = 0.64) |
Axonal injury |
≥4 foci (DAI) (14) |
3.0* (1.1-8.3, p = 0.03) |
| Male(97) | 1.0 (reference) |
Subdural hematoma |
Yes (18) | 2.0 (0.9-5.9, p = 0.12) |
1 to 3 foci (TAI) (26) |
1.1 (0.98-1.3, p = 0.11) |
||
| GCS (ED arrival) |
13 (3) | 2.6(0.3-20.5, p = 0.35) |
Subarachnoid hemorrhage |
Yes (17) | 2.5* (0.99-6.2, p = 0.05) |
None (95) | 1.0 (reference) |
|
| 14 (26) | 1.8 (0.8-4.0, p = 0.16) |
Contusion | Yes (14) | 2.5 (0.9-6.8, p = 0.07) |
||||
| 15 (106) | 1.0 (reference) |
Axonal injury | 1 to 3† foci (TAI) (3) |
0.9 (0.1-7.5, p=0.95) |
||||
| None(132) | 1.0 (reference) | |||||||
| LOC or PTA | Yes/ Suspected (108) |
0.9 (0.4-1.9, p = 0.77) |
Any one or more CT abnormalities |
Yes (37) | 1.4 (0.7-2.7, p = 0.35) |
|||
| Prior TBI resulting in acute medical evaluation |
Yes (45) | 2.4* (1.2-4.6, p=0.01) |
||||||
| Education | Adults ≥19 years old with less than diploma/ G.E.D. (12) |
3.2* (1.1-9.4, p=0.03) |
||||||
| Diploma/ G.E.D., bachelor’s, advanced degree(121) |
1.0 (reference) |
|||||||
| Employment status |
Unemployed (24) |
3.2**(1.4-7.2, p=0.005) |
||||||
| Part- or full-time employed; student; retiree (110) |
1.0 (reference) |
|||||||
Light-gray and dark-gray shaded boxes denote statistically significant univariate predictors at p ≤ 0.05 and p ≤ 0.01 respectively.
p ≤ 0.05
p ≤ 0.01 CI – confidence interval GOS-E – Extended Glasgow Outcome Scale GCS – Glasgow Coma Scale ED – Emergency Department LOC – Loss of consciousness PTA – Posttraumatic amnesia G.E.D. – Graduate equivalency degree TAI – Traumatic axonal injury (1 to 3 foci) DAI – Diffuse axonal injury (≥ 4 foci)
CT showed evidence of hemorrhagic axonal injury in 3 study participants, all with 1 to 3 foci of injury.
Next, we performed univariate ordinal logistic regression of 3-month GOS-E upon 8 TBI-CDE-defined types of pathoanatomic injuries observed in our study population (Table 2). Presence of one or more brain contusions on MRI was associated with a statistically significant reduction of 3-month GOS-E, with an odds ratio of 3.5 per unit decrease in 3-month GOS-E (p=0.004). Presence of ≥4 foci of hemorrhagic axonal injury on MRI was associated with a statistically significant odds ratio of 3.0 (p=0.03). Subarachnoid hemorrhage (SAH) on CT was associated with a statistically significant odds ratio of 2.5 (p=0.05). Abnormal head CT, defined as presence of any TBI-CDE abnormality (i.e., not stratified according to individual TBI-CDE pathoanatomic features), was not a statistically significant predictor (p=0.35).
To test for a main effect of patient recruitment site upon 3-month GOS-E, we performed the Kruskal-Wallis test, which demonstrated no statistically significant difference in 3-month GOS-E across the three recruitment sites, (χ2 =0.75, df = 2, p=0.69, 135 subjects). We also performed univariate ordinal logistic regression of 3-month GOS-E upon site, in which site was found to not be a statistically significant predictor (p=0.67).
Finally, to investigate the possibility of interactions among patient recruitment site and the predictors, logistic regression of 3-month GOS-E upon each predictor in Table 2 plus the interaction term, site*predictor, was performed. The odds ratios and significance levels for each interaction term, site*predictor, when added to the main effect of the predictor, were calculated. No interaction term, site*predictor, was statistically significant when added to the main effect of the predictor.
Multivariate ordinal logistic regression
Next, we evaluated three different multivariate models of the 3-month GOS-E, based on the following different sets of predictive variables: 1) clinical and demographic/socioeconomic features only, 2) clinical, demographic/socioeconomic, and head CT features, and 3) clinical, demographic/socioeconomic, head CT, and brain MRI features. In all three models, only clinical, demographic/socioeconomic, CT and MRI features that were statistically significant univariate predictors at p≤0.05 were included.
Model 1: Clinical and demographic/socioeconomic features only
Our simplest multivariate model was based solely on clinical and demographic/socioeconomic features that were statistically significant univariate predictors at p≤0.05. These were history of prior TBI, educational background, and employment status. This model was statistically significant (χ2=12.7, p=0.005), and explained between 9.5% (Cox and Snell pseudo-R2) and 10.2% (Nagelkerke pseudo-R2) of the variability in 3-month GOS-E (Table 3, Model 1).
Table 3.
Multivariate ordinal logistic regression of 3-month GOS-E upon clinical, demographic, socioeconomic, CT, and MRI predictors that are statistically significant univariate predictors at p ≤ 0.05
| Model | Predictor | Multivariate odds ratio of predictor |
Overall model significance |
Cox and Snell pseudo-R2 |
Nagelkerke pseudo-R2 |
|---|---|---|---|---|---|
| Model 1. Clinical and demographic/ socioeconomic predictors only |
Prior TBI resulting in acute medical evaluation |
1.8 (p=0.10) | p=0.005** | 9.5%** | 10.2%** |
| Adults ≥19 years old with less than diploma/G.E.D. |
2.6 (p=0.09) | ||||
| Unemployed | 2.4* (p=0.04) | ||||
| Model 2. Clinical, demographic/ socioeconomic, and CT predictors |
Prior TBI resulting in acute medical evaluation |
2.0 (p=0.07) | p = 0.0006*** | 14.4%*** | 15.3%*** |
| Adults ≥19 years old with less than diploma/G.E.D. |
2.7 (p=0.08) | ||||
| Unemployed | 2.6* (p=0.03) | ||||
| CT: Subarachnoid hemorrhage |
3.5* (p=0.01) | ||||
| Model 3. Clinical, demographic/ socioeconomic, CT, and MRI predictors |
Prior TBI resulting in acute medical evaluation |
2.0 (p=0.06) | p = 0.00005**** | 20.6%%**** | 21.9%**** |
| Adults ≥19 years old with less than diploma/G.E.D. |
3.2* (p=0.05) | ||||
| Unemployed | 2.9* (p=0.02) | ||||
| CT: Subarachnoid hemorrhage |
1.3 (p= 0.70) | ||||
| MRI: ≥1 contusion | 4.5** (p= 0.01) | ||||
| MRI: ≥4 foci axonal injury |
3.2* (p= 0.03) |
GOS-E – Extended Glasgow Outcome Scale G.E.D. – Graduate Equivalency Degree
p ≤ 0.05
p ≤ 0.01
p ≤ 0.001
p ≤ 0.0001
Model 2: Clinical, demographic/socioeconomic, and CT features
Next, we considered a multivariate model employing clinical, demographic/socioeconomic, and CT features that were statistically significant univariate predictors. The predictors in this model were therefore CT evidence of SAH, plus the clinical and socioeconomic predictors used in Model 1. Model 2 was statistically significant (χ2=19.7, p=0.0006), explaining between 14.4% (Cox and Snell pseudo-R2) and 15.3% (Nagelkerke pseudo-R2) of the variability in 3-month GOS-E (Table 3, Model 2). The strongest predictor of outcome in this model was CT evidence of SAH, with multivariate odds ratio of 3.5 (p=0.01).
Model 3: Clinical, demographic/socioeconomic, CT, and MRI features
Finally, we considered a more comprehensive multivariate model based on clinical, demographic/socioeconomic, CT and MRI features that were statistically significant univariate predictors at p≤0.05. These were MRI evidence of hemorrhagic axonal injury and MRI evidence of brain contusion, in addition to the CT, clinical, and socioeconomic predictors included in Model 2. This model (Table 3, Model 3) was highly statistically significant (χ2=29.3, p=0.00005), accounting for 20.6% (Cox and Snell pseudo-R2) to 21.9% (Nagelkerke pseudo-R2) of the variability in 3-month GOS-E, a greater than two-fold increase from Model 1 that was based solely on clinical and socioeconomic features. The strongest predictor of poor outcome in Model 3 was presence of one or more brain contusions, with a multivariate odds ratio of 4.5 (p=0.01). This indicates that patients with one or more brain contusions were 4.5 times more likely to have 3-month GOS-E≤7 than those without brain contusion. Presence of ≥4 foci of axonal injury was also a statistically significant predictor in this model, with multivariate odds ratio of 3.2 (p=0.03) per unit reduction of 3-month GOS-E.
In Model 3, the odds ratio for CT evidence of SAH dropped to 1.3 from its univariate value of 2.5, and it was no longer statistically significant (p=0.70 as a multivariate predictor in Model 3, compared to p=0.05 as univariate predictor). This raised suspicion for collinearity between CT evidence of SAH and one or more other predictors. We therefore performed Spearman correlation analysis of the predictors (Supplementary Table 4). This indeed revealed a strong, highly significant correlation (ρ=0.58, p=3×10-13) between MRI evidence of contusion and CT evidence of SAH. In contrast, hemorrhagic axonal injury demonstrated only weak correlation with other imaging features. No imaging feature demonstrated significant correlation with demographic, clinical or socioeconomic predictors. Of note, number of foci of hemorrhagic axonal injury on MRI was not significantly correlated with age or history of prior TBI, and thus unlikely to be mostly attributable to old TBI, hypertensive vasculopathy, or cerebral amyloid angiopathy, rather than the acute TBI event leading to participation in the current study. Finally, weak correlations among history of prior TBI, education, and employment status were seen, accounting for the slight drop in odds ratios for these features in the multivariate models (Table 3) compared to their univariate odds ratios (Table 2).
ROC analysis
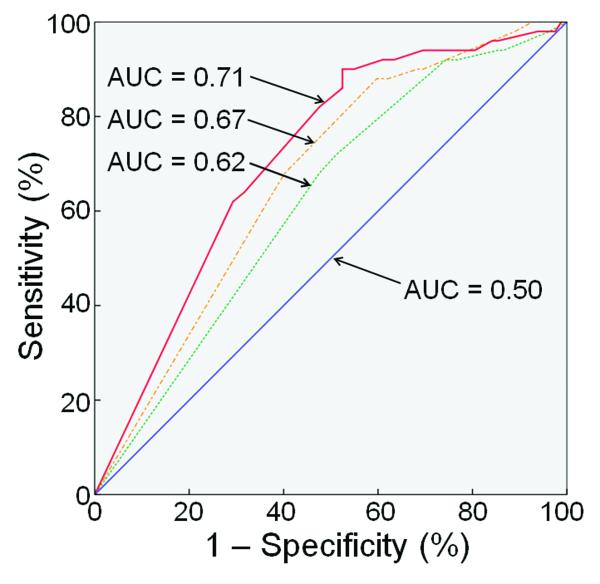
Figure 3 shows ROCs for binary logistic regression analogs of the multivariate ordinal logistic regression models from Table 3, for a dichotomization of the 3-month GOS-E into scores of 8 versus 7 and below. As expected from the results in Table 3, Figure 3 shows that the AUC increases progressively for Models 1, 2 and 3. Dichotomization of the GOS-E into scores 7 and 8 versus 6 and below, and scores 6 to 8 versus 5 and below, yielded qualitatively similar results.
Figure 3.
All three binary logistic models in Figure 3 were significantly superior to random guessing at p≤0.05 (Model 1, AUC = 0.620, p=0.02; Model 2, AUC = 0.669, p=0.001; Model 3, AUC=0.712, p=0.00005). Using the method of Delong et al.,33 the AUC for Model 3 significantly exceeded the AUC for Model 2 (p = 0.04) and the AUC for Model 1 (p = 0.007). The AUC for Model 2 was not significantly greater than that for Model 1 at p≤0.05, though there was a trend toward significance (p=0.10); in light of the results in Table 3, this may be attributable to the loss of ordinal information contained in the GOS-E, and decreased power of the binary models.
DISCUSSION
There is evidence that a subset of MTBI patients have significant alterations in neuropsychiatric functioning within weeks to months of injury, and approximately 15% have measurable deficits at 1 year.5-9,34 There is also growing recognition that current classification schemes for TBI based on GCS are severely limited, with small mean effect sizes in long-term impairment potentially obscuring differences among diverse subgroups of TBI patients with very different prognoses.21, 35
To date, no consensus exists on the clinical relevance, if any, of traumatic focal lesions on brain imaging studies in MTBI. Regarding CT, most studies have demonstrated a correlation between intracranial hemorrhage on admission head CT and acute and long-term neuropsychiatric deficits in MTBI,34, 36, 37 while a few studies have found no correlation,38 only a weak correlation far outweighed by demographic factors,39 or even a better long-term outcome associated with intracranial hemorrhage.40
Regarding MRI, it has been shown that MRI at both 1.5 and 3 Tesla has far superior sensitivity to CT for small, focal traumatic intracranial lesions in TBI.6,41-44 However, no consistent relationship between such lesions and long-term outcome in MTBI has been demonstrated. For example, a study of focal intracranial lesions in MTBI using 3 Tesla MRI found that MTBI patients performed significantly worse on acute neurocognitive tests, with milder but detectable deficits at 1 year.6 However, there was no significant correlation between focal intracranial lesions on conventional MRI sequences and neurocognitive deficits at any time point (2 weeks, 1 month, and 1 year post-injury). Another study showed no difference between MTBI patients with normal versus abnormal MRI, on the Rivermead Postconcussion Symptoms Questionnaire or in return-to-work status, at 6 months post-injury.12 A third study showed correlation between outcome at 5-18 months with evidence of brain atrophy on late MRI, but little or no relationship with traumatic intracranial lesions on early CT or MRI.15 Other studies demonstrating a correlation between intracranial MRI findings and intermediate-to-long-term outcome in mild-to-severe14, 45 or moderate-to-severe TBI46, 47 did not adjust for important, previously validated outcome predictors in moderate-to-severe TBI,48, 49 including age, GCS, pupillary reactivity, and admission head CT features; thus, the differential predictive power of MRI was unknown. Finally, advanced MRI techniques including diffusion tensor imaging and resting-state functional MRI hold great promise for characterization and outcome prediction in MTBI;50-52 although group differences between MTBI patients and controls have been demonstrated, no consensus yet exists on the practical application of these techniques to outcome prediction in the individual patient.
That MTBI patients with abnormalities on MRI have poorer outcomes is not especially surprising, as “complicated” MTBI (usually defined as acute intracranial hemorrhage on head CT, with skull fracture also included by some researchers) has been associated with poorer outcome in several prior studies.34,36,37 What is unique about this study is the greater specification of types of lesions that may be predictive, the control for other predictors, the careful use of the TBI-CDEs to categorize the imaging findings, and the multicenter nature of the patient sample. We redemonstrate the exquisite sensitivity of MRI for small cortical contusions and hemorrhagic axonal injury, and show for the first time that such MRI features improve MTBI outcome prediction after controlling for demographic/socioeconomic, clinical, and CT features. The addition of both CT and MRI pathoanatomic features of SAH, contusion and hemorrhagic axonal injury to a prognostic model of MTBI based on demographic/socioeconomic and clinical predictors alone results in a doubling of the explained variance in 3-month GOS-E.
Our results agree with prior work4, 17, 18 that demonstrated the influence of socioeconomic factors on outcome in MTBI. Although we did not confirm age as a statistically significant predictor of outcome (odds ratio 1.01/year, 95% confidence interval 0.99-1.02/year, Table 2), this may be attributable to the smaller number of patients in our study (135 patients) compared, for example, to a recent study of 2,784 MTBI patients that demonstrated a mild age effect (odds ratio 1.02/year) on long-term outcome.39 Our finding that specific imaging markers are stronger predictors in MTBI than demographic factors such as age is a new finding.
The finding that CT evidence of SAH and MRI evidence of contusion are strongly correlated suggests they are mechanistically associated, in contrast to MRI evidence of axonal injury, which was only weakly correlated with the other two imaging features. It is interesting to recall Strich’s53, 54 and Holbourn’s55 theoretical work, supported by postmortem observations56, 57 and experiments by Gennarelli,57 which showed that traumatic axonal injuries result from rotational acceleration and ensuing shear-strain deformation at interfaces between tissues of different density (e.g., gray/white matter), in contradistinction to contusions, which were attributed to a different mechanism – transient, sudden inbending of the skull with direct impact to the brain surface.58 Our results support these different mechanisms for axonal injuries and contusions, and furthermore suggest the latter mechanism also causes SAH.
Our multicenter study follows a cohort of 135 MTBI patients with highly diverse socioeconomic backgrounds and few exclusion criteria. Our approach is thus distinct from studies that have stringently excluded patients with potential confounding influences on outcome, such as history of prior head injury or advanced age. Although such studies are important, the high incidence of these features in the general population, and even greater incidence in those at high risk for TBI, may severely limit the generalizability of results from such studies. Our results on a natural cross section of MTBI patients at three Level I trauma centers is complementary to such highly controlled studies. We analyzed factors across a range of domains, including socioeconomic, clinical, and demographic factors, using a truly multivariate approach in order to mitigate any spurious inferences of causality between outcome and any single predictive feature.
Triage of patients to undergo head CT was an inclusion criterion at all 3 enrollment sites. The 2008 ACEP/CDC evidence-based practice guideline (Supplementary Table 1),23 incorporating both Canadian CT Head Rule59 and New Orleans Criteria,60 is applied by many ED physicians. However, there is undoubtedly variation in the practical application of these criteria. A major strength of our study is recruitment at geographically diverse Level I trauma centers, which affords a better cross-sectional representation of average criteria employed across different hospitals. Our results should be viewed as relevant primarily to MTBI patients who meet ACEP/CDC ED criteria for head CT, and who thus generally have more severe injuries than MTBI patients who are not triaged to head CT. This is reflected in the 80% rate of LOC/PTA and 27% positive CT rate in our study population.
We emphasize that many patients with abnormal MRI findings nonetheless have good outcomes, with 27% of patients with one or more brain contusions and/or at least one focus of hemorrhagic axonal injury demonstrating a 3-month GOS-E of 8 (“upper good recovery”) and another 28% demonstrating a 3-month GOS-E of 7 (“lower good recovery”) (Supplementary Figure 3). We have shown that this is due in part to the contributions of predictors in other domains that may mitigate the negative effects of a structural brain injury on outcome after MTBI. It is also likely at least partly attributable to imperfect sensitivity of the GOS-E for subtle dysfunction.
In this study, identification of individual pathoanatomic features and their relationship to outcome constitutes progress toward evidence-based classification of injury severity, with improved categorization of diverse subgroups within the traditional “MTBI” population. We show for the first time that traumatic intracranial findings on conventional CT and MRI account for a significant portion of the variability in outcome in MTBI. Routine performance of brain MRI on MTBI patients may not currently be cost-effective. However, smaller, less-costly head-only MRI scanners are under development. These among other continuing advances in MRI technology may ultimately render the expense and logistics of acute MRI scans less prohibitive. Finally, our results are a step toward standardized reporting of pathoanatomic features, employing the TBI-CDEs. Such standardization is a key prerequisite for progress in this field beyond mere definition of MTBI, toward evidence-based diagnosis based on proven correlations of objective biomarkers with patient outcome.19-22
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grant NS069409.
APPENDIX. TRACK-TBI INVESTIGATORS
Scott S. Casey, B.A. (Brain and Spinal Injury Center, and Department of Neurosurgery, University of California, San Francisco, CA), Maxwell Cheong, B.S. (Department of Radiology and Biomedical Imaging, University of California, San Francisco, CA), Shelly R. Cooper, B.A. (Brain and Spinal Injury Center, and Department of Neurosurgery, University of California, San Francisco, CA), Kristen Dams-O’Connor, Ph.D. (Department of Rehabilitation Medicine, Mount Sinai School of Medicine, New York, NY), Allison J. Hricik, M.S. (Department of Psychology, University of Texas, Austin, TX), Tomoo Inoue, M.D., Ph.D. (Brain and Spinal Injury Center, and Department of Neurosurgery, University of California, San Francisco, CA), Emily E. Knight, B.S. (Department of Psychology, University of Texas, Austin, TX), Kerri Lawless, R.N. (Department of Neurological Surgery and Neurotrauma Clinical Trials Center, University of Pittsburgh Medical Center, Pittsburgh, PA), David K. Menon, M.D., Ph.D. (Division of Anaesthesia, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK), Jennifer L. Pacheco, Ph.D. (Department of Psychology, University of Texas, Austin, TX), Ava M. Puccio, R.N., Ph.D. (Department of Neurological Surgery and Neurotrauma Clinical Trials Center, University of Pittsburgh Medical Center, Pittsburgh, PA), Tuhin K. Sinha, Ph.D. (Department of Radiology and Biomedical Imaging, University of California, San Francisco, CA), and Mary J. Vassar, R.N., M.S. (Brain and Spinal Injury Center, and Department of Neurosurgery, University of California, San Francisco, CA)
REFERENCES
- 1.Mild Traumatic Brain Injury Committee Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86–7. [Google Scholar]
- 2.National Center for Injury Prevention and Control Report to Congress on mild traumatic brain injury in the United States: Steps to prevent a serious public health problem. Centers for Disease Control and Prevention; Atlanta, GA. 2003. [Google Scholar]
- 3.Carroll LJ, Cassidy JD, Holm L, Kraus J, Coronado VG, Injury WCCTFoMTB Methodological issues and research recommendations for mild traumatic brain injury: The WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43(Suppl.):113–25. doi: 10.1080/16501960410023877. [DOI] [PubMed] [Google Scholar]
- 4.Carroll LJ, Cassidy JD, Peloso PM, et al. Prognosis for mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43(Suppl.):84–105. doi: 10.1080/16501960410023859. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein DM. Recovery from mild head injury. Brain Inj. 1999;13(3):151–72. doi: 10.1080/026990599121683. [DOI] [PubMed] [Google Scholar]
- 6.Lee H, Wintermark M, Gean AD, Ghajar J, Manley GT, Mukherjee P. Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J Neurotrauma. 2008;25(9):1049–56. doi: 10.1089/neu.2008.0566. [DOI] [PubMed] [Google Scholar]
- 7.Hessen E, Nestvold K. Indicators of complicated mild TBI predict MMPI-2 scores after 23 years. Brain Inj. 2009;23(3):234–42. doi: 10.1080/02699050902748349. [DOI] [PubMed] [Google Scholar]
- 8.Thornhill S, Teasdale GM, Murray GD, McEwen J, Roy CW, Penny KI. Disability in young people and adults one year after head injury: prospective cohort study. BMJ. 2000;320(7250):1631–5. doi: 10.1136/bmj.320.7250.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dikmen S, Machamer J, Fann JR, Temkin NR. Rates of symptom reporting following traumatic brain injury. J Int Neuropsychol Soc. 2010;16(3):401–11. doi: 10.1017/S1355617710000196. [DOI] [PubMed] [Google Scholar]
- 10.Borg J, Holm L, Peloso PM, et al. Non-surgical intervention and cost for mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on mild traumatic brain injury. J Rehabil Med. 2004;43(Suppl.):76–83. doi: 10.1080/16501960410023840. [DOI] [PubMed] [Google Scholar]
- 11.Ponsford J, Willmott C, Rothwell A, et al. Impact of early intervention on outcome following mild head injury in adults. J Neurol Neurosurg Psychiatry. 2002;73(3):330–2. doi: 10.1136/jnnp.73.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes DG, Jackson A, Mason DL, Berry E, Hollis S, Yates DW. Abnormalities on magnetic resonance imaging seen acutely following mild traumatic brain injury: correlation with neuropsychological tests and delayed recovery. Neuroradiology. 2004;46:550–8. doi: 10.1007/s00234-004-1227-x. [DOI] [PubMed] [Google Scholar]
- 13.Scheid R, Preul C, Gruber O, Wiggins C, von Cramon DY. Diffuse axonal injury associated with chronic traumatic brain injury: Evidence from T2*-weighted gradient-echo imaging at 3 T. AJNR Am J Neuroradiol. 2003;24:1049–56. [PMC free article] [PubMed] [Google Scholar]
- 14.Scheid R, Walther K, Guthke T, Preul C, von Cramon DY. Cognitive sequelae of diffuse axonal injury. Arch Neurol. 2006;63:418–24. doi: 10.1001/archneur.63.3.418. [DOI] [PubMed] [Google Scholar]
- 15.Wilson JT, Wiedmann KD, Hadley DM, Condon B, Teasdale G, Brooks DM. Early and late magnetic resonance imaging and neuropsychological outcome after head injury. J Neurol Neurosurg Psychiatry. 1988;51(3):391–6. doi: 10.1136/jnnp.51.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bazarian JJ, McClung J, Cheng YT, Flesher W, Schneider SM. Emergency department management of mild traumatic brain injury in the USA. Emerg Med J. 2005;22:473–7. doi: 10.1136/emj.2004.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dikmen S, Machamer J, Temkin N. Mild head injury: facts and artifacts. J Clin Exp Neuropsychol. 2001;23:729–38. doi: 10.1076/jcen.23.6.729.1019. [DOI] [PubMed] [Google Scholar]
- 18.Iverson GL. Outcome from mild traumatic brain injury. Curr Opinion Psychiatry. 2005;18:301–17. doi: 10.1097/01.yco.0000165601.29047.ae. [DOI] [PubMed] [Google Scholar]
- 19.Duhaime AC, Gean AD, Haacke EM, et al. Common data elements in radiologic imaging of traumatic brain injury. Arch Phys Med Rehabil. 2010;91(11):1661–6. doi: 10.1016/j.apmr.2010.07.238. [DOI] [PubMed] [Google Scholar]
- 20.Haacke EM, Duhaime AC, Gean AD, et al. Common data elements in radiologic imaging of traumatic brain injury. J Magn Reson Imaging. 2010;32(3):516–43. doi: 10.1002/jmri.22259. [DOI] [PubMed] [Google Scholar]
- 21.Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719–38. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whyte J, Vasterling J, Manley GT. Common data elements for research on traumatic brain injury and psychological health: current status and future development. Arch Phys Med Rehabil. 2010;91(11):1692–6. doi: 10.1016/j.apmr.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Jagoda AS, Bazarian JJ, Bruns JJ, et al. Clinical policy: Neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann Emerg Med. 2008;52(6):714–48. doi: 10.1016/j.annemergmed.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Nichol AD, Higgins AM, Gabbe BJ, Murray LJ, Cooper DJ, Cameron PA. Measuring functional and quality of life outcomes following major head injury: Common scales and checklists. Injury. 2011;42(3):281–7. doi: 10.1016/j.injury.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 25.Wilson J, Pettigrew L, Teasdale G. Emotional and cognitive consequences of head injury in relation to the Glasgow outcome scale. J Neurol Neurosurg Psychiatry. 2000;69(2):204–9. doi: 10.1136/jnnp.69.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin HS, Boake C, Song J, et al. Validity and sensitivity to change of the Extended Glasgow Outcome Scale in Mild to Moderate Traumatic Brain Injury. J Neurotrauma. 2004;18(6):575–84. doi: 10.1089/089771501750291819. [DOI] [PubMed] [Google Scholar]
- 27.Hudak AM, Caesar RR, Frol AB, et al. Functional outcome scales in traumatic brain injury: a comparison of the Glasgow Outcome Scale (Extended) and the Functional Status Examination. J Neurotrauma. 2005;22(11):1319–26. doi: 10.1089/neu.2005.22.1319. [DOI] [PubMed] [Google Scholar]
- 28.Shukla D, Devi I, Agrawal A. Outcome measures for traumatic brain injury. Clin Neurol Neurosurg. 2011;113(6):435–41. doi: 10.1016/j.clineuro.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Kleinbaum DG, Klein M. Ordinal logistic regression. Logistic Regression. 3rd ed Springer; New York: 2010. pp. 463–88. [Google Scholar]
- 30.Roozenbeek B, Lingsma HF, Perel P, et al. The added value of ordinal analysis in clinical trials: an example in traumatic brain injury. Crit Care. 2011;15(3):R127. doi: 10.1186/cc10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maas AIR, Steyerberg EW, Marmarou A, et al. IMPACT recommendations for improving the design and analysis of clinical trials in moderate to severe traumatic brain injury. Neurotherapeutics. 2010;7(1):127–34. doi: 10.1016/j.nurt.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332:1080. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 34.Kashluba S, Hanks RA, Casey JE, Millis SR. Neuropsychologic and functional outcome after complicated mild traumatic brain injury. Arch Phys Med Rehabil. 2008;89:904–11. doi: 10.1016/j.apmr.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 35.Iverson GL. Mild traumatic brain injury meta-analyses can obscure individual differences. Brain Inj. 2010;24:1246–55. doi: 10.3109/02699052.2010.490513. [DOI] [PubMed] [Google Scholar]
- 36.Sadowski-Cron C, Schneider J, Senn P, Radanov BP, Ballinari P, Zimmermann H. Patients with mild traumatic brain injury: immediate and long-term outcome compared to intracranial injuries on CT scan. Brain Inj. 2006;20:1131–7. doi: 10.1080/02699050600832569. [DOI] [PubMed] [Google Scholar]
- 37.Williams DH, Levin HS, Eisenberg HM. Mild head injury classification. Neurosurgery. 1990;27:422–8. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- 38.McCauley SR, Boake C, Levin HS, Contant CF, Song JX. Postconcussional disorder following mild to moderate traumatic brain injury: Anxiety, depression, and social support as risk factors and comorbidities. J Clin Exp Neuropsychol. 2001;23:792–808. doi: 10.1076/jcen.23.6.792.1016. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs B, Beems T, Stulemeijer M, et al. Outcome prediction in mild traumatic brain injury: age and clinical variables are stronger predictors than CT abnormalities. J Neurotrauma. 2010;27:655–68. doi: 10.1089/neu.2009.1059. [DOI] [PubMed] [Google Scholar]
- 40.Zumstein MA, Moser M, Mottini M, et al. Long-term outcome in patients with mild traumatic brain injury: A prospective observational study. J Trauma. 2011;71:120–7. doi: 10.1097/TA.0b013e3181f2d670. [DOI] [PubMed] [Google Scholar]
- 41.Gentry LR, Godersky JC, Thompson B, Dunn VD. Prospective comparative study of intermediate-field MR and CT in the evaluation of closed head trauma. AJR Am J Roentgenol. 1988;150:673–82. doi: 10.2214/ajr.150.3.673. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins A, Hadley MDM, Teasdale G, Macpherson P, Rowan JO. Brain lesions detected by magnetic resonance imaging in mild and severe head injuries. Lancet. 1986;2(8504):445–6. doi: 10.1016/s0140-6736(86)92145-8. [DOI] [PubMed] [Google Scholar]
- 43.Mittl RL, Grossman RI, Hiehle JF, et al. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR Am J Neuroradiol. 1994;15:1583–9. [PMC free article] [PubMed] [Google Scholar]
- 44.Orrison WW, Gentry LL, Stimac GK, Tarrel RM, Espinosa MC, Cobb LC. Blinded comparison of cranial CT and MRI in closed head injury evaluation. AJNR Am J Neuroradiol. 1994;15:351–6. [PMC free article] [PubMed] [Google Scholar]
- 45.Chastain CA, Oyoyo UE, Zipperman M, et al. Predicting outcomes of traumatic brain injury by imaging modality and injury distribution. J Neurotrauma. 2009;26:1183–96. doi: 10.1089/neu.2008.0650. [DOI] [PubMed] [Google Scholar]
- 46.Mannion RJ, Cross J, Bradley P, et al. Mechanism-based MRI classification of traumatic brainstem injury and its relationship to outcome. J Neurotrauma. 2007;24:128–35. doi: 10.1089/neu.2006.0127. [DOI] [PubMed] [Google Scholar]
- 47.van der Naalt J, Hew JM, van Zomeren AH, Sluiter WJ, Minderhous JM. Computed tomography and magnetic resonance imaging in mild to moderate head inury: Early and late imaging related to outcome. Ann Neurol. 1999;46:70–8. doi: 10.1002/1531-8249(199907)46:1<70::aid-ana11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 48.Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: Development and international validation of prognostic scores based on admission characteristics. PLOS Med. 2008;5:1251–61. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marshall LF, Eisenberg HM, Jane JA, Marshall SB, Klauber MR. A new classification of head injury based on computerized tomography. J Neurosurgery. 1991;75:S14–S20. [Google Scholar]
- 50.Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: A pilot study. J Neurotrauma. 2007;24:1447–59. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- 51.Niogi SN, Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil. 2010;25:241–55. doi: 10.1097/HTR.0b013e3181e52c2a. [DOI] [PubMed] [Google Scholar]
- 52.Van Boven RW, Harrington GS, Hackney DB, et al. Advances in neuroimaging of traumatic brain injury and posttraumatic stress disorder. J Rehabil Res Dev. 2009;46:717–57. doi: 10.1682/jrrd.2008.12.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strich SJ. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J Neurol Neurosurg Psychiatry. 1956;19:163–85. doi: 10.1136/jnnp.19.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strich SJ. Shearing of nerve fibers as a cause of brain damage due to head injury, a pathological study of twenty cases. Lancet. 1961;2:443–8. [Google Scholar]
- 55.Holbourn AHS. Mechanics of head injuries. Lancet. 1943;2:438–41. [Google Scholar]
- 56.Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to non-missile head injury in humans: an analysis of 45 cases. Ann Neurol. 1982;12:557–63. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- 57.Adams JH, Gennarelli TA, Graham DI. In: Brain damage in non-missile head injury: observations in man and subhuman primates. Smith WT, Cavanaugh JB, editors. Churchill Livingston; Edinburgh: 1982. [Google Scholar]
- 58.Gennarelli TA. Mechanisms of brain injury. J Emerg Med. 1993;11(Suppl. 1):5–11. [PubMed] [Google Scholar]
- 59.Stiell IG, Wells GA, Vandemheen K, et al. The Canadian CT Head Rule for patients with minor head injury. Lancet. 2001;357:1391–6. doi: 10.1016/s0140-6736(00)04561-x. [DOI] [PubMed] [Google Scholar]
- 60.Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PM. Indications for computed tomography in patients with minor head injury. New Engl J Med. 2000;343:100–5. doi: 10.1056/NEJM200007133430204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.