Abstract
Background
Cigarette smoke-induced chronic obstructive pulmonary disease (COPD) is a life-threatening inflammatory disorder of the lung. The development of effective therapies for COPD has been hampered by the lack of an animal model that mimics the human disease in a short time-frame.
Objectives
To create an early onset mouse model of cigarette smoke-induced COPD that develops the hallmark features of the human condition in a short time-frame. To use this model to better understand pathogenesis and the roles of macrophages and mast cells (MCs) in COPD.
Methods
Tightly controlled amounts of cigarette smoke were delivered to the airways of mice, and the development of the pathological features of COPD was assessed. The roles of macrophages and MC tryptase in pathogenesis were evaluated using depletion and in vitro studies and MC protease-6 deficient mice.
Results
After just 8 weeks of smoke exposure, wild-type mice developed chronic inflammation, mucus hypersecretion, airway remodeling, emphysema, and reduced lung function. These characteristic features of COPD were glucocorticoid-resistant and did not spontaneously resolve. Systemic effects on skeletal muscle and the heart, and increased susceptibility to respiratory infections also were observed. Macrophages and tryptase-expressing MCs were required for the development of COPD. Recombinant MC tryptase induced pro-inflammatory responses from cultured macrophages.
Conclusion
A short-term mouse model of cigarette smoke-induced COPD was developed in which the characteristic features of the disease were induced more rapidly than existing models. The model can be used to better understand COPD pathogenesis, and we show a requirement for macrophages and tryptase-expressing MCs.
Keywords: cigarette smoke, COPD, inflammation, emphysema, airway remodeling, lung function, macrophage, mast cell, protease, mMCP-6, hTryptase-β
INTRODUCTION
Cigarette smoke-induced chronic obstructive pulmonary disease (COPD) is a debilitating disorder of the lung. It is the 4th leading cause of chronic morbidity and death worldwide, and its prevalence is increasing.1 The disease is characterized by chronic airway inflammation, mucus hypersecretion, airway remodeling, and emphysema that lead to reduced lung function and breathlessness.2,3 Systemic effects also are observed in the skeletal muscle, heart, and other organs. Moreover, COPD patients are more susceptible to respiratory infections. Because the mechanisms that lead to COPD and its sequelae are poorly understood at the molecular level, there are no effective treatments.
A major factor that has hampered the study of COPD is the lack of a small animal model that recapitulates the hallmark features of the disease in a reasonable time frame. While lipopolysaccharide and elastase have been used to induce lung damage in rodents that somewhat resemble COPD in humans, such single-factor approaches are not representative of the complex pathology that occurs in those patients who smoke for many years.3 Current models of smoke-induced COPD involve whole body or nose-only exposure of mice to cigarette smoke.3 Acute models of 4 days to 4 weeks duration have been valuable for evaluating the early smoke-induced inflammatory responses in the lung. However, the smoke-exposed mice in these models do not develop emphysema or have diminished lung function.4–8 Chronic models of >6 months duration result in airway remodeling and emphysema, but only induce mild alterations in lung function,4,9–12 and the prolonged time needed to induce these features greatly restricts the use of these models for extensive therapeutic and mechanistic research. This is especially relevant considering the short life-span of the mouse and the substantial animal and labor expenses needed for 6-month experiments. Thus, there is a need for a mouse model of cigarette smoke-induced COPD that has all of the major features of the human condition that are induced in a shorter time frame.
Although mast cells (MCs) have been casually linked to the pathogenesis of COPD, the relevant factors exocytosed from these immune cells have not been identified.13 MC numbers are increased in inflammatory infiltrates in COPD patients, which is associated with reduced lung function, airway remodeling and emphysema.14,15 The levels of MC-derived human (h)Tryptase-β in sputum correlated with the severity of COPD in one study,16 and the exposure of IL-3-dependent MCs to cigarette smoke-treated culture medium resulted in increased expression of mouse MC protease-6 (mMCP-6).17 mMCP-6 is also known to promote inflammation, chemokine expression and macrophage and neutrophil chemotaxis,18 which are all hallmark features of COPD. Nevertheless, the roles of hTryptase-β and mMCP-6 in COPD pathogenesis have not been investigated in depth.
Here we report the development of a mouse model of COPD in which we deliver tightly controlled amounts of cigarette smoke directly into the airways. The exposed mice exhibit the major characteristic features of COPD observed in humans after only 8 weeks of smoke exposure, thereby facilitating the discovery and/or testing of the efficacy of new therapeutics. The model also enables us to elucidate the cellular, biochemical and molecular mechanisms that underpin the pathogenesis of COPD. In that regard, we now show for the first time detrimental roles for a MC-restricted tetramer-forming tryptase in experimental COPD.
METHODS
Additional details are described in the Journal’s Online Repository at www.jacionline.org.
Smoke exposure
Wild-type (WT) BALB/c, WT C57BL/6 (B6), and mMCP-6−/− B6 mice18 were used in the study. In each experiment, 12 mice were simultaneously exposed to cigarette smoke [twelve 3R4F reference cigarettes (University of Kentucky, Lexington, Ky) twice/day, 5 times/week, for 1–12 weeks] using a custom-designed and purpose-built nose-only, directed flow inhalation and smoke-exposure system (CH Technologies, Nj) housed in a fume and laminar flow hood (Fig S1). Each exposure lasted 75 minutes. All experiments were approved by our institutional animal ethics committee.
Airway and lung inflammation, airway remodeling, and emphysema
Airway inflammation was assessed by differential enumeration of inflammatory cells in bronchoalveolar lavage fluid (BALF).19–22 Parenchymal inflammation was assessed by counting the inflammatory cells in 10 randomized fields (x100 magnification) of whole lung sections.23 RNA was extracted and transcript levels were assessed by standard real-time quantitative (q)PCR assays,24 using the primers described in Table S1.
The numbers of macrophages and MCs in lung homogenates were assessed by flow cytometry and histochemistry.25–27 Airway remodeling was determined by measuring the number of mucus-expressing goblet cells around the airways and by assessing airway epithelium thickening.22,23,28–30 Emphysema was assessed using the mean linear intercept technique, which is a standard method of assessing alveolar diameter and emphysema in mice.28
Lung function
Forced oscillation and forced maneuver techniques were used to assess lung function parameters.25,31
Glucocorticoid treatment
Dexamethasone (1 mg/kg in 50 µL sterile water, Sigma, St Louis, MO) was administered intranasally 3 times/week.32 Controls were sham-treated with sterile water.
Respiratory infections
Mice were infected with mouse-adapted strains of Streptococcus pneumoniae intratracheally or influenza virus intranasally. Pathogen load was determined by culture or plaque assays of lung homogenates, respectively.33–36
Macrophage and neutrophil depletion
Lung macrophages and neutrophils were depleted by intranasal administration of liposome-encapsulated clodronate or intraperitoneal injection of anti-Ly6G antibody (1A8, Bioxcell, Lebannon, Nh), respectively, 3 times/week.25,37
Transcript expression in tryptase-treated macrophages
B6 mouse bone marrow-derived macrophages were cultured in the absence or presence of recombinant hTryptase-β (0.8 µg/ml, 25 nM, Promega, Madison, Wi). RNA was isolated, and qPCR assays were used to evaluate the levels of tumor necrosis factor-α (TNF-α), Cxcl1/KC, and interleukin (IL)-1β transcripts.
Statistical analyses
Data are presented as mean±SEM (n=6–8). Comparisons between two groups were made using a two-tailed Mann-Whitney Test. Multiple comparisons were made by one-way ANOVA with Tukey’s post-test, or Kruskal-Wallis with Dunn’s post-test, where non-parametric analyses were appropriate. Weights were assessed using one-way ANOVA (repeated measures). Analyses used GraphPad Prism Software (San Diego, CA).
RESULTS
Nose-only exposure of WT BALB/c mice to cigarette smoke induces the hallmark features of COPD
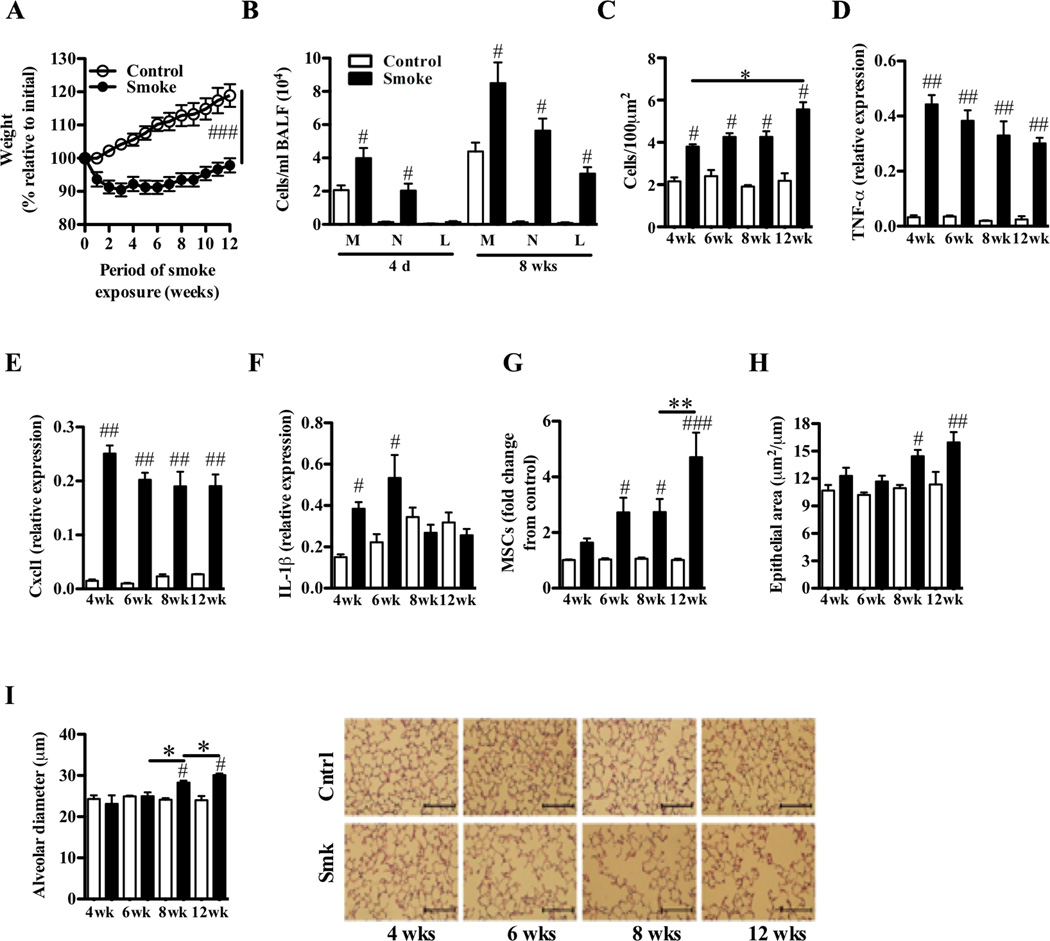
We delivered tightly controlled amounts of cigarette smoke into the nares of WT BALB/c mice for 1–12 weeks and assessed the hallmark features of COPD. Weight loss was evident within the 1st week of exposure (Fig 1, A). Animals lost 10% of their initial weight after 3 weeks, and only re-gained 5% of this initial weight over the remaining exposure period. In contrast, age-matched non-exposed mice steadily gained weight. Four days of exposure to cigarette smoke led to acute inflammation in the airways, characterized by increased numbers of macrophages and neutrophils in the BALF (Fig 1, B). Inflammation persisted and increased with the additional involvement of lymphocytes (particularly CD8+ T cells) after 8-weeks. Smoke exposure also induced progressive increases in chronic parenchymal inflammation (Fig 1, C). This was accompanied by increased expression in the lung of the transcripts that encode TNF-α (Fig 1, D), Cxcl1 (Fig 1, E), and IL-1β (Fig 1, F), but not IL-6, IL-10, IL-13, or interferon-γ (IFN-γ) (data not shown). There were signs of airway remodeling with increased numbers of MSCs in the airways from week 6 and thickening of the airway epithelium from week 8 (Fig 1, G and H). Airway remodeling was accompanied by alveolar enlargement (increases in alveolar diameter, representative of emphysematous tissue destruction) after 8 weeks, which increased in severity by week 12 (Fig 1, I). Although there likely was a steady continuum of pathologic changes taking place in the smoke-exposed lungs of the WT mice, the results revealed the induction and progression of the disease over weeks 4–6 and 8–12, respectively.
FIG 1.
Nose-only exposure of the lungs of BALB/c mice to cigarette smoke induces the hallmark features of human COPD. A–I, WT BALB/c mice were exposed to cigarette smoke or normal air for 1–12 weeks. Relative to control mice, smoke-exposed mice had (A) reduced weight-gain relative to initial weight; (B) acute (after 4 days) and chronic (after 8 weeks) increases in the numbers of macrophages (M), neutrophils (N) and lymphocytes (L) in BALF; (C) increased cellular infiltrates in the parenchyma; increased levels of the transcripts that encode (D) TNF-α, (E) Cxcl1, and (F) IL-1β in lung homogenates; (G) increased number of mucus-secreting goblet cells (MSCs) in the airways; (H) airway epithelium thickening; and (I) alveolar enlargement (scale bar on micrographs = 100 µm). Data are means±SEM of 6–8 mice/group, # P<0.05, ## P<0.01, ### P<0.001 compared to mice that breathed normal air, * P<0.05, ** P<0.01 compared to other groups indicated. Statistical significance of the reduced weight gain is for the whole curve.
Smoke exposure of the airways of mice resulted in reduced lung function similar to that observed in humans with COPD
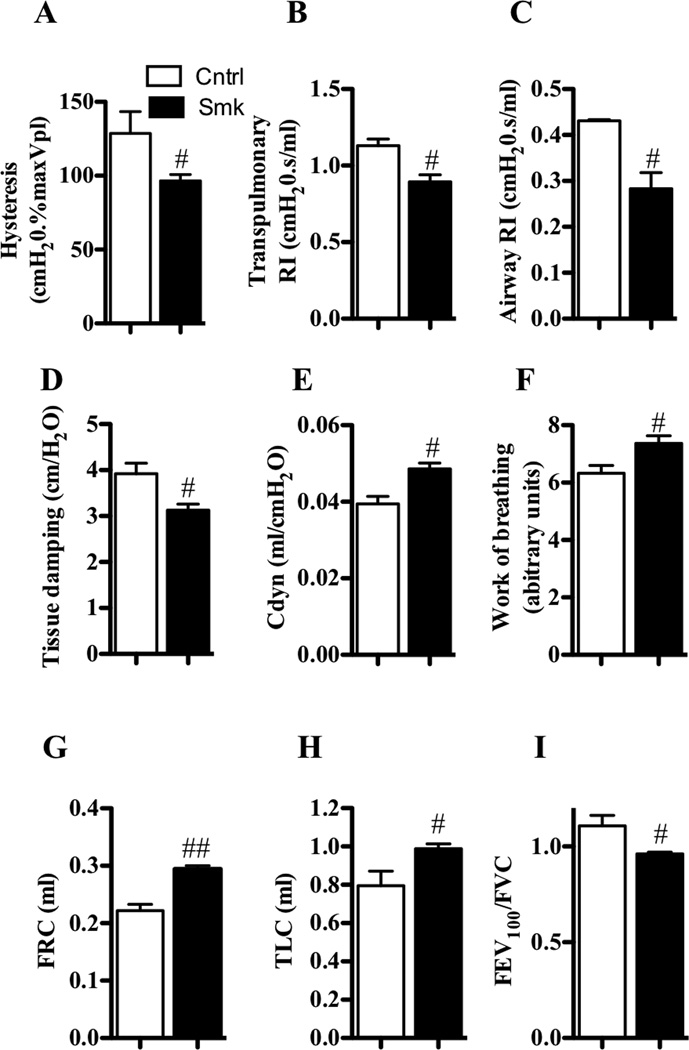
Since 8 weeks of exposure was required to induce key features of COPD, other parameters were assessed at this time-point. We next investigated the effects of smoke exposure on parameters of lung function. Exposure decreased hysteresis, transpulmonary and airway-specific resistance (RI), tissue damping, and forced expiratory volume in 100 milliseconds/functional vital capacity ratio (FEV100/FVC, representative of FEV1/FVC ratio in humans), but increased dynamic compliance (Cdyn), work of breathing, functional residual capacity (FRC), and total lung capacity (TLC) (Fig 2, A–I). These adverse changes in lung function likely resulted from the combination of chronic inflammation, airway remodeling, and emphysematous lesions with associated reductions in alveolar tissue and supporting airway attachments.
FIG 2.
Nose-only cigarette smoke exposure leads to changes in lung function that are similar to that in humans with COPD. A–I, WT BALB/c mice were exposed to cigarette smoke or normal air for 8 weeks. Relative to control mice, smoke-exposed mice had decreased (A) hysteresis, (B) transpulmonary and (C) airway-specific resistance (RI) and (D) tissue damping, but increased (E) dynamic compliance (Cdyn), (F) work of breathing, (G) functional residual capacity (FRC), and (H) total lung capacity (TLC), and (I) reduced ratio of forced expiratory volume in 100 milliseconds/forced vital capacity (FEV100/FVC). Data are means±SEM of 6–8 mice/group, # P<0.05, compared to mice that breathed normal air.
COPD features in the experimental model are glucocorticoid-resistant
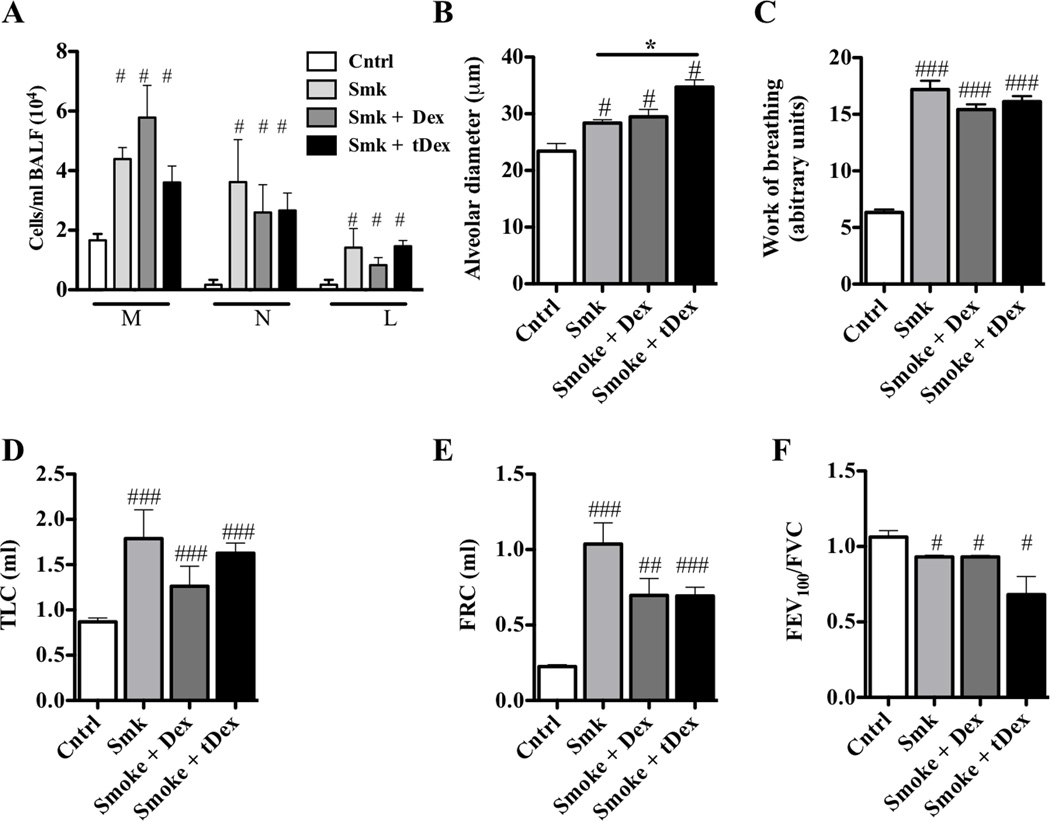
While glucocorticoids are used to treat patients with COPD to reduce acute inflammation and the frequency of acute exacerbations,38,39 they have limited efficacy.40–42 Glucocorticoid treatment (either throughout, or for the last 6 weeks of 12 weeks of smoke exposure) did not prevent chronic inflammation or emphysema, nor did they suppress declines in lung function (Fig 3, A–F).
FIG 3.
Experimental COPD is glucocorticoid-resistant. A–F, WT BALB/c mice were exposed to cigarette smoke or normal air for 12 weeks and were treated with dexamethasone or sham-treated with sterile distilled water either prophylactically throughout (Dex), or therapeutically (tDex) for the last 6 weeks, of the smoking protocol. Steroid treatment had no effect on (A) macrophage (M), neutrophil (N) and lymphocyte (L) numbers in the BALF, (B) alveolar enlargement or changes in lung function; (C) work of breathing, (D) total lung capacity (TLC), (E) functional residual capacity (FRC) or (F) forced expiratory volume in 100 milliseconds/forced vital capacity (FEV100/FVC) ratio. Data are means±SEM of 6–8 mice/group, # P<0.05, ## P<0.01, ### P<0.001 compared to mice that breathed normal air. There were no differences between other groups.
Smoke exposure of the airways has systemic effects
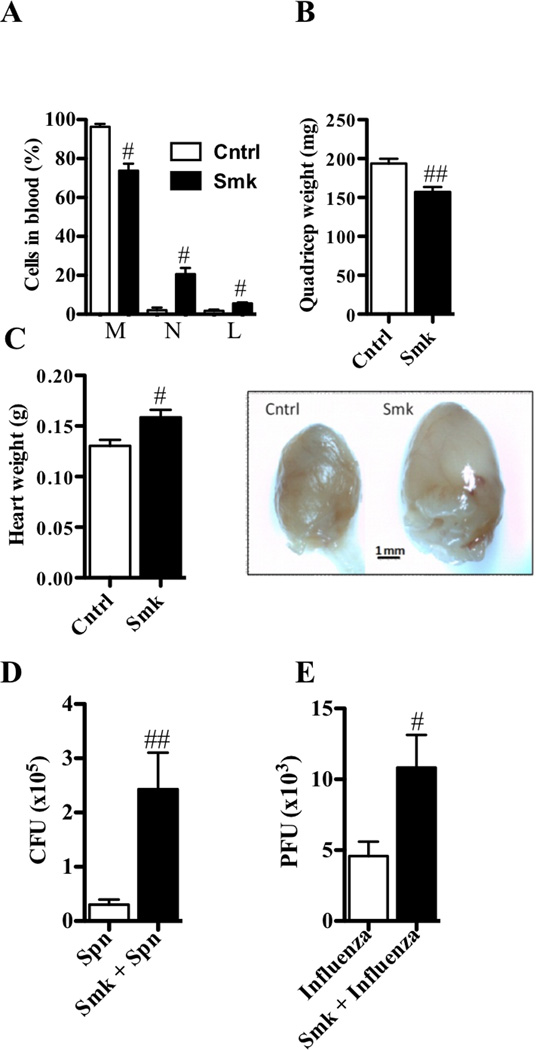
The lung is not the only organ adversely affected in humans with COPD, and systemic inflammation, loss in skeletal muscle mass, and cardiovascular disease often occur in this disease.43 In our model, 8 weeks of exposure also induced systemic changes with significant alterations in the proportion of leukocytes in the blood. The percentage of monocytes decreased but the percentage of neutrophils and lymphocytes increased (Fig 4, A). Furthermore, skeletal muscle mass (i.e., the quadriceps) was reduced (Fig 4, B). Moreover, the hearts of smoke-exposed mice also were enlarged by ~25% and had more surrounding fatty tissue (Fig 4, C).
FIG 4.
Experimental COPD has systemic involvement and exacerbates respiratory infections. A–E, WT BALB/c mice were exposed to cigarette smoke or normal air for 8 weeks. Relative to control mice, smoke-exposed mice had (A) alterations in the proportions of monocytes (M, decreased), neutrophils (N, increased), and lymphocytes (L, increased) in blood; (B) reduced quadriceps weight; (C) increased heart weight, size, and fatty deposits; and decreased (D) clearance of S. pneumoniae (after 48 hours of infection) and (E) influenza virus (after 7 days of infection). Data are means±SEM of 6–8 mice/group, # P<0.05, ## P<0.01, compared to WT mice that breathed normal air.
Induction of experimental COPD exacerbates respiratory infections
Chronic microbial colonization of the airways and infection-induced exacerbations are common in COPD patients.44 When we infected mice previously exposed to smoke for 8 weeks with S. pneumoniae or influenza virus (and smoke exposure discontinued), pathogen burden increased ~10- and ~2.5-fold, respectively (Fig 4, D and E).
Experimental COPD does not rapidly resolve following smoking cessation
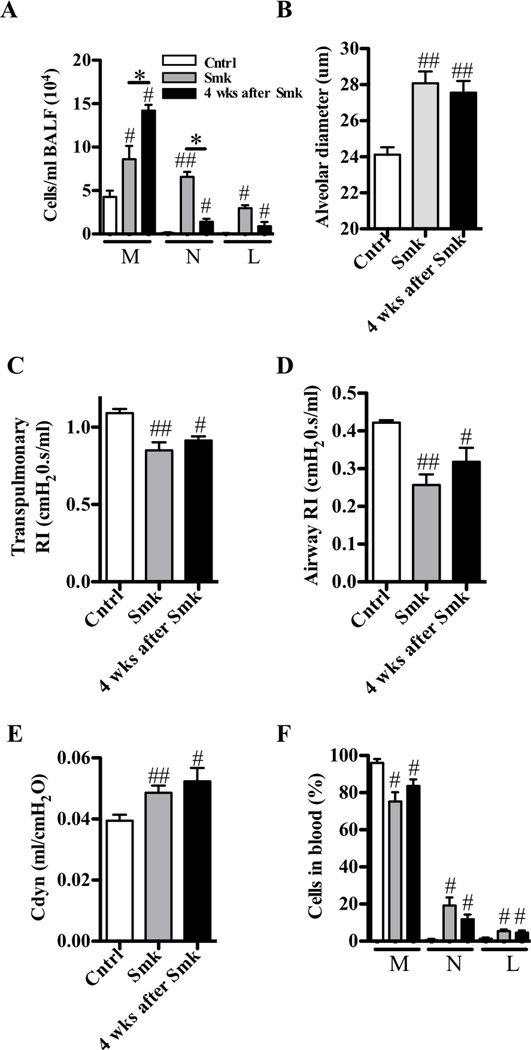
Once patients develop COPD, their clinical conditions generally do not improve significantly after smoking cessation, and often lung function further deteriorates.2 In our model, smoke exposure for 8 weeks followed by cessation for 4 weeks did not improve airway inflammation, emphysema, lung function, or circulating leukocyte abnormalities (Fig 5, A–F). In regard to airway inflammation (Fig 5, A), the numbers of macrophages continued to increase, suggesting the presence of a macrophage-rich pro-inflammatory environment in the lung that underpins the progression of disease.
FIG 5.
Experimental COPD does not rapidly resolve following cessation of smoke exposure. A–F, WT BALB/c mice were exposed to cigarette smoke or normal air for 8 weeks. Smoke exposed mice were evaluated immediately after the cessation of smoking or 4 weeks later. Relative to control mice, both groups of smoke-exposed mice had (A) increased airway inflammation; (B) alveolar enlargement; and changes in lung function; decreased (C) transpulmonary and (D) airway-specific resistance (RI); (E) increased dynamic compliance (Cdyn); as well as (F) altered leukocyte populations in blood. Mice that had ceased smoking 4 weeks earlier had more macrophages, but fewer neutrophils, in their BALF. Data are means±SEM of 6–8 mice/group, # P<0.05, ## P<0.01, compared to mice that breathed normal air, * P<0.05, compared to other groups indicated.
Experimental COPD is macrophage dependent
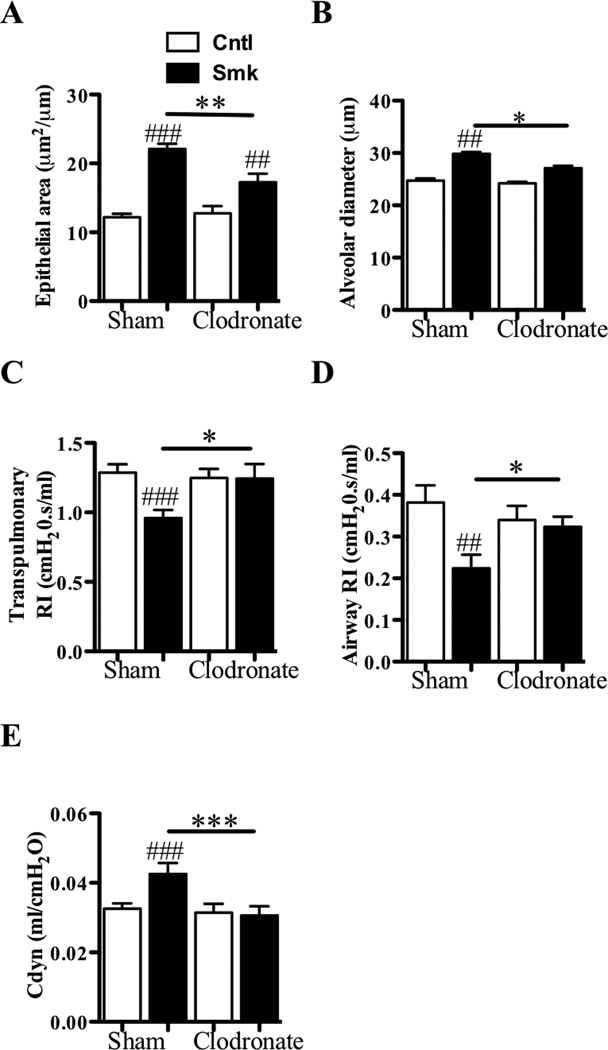
Numerous studies have indicated that pulmonary macrophages have prominent pathologic roles in humans with COPD.7,9,45,46 Since increased numbers of macrophages were found in the lungs of our smoked-exposed WT mice (Fig 1, B), we assessed the importance of these phagocytes in the development of experimental COPD by reducing their numbers during smoke exposure using the clodronate-depletion method. Liposome-encapsulated clodronate were administered into the airways 3 times/week throughout 8 weeks of smoke exposure. Control animals were sham-treated with empty liposomes. The numbers of macrophages were then quantified in the BALF by morphology and in dispersed whole lung by flow cytometry (F4/80+). Macrophages were depleted by 61±4% and 73±5% in smoke-exposed mice and by 25±3% and 36±2% in non-exposed controls in BALF and lung tissue, respectively. In contrast, the percentage of monocytes in the peripheral blood was unaffected. Macrophage depletion in the lung resulted in reduced smoke-induced epithelial thickening and emphysema, and protection against alterations in lung function (Fig 6, A–E). In contrast, depletion of neutrophils using the anti-Ly6G antibody approach did not suppress the effects of smoke exposure (Fig S2). The accumulated data suggest a central role for pulmonary macrophages in our animal model of cigarette smoke-induced COPD, thereby supporting the clinical data of others that have implicated these cells in the pathogenesis of COPD in humans.7
FIG 6.
Depletion of pulmonary macrophages suppresses the development of experimental COPD. A–E, WT BALB/c mice were exposed to cigarette smoke or normal air for 8 weeks. These two groups of mice also were treated with either liposome encapsulated clodronate or empty liposomes (Sham) 3 times/week for the duration of the experiment commencing on the 1st day of smoking. Relative to smoke-exposed macrophage-sufficient mice, smoke-exposed macrophage-depleted mice had reduced (A) airway epithelium thickening; and (B) alveolar enlargement; and altered lung function; increased (C) transpulmonary and (D) increased airway-specific resistance (RI), and (E) reduced dynamic compliance (Cdyn). The smoke-exposed macrophage-depleted mice had no alveolar enlargement or changes in lung function compared to non-smoke exposed control mice. Data are means±SEM of 6–8 mice/group, ## P<0.01, ### P<0.001 compared to mice that breathed normal air, * P<0.05, ** P<0.01 *** P<0.001 compared to other groups indicated.
Exposure of WT B6 mice to cigarette smoke also induces COPD
WT BALB/c and B6 mice respond differently in numerous disease models. To assess the general applicability of our model to another commonly used mouse strain, we examined the effects of smoke exposure on the lungs of WT B6 mice. Exposure for 8 weeks resulted in a similar profile and magnitude of weight loss, parenchymal inflammation, airway remodeling, emphysematous destruction (alveolar enlargement), and altered lung function in B6 mice compared to BALB/c mice (Fig S3, A–N).
The tetramer-forming tryptase mMCP-6 contributes to macrophage accumulation and inflammation in the airways, and is required for experimental COPD
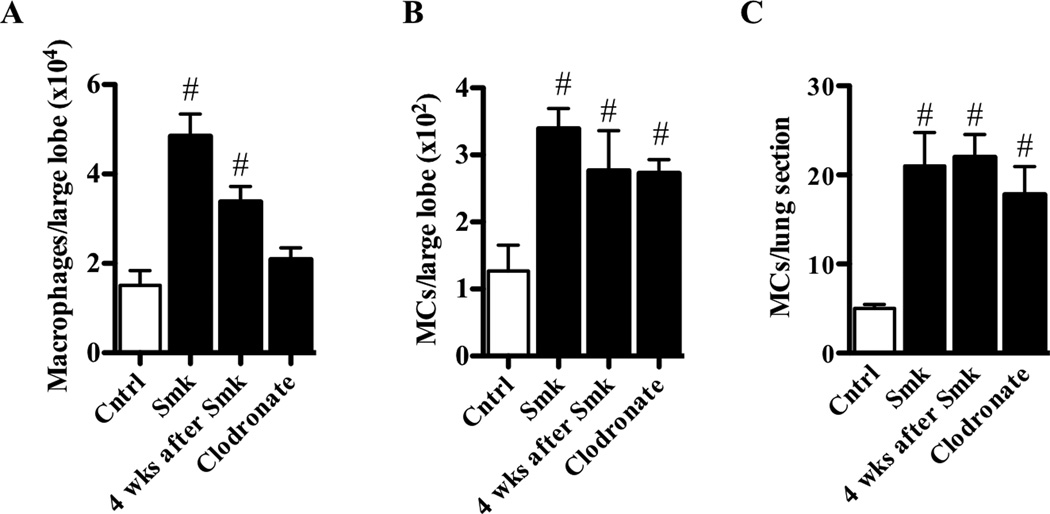
Macrophage accumulation in the lungs of smoke-exposed mice could be caused by unknown factors released from activated MCs. Although MCs also have been implicated in the pathogenesis of COPD, the link between these two cell types and smoke-induced COPD has not been demonstrated, and the relevant MC-derived factors have not been identified.13 Following 8 weeks of smoke exposure, the numbers of macrophages and MCs in the lung of WT B6 mice (i.e., the largest lobe in the multi-lobed right lung by flow cytometry or in the single-lobed left lung by histochemistry) increased ~3 fold in both instances (Fig 7, A–C and Fig S4).
FIG 7.
Experimental COPD increases the numbers of pulmonary macrophages and MCs. A–C, WT B6 mice were exposed to cigarette smoke or normal air for 8 weeks. Some mice were then rested for 4 weeks after smoking. Other mice were treated with clodronate. Relative to control mice, smoke-exposed mice had increased numbers of (A) F4/80+ macrophages and (B) Kit+/FcεRI+/IgE+ (by flow cytometry in the largest lobe of the multi-lobed right lung) or (C) toluidine blue+ (by histochemistry in the single-lobed left lung) MCs in the lungs. Smoking cessation did not alter macrophage or MC numbers. Clodronate specifically attenuated macrophage numbers. Data are means±SEM of 6–8 mice/group, # P<0.05, compared to mice that breathed normal air.
To provide further evidence for the importance of macrophages and MCs in pathogenesis we also assessed their levels in the smoking cessation and clodronate depletion studies. In the cessation studies, the numbers of both of these cells types were elevated concomitant with the maintenance of disease (Fig 7, A–C). In the depletion studies, the suppression of macrophages but not MCs in the lung correlated with the prevention of COPD (Fig 7, A–C). These studies provide further evidence for the pivotal role of macrophages in the pathogenesis of experimental COPD.
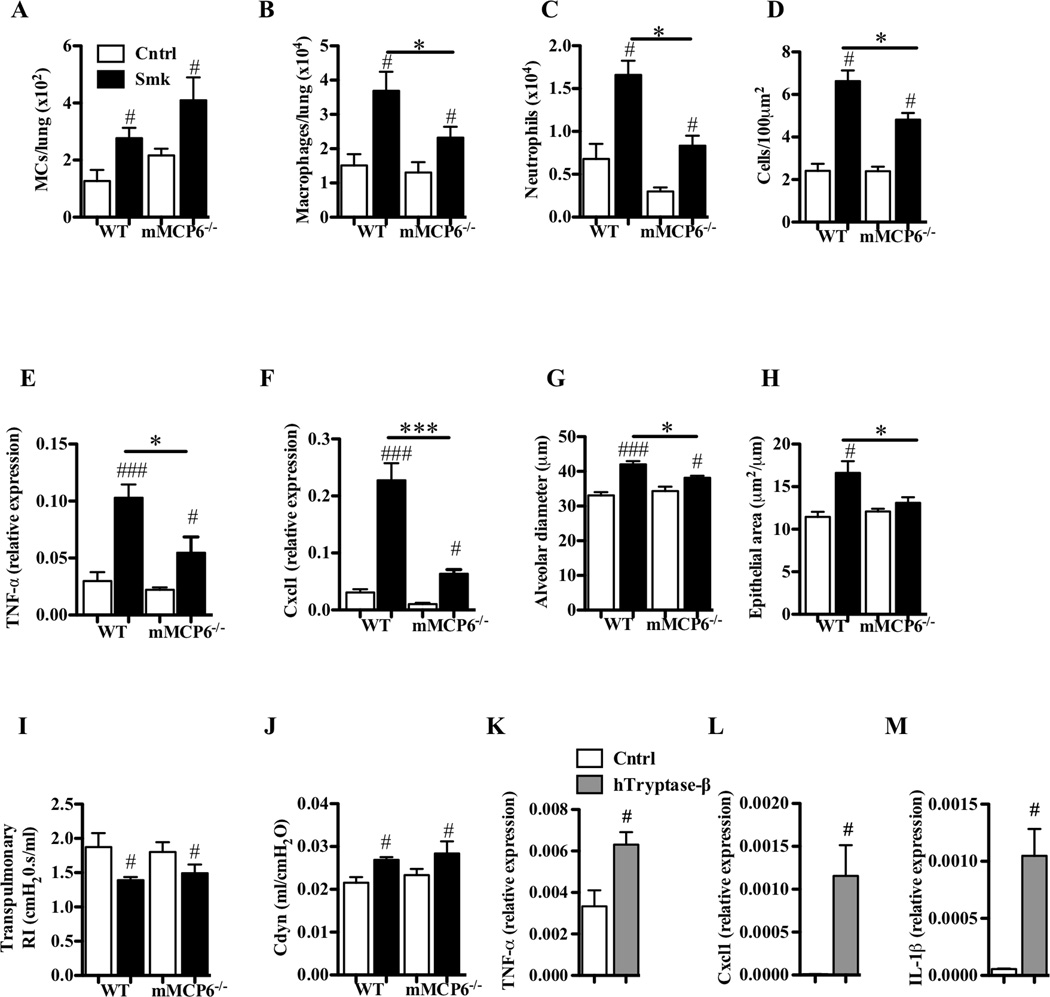
mMCP-6 and hTryptase-β have prominent roles in innate immunity and inflammation.18,47 Thus, we hypothesized that mMCP-6 might play a critical role in experimental COPD, as occurs in experimental arthritis and colitis. We therefore subjected WT and mMCP-6 null (−/−) B6 mice18 to smoke exposure. We could not detect mMCP-6 mRNA by qPCR in the lungs of non-treated and smoke-treated WT mice, and the levels of mMCP-6 protein were below the limits of detection by SDS-PAGE immunoblot analysis. The numbers of MCs in the lungs of smoke-exposed mMCP-6−/− mice were not affected and were similar to those in the lungs of smoke-exposed WT mice. However, smoke-exposed mMCP-6-null mice had significantly fewer macrophages (Fig 8, A and B). The reduction in macrophage accumulation in the airways of the tryptase-deficient mice correlated with reductions in neutrophil numbers in the BALF, inflammation of the parenchyma, pro-inflammatory cytokine (e.g., TNF-α and IL-1β) and chemokine (e.g., Cxcl1) mRNA levels, and emphysematous lesions (Fig 8, C–H). While airway remodeling also was abrogated, there were no obvious differences in the lung function parameters measured between smoke-treated WT and mMCP-6−/− mice (Fig 8, I and J). While our accumulated data revealed adverse roles for mMCP-6 in the COPD model, other factors that remain to be identified must contribute to the deterioration in lung function in the smoke-exposed mice.
FIG 8.
The tryptase mMCP-6 contributes to pulmonary macrophage accumulation and parenchymal inflammation, and is required for airway remodeling and alveolar enlargement in experimental COPD. A–J, WT and mMCP-6−/− B6 mice were exposed to cigarette smoke or normal air for 8 weeks. Relative to smoke-exposed WT mice, smoke-exposed mMCP-6−/− mice had (A) no change in the number of Kit+/FcεRI+/IgE+ MCs but had reduced (B) numbers of F4/80+ macrophages and (C) neutrophils in the lung, (D) less cellular infiltrates in the parenchyma, attenuated (E) TNF-α and (F) Cxcl1 mRNA levels in lung homogenates, (G) diminished alveolar enlargement, (H) no airway remodelling and no differences in lung function [e.g. (I) transpulmonary resistance (RI) or (J) dynamic compliance (Cdyn)]. K–M, B6 mouse bone marrow-derived macrophages were cultured in the absence or presence of recombinant hTryptase-β. Relative to untreated cells, hTryptae-β-treated cells had increased levels of the transcripts that encode (K) TNF-α, (L) Cxcl1 and (M) IL-1β. Data are means±SEM of 6–8 mice/group, or of 3 cell cultures in triplicate (representative of 4 repeat experiments), # P<0.05, ## P<0.01, ### P<0.001 compared to mice that breathed normal air (A–J) or compared to sham-treated macrophages (K–M), * P<0.05, compared to other groups indicated.
hTryptase-β induces macrophages to increase their expression of pro-inflammatory cytokines and chemokines
To confirm the link between MC tryptases and the pro-inflammatory activity of activated macrophages, we next treated cultured mouse bone marrow-derived macrophages with recombinant hTryptase-β. The tryptase induced these macrophages to markedly increase the levels of the transcripts that encode TNF-α, Cxcl1, and IL-1β (Fig 8, K–M). These changes did not occur if the tryptase was boiled for 5 minutes before treatment. Although the mechanism by which MC tetramer-forming tryptases induce macrophages to increase the expression of these cytokines and chemokines remains to be determined at the molecular level, the collective data suggest that mMCP-6 and hTryptase-β are associated with macrophage accumulation and macrophage-dependent inflammation, remodeling, and emphysema in COPD.
DISCUSSION
COPD is a major respiratory health problem worldwide2 that is usually caused by the chronic inhalation of cigarette smoke.48,49 Although a heterogeneous disease, COPD is characterized by chronic airway inflammation (bronchitis) and/or emphysema, as well as reduced lung function.49 The chronic inflammation that occurs in the smoke-exposed lung is thought to drive the progressive mucus hypersecretion, airway remodeling, and destruction of alveolar tissue that synergize to reduce pulmonary function.48,49 While inhaled glucocorticoids and bronchodilators are used therapeutically to treat the symptoms and exacerbations of COPD,39 there is no effective treatment that prevents the induction of the disease or halts its progression.41
The lack of a cigarette smoke-induced animal model of COPD of short duration that recapitulates the major features of the human condition has hindered our understanding of the disease. To address this deficiency, we developed a short-term mouse model of cigarette smoke-induced COPD that has most of the key pathological and clinical features of the human disease. Importantly, our in vivo model gradually progresses to overt disease over 6–8 weeks (Fig 1) and does not rapidly resolve (Fig 5). The hallmark features of the disease are induced within 8 weeks (Figs 1, 2, and S3), providing opportunities to identify therapeutic targets for both the induction and progression phases of the disease. As occurs in humans who smoke,14,47,50 the direct delivery of smoke to the airways of WT BALB/c and B6 mice resulted in acute and chronic inflammation that was dominated by neutrophils, macrophages, and eventually CD8+ T cells (Figs 1 and S3). The disease also had a MC component (Figs 7 and 8).
Inflammation was associated with airway remodeling, emphysematous changes, and reduced lung function (Figs 1, 2, S2, and S3). The airway remodeling, alveolar enlargement and emphysema were likely due to inflammation-induced damage of the parenchymal walls. Together, the airway remodeling and emphysema in our model led to reduced lung function, as occurs in humans with COPD (Figs 2 and S3).
The only feature not consistent with human COPD was a decrease in airway resistance (Fig 2, B and C). This finding suggests an absence of obstruction despite the presence of goblet cell metaplasia and airway remodeling. Because mice have a greater proportion of parenchyma to airway tissue compared to humans, the reduced resistance might be the result of emphysema and associated reductions in tissue attachments. Alveolar distension and hyperinflation led to widening of the airways during ventilation and reduced resistance in other mouse models of emphysema.51
Glucocorticoids have limited efficacy in treating COPD symptoms in humans. They partially suppress chronic inflammation, but do not reverse the tissue lesions or modify factors that drive chronic disease, and have no effect on the decline in lung function.40–42 As occurs in COPD patients, dexamethasone did not suppress inflammation, emphysema, or alterations in lung function in our model (Fig 3). COPD has a systemic component with changes in circulating leukocyte populations and loss of skeletal muscle mass.43 Both of these features were observed in our model (Fig 4, A and B). COPD is also linked to cardiovascular disease, and the hearts of smoke-exposed WT mice had pathologic changes (Fig 4, C).
COPD patients have a 3–6 fold increased risk of S. pneumoniae-induced pneumonia.52 They also are more susceptible to influenza virus and suffer more severe symptoms when infected.53 Respiratory infections induce further inflammation and acute exacerbations of COPD symptoms which, in turn, increase the rate of disease progression.54 The development of experimental COPD was associated with enhanced respiratory infection by S. pneumoniae and influenza virus (Fig 4, D and E). The numbers of CD8+ T cells that infiltrated the lungs of smoke-exposed mice were elevated but these lymphocytes had reduced expression of the activation marker CD98 (data not shown), which may contribute to impaired pathogen clearance. Although inflammation was increased, the latter findings suggest that immune function was suppressed, thereby predisposing the diseased mice to more severe pulmonary infections.
Once COPD develops in humans, the clinical condition and features of disease often deteriorate further even after the cessation of smoking.2 After 8 weeks of smoking and 4 weeks of cessation in our model, macrophage accumulation in the airways of WT mice actually increased compared to that observed immediately after 8 weeks of smoking (Fig 5, A). It is possible that the increase in the number of macrophages in the lungs underpins the progression of COPD even after smoking cessation. This macrophage accumulation may be additionally exacerbated by infection that could further increase the rate of disease progression. There were no signs of resolution of any features of disease, at least 4 weeks after smoking cessation (Fig 5).
We then performed macrophage-depletion studies in WT mice to demonstrate that these phagocytes were essential for smoke-induced emphysema and reduced lung function (Fig 6). The clodronate method is the only method that can be used to reliably deplete the number of macrophages in the lungs of mice. Although clodronate might deplete some dendritic cells, there was no reduction in neutrophils by the method. Furthermore, the direct depletion of neutrophils did not suppress changes in lung structure or function (Fig S3).
The mechanisms that drive COPD pathogenesis are incompletely understood. Chronic influx of inflammatory cells into the lungs in COPD leads to the generation and release of pro-inflammatory cytokines, chemokines, and leukotrienes55 that are thought to contribute to tissue destruction and the development of COPD. However, abnormalities in the protease:protease inhibitor balance in the lung also is important.56 In that regard, MCs and their tryptase•serglycin proteoglycan complexes promote inflammation in numerous diseases,47,57,58 and MCs have been implicated in COPD pathogenesis.13 MCs are common in inflammatory infiltrates in COPD, and increases in their numbers correlate with reduced lung function and airway remodeling.14 The numbers of MCs detected in smoke-exposed mice were not high relative to other cell types (Fig 7). Nevertheless, it is well known that MCs release potent pro-inflammatory mediators, and that even small numbers of these cells can have devastating effects in vivo as occurs in systemic anaphylaxis.
hTryptase-β59,60 comprises up to 50% of the protein content of a human MC. Its ortholog mMCP-661 has beneficial roles in the control of infections,18,62 but adverse roles in MC-dependent inflammation of the airways,53 joints,47,57 and colon in mice.58,63 However, the roles of MCs and their granule mediators in COPD pathogenesis have not been elucidated. In our model, there were no detectable increases in mMCP-6 mRNA or protein levels. This was not surprising since MC tryptases are pre-formed and stored in the cell’s granules. Thus, changes in their mRNA levels are not as important as their release or the activation state of the MC. Only low levels of MCs were observed and mMCP-6 protein levels were below the limits of detection by immunoblot in whole lung tissues. There are no other more sensitive tests, such as ELISA, available. Nevertheless, Mortaz and coworkers, have demonstrated that cigarette smoke conditioned media induced the expression of mMCP-6 protein in primary cultured mast cells.17
Despite these data, the generation of mMCP-6−/− B6 mice18 allowed us the opportunity for the first time to definitively evaluate the importance of this MC-restricted tryptase in experimental COPD. The MCs in WT BALB/c mice express mMCP-661 and the other tryptase family member mMCP-7.64 In contrast, the MCs in WT B6 mice lack mMCP-7 due to a splice-site mutation in its gene.65 The finding that all the features of COPD were similar in WT BALB/c (Fig 1) and B6 (Fig S3) mice indicates that mMCP-7 is not essential in our experimental model.
Smoke exposure resulted in enhanced pro-inflammatory cytokine and chemokine expression (Fig 1, D–F and 8, E and F) and increased the numbers of macrophages and MCs in the lungs of WT B6 mice (Fig 7). We then employed our mMCP-6−/− B6 mice to demonstrate that the presence of this tryptase is required for smoke-induced pro-inflammatory cytokine and chemokine expression and accumulation of macrophages in the lung, and for airway epithelial thickening and emphysematous damage (Fig 8). There were no statistically significant differences between neutrophil numbers or chemokine mRNA levels in the lung (Fig 8, C and F) or the percentage of leukocytes that were neutrophils in the blood (data not shown) at baseline. Thus, the decreased cellular infiltration in the lungs of the smoke-treated mMCP-6-null mice was not due to a baseline defect. We then showed that recombinant hTryptase-β induced mouse macrophages to increase their expression of the transcripts that encode the cytokines and chemokines that are increased in the lungs of mice with experimental COPD. These findings suggest the tryptase contributes to COPD, at least in part, by directly activating the macrophages in the lungs of the diseased animals. Our discovery that macrophages and tryptase+ MCs participate in the pathogenesis of COPD in our model raise the possibility that these cells and their granule tetramer-forming tryptases have comparable adverse roles in human COPD. Thus, the next generation of hTryptase-β inhibitors might have efficacy in the treatment of patients with COPD.
In summary, we developed a short-term cigarette smoke-induced mouse model that has the hallmark features of COPD. This animal model enables the study of the pathogenesis of COPD in a significantly shorter time-frame than existing cigarette smoke-induced models, thereby providing opportunities for pharmacologic intervention. Our model also enables the evaluation of the consequences of COPD on the ability of the lung to combat infections. COPD is the result of a complex interplay of immune dysregulation, and our model allows the evaluation of other important parameters (e.g. systemic consequences) in COPD in the mouse. This study raises the possibility that macrophages and tryptase+ MCs might have similar adverse roles in patients with COPD.
Clinical implications.
We describe a short-term mouse model with the hallmark features of cigarette smoke-induced COPD. We then show that the model can be used to further our understanding of the pathogenesis of COPD. We demonstrate for the first time that a MC-restricted tetramer-forming tryptase has a prominent adverse role in experimental COPD.
Acknowledgments
Supported by grants from the National Health and Medical Research Council of Australia (510762, 569219, 1003565, 1003593, 1023131), the National Institutes of Health USA (AI065858 and AI059746), and the Harvard Club of Australia Foundation. We thank Dr. Julia Charles (Brigham and Women’s Hospital, Boston, Mass) for her assistance in the generation of mouse bone marrow-derived macrophages.
Abbreviations used
- BALF
bronchoalveolar lavage fluid
- B6
C57BL/6
- Cdyn
dynamic compliance
- CFU
colony forming unit
- COPD
chronic obstructive pulmonary disease
- FEV100
forced expiratory volume in 100 milliseconds
- FRC
functional residual capacity
- FVC
functional vital capacity
- IFN-γ
interferon-γ
- MC
mast cell
- mMCP
mouse MC protease
- MSC
mucus-secreting goblet cell
- qPCR
quantitative polymerase chain reaction
- PFU
plaque forming unit
- PV
pressure/volume
- RI
resistance
- TLC
total lung capacity
- TNF-α
tumor necrosis factor-α
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interests.
REFERENCES
- 1.WHO. Global Burden of Disease: 2004 Update. 2008
- 2.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 3.Vlahos R, Bozinovski S, Gualano RC, Ernst M, Anderson GP. Modelling COPD in mice. Pulm Pharmacol Ther. 2006;19:12–17. doi: 10.1016/j.pupt.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Bracke KR, D'hulst AI, Maes T, Moerloose KB, Demedts IK, Lebecque S, et al. Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J Immunol. 2006;177:4350–4359. doi: 10.4049/jimmunol.177.7.4350. [DOI] [PubMed] [Google Scholar]
- 5.Gaschler GJ, Zavitz CCJ, Bauer CMT, Skrtic M, Lindahl M, Robbins CS, et al. Cigarette smoke exposure attenuates cytokine production by mouse alveolar macrophages. Am J Respir Cell Mol Biol. 2007;38:218–226. doi: 10.1165/rcmb.2007-0053OC. [DOI] [PubMed] [Google Scholar]
- 6.Motz GT, Eppert BL, Wortham BW, Amos-Kroohs RM, Flury JL, Wesselkamper SC, et al. Chronic cigarette smoke exposure primes NK cell activation in a mouse model of chronic obstructive pulmonary disease. J Immunol. 2010;184:4460–4469. doi: 10.4049/jimmunol.0903654. [DOI] [PubMed] [Google Scholar]
- 7.Hodge S, Matthews G, Mukaro V, Ahern J, Shivam A, Hodge G, et al. Cigarette smokeinduced changes to alveolar macrophage phenotype and function are improved by treatment with procysteine. Am J Respir Cell Mol Biol. 2011;44:673–681. doi: 10.1165/rcmb.2009-0459OC. [DOI] [PubMed] [Google Scholar]
- 8.Atkinson JJ, Lutey BA, Suzuki Y, Toennies HM, Kelley DG, Kobayashi DK, et al. The role of matrix metalloproteinase-9 in cigarette smoke-induced emphysema. Am J Respir Crit Care Med. 2011;183:876–884. doi: 10.1164/rccm.201005-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 10.Wright JL, Churg A. Smoking cessation decreases the number of metaplastic secretory cells in the small airways of the guinea pig. Inhal Toxicol. 2002;14:1153–1159. doi: 10.1080/08958370290084836. [DOI] [PubMed] [Google Scholar]
- 11.Huvenne W, Pérez-Novo CA, Derycke L, De Ruyck N, Krysko O, Maes T, et al. Different regulation of cigarette smoke induced inflammation in upper versus lower airways. Respir Res. 2010;11:100–109. doi: 10.1186/1465-9921-11-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruwanpura SM, McLeod L, Miller A, Jones J, Bozinovski S, Vlahos R, et al. Interleukin-6 promotes pulmonary emphysema associated with apoptosis in mice. Am J Respir Cell Mol Biol. 2011;45:720–730. doi: 10.1165/rcmb.2010-0462OC. [DOI] [PubMed] [Google Scholar]
- 13.Mortaz E, Folkerts G, Redegeld F. Mast cells and COPD. Pulm Pharmacol Ther. 2011;24:367–372. doi: 10.1016/j.pupt.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Andersson CK, Mori M, Bjermer L, Lofdahl CG, Erjefalt JS. Alterations in lung mast cell populations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:206–217. doi: 10.1164/rccm.200906-0932OC. [DOI] [PubMed] [Google Scholar]
- 15.Ballarin A, Bazzan E, Zenteno RH, Turato G, Baraldo S, Zanovello D, et al. Mast cell infiltration discriminates between histopathological phenotypes of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:233–239. doi: 10.1164/rccm.201112-2142OC. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Zheng H, Ma W, Wang F, Zeng X, Liu C, et al. Tryptase enzyme activity is correlated with severity of chronic obstructive pulmonary disease. Tohoku J Exp Med. 2011;224:179–187. doi: 10.1620/tjem.224.179. [DOI] [PubMed] [Google Scholar]
- 17.Mortaz E, Givi ME, Da Silva CA, Folkerts G, Redegeld FA. A relation between TGF-β and mast cell tryptase in experimental emphysema models. Biochim Biophys Acta. 2012;1822:1154–1160. doi: 10.1016/j.bbadis.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, et al. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 19.Thorburn AN, O'Sullivan BJ, Thomas R, Kumar RK, Foster PS, Gibson PG, et al. Pneumococcal conjugate vaccine-induced regulatory T cells suppress the development of allergic airways disease. Thorax. 2010;65:1053–1060. doi: 10.1136/thx.2009.131508. [DOI] [PubMed] [Google Scholar]
- 20.Essilfie AT, Simpson JL, Horvat JC, Preston JA, Dunkley ML, Foster PS, et al. Haemophilus influenzae infection drives IL-17-mediated neutrophilic allergic airways disease. PLoS Pathog. 2011;7:e1002244. doi: 10.1371/journal.ppat.1002244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preston JA, Essilfie AT, Horvat JC, Wade MA, Beagley KW, Gibson PG, et al. Inhibition of allergic airways disease by immunomodulatory therapy with whole killed Streptococcus pneumoniae. Vaccine. 2007;25:8154–8162. doi: 10.1016/j.vaccine.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 22.Thorburn AN, Foster PS, Gibson PG, Hansbro PM. Streptococcus pneumoniae components suppress allergic airways disease and natural killer T cells by inducing regulatory T cells. J Immunol. 2012;188:4611–4620. doi: 10.4049/jimmunol.1101299. [DOI] [PubMed] [Google Scholar]
- 23.Horvat JC, Beagley KW, Wade MA, Preston JA, Hansbro NG, Hickey DK, et al. Neonatal chlamydial infection induces mixed T-cell responses that drive allergic airway disease. Am J Respir Crit Care Med. 2007;176:556–564. doi: 10.1164/rccm.200607-1005OC. [DOI] [PubMed] [Google Scholar]
- 24.Starkey MR, Essilfie AT, Horvat JC, Kim RY, Nguyen DH, Beagley KW, et al. Constitutive production of IL-13 promotes early-life Chlamydia respiratory infection and allergic airway disease. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.99. in press. [DOI] [PubMed] [Google Scholar]
- 25.Li JJ, Wang W, Baines KJ, Bowden NA, Hansbro PM, Gibson PG, et al. IL-27/IFN-γ induce MyD88-dependent steroid-resistant airway hyperresponsiveness by inhibiting glucocorticoid signaling in macrophages. J Immunol. 2010;185:4401–4409. doi: 10.4049/jimmunol.1001039. [DOI] [PubMed] [Google Scholar]
- 26.Asquith KL, Horvat JC, Kaiko GE, Carey AJ, Beagley KW, Hansbro PM, et al. Interleukin-13 promotes susceptibility to chlamydial infection of the respiratory and genital tracts. PLoS Pathog. 2011;7:e1001339. doi: 10.1371/journal.ppat.1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu M, Rhee I, Liu Y, Zhang W. Negative regulation of FcεRI-mediated signaling and mast cell function by the adaptor protein LAX. J Biol Chem. 2006;281:18408–18413. doi: 10.1074/jbc.M601535200. [DOI] [PubMed] [Google Scholar]
- 28.Horvat JC, Starkey MR, Kim RY, Phipps S, Gibson PG, Beagley KW, et al. Early-life chlamydial lung infection enhances allergic airways disease through age-dependent differences in immunopathology. J Allergy Clin Immunol. 2010;125:617–625. doi: 10.1016/j.jaci.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Horvat JC, Starkey MR, Kim RY, Beagley KW, Preston JA, Gibson PG, et al. Chlamydial respiratory infection during allergen sensitization drives neutrophilic allergic airways disease. J Immunol. 2010;184:4159–4169. doi: 10.4049/jimmunol.0902287. [DOI] [PubMed] [Google Scholar]
- 30.Kumar RK, Herbert C, Kasper M. Reversibility of airway inflammation and remodelling following cessation of antigenic challenge in a model of chronic asthma. Clin Exp Allergy. 2004;34:1796–1802. doi: 10.1111/j.1365-2222.2004.02097.x. [DOI] [PubMed] [Google Scholar]
- 31.Harris RS. Pressure-volume curves of the respiratory system. Respir Care. 2005;50:78–98. [PubMed] [Google Scholar]
- 32.Essilfie AT, Simpson JL, Dunkley ML, Morgan LC, Oliver BG, Gibson PG, et al. Combined Haemophilus influenzae respiratory infection and allergic airways disease drives chronic infection and features of neutrophilic asthma. Thorax. 2012;67:588–599. doi: 10.1136/thoraxjnl-2011-200160. [DOI] [PubMed] [Google Scholar]
- 33.Preston JA, Beagley KW, Gibson PG, Hansbro PM. Genetic background affects susceptibility in nonfatal pneumococcal bronchopneumonia. Eur Respir J. 2004;23:224–231. doi: 10.1183/09031936.03.00081403. [DOI] [PubMed] [Google Scholar]
- 34.Preston JA, Thorburn AN, Starkey MR, Beckett EL, Horvat JC, Wade MA, et al. Streptococcus pneumoniae infection suppresses allergic airways disease by inducing regulatory T-cells. Eur Respir J. 2011;37:53–64. doi: 10.1183/09031936.00049510. [DOI] [PubMed] [Google Scholar]
- 35.Hsu AC, Barr I, Hansbro PM, Wark PA. Human influenza is more effective than avian influenza at antiviral suppression in airway cells. Am J Respir Cell Mol Biol. 2011;44:906–913. doi: 10.1165/rcmb.2010-0157OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu AC, Parsons K, Barr I, Lowther S, Middleton D, Hansbro PM, et al. Critical role of constitutive type I interferon response in bronchial epithelial cell to influenza infection. PLoS One. 2012;7:e32947. doi: 10.1371/journal.pone.0032947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 38.Singh S, Amin AV, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch Intern Med. 2009;169:21929. doi: 10.1001/archinternmed.2008.550. [DOI] [PubMed] [Google Scholar]
- 39.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 40.Séguin RM, Ferrari N. Emerging oligonucleotide therapies for asthma and chronic obstructive pulmonary disease. Expert Opin Investig Drugs. 2009;18:1505–1517. doi: 10.1517/13543780903179294. [DOI] [PubMed] [Google Scholar]
- 41.Yang IA, Fong KM, Sim EH, Black PN, Lasserson TJ. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007:CD002991. doi: 10.1002/14651858.CD002991.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29–43. doi: 10.1111/j.1476-5381.2010.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 44.Miravitlles M, Marín A, Monsó E, Vilà S, de la Roza C, Hervás R, et al. Colour of sputum is a marker for bacterial colonisation in chronic obstructive pulmonary disease. Respir Res. 2010;11:58–67. doi: 10.1186/1465-9921-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, et al. Loss of integrin αvβ6-mediated TGF-β activation causes MMP12-dependent emphysema. Nature. 2003;422:169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- 46.Ofulue AF, Ko M, Abboud RT. Time course of neutrophil and macrophage elastinolytic activities in cigarette smoke-induced emphysema. Am J Physiol. 1998;275:L1134–L1144. doi: 10.1152/ajplung.1998.275.6.L1134. [DOI] [PubMed] [Google Scholar]
- 47.McNeil HP, Shin K, Campbell IK, Wicks IP, Adachi R, Lee DM, et al. The mouse mast cell-restricted tetramer-forming tryptases mouse mast cell protease 6 and mouse mast cell protease 7 are critical mediators in inflammatory arthritis. Arthritis Rheum. 2008;58:2338–2346. doi: 10.1002/art.23639. [DOI] [PubMed] [Google Scholar]
- 48.Houghton AM, Mouded M, Shapiro SD. Common origins of lung cancer and COPD. Nat Med. 2008;14:1023–1024. doi: 10.1038/nm1008-1023. [DOI] [PubMed] [Google Scholar]
- 49.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 50.Battaglia S, Mauad T, van Schadewijk AM, Vignola AM, Rabe KF, Bellia V, et al. Differential distribution of inflammatory cells in large and small airways in smokers. J Clin Pathol. 2007;60:907–911. doi: 10.1136/jcp.2006.037002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanoirbeek JA, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, et al. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol. 2010;42:96–104. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]
- 52.Welte T, Köhnlein T. Global and local epidemiology of community-acquired pneumonia: the experience of the CAPNETZ Network. Semin Respir Crit Care Med. 2009;30:127–135. doi: 10.1055/s-0029-1202941. [DOI] [PubMed] [Google Scholar]
- 53.Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 54.Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1090–1095. doi: 10.1164/rccm.200210-1179OC. [DOI] [PubMed] [Google Scholar]
- 55.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-α in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153:530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 56.Keely S, Talley NJ, Hansbro PM. Pulmonary-intestinal crosstalk in mucosal inflammatory disease. Mucosal Immunol. 2012;5:7–18. doi: 10.1038/mi.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin K, Nigrovic PA, Crish J, Boilard E, McNeil HP, Larabee KS, et al. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J Immunol. 2009;182:647–656. doi: 10.4049/jimmunol.182.1.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamilton MJ, Sinnamon MJ, Lyng GD, Glickman JN, Wang X, Xing W, et al. Essential role for mast cell tryptase in acute experimental colitis. Proc Natl Acad Sci U S A. 2011;108:290–295. doi: 10.1073/pnas.1005758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz LB, Lewis RA, Austen KF. Tryptase from human pulmonary mast cells. Purification and characterization. J Biol Chem. 1981;256:11939–11943. [PubMed] [Google Scholar]
- 60.Miller JS, Moxley G, Schwartz LB. Cloning and characterization of a second complementary DNA for human tryptase. J Clin Invest. 1990;86:864–870. doi: 10.1172/JCI114786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynolds DS, Stevens RL, Lane WS, Carr MH, Austen KF, Serafin WE. Different mouse mast cell populations express various combinations of at least six distinct mast cell serine proteases. Proc Natl Acad Sci U S A. 1990;87:3230–3234. doi: 10.1073/pnas.87.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, et al. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J Immunol. 2008;180:4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oh SW, Pae CI, Lee DK, Jones F, Chiang GK, Kim HO, et al. Tryptase inhibition blocks airway inflammation in a mouse asthma model. J Immunol. 2002;168:1992–2000. doi: 10.4049/jimmunol.168.4.1992. [DOI] [PubMed] [Google Scholar]
- 64.McNeil HP, Reynolds DS, Schiller V, Ghildyal N, Gurley DS, Austen KF, et al. Isolation, characterization, and transcription of the gene encoding mouse mast cell protease 7. Proc Natl Acad Sci U S A. 1992;89:11174–11178. doi: 10.1073/pnas.89.23.11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hunt JE, Stevens RL, Austen KF, Zhang J, Xia Z, Ghildyal N. Natural disruption of the mouse mast cell protease 7 gene in the C57BL/6 mouse. J Biol Chem. 1996;271:2851–2855. doi: 10.1074/jbc.271.5.2851. [DOI] [PubMed] [Google Scholar]