Abstract
IL-27, an IL-12 family cytokine, has pleiotropic functions in the differentiation and expansion of CD4+ T cell subsets. Here, we discovered a novel function of IL-27. CD4+CD45RBhigh T cells from mice deficient for the α chain of IL-27 receptor failed to induce colitis in Rag−/− recipients, because of an inability of activated donor cells to survive. Interestingly, IL-27 was indispensable for prevention of colitis by regulatory T cells, also because of a defect in long-term cell survival. IL-27 affected the survival of activated T lymphocytes, rather than promoting cell proliferation, by inhibiting Fas-mediated activation-induced T cell death, acting through the STAT3 signaling pathway. The addition of IL-27 during activation resulted in an increased cell number, which was correlated with decreased activation of both caspases 3 and 8. This pro-survival effect was attributed to downregulation of FasL, as well as to the induction of the anti-apoptotic protein cFLIP. While activation induced cell death is an important mechanism for the maintenance of immunological homeostasis, protection of lymphocytes from excessive cell death is essential for effective immunity. Our data indicate that IL-27 plays a crucial role in the inhibition of activation-induced cell death, thereby permitting antigen-driven T cell expansion.
Introduction
Priming T lymphocytes through the TCR and CD28 elicits a series of events that drives cell proliferation. The induction of cell death is an important mechanism for limiting the expansion of activated T lymphocytes, a result not only of passive mechanisms, such as nutrient deprivation, but also resulting from the increased expression of molecules that trigger programmed cell death. Among the molecules involved in triggering cell death, the importance of the death receptor Fas (CD95) is demonstrated by the phenotype of the lymphoproliferation spontaneous mutation mouse strain (Faslpr mutant) (1), in which accumulated, activated T lymphocytes cause autoimmune disease. Because the activation of T cells induces both Fas and its ligand, FasL (2, 3), the induction of Fas-dependent activation induced cell death (AICD) occurs concomitantly with T cell activation. Therefore, protection of activated T cells from excessive Fas-mediated AICD is crucial for sustaining T lymphocyte expansion.
The cellular form of FLICE inhibitory protein (cFLIP) is a direct inhibitor of Fas-mediated signaling (4). cFLIP is recruited to the Fas Death Inducing Signaling Complex (DISC) and inhibits the activation of caspase 8, thereby preventing Fas-mediated AICD. cFLIP protein is expressed by various cell types, including naïve T cells (4), and as predicted from its biochemical function, cFLIP deficient T lymphocytes are more susceptible to Fas-mediated cell death (5). Furthermore, cFLIP overexpression is a proposed mechanism of the abnormal accumulation of CD4+ T cells in IL-2−/− mice (6). Collectively, these data indicate that the amount of cFLIP is a key element regulating the population size of activated T cells.
IL-27 is a heterodimeric IL-12 family cytokine composed of p28 and Epstein-Barr virus induced (EBI3) chains (7). It binds to a receptor, expressed by most hematopoietic cells, composed of IL-27 receptor (IL-27R) α (also known as TCCR or WSX-1) and a gp130 chain, which is shared by many cytokines (8-10). IL-27 has been reported to influence the differentiation of several functional CD4 T cell subtypes, including the stimulation of IL-10 producing cells and Th1 cells (7, 9, 11-14), and the inhibition of Th17 cells and Th2 cells (15-17), in addition to suppressing IL-2 expression (18). In addition to these IL-27 functions in T helper cell differentiation, early studies demonstrated its function as a growth factor for CD4+ T lymphocytes (7). Because of these important and diverse effects, we initiated an investigation of the role of IL-27-mediated signals in a T cell-dependent colitis model. As a result, we discovered an indispensable role for IL-27 in the expansion of activated CD4+ T lymphocytes, predominantly by inhibiting AICD, which we attribute not only to the inhibition of FasL expression, but also to the induction of cFLIP expression through the activation of STAT3 pathway. Interestingly, the anti-AICD function of IL-27 was also required for the maintenance of the regulatory T cell population. Together these data suggest that a major role of IL-27 in vivo in T lymphocytes is related to the survival of activated cells.
Materials and Methods
Mice
IL-27Rα−/− mice reported previously were provided by Amgen, Inc. (Thousand Oaks, CA) (19). STAT1−/−, STAT3flox/flox and gp130flox/flox mice were used with the permission of Drs. David Levy (New York University), Kiyoshi Takeda (Osaka University, Japan) and Kirk Knowlton (U.C. San Diego), respectively. CD4 Cre recombinase transgenic mice were purchased from Taconic. All other mouse strains were purchased from the Jackson Laboratory (Bar Harbor, ME). Triple knock out IL-2−/−B7-1−/−B7-2−/− mice were bred from single and double knockouts at the La Jolla Institute for Allergy and Immunology. Animal care and experimentation were consistent with the NIH guidelines and were approved by the Institutional Animal Care and Use Committee at the La Jolla Institute for Allergy and Immunology.
Antibodies and reagents
Monoclonal antibodies (mAbs) directed against the following target proteins were purchased from BD Biosciences: CD4, CD45RB, TGFβ, FasL, CD45.1, CD45.2, CD25 (3C7, used for blocking), FasL (MFL3, used for blocking) and CD28 (37.51, used for stimulation). Anti-Foxp3, anti-IL-2 neutralizing mAb (JES6-1A12), anti-CD3ε mAb (145-2C11) and biotinylated mAbs for negative selection were purchased from eBiosciences. Brilliant Violet 421 streptavidin was purchased from Biolegend. A biotinylated anti-Armenian hamster polyclonal Ab was purchased from Jackson ImmunoResearch. Alexa Fluor 488-conjugated anti-cleaved caspase 3 and rabbit IgG isotype control were purchased from Cell Signaling Technology. The FLICA caspase 8 assay kit was purchased from ImmunoChemistry Technologies. Anti-FLIP mAb, HRP conjugated anti-rabbit polyclonal Ab and recombinant mouse IL-27 were purchased from R&D Systems.
Transfer of CD4+ T cells
CD4+ cells were purified from spleens using CD4 (L3T4) microbeads (Miltenyi Biotec) according to the manufacturer’s protocol. Purified CD4+ cells were further sorted for the CD4+CD45RBhigh or CD4+CD25high subsets on a FACSVantage SE with FACSDiVa option (BD Biosciences). Groups of recipients were injected i.v. with 5-10 × 105 donor lymphocytes.
Histological scoring for colonic inflammation in the recipients
Samples of 2–3 mm tissue, obtained from the distal, middle, and proximal portions of the colon and cecum, were processed for hematoxylin and eosin (H&E) staining. Samples were coded and scored by a pathologist blinded to the conditions under which the experiment was conducted. A previously described scoring system (maximum score, 14) was used for the tissue sections (20). Scores (maximum =14) fromthree parts were averaged to represent the severity of disease.
Ex vivo FACS analyses
Large intestinal lymphocytes were isolated as described before (21), except we used collagenase type VIII instead of type IV. For ex vivo FasL staining, cells were stained by serial incubation with anti-FasL mAb, biotinylated anti-hamster Ab and Allophycocyanin-conjugated streptavidin. For in vitro FasL staining, cells were incubated with biotinylated anti-FasL mAb followed by detection with Brilliant Violet dye-conjugated streptavidin.
In vitro induction of AICD
Naïve CD4+ T lymphocytes were isolated from either B7-1−/−B7-2−/− or IL-2−/−B7-2−/−B7-2−/− mouse spleens by negative selection using anti-biotin MACS microbeads (Miltenyi Biotec), after the incubation with an antibody cocktail containing anti-CD8α (53-6.7), anti-CD11b (M1/70), anti-CD11c (N418), anti-NK1.1 (PK136), anti-Ter119 (TER-119), anti-CD45R (RA3-6B2) and anti-CD25 (7D4). CD4+CD25− cells selected from B7-1−/− B7-2−/− mice were >97% CD4+CD45RBhigh, containing <0.05% CD4+CD25+ cells (data not shown). For isolating naïve CD4+ cells from various gene deficient mice, CD4+CD45RBhigh cells were sorted, as described above. Isolated CD4+ T cells were stimulated in a high-bind microplate (Corning #3361) coated with 1.0μg/ml anti-CD3ε mAb. 0.5μg/ml soluble anti-CD28 mAb was added for costimulation. After the culture, cells were stained with 7-AAD (BD Biosciences) according to the manufacturer’s protocol.
Western blotting
Cells were stimulated in 96-well high bind microplates as described for the indicated times. Harvested cells were counted and cytoplasmic protein was extracted using the EpiQuik Nuclear Extraction Kit (Epigentek, Brooklyn, NY). To perform a cell equivalent comparison, one hundred μl per 1×107 counted cells in lysis buffer was used for each sample, and 15μl lysate was loaded onto a 12% Ready-Gel Tris-HCl gel (Biorad). Blotted polyvinylidene difluoride (PVDF) membranes were blocked with 5% skim milk and incubated with anti-FLIP mAb (1:1000) overnight at 4°C followed by an incubation with HRP conjugated-anti-rabbit Ab for 1 h and visualization with Western Blotting Luminal Reagent (Santa Cruz).
Retroviral induction of cFLIP
The plasmids for packaging the retrovirus (RV) coding cFLIP (cFLIP-RV) were generated in pMSCV plasmids co-expressing human nerve growth factor receptor (hNGFR) or GFP as infection markers (pMSCV-cFLIP-IRES-hNGFR, or pMSCV-cFLIP-IRES-GFP, respectively). For generating retroviruses, 3×105 Platinum-E retroviral packaging cells (Cell Biolabs, Inc.) were transfected with pMSCV-cFLIP, or pMSCV mock plasmid expressing only infection markers in a 6-well plate using TransIT-LT1 transfection reagent (Mirus Bio). Three day later, the culture supernatant was filtered and used for infection. For retroviral infection, a 96-well high-bind microplate was serially coated with 1.0μg/ml anti-CD3ε mAb and 50μg/ml RetroNectin (Takara Bio). After rinsing the wells with culture media, the coated wells were incubated with 50μl retrovirus-containing supernatant for at least 1 h before adding 1×105 CD4+CD45RBhigh (naïve) T cells and 0.5μg/ml anti-CD28 mAb. After 4 days of stimulation and infection, cFLIP transduced cells were identified by detection of hNGFR or GFP. On average, 25 to 30% of the harvested cells were positive for an infection marker.
Results
IL-27Rα−/− T cells failed to induce colitis
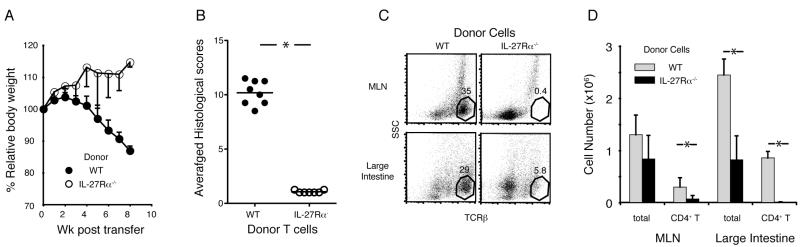
The colitis model induced by the transfer of CD4+CD45RBhigh cells to immune deficient mice has been often used to test the roles of CD4 T lymphocytes in inducing or preventing intestinal inflammation (22-24). The transferred T lymphocytes undergo expansion driven by the recognition of antigens from the microbial flora, and they differentiate into pathogenic T helper cells (25, 26). Therefore, this is a useful model for analyzing how naïve T cells go through the process of activation, expansion, differentiation and migration, leading to inflammation in the large intestine. Using IL-27Rα−/− donor CD4+CD45RBhigh cells in this model, we tested the function of IL-27-mediated signals in the induction of T cell-mediated colitis. As reported previously, recipients that received wild type (WT) CD4+CD45RBhigh cells developed chronic colitis by 8 weeks post transfer, as demonstrated by body weight loss and high histology scores (Fig. 1A and 1B). Interestingly, the recipients of IL-27Rα−/− donor cells did not exhibit symptoms of colitis, including body weight loss (Fig. 1A), diarrhea and rectal prolapse (data not shown). Histological analyses confirmed the absence of inflammation in recipients of IL-27Rα−/− CD4+CD45RBhigh cells (Fig. 1B). Separate transfer experiments revealed a profound defect in the accumulation of IL-27Rα−/− donor cells. Donor IL-27Rα−/− T lymphocytes, gated in the FACS analysis as TCRβ+ cells, were barely detectable either in the mesenteric lymph nodes (MLN) or the large intestine at 4 weeks post transfer (Figs. 1C, D). These data suggest that the deficiency of IL-27 signaling in donor T cells caused a defect in the proliferation and/or survival of the donor T cell population, which prevented the induction of colitis.
Figure 1.
IL-27Rα−/− CD4+CD45RBhigh T cells failed to induce colitis. CD4+CD45RBhigh T cells isolated from WT or IL-27Rα−/− spleens were transferred to Rag1−/− mice. The analyses of the recipients at 8 weeks (A, B) and 4 weeks (C, D) post transfer are shown. (A) The percentage bodyweight at each time point compared to day 0. Data from 8 recipients of WT T cells (filled circles) and 7 recipients of IL-27Rα−/− T cells (open circles), combined from two independent experiments, are shown. (B) Histological scores of specimens obtained from the large intestine from the same transfers as in (A). Each dot represents an average score from 4 different parts of the large intestine from a single recipient (maximum score =14). Horizontal lines indicate the mean. * P < 0.001 (two-tailed Student’s t-test). (C) Representative dot plot analyses detecting TCRβ+ cells in the MLN (top) and large intestine (bottom) from recipients of either WT (left) or IL-27Rα−/− (right) donor cells. Numbers in each plot represent the percentage of TCRβ+ side scatter low (SSClow) cells from the total cells recovered. (D) Average total cell and T lymphocyte numbers in MLN and large intestine. The CD4+TCRβ+ cell numbers were calculated from the total cell count multiplied by the percentage of T lymphocytes, obtained from flow cytometry analyses. Averages from 4 recipients each transferred with WT or IL-27Rα−/− CD4+CD45RBhigh donor cells are shown (error bars; s.d.). *P<0.005 (two-tailed Student’s t-test).
IL-27 signaling is required for the function of regulatory T cells
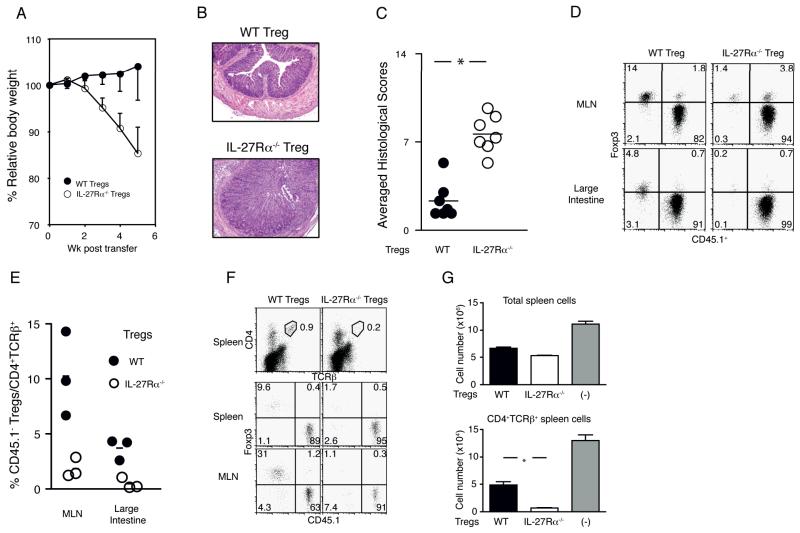
To investigate if IL-27 also affects the population size of CD4+CD25+ Foxp3+ regulatory T cells (Treg) after transfer, we compared the disease preventive effect of IL-27Rα WT and IL-27Rα−/− Treg in the colitis model. Consistent with the previous experience of many groups, co-transfer of CD4+CD45RBhigh cells with WT Treg at a 4:1 ratio was sufficient for preventing colitis (27). By contrast, all the recipients that received IL-27Rα−/− Treg, developed colitis by 5 weeks post transfer, as manifested by increased weight loss (Fig. 2A) and analysis of tissue sections (Figs. 2B). The average histology scores demonstrated severe colonic mucosal inflammation in the recipients of IL-27Rα−/− Treg, unlike the outcome when an equal number of WT Treg was transferred (Fig. 2C). To determine if there was a loss of regulatory function and Foxp3 expression following transfer, as has been reported previously in other contexts (28), we co-transferred CD45.1+ congenic CD4+CD45RBhigh cells with CD45.2+ WT or IL-27Rα−/− Treg. Four weeks post transfer, the percentage of CD45.1− Foxp3+ Treg in the total population of CD4+TCRβ+ donor-derived cells was significantly lower in the recipients of IL-27Rα−/− Treg (Fig. 2D and 2E), but there was no evidence for increased Foxp3 loss in the IL-27Rα−/− Treg (Fig. 2D). In particular, IL-27Rα−/− Treg were barely detectable in the large intestine, where activated pathogenic T cells and WT Treg migrate (29, 30). Therefore the inability of IL-27Rα−/− Treg to accumulate following transfer is not secondary to their inability to prevent intestinal inflammation. This was confirmed in a short-term experiment performed in the same context. In this case, analyzed at only 3 days post transfer, the percentage of IL-27Rα−/− CD45.1− Foxp3+ Treg in the total donor cell population was greatly decreased in the spleen and the MLN, while the percentage of WT Treg was maintained closer to the starting ratio (Fig. 2F).
Figure 2.
IL-27 is required for the maintenance of Treg population. (A-C) 4×105 CD45.1+ WT CD4+CD45RBhigh cells were co-injected to Rag1−/− mice with 1×105 CD45.2 CD4+CD25high cells sorted from WT (filled symbols) or IL-27Rα−/− (open) spleens. (A) Relative body weight compared to the original body weight at each time point, averaged from a total of seven recipients. Data shown are combined from two independent experiments. (B) Representative H&E staining of the large intestine of recipients that received CD4+CD45RBhigh cells and WT (top) or IL-27Rα−/− (bottom) Treg at 4:1 ratio. (C) Histological scores of large intestine. Each dot represents the average of 4 specimens obtained from a single recipient. Horizontal lines indicate the mean. * P < 0.001 (two-tailed Student’s t-test). (D-E) T lymphocytes were transferred as in (A). Four weeks post transfer, cells isolated from MLN and large intestine were stained for Foxp3. A representative dot plot showing Foxp3 and CD45 staining of CD4+TCRβ+ donor cells isolated from the recipients that received a 4:1 ratio of CD45.1+ CD4+CD45RBhigh T cells and either WT (left) or IL-27Rα−/− (right) CD45.2+ Treg. Numbers indicate the percentage of the cells in each quadrant. (E) Compilation of the percentages of CD45.1− cells in CD4+TCRβ+ total donor cells isolated from MLN and large intestine of the recipients that received WT (filled circles) or IL-27Rα−/− (open) Treg at the indicated ratio with CD4+CD45RBhigh naïve T cells. Small horizontal lines indicate the mean. Differences between WT and IL-27Rα−/− Treg were significant. * P < 0.001 (two-tailed Student’s t-test). (F-G) Analysis at three days after transfer to Rag1−/− recipients of 8×105 CD45.1+ CD4+CD45RBhigh cells with 2×105 WT or IL-27Rα−/− Treg (CD45.1−). (F) Top panels represent CD4 and TCRβ staining of total spleen cells at 3 days post transfer. Numbers in the plots indicate the percentage of CD4+TCRβ+ cells gated as shown. CD4+TCRβ+ splenic (middle panels) or MLN (bottom panels) cells gated as above were further analyzed for Foxp3 expression by CD45.1+ or CD45.1− cells. Percentages are indicated in each quadrant. (G) Total number of spleen cells (top) or CD4+ T cells (bottom) isolated from the recipients of either WT Treg (black bar) or IL-27Rα−/− Treg (blank bar) with WT CD45.1+ naïve CD4+ T cells, or naïve CD4+ T cells only (gray bar). Each bar indicates the average of 4 recipients in each condition (error bar; s.d.). *P < 0.001 (two-tailed Student’s t-test).
Interestingly, despite their reduced accumulation, at a very early time after transfer, the IL-27Rα−/− Treg suppressed the expansion of CD45.1+ CD4+CD45RBhigh population more potently than WT Treg (Fig. 2G). The addition of WT Treg suppressed the donor T lymphocyte population by only approximately 60% in the spleen, while IL-27Rα−/− Treg limited the expansion of the donor T cell population by 95%. These data indicate that IL-27 is involved in the expansion of Treg following transfer, although the deficiency of IL-27 signaling does not inhibit, and in fact may enhance, the regulatory function of Treg in the short term, before the population collapses.
IL-27 inhibits AICD of CD4 T lymphocytes
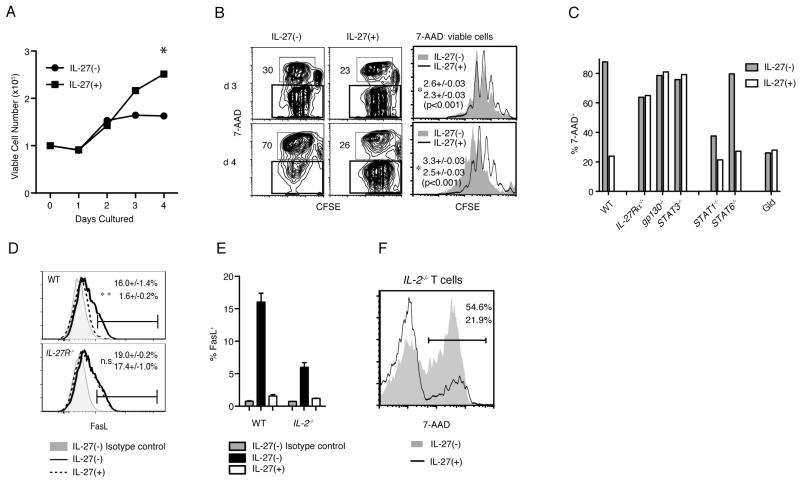
To address how IL-27 acts to facilitate T cell accumulation after transfer, we carried out in vitro culture experiments, in which purified naïve CD4 T lymphocytes were stimulated with a plate-bound anti-CD3ε mAb in the presence of a soluble anti-CD28 mAb. As shown in Fig. 3A, the addition of recombinant IL-27 (rIL-27) significantly enhanced the recovery of viable T cells after 3 days post activation. To identify a mechanism for the increased number of activated T cells in the presence of rIL-27, CFSE-labeled cells were stimulated as above, and stained with the DNA-binding dye 7-AAD. This allowed us to simultaneously analyze both proliferation and survival of the activated T lymphocytes. The degree of AICD was determined by the percentage of 7-AAD+ cells in the population of cells that underwent at least one division. As shown in Fig. 3B, while the percentage of the cells that underwent AICD was increased between day 3 and day 4 of the culture, and reached 70%, the addition of rIL-27 greatly reduced this percentage. On the other hand, the analysis of CFSE dilution for the 7-AAD− viable cells demonstrated a moderate suppressive effect of rIL-27 on T cell proliferation (Fig. 3B, right panel). These data indicate that although IL-27 has a suppressive effect on T cell proliferation, it is able to support T cell expansion by inhibiting AICD. Consistent with the in vivo results, IL-27 also had a pro-survival effect on Treg in vitro. When Treg were co-cultured with naïve CD4+ T cells and activated as described above, the percentage of 7-AAD− viable Treg was increased when IL-27 was included in the culture medium (Supplementary Fig. 1).
Figure 3.
IL-27 inhibits AICD through STAT3 signaling upstream of FasL-Fas. 1×105 CFSE-labeled naïve CD4+ T cells were stimulated with plate-bound anti-CD3ε and soluble anti-CD28 mAbs in the absence or presence of 10ng/ml rIL-27, as indicated in each panel. (A) Viable cell numbers identified with trypan blue, averaged from 3 culture wells at each time point. The figure represents data compiled from 3 independent experiments. (B) Representative contour plots at day 3 (top) and day 4 (bottom) analyzing the proliferation, measured by CFSE dilution, and cell death, by staining with 7-AAD, of the activated T cells. Numbers in each plot represent the percentage of 7AAD+ cells that underwent at least one division. Representative histograms (right) show the CFSE dilution of 7-AAD− viable cells gated in the contour plots. The numbers in the histograms are the averaged proliferation indexes of activated cells in the absence (top number in both panels) and presence (bottom numbers) of rIL-27. (C) Sorted CD4+CD45RBhigh cells from the indicated mouse spleens were stimulated as above. The percentages of 7-AAD+ cells, identified as in (B), are shown at day 4 of culture. Data are representative of 2 independent experiments. (D) Flow cytometric analyses showing FasL expression by WT (top) and IL-27R−/− (bottom) cells at day 2 of stimulation. Numbers represent the percentage of FasL+ cells in the absence (top) and presence (bottom) of rIL-27, averaged from 3 independent experiments. (E) FasL expression by WT and IL-2−/− cells, which was determined as Fig. 3D. (F) 7-AAD staining of activated IL-2−/− CD4+ T cells in the presence (solid line) and absence (shaded contour, no line) of rIL-27. Numbers represent the percentage of 7-AAD+ cells among the stimulated cells.
IL-27 inhibits Fas-mediated cell death through activation of STAT3
IL-27 has been reported to activate multiple STAT pathways (31). To determine which pathway(s) are involved in the IL-27-mediated inhibition of AICD, we stimulated sorted CD4+CD45RBhigh T cells obtained from the indicated gene deficient mice (Fig. 3C) in the presence or absence of IL-27, and measured the percent that were 7-AAD+. Cells deficient for either subunit of the IL-27 receptor provided negative controls for the effect of IL-27, consistent with a specific, receptor-mediated effect of the cytokine. IL-27 absolutely required STAT3 for inhibiting AICD, as the pro-survival effect of rIL-27 was negligible when we stimulated CD4 Cre+ STAT3flox/flox (STAT3−/−) T cells. On the other hand, STAT1 and STAT6 were dispensable for the pro-survival function of IL-27, although a lower percentage of 7-AAD+ cells in the cultures of activated STAT1−/− cells without Il-27 suggested a role for STAT1 for the induction of AICD.
Importantly, there was decreased cell death in cultures of T cells carrying a Faslgld mutation, and no further decrease when rIL-27 was added (Fig. 3C), consistent with the hypothesis that IL-27 acts through the Fas pathway. Additionally, we tested if IL-27 modifies the expression of FasL, which is induced by activation, and/or Fas. While the Fas receptor is not affected by rIL-27 (data not shown), there was activation-induced expression of FasL at day 2 that was abolished by the addition of rIL-27 in WT cells but not in IL-27R−/− cells (Fig. 3D).
The pro-survival function of IL-27 is independent of IL-2
Autocrine or paracrine IL-2 signaling is a key factor for mediating AICD and for upregulating FasL expression by activated T lymphocytes. Because IL-27 suppresses IL-2 expression (18, 32), we hypothesized that IL-27 might downregulate FasL through inhibition of IL-2 synthesis by T cells. We tested for an IL-2 independent function of IL-27 by analyzing naïve CD4 T cells from IL-2−/− mice that were also deficient for both the B7-1 and B7-2 costimulatory molecules. Deficiency for both B7 molecules prevents T cell hyperactivation in the IL-2−/− mice and therefore allows them to remain healthy. FasL induction was impaired in vitro in these activated, IL-2−/− T lymphocytes compared to controls (Fig. 3E). However, the induction of AICD in these IL-2−/− cells still was reduced by rIL-27 (Fig. 3F). Furthermore, cell death when IL-2 signaling was blocked with mAbs was also Fas mediated, and the action of IL-27 in reducing it also was STAT3 dependent (Supplementary Fig. 2). The data therefore indicate that while the inhibition of increased expression of FasL by IL-27 may contribute to its pro-survival effect, IL-27 has additional modes of action for preventing AICD, which are independent of IL-2 regulation.
T cell intrinsic pro-survival function of IL-27
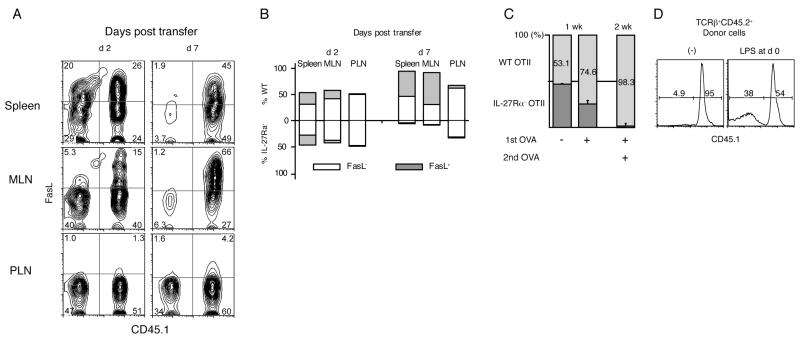
We explored if Fas-mediated signals leading to AICD also could be downregulated by IL-27 via a T cell intrinsic mechanism. To test if IL-27 regulates the Fas-mediated pathway in a cell intrinsic manner, we carried out co-transfer experiments using a mixture of WT (CD45.1+) and IL-27Rα−/− (CD45.2+) donor cells. As shown in Fig. 4A, left panels, the ratios of the co-transferred populations in each organ were maintained at approximately 1:1 in the spleen and MLN by 2 days post transfer. FasL was expressed by both WT and IL-27Rα−/− cells, although to an even greater extent by WT cells in MLN. By day 7, however, the numbers of TCRβ+ cells were significantly skewed toward the CD45.1+ WT population in the spleen and MLN (Fig. 4A, right panels), and at 2 weeks post transfer, IL-27Rα−/− donor cells were barely detectable in the MLN and the large intestine (data not shown). The difference was due to the accumulation of FasL+ activated T cells only in the IL-27Rα WT (CD45.1+) compartment, constituting 50 to 70% of the expanded WT cells (Fig. 4B). FasL+ cells were barely detectable in the surviving cells in the IL-27Rα−/− population, although FasL expressed by the WT donor cells should bind equally to the Fas receptor on cells from either population to initiate a death signal. Therefore, we conclude that activation of IL-27Rα−/− T cells, leading to induction of FasL expression, puts these cells at a strong survival disadvantage because of the absence of IL-27 signals. Interestingly, because activation of donor T cells in the Rag1−/− recipients mostly depends upon antigens originating from the intestinal microflora, T cells of either genotype that migrated to the non-draining peripheral lymph nodes (PLN) did not express FasL (Fig. 4B), consistent with the notion that FasL expression reflected the antigen-dependent activation of donor T cells. Furthermore, at least up to day 7, the ratio between the IL-27Rα−/− and IL-27Rα WT populations did not deviate far from 1:1 in the PLN, where T cell activation was not sufficient for upregulating FasL (Fig. 4B). These data suggest that IL-27-mediated signals are involved in a cell intrinsic fashion in the inhibition of AICD, but not in the maintenance of a steady state T cell population.
Figure 4.
IL-27 mediated enhancement of CD4 T cell survival involves a cell intrinsic mechanism. (A-C) 5×105 each of WT (CD45.1+) and IL-27Rα−/− (CD45.1−) cells were co-injected intravenously to Rag1−/− mice. Cells were harvested from spleen, MLN and PLN at days 2, 7 and 14. (A) Representative contour plots of CD4+TCRβ+ cells stained for FasL and CD45.1. Numbers represent the percentage of the cells in each quadrant. Data are representative of one of two independent experiments with three recipients each time. (B) Skewed expansion of the donor cell populations by 7 days post transfer. Bars represent the total (= 100%) of CD45.1+ WT (above 0) and CD45.1− IL-27Rα−/− (below 0) cells averaged from 3 recipients. The percentage of FasL+ cells in each cell population is shown as the shaded portion of the bars (C, D) 5×105 each of an equal number of sorted, naïve CD45.1+CD45.2+ WT and CD45.1−CD45.2+ IL-27Rα−/− OTII T cells were co-injected to CD45.1+CD45.2− recipients in the absence and presence of OVA as indicated. (C) Bars represent the ratios of WT and IL-27Rα−/− cells in splenic CD4+CD45.2+ donor T cells analyzed 1 week (left and middle) and 2 weeks (right) post transfer (error bars; s.d.). Mice analyzed 2 weeks post transfer received an additional intraperitoneal OVA injection at day 7. Numbers represent the percentage of WT cells in the CD4+CD45.2+ donor population. (D) Histograms (bottom panels) represent CD45.1 staining of CD45.2+TCRβ+ donor cells. Some recipients (right panel) received LPS at the same time as the donor cells. Numbers represent the percentage of CD45.1+ (WT) and CD45.1− (IL-27Rα−/−) cells, averaged from 3 mice.
To prove that the IL-27-mediated survival of activated T cells is not a phenomenon confined to immune deficient recipients, we carried out similar experiments monitoring antigen-specific OTII TCR transgenic T cells in immune competent mice. Ovalbumin (OVA)-specific IL-27Rα WT (CD45.1+CD45.2+) and IL-27Rα−/− (CD45.1− CD45.2+) OTII T cells were co-transferred to WT recipients (CD45.1+CD45.2−). OVA protein was injected together with donor cells, and 1 week post transfer, the ratio of the co-transferred donor cell populations was skewed toward CD45.1+ IL-27Rα WT donor cells in the spleen. Following a re-injection of OVA antigen, the IL-27Rα−/− cells were even more greatly out-numbered by the WT donor cells (Fig. 4C), due to a failure of the IL-27Rα−/− cells to expand (data not shown). Interestingly, the injection of LPS at the same time with donor cells partially supported the expansion of IL-27Rα−/− cells (Fig. 4D), which suggests that IL-27 function might be compensated for by other molecules that are induced by a strong activation of the innate immune system. In the absence of OVA injection, the deficiency in IL-27 signaling did not cause a difference in the maintenance of the IL-27Rα−/− OTII T cells (Fig. 4C), consistent with the hypothesis that IL-27 is important for T cell survival only following antigen-dependent activation. Furthermore, these data confirm that IL-27 inhibits the induction of AICD in a T cell intrinsic fashion, because the co-transferred IL-27Rα WT and IL-27Rα−/− populations likely were exposed to the same amount of FasL.
IL-27 inhibits Fas-downstream signaling mediated through caspases
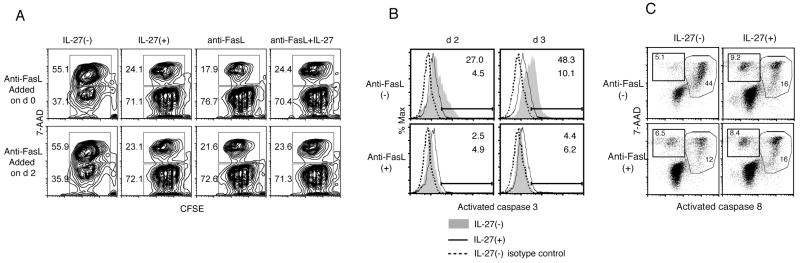
Interestingly, the addition of anti-FasL antibody at day 2 of an in vitro activation culture still inhibited AICD as effectively as IL-27 addition (Fig. 5A), indicating that Fas-mediated signaling is still required at this time. To demonstrate which aspects of the cell death process downstream of Fas are inhibited by IL-27, we first analyzed the activation of caspase 3, the general executioner caspase for programmed cell death. Both death receptor-mediated and the mitochondrial apoptotic pathways lead to activation of caspase 3 by proteolytic cleavage (33). Intracellular staining experiments demonstrated an increased amount of the activated form of caspase 3 in CD4 T cells in the absence of IL-27 (Fig. 5B). This increase was completely inhibited by the addition of an anti-FasL mAb. As we expected from the previous results, rIL-27 dramatically inhibited the activation of caspase 3, providing direct evidence for IL-27-mediated inhibition of caspase-mediated apoptotic cell death. We further analyzed the activation of caspase 8, an initiator caspase for Fas-mediated cell death, by FACS analyses using a cell-permeable compound that specifically binds to activated caspase 8. As shown in Fig. 5C, while CD4 T cells that were activated in vitro for 3 days exhibited an increased percentage of activated caspase 8+ cells, this increase was significantly inhibited by the addition of either rIL-27 or anti-FasL blocking mAb. These data clearly indicate that IL-27 inhibits the Fas-mediated signaling cascade for inducing AICD at or before the activation of caspase 8.
Figure 5.
IL-27 inhibits the activation of caspases. CFSE-labeled WT naïve CD4+ T cells were stimulated as in Figure 2 in the presence or absence of rIL-27. Anti-FasL blocking antibody was added where indicated either on day 0 (top row) or day 2 (bottom row). Numbers indicate the percentages in the respective gates for 7-AAD+ or 7-AAD− cells. (B) Intracellular staining for activated caspase 3 in cells stimulated for 2 or 3 days, in the absence or presence of rIL-27, and/or anti-FasL antibody added on day 0. Broken lines represent negative control stained with an isotype control antibody. (C) Staining for activated caspase 8 and 7-AAD in cells stimulated for 3 days, in the absence or presence of rIL-27, and/or anti-FasL antibody on day 0. Numbers in dot plots represent the percentage cells in respective gates to total cells. Data are representative from one of at least two experiments.
IL-27 induces cFLIP expression through the activation of STAT3
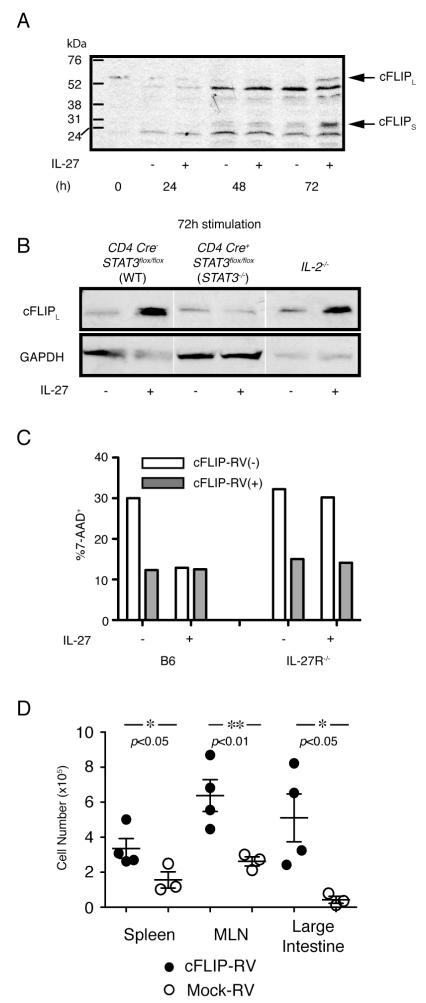
Upon ligation by FasL, the Fas receptor forms a DISC with an adaptor molecule, Fas-Associated protein with Death Domain (FADD), which recruits the inactivated form of caspase 8 (procaspase 8 or FLICE) (34). cFLIP is a homologue of caspase 8 that inhibits caspase 8 activation by competing for the binding by procaspase 8 to FADD (35). Based upon the data demonstrating suppressed activation of caspase 8 in the presence of rIL-27, despite a comparable expression of Fas receptor in the stimulated T cells, we analyzed the expression of cFLIP. Consistent with previous reports (3), cFLIP mRNA was expressed by CD4 T cells before activation, and was quickly downregulated (data not shown). Interestingly, by day 3 when FAS-mediated AICD is occurring, the addition of rIL-27 significantly restored the expression of cFLIP protein, detectable by Western blot as two forms, cFLIPL (58 kDa) and cFLIPS (30 kDa) (Fig. 6A). The amount of cFLIP protein at day 3 in the presence of IL-27 was even higher than the amount before activation. To determine if the enhanced induction of cFLIP by IL-27 requires STAT3, we activated STAT3−/− naïve CD4+CD45RBhigh cells in the presence or absence of rIL-27. As shown in Fig. 6B, the addition of rIL-27 did not restore the cFLIP protein level by 72 h of activation in STAT3−/− T cells.
Figure 6.
IL-27 increases cFLIP expression through a STAT3-mediated pathway. (A) Western blotting for detections of cFLIP protein. MACS sorted CD4+CD25− cells were stimulated as in previous figures, in the presence or absence of rIL-27. Cells were harvested at the indicated time points, washed, counted, lysed and cell lysates were loaded for electrophoresis. Arrows in (A) indicate the bands for the two alternative forms of cFLIP, cFLIPL (59kDa) and cFLIPs (30kDa) (B) Western blotting analyses for detection of cFLIPL expressed by WT, STAT3−/− and IL-2−/− T cells, in the absence or presence of rIL-27. Bands representing cFLIP expression in each condition on the same gel exposure are shown. Cells were isolated, stimulated as above and harvested at 72 h. (A, B) Representative data from at least two experiments are shown. (C) WT B6 (left) or IL-27Rα−/− (right) naïve CD4+ T cells were stimulated in the presence or absence of IL-27 as described in Fig. 3, and infected with the pMSCV-cFLIP-IRES-hNGFR RV. Bars represent the percentage of 7-AAD+ cells in the cFLIP-RV− population (open bars; hNGFR−) and cFLIP-RV infected cells (gray bars: hNGFR+) cultured in the same wells. Data are representative from 2 independent experiments. (D) IL-27Rα−/− naïve CD4+ T cells were stimulated for 4 days and infected with pMSCV-cFLIP-IRES-GFP or Mock-RV. After the stimulation, 1.5×106 FACS-sorted cFLIP-RV infected (n=4) or Mock-RV infected cells (n=3), detected as GFP+, were injected to Rag1−/− mice. Two weeks post transfer, the number of cFLIP-RV infected (closed circles) and Mock-RV infected (open circles) donor T cells in spleen, MLN and large intestine were calculated from the total cell number and the percentage of CD4+TCRβ+ cells. Horizontal bars represent the average number of 4 cFLIP-RV infected, and 3 Mock-RV infected donor cells in each organ. The p values calculated by Student’s t-tests are shown.
It has been reported that IL-2 has a role in the degradation of cFLIP protein (6), and therefore we analyzed the relationship between the expression of cFLIP and IL-27 in activated IL-2−/− CD4 T cells. In the absence of IL-27, the level of cFLIP protein at day 3 in activated IL-2−/− CD4 T cells was only slightly higher than those in WT cells (Fig. 6B). However, the presence of rIL-27 caused a significantly higher level of cFLIP protein at day 3 of the culture of IL-2−/− T lymphocytes. Finally, to prove that the lack of cFLIP induction by IL-27 was the major cause of attenuated survival of IL-27R−/− T cells, we tested if the induction of cFLIP in IL-27R−/− T cells is sufficient for preventing AICD and supporting cell expansion. As shown in Fig 6C, retroviral (RV) induction of cFLIP during in vitro stimulation reduced the percentage of 7-AAD+ cells as efficiently as IL-27 did in WT T cells. Moreover, the cells infected with the cFLIP expressing RV, but not with the empty mock RV, supported the expansion of IL-27R−/− T cells in vivo in all 3 organs analyzed 2 weeks post transfer of RV-infected cells to Rag1−/− recipients (Fig. 6D). Overall, these data further support our hypothesis that the anti-apoptotic effects on activated CD4+ T cells of IL-27 can be attributed to the induction of cFLIP synthesis, which requires STAT3 acting downstream of IL-27, processes independent of the inhibition of IL-2 synthesis by IL-27.
Discussion
IL-27 is a unique IL-12 family cytokine, in part because naïve CD4 T cells constitutively express IL-27R, and also, because downstream of the receptor multiple STAT transcription factors are activated. Perhaps because of its expression pattern, and/or its ability to activate multiple signaling pathways, IL-27 has been reported to influence several types of CD4 T cell responses, including enhancement of IFNγ and IL-10 production and inhibition of Th17 cell differentiation (7, 9, 11-16). Besides the functions influencing T helper differentiation, IL-27 also has been reported as a growth factor for CD4+ T lymphocytes. This function was demonstrated mostly in two different settings. Pflanz et al. showed that IL-27 promotes naïve CD4+ T lymphocyte proliferation when cells were stimulated in the absence of IL-2 (7). Another study demonstrated that a mouse strain that overexpresses IL-27Rα chain exhibited accumulation and hyperactivation of CD4+ T lymphocytes (36). However, neither study determined if the expansion of the CD4+ population was due to enhanced proliferation or survival. Here, however, we have reported a novel function of IL-27 in promoting the survival of activated CD4 T lymphocytes while it moderately suppressed proliferation. We observed an effect of IL-27 on activated CD4 T lymphocyte survival using in vitro cultures, and in vivo using a colitis model initiated by T cell transfer into lymphopenic mice, in which IL-27Rα−/− CD4+ CD45RBhigh T cells were unable to cause disease. IL-27Rα−/− CD4 T cells also were at a disadvantage following immunization of immune competent mice. Our experiments clearly demonstrated the importance of IL-27 also for the expansion of Foxp3+ Treg. Although at early times after transfer, IL-27Rα−/− Treg functioned in vivo to inhibit effector cell expansion, the Treg population was not maintained in the absence of IL-27-mediated signals. Because the efficiency of Treg-mediated suppression of bystander T cell expansion and effector cell differentiation are highly dependent upon the frequency of Treg in the microenvironment, recipients of IL-27Rα−/− Treg were not protected from colitis induction in the weeks after cell transfer.
While this manuscript was in preparation, another group also reported that IL-27Rα−/− CD4+ CD45RBhigh T cells were deficient in their ability to induce colitis and effectors accumulated to a reduced extent (37). In this report, Cox et al. concluded that the failure of colitis induction was attributed to the increased percentage of Foxp3+ cells converted from the naïve donor cell compartment, consistent with studies indicating that IL-27 signals inhibit expression of Foxp3 (38). Although they also demonstrated significantly less expansion of IL-27Rα−/− donor cells in the large intestine, we found a more profound effect of IL-27 on cell survival, such that donor cells were barely detectable, especially in the large intestine, regardless of Foxp3 expression. We further demonstrated that the IL-27 mediated survival effect also was required for Foxp3+ Treg expansion, consistent with other reports indicating that activated Treg can be subject to Fas-mediated AICD (39, 40). By contrast, surprisingly Cox et al. found that IL-27R−/− Treg were unimpaired following transfer to Rag−/− mice, not only for regulatory function, but even in terms of expansion in the hosts. The reason for this discrepancy is uncertain, but a strain difference could be one important factor, as our experiments were carried out in C57BL/6 mice, while the earlier studies were performed with BALB/c animals. Differences in the microflora in different animal colonies could be another factor, and these could relate to the production of IL-27 or other stimulatory cytokines by the innate immune system.
Our experiments show that the mechanism of IL-27 action depends to a large degree on the inhibition of Fas-mediated cell death. It has been well documented that the Fas-mediated induction of cell death is essential for the maintenance of immune homeostasis. We considered it possible that the pro-survival effect of IL-27 is due primarily to the inhibition of IL-2 synthesis, causing reduced FasL expression after activation. However, even the minimal induction of FasL in the absence of IL-2 was sufficient for Fas-dependent AICD, which could be completely inhibited by IL-27. These data therefore indicate a broader role for IL-27 in promoting the survival of activated CD4 T lymphocytes, in addition to the inhibition of IL-2 synthesis. Our in vivo co-transfer experiments clearly indicated a T cell intrinsic mechanism whereby IL-27 inhibits the Fas-mediated signaling pathway. In these co-transfers, we could not confirm a higher expression of FasL by IL-27Rα−/− donor cells, perhaps because of the rapid induction of AICD leading to the selective depletion of FasL+ IL-27Rα−/− activated T cells. In this study, the accumulation of WT CD4+ T cells that expressed increased amounts of FasL, particularly at the sites of T cell activation, indicated that the WT but not IL-27Rα−/− cells were protected from AICD, regardless of the expression level of FasL or the mode of Fas ligation, in cis or in trans. This led us to explore alterations in the Fas signaling pathway dependent on IL-27 that could explain the cell-intrinsic defect in the accumulation of IL-27Rα−/− T cells.
In accordance with an AICD process inhibited by IL-27 in a T cell intrinsic manner, we found that IL-27 enhanced the expression of cFLIP protein, an inhibitor of death-receptor mediated AICD, acting at late times (>2 days) after activation. Consistent with this, the activation of both caspase 8 and caspase 3 was attenuated by the addition of IL-27. In agreement with an important biologic effect of IL-27 in augmenting cFLIP synthesis, even in the absence of IL-2-dependent cFLIP degradation, IL-27 still increased cFLIP protein, which occurred relatively late, at 72h, consistent with the FasL blocking antibody results. Moreover, consistent with the findings on AICD, the effect of IL-27 on cFLIP protein was STAT3 dependent and independent of its effects on IL-2 synthesis.
In the earlier studies that explored the multivalent functions of IL-27, the survival promoting effect of IL-27 may have been missed because of the anti-apoptotic functions of several other STAT activating cytokines. For example, IL-6, which shares the gp130 chain with the IL-27R complex, prevents the apoptotic death of hepatocytes induced by Fas ligation (41, 42). Furthermore, IL-12 inhibits Fas-mediated cell death in CD8+ T cells (43). In our in vivo models in the absence of strong stimulation of innate immunity, however, the selective loss of IL-27Rα−/− T cells was very dramatic, despite the intact functions of other cytokine and cytokine receptor genes. This unique importance of IL-27 could be related to the constitutive expression of the IL-27R by naïve T cells (7), in contrast, for example, to the IL-12R, which is induced during T cell differentiation. In addition, compared to IL-6 that is induced during inflammation, IL-27 might be efficiently induced by T-DC contact through the ligation of CD40 (7). Therefore, the rapid coexpression of both IL-27 and IL-27R could be a key for the non-redundant function of IL-27 for preventing AICD in our models. In fact, the lack of antigen-induced expansion of transferred IL-27Rα−/− OTII T cells was greatly restored by the injection of LPS at the same time with OVA. This suggests that in infections providing strong activation of the innate immune system, the induction of cytokines such as IL-6 or IL-12 might provide a redundant function for IL-27-mediated survival of antigen stimulated T lymphocytes. Correspondingly, IL-27 function for the expansion of T lymphocytes might be more critical when T cells are activated with a less vigorous stimulation of the innate immune system.
The critical role for IL-27-mediated signals for the survival of antigen activated CD4 T lymphocytes, while having a minimal or no effect on the homeostasis of bulk CD4 T cell populations, suggests that this pathway could provide important targets for the manipulation of the immune response. For example, in the context of chronic autoimmunity, blockade of IL-27-mediated signals could be beneficial, while augmenting IL-27 or IL-27R signals could enhance the potency of vaccines.
Supplementary Material
Acknowledgement
We are grateful to Ms. Venetia Morris and Dr. Olga Turovskaya for technical assistance.
Supported by NIH grant P01AI089624 (M.K., H.C.). This is publication number 1343 from the La Jolla Institute for Allergy & Immunology.
Abbreviations
- (AICD)
activation induced cell death
- (DISC)
death inducing signaling complex
- (FADD)
Fas-associated protein with death domain
- (cFLIP)
FLICE inhibitory protein
- (hNGFR)
human nerve growth factor receptor
- (RV)
retroviral
Footnotes
Current affiliation of R.S.; Laboratory of Lymphocyte Differentiation, Riken Research Center for Allergy and Immunology, Yokohama, Japan.
References
- 1.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 2.Suda T, Okazaki T, Naito Y, Yokota T, Arai N, Ozaki S, Nakao K, Nagata S. Expression of the Fas ligand in cells of T cell lineage. J Immunol. 1995;154:3806–3813. [PubMed] [Google Scholar]
- 3.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–623. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 4.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 5.Zhang N, He YW. An essential role for c-FLIP in the efficient development of mature T lymphocytes. J Exp Med. 2005;202:395–404. doi: 10.1084/jem.20050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chastagner P, Reddy J, Theze J. Lymphoadenopathy in IL-2-deficient mice: further characterization and overexpression of the antiapoptotic molecule cellular FLIP. J Immunol. 2002;169:3644–3651. doi: 10.4049/jimmunol.169.7.3644. [DOI] [PubMed] [Google Scholar]
- 7.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 8.Sprecher CA, Grant FJ, Baumgartner JW, Presnell SR, Schrader SK, Yamagiwa T, Whitmore TE, O’Hara PJ, Foster DF. Cloning and characterization of a novel class I cytokine receptor. Biochem Biophys Res Commun. 1998;246:82–90. doi: 10.1006/bbrc.1998.8576. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 10.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 11.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 13.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 14.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, Yoshimura A, Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 15.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 16.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 17.Artis D, Villarino A, Silverman M, He W, Thornton EM, Mu S, Summer S, Covey TM, Huang E, Yoshida H, Koretzky G, Goldschmidt M, Wu GD, de Sauvage F, Miller HR, Saris CJ, Scott P, Hunter CA. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 18.Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. J Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, Xia M, Wakeham AC, Nishina H, Potter J, Saris CJ, Mak TW. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 20.Aranda R, Sydora BC, McAllister PL, Binder SW, Yang HY, Targan SR, Kronenberg M. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol. 1997;158:3464–3473. [PubMed] [Google Scholar]
- 21.Kim G, Turovskaya O, Levin M, Byrne FR, Whoriskey JS, McCabe JG, Kronenberg M. Spontaneous colitis occurrence in transgenic mice with altered B7-mediated costimulation. J Immunol. 2008;181:5278–5288. doi: 10.4049/jimmunol.181.8.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 24.Kim G, Levin M, Schoenberger SP, Sharpe A, Kronenberg M. Paradoxical effect of reduced costimulation in T cell-mediated colitis. J Immunol. 2007;178:5563–5570. doi: 10.4049/jimmunol.178.9.5563. [DOI] [PubMed] [Google Scholar]
- 25.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 26.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009 doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunological reviews. 2005;204:184–194. doi: 10.1111/j.0105-2896.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 30.Denning TL, Kim G, Kronenberg M. Cutting edge: CD4+CD25+ regulatory T cells impaired for intestinal homing can prevent colitis. Journal of immunology. 2005;174:7487–7491. doi: 10.4049/jimmunol.174.12.7487. [DOI] [PubMed] [Google Scholar]
- 31.Villarino AV, Huang E, Hunter CA. Understanding the pro- and anti-inflammatory properties of IL-27. J Immunol. 2004;173:715–720. doi: 10.4049/jimmunol.173.2.715. [DOI] [PubMed] [Google Scholar]
- 32.Owaki T, Asakawa M, Kamiya S, Takeda K, Fukai F, Mizuguchi J, Yoshimoto T. IL-27 suppresses CD28-mediated [correction of medicated] IL-2 production through suppressor of cytokine signaling 3. J Immunol. 2006;176:2773–2780. doi: 10.4049/jimmunol.176.5.2773. [DOI] [PubMed] [Google Scholar]
- 33.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH, Peter ME. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thome M, Tschopp J. Regulation of lymphocyte proliferation and death by FLIP. Nat Rev Immunol. 2001;1:50–58. doi: 10.1038/35095508. [DOI] [PubMed] [Google Scholar]
- 36.Takeda A, Hamano S, Shiraishi H, Yoshimura T, Ogata H, Ishii K, Ishibashi T, Yoshimura A, Yoshida H. WSX-1 over-expression in CD4(+) T cells leads to hyperproliferation and cytokine hyperproduction in response to TCR stimulation. Int Immunol. 2005;17:889–897. doi: 10.1093/intimm/dxh268. [DOI] [PubMed] [Google Scholar]
- 37.Cox JH, Kljavin NM, Ramamoorthi N, Diehl L, Batten M, Ghilardi N. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med. 208:115–123. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neufert C, Becker C, Wirtz S, Fantini MC, Weigmann B, Galle PR, Neurath MF. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 39.Fritzsching B, Oberle N, Eberhardt N, Quick S, Haas J, Wildemann B, Krammer PH, Suri-Payer E. In contrast to effector T cells, CD4+CD25+FoxP3+ regulatory T cells are highly susceptible to CD95 ligand-but not to TCR-mediated cell death. J Immunol. 2005;175:32–36. doi: 10.4049/jimmunol.175.1.32. [DOI] [PubMed] [Google Scholar]
- 40.Reardon C, Wang A, McKay DM. Transient local depletion of Foxp3+ regulatory T cells during recovery from colitis via Fas/Fas ligand-induced death. J Immunol. 2008;180:8316–8326. doi: 10.4049/jimmunol.180.12.8316. [DOI] [PubMed] [Google Scholar]
- 41.Haga S, Terui K, Zhang HQ, Enosawa S, Ogawa W, Inoue H, Okuyama T, Takeda K, Akira S, Ogino T, Irani K, Ozaki M. Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J Clin Invest. 2003;112:989–998. doi: 10.1172/JCI17970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovalovich K, Li W, DeAngelis R, Greenbaum LE, Ciliberto G, Taub R. Interleukin-6 protects against Fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2, and Bcl-xL. J Biol Chem. 2001;276:26605–26613. doi: 10.1074/jbc.M100740200. [DOI] [PubMed] [Google Scholar]
- 43.Lee SW, Park Y, Yoo JK, Choi SY, Sung YC. Inhibition of TCR-induced CD8 T cell death by IL-12: regulation of Fas ligand and cellular FLIP expression and caspase activation by IL-12. J Immunol. 2003;170:2456–2460. doi: 10.4049/jimmunol.170.5.2456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.