Abstract
The hypothesis that respiratory reflexes, such as cough, reflect the net and often opposing effects of activation of multiple afferent nerve subpopulations throughout the airways was evaluated. Laryngeal and tracheal mucosal challenge with either citric acid or mechanical probing reliably evoked coughing in anesthetized guinea pigs. No other stimulus reliably evoked coughing in these animals, regardless of route of administration and despite some profound effects on respiration. Selectively activating vagal C-fibers arising from the nodose ganglia with either adenosine or 2-methyl-5-HT evoked only tachypnea. Selectively activating vagal afferents arising from the jugular ganglia induced respiratory slowing and apnea. Nasal afferent nerve activation by capsaicin, citric acid, hypertonic saline, or histamine evoked only respiratory slowing. Histamine, which activates intrapulmonary rapidly adapting receptors but not airway or lung C-fibers or tracheal bronchial cough receptors induced bronchospasm and tachypnea, but no coughing. The results indicate that the reflexes initiated by stimuli thought to be selective for some afferent nerve subtypes will likely depend on the net and potentially opposing effects of multiple afferent nerve subpopulations throughout the airways. The data also provide further evidence that the afferent nerves regulating cough in anesthetized guinea pigs are distinct from either C-fibers or intrapulmonary rapidly adapting receptors.
Keywords: vagal, capsaicin, apnea, trigeminal, laryngeal
the cough reflex is initiated in animals and in human subjects following activation of one of perhaps several vagal afferent nerve subtypes innervating the larynx, trachea, and bronchi (10). Bronchopulmonary C-fibers and rapidly adapting receptors (RARs) are most often implicated (2, 5, 9, 17–19, 22, 35, 69, 73–75). But many well-established stimulants of intrapulmonary RARs, such as bronchial smooth muscle contraction and inspiratory efforts against a closed glottis, are very ineffective at evoking cough in most species, including humans (10). Similarly, in anesthetized animals, C-fiber activation is very ineffective at evoking cough and may even inhibit cough under some conditions (9, 10, 36, 68, 69). More recent studies in anesthetized guinea pigs implicate an afferent nerve subtype distinct from both RARs and C-fibers in cough (8, 9).
The conflicting conclusions drawn from the studies summarized above likely arise from the many different experimental designs used to study cough in animals (e.g., awake, anesthetized, decerebrate), and perhaps species differences. Semantics may also be an issue. The terms “irritant” and “rapidly adapting” have been used interchangeably to describe populations of afferent nerves with vastly different physiological properties and distinct sites of termination in the airways. We and Widdicombe previously differentiated tracheal irritant receptors (“cough receptors”) from lung irritant receptors, and yet both are often grouped into a single class and called rapidly adapting receptors (9, 66, 73). C-fiber subtypes have also been described (72). Whatever the explanations for the conflicting reports in the literature, it is certain that cough thresholds, cough intensity, and the patterning of cough are determined by the coordinated actions of multiple afferent nerve subtypes innervating the airways and lungs and likely those innervating the respiratory muscles lining the thoracic cavity (10). This is especially true in studies of cough initiated by aerosol challenge. Aerosols will activate in sequence, depending on the stimulus and the mode of delivery, upper airway afferent nerves arising from the trigeminal ganglia, pharyngeal afferent nerves, arising from vagal and glossopharyngeal sensory ganglia, laryngeal and tracheal vagal afferent nerves, bronchial and pulmonary afferent nerves of both vagal and spinal origin, and subsequently the vagal and spinal afferent nerves activated as a consequence of the thoracic, lung, and airway pressures generated during cough. It thus remains possible that C-fiber and RAR-selective stimulants may evoke coughing in anesthetized animals, provided the appropriate subpopulations are selectively activated.
In guinea pigs, afferent nerve subtypes innervating the nasal mucosa, larynx, trachea, bronchi, and intrapulmonary airways and lungs have been reasonably well defined (3, 4, 9, 20, 21, 41, 51, 59, 60, 62–65, 70, 72). Stimuli that are relatively selective for these subtypes have also been described (3, 9, 14, 15, 51, 53, 70). We hypothesize that coughing is regulated by the coordinated actions of afferent nerves throughout the airways and the thoracic afferent nerves innervating the respiratory muscles and surrounding tissues. As a first step toward better defining the role of these various airway afferents in regulating the cough reflex, we have evaluated the ability of selectively activating these various nerve subtypes on respiratory pattern and on coughing.
MATERIALS AND METHODS
Experiments were performed after approval from the institutional animal care and use committee. Male Hartley guinea pigs (250–350 g) were anesthetized with urethane (1.5 g/kg ip) and secured supine on a heating pad. A midline-incision in the neck exposed the extrathoracic trachea, which was cannulated at its caudal-most end with a bent luer stub adaptor. The tracheal cannula was attached to a length of tubing that terminated inside a water-jacketed organ bath continuously filled with humidified and warmed air. Respiratory efforts were monitored and recorded by a calibrated pressure transducer attached to a side port of the tracheal cannula. Animals were allowed to breathe spontaneously and wrapped in surgical mats upon completion of the dissection. At the end of each experiment, animals were euthanized in a chamber filled with 100% CO2. All output from the tracheal and arterial pressure transducers was digitally collected using a Biopac MP100 (Santa Barbara, CA), and stored for further analysis.
The drugs used in this study (capsaicin, bradykinin, adenosine, histamine, methacholine, albuterol, ruthenium red, citric acid, hypertonic saline, ala β-Ala8-NKA4-10, indomethacin, and propranolol) were purchased from Sigma (St. Louis, MO). The ability of stimuli to activate the various afferent nerve subtypes innervating the airways and lungs is summarized in Fig. 1.
Fig. 1.
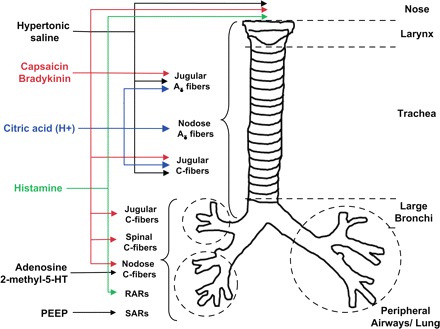
The distribution and responsiveness of airway afferent nerve subtypes are identified in guinea pigs. RARs, rapidly acting receptors; SARs, slowly adapting receptors. See text and references therein for further details.
Nasal, laryngeal and tracheal challenges.
Unless otherwise stated, challenge agents were dissolved in warm saline and applied in 100-μl aliquots to the nasal, laryngeal, or tracheal mucosa. A 1-cm length of polyethylene (PE)-60 tubing guided into the nose and attached to a syringe was used for nasal challenges. Laryngeal challenges were delivered through PE-60 tubing attached to a bent 30-gauge needle with the tip suspended in the laryngeal lumen (47). Tracheal challenges were performed by either topically applied agent or by perfusing the challenge solution continuously over the tracheal mucosa, as described previously (8, 9). In most of these experiments, the extrathoracic trachea was continuously perfused (3 ml/min) with Krebs bicarbonate buffer comprising (in mM) 118 NaCl, 5.4 KCl, 1 NaHPO4, 1.2 MgSO4, 1.9 CaCl2, 25 NaHCO3, and 11.1 dextrose (pH = 7.4). The buffer was warmed to 37°C by passing it through a water-jacketed heating coil using a syringe pump and was introduced into the trachea via a small slit, one cartilage ring rostral to the tracheal cannula. The buffer was removed at the rostral end of the trachea by gentle suction applied to a length of PE-60 tubing advanced through the larynx and out the nose. The buffer contained 3 μM of indomethacin to block formation of neuromodulatory prostanoids (46). Vehicle control experiments for both nasal and laryngeal challenges were carried out in parallel in separate animals (an unpaired experimental design), and for every intervention studied.
Intra-arterial and intravenous challenges.
The left carotid artery and right jugular vein were cannulated with heparinized PE-60 for mean arterial pressure measurements and intra-arterial administration of drugs, and for intravenous drug challenges, respectively. All challenge agents were dissolved in saline and administered in 100-μl aliquots. Concentration response curves were constructed in an ascending fashion, with doses administered at 3-or 5-min intervals.
Inhalation challenges.
Aliquots of concentrated NaCl, citric acid, and capsaicin were added to or dissolved in 2 ml of sterile diluent (distilled water or NaCl solution) to make solutions with final concentrations of 6% NaCl, 0.1 M citric acid, 0.1 mM capsaicin, and 0.1 M adenosine, respectively. Aerosols of these challenge agents were generated using an ultrasonic nebulizer (∼5-μm particle size) and delivered to the animals for 10 min. Animals were prepared as described above and placed in a Plexiglas exposure chamber continuously filled with room air. Respiratory mechanics were monitored and recorded by a calibrated pressure transducer attached to a side port of the tracheal cannula and fed to the outside of the chamber via luer stub.
Effects of experimental challenges on pulmonary inflation pressure.
Animals were first prepared with both intravenous and intra-arterial lines and with tracheal cannulation, as mentioned above, and were then paralyzed with succinylcholine chloride (1 mg/kg sc). The animal was then connected to a small animal respirator and ventilated artificially with humidified air at a rate of 60 breaths/min (46). A calibrated pressure transducer attached to a side port of the tracheal cannula, monitored tracheal pressure (pulmonary inflation pressure, PIP) during mechanical ventilation. The effects of several of the interventions described above on PIP were evaluated. The baseline PIP was 8–11 cm H2O, and results are expressed as a percentage increase in PIP.
Statistical analysis.
We used an upaired experimental design for all of the interventional and comparative studies described below. Dose-dependent effects of stimuli administered intravenously were assessed in paired experimental designs. The results are presented as means ± SE of n experiments, where n refers to a single animal. Differences among group means were assessed by unpaired t-test or by one-way ANOVA, followed by Scheffe's F-test for unplanned comparisons. A P value <0.05 was considered significant.
RESULTS
Basal respiratory rate, the effects of vagotomy, and cough responses.
The average baseline respiratory rate was 71 ± 1 breaths/min in animals with intact vagus nerves (n = 150), and was 33 ± 1 breaths/min following vagotomy (n = 14). Representative traces of the various respiratory reflexes seen in this study are shown in Fig. 2. With respect to cough (all animals studied coughed during surgical preparation of the airways), only citric acid applied topically to either the tracheal or laryngeal mucosa reliably evoked coughing in these anesthetized guinea pigs. Hypertonic saline also evoked coughing when applied topically to the laryngeal mucosa but not so readily when administered to the trachea. No other stimulus studied, regardless of route of administration and despite some profound effects on respiration, evoked coughing in anesthetized guinea pigs. Sneezing was also not observed in response to any of the stimuli or in response to any of the surgical procedures.
Fig. 2.
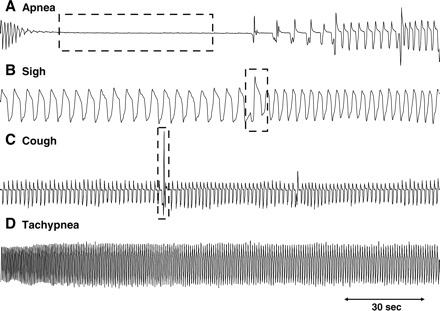
Representative traces of respiratory reflexes evoked in anesthetized guinea pigs. Depending upon the stimulus and the route of administration: apnea (A), sighs or augmented breaths (B), cough (C), and/or tachypnea (D) were evoked.
Capsaicin.
Capsaicin applied topically to the nasal mucosa (10 μM, 100 μl) evoked a marked slowing of respiratory rate in anesthetized guinea pigs, an effect that peaked within 2 min of challenge and took more than 5 min to resolve (Fig. 3A). Vagotomy greatly reduced basal respiratory rate but did not prevent the slowing of respiration evoked by nasal capsaicin challenge. Laryngeal capsaicin challenge also slowed respiratory rate with a magnitude and duration similar to that seen with nasal capsaicin challenge (Fig. 3B). As expected, on the basis of previous anatomical and physiological analyses (9, 47), the reflexes evoked from the larynx by capsaicin were not altered by bilateral cuts of either the recurrent or superior laryngeal nerves, while cutting both the recurrent and superior laryngeal nerves abolished the response. Vagotomy caudal to the nodose ganglia (leaving the superior laryngeal nerves intact) greatly reduced basal respiratory rate but did not prevent the respiratory slowing induced by laryngeal capsaicin challenge (n = 5; data not shown).
Fig. 3.
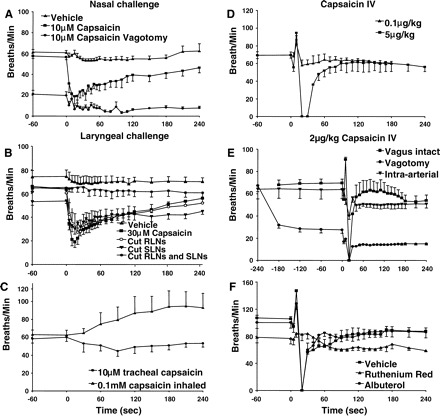
Effects of capsaicin on respiratory rate in anesthetized guinea pigs. Capsaicin was applied directly to the nasal (n = 6) (A), laryngeal (n = 5) (B), or tracheal (n = 5) (C) mucosa. Vehicle control experiments (saline) were carried out in parallel for each treatment studied (n ≥ 6). The effects of inhaled capsaicin (10 μM; n = 5) (C), intravenous capsaicin (0.1–5 mg/kg; n = 6) (D–F), and intra-arterial capsaicin (0.1–5 μg/kg; n = 5) (E) were also studied. Vagotomy markedly reduced respiratory rate (A and E) but did not prevent the respiratory slowing evoked by either nasal or intravenous capsaicin challenge (n = 4 or 5). The tachypnea associated with capsaicin challenge was, however, abolished (E; n = 5). Bilateral transection of the recurrent (RLNs) and superior (SLNs) laryngeal nerves (B) was required to abolish the respiratory slowing evoked by laryngeal capsaicin challenge (n = 5; P < 0.01). Ruthenium red (5 mg/kg iv; n = 5) but not albuterol (300 μg/ kg iv; n = 5) essentially abolished the respiratory rate changes evoked by iv 2 μg/kg capsaicin (F; n = 5; P < 0.01). None of these capsaicin challenges evoked coughing.
In contrast to the rapid and marked falls in respiratory rate evoked by nasal or laryngeal capsaicin challenge, capsaicin applied topically to the tracheal mucosa produced very modest changes in respiratory rate (Fig. 3B), with some animals displaying a fall in rate, others a slight increase in rate, and still others with little or no change in respiratory rate or pattern despite continuous perfusion of the trachea with a supramaximal concentration (30 μM) of the transient receptor potential vanilloid type 1 (TRPV1) receptor agonist. These data may imply competing reflexes evoked by subpopulations of capsaicin-sensitive vagal afferent nerves, with some, such as those innervating the larynx, promoting a slowing of respiratory rate and others producing tachypnea upon activation. Consistent with the notion that a separate population of capsaicin-sensitive tracheal afferent nerves initiates tachypnea upon activation, capsaicin inhalation (the extrathoracic trachea was cannulated for respiration at its caudal most end) consistently produced tachypnea in anesthetized guinea pigs (Fig. 3C). In contrast, selectively injecting a 100–200 μl bolus of 30 μM capsaicin into the esophageal lumen had no effect on respiration and did not evoke coughing (n = 10; data not shown).
Intravenous bolus injections of capsaicin (0.1, 1, 2, and 5 μg/kg) evoked dose-dependent, multiphasic alterations in respiratory rate, consisting of an abrupt drop in rate followed by tachypnea, then followed by a precipitous fall in respiratory rate sometimes with an associated apnea (about 10 s in duration) and then a slow return to basal rate, occasionally with a rebound tachypnea (Fig. 3, D and E). The magnitude of each of these phases of the response to intravenous capsaicin was dose dependent. Low doses of intravenous capsaicin produced mostly increases in respiratory rate, with little or no slowing of rate either before or after the short-lived tachypnea. Apnea was not seen except in response to 5 μg/kg capsaicin [higher doses were not studied to avoid the pronounced bronchospasm that would have been evoked; (51)]. Bilateral vagotomy rendered the response to intravenous capsaicin monophasic, with only modest decreases in rate observed (Fig. 3E). Ruthenium red (5 mg/kg iv) pretreatment nearly abolished the respiratory reflex effects evoked by intravenous capsaicin, while albuterol pretreatment (300 μg/ kg iv) was without effect (Fig. 3F). Similar modest, monophasic responses (decreases in rate) were seen upon intra-arterial administration of capsaicin (Fig. 3F). In separate experiments, we observed that all doses of intravenous capsaicin studied (≤5 μg/kg) induced a transient and small increase in pulmonary inflation pressure, and a decrease in mean arterial blood pressure (Table 1).
Table 1.
Effects of various stimuli administered intravenously on pulmonary inflation pressure and mean arterial blood pressure in anesthetized guinea pigs
| Stimulus | %Increase in PIP | %Decrease in MABP |
|---|---|---|
| Capsaicin | ||
| 1 μg/kg | 13±3 | −13±2 |
| 2 μg/kg | 16±3 | −24±5 |
| 5 μg/kg | 30±4 | −33±5 |
| Histamine | ||
| 2 μg/kg | 20±4 | −12±2 |
| 5 μg/kg | 34±4 | −21±5 |
| Adenosine | ||
| 1 mg/kg | 1±1 | −10±4 |
| 2 mg/kg | 1±1 | −16±3 |
| Methacholine | ||
| 2 μg/kg | 27±5 | −38±10 |
| 5 μg/kg | 35±3 | −47±10 |
Results are presented as the means ± SE percentage increase in pulmonary inflation pressure (PIP) and decrease in mean arterial blood pressure (MABP), respectively (n = 4 each). Intra-arterial histamine (5 μg/kg) had little or no effect on either PIP or MABP (n = 3; data not shown). Capsaicin, histamine, and adenosine, at the doses studied, evoked modest, statistically insignificant effects on heart rate while, as expected, methacholine evoked an acute and precipitous fall in heart rate (data not shown).
Histamine.
Nasal histamine challenge (30 μM, 100 μl) produced a short-lived and slight slowing of respiratory rate considerably smaller in magnitude and duration relative to that evoked by capsaicin (Fig. 4A). Continuously perfusing the tracheal lumen with histamine was without effect on respiration. By contrast, intravenous bolus injection of histamine (1–10 μg/kg) evoked dose-dependent tachypnea (Fig. 4B). At a dose (10 μg/kg) known to be maximal for producing bronchospasm (11), the tachypnea produced by intravenous histamine was quite pronounced (>100% increase), albeit short lived, returning to baseline within 30 s of administration. No dose of intravenous histamine produced a slowing of respiratory rate. Intra-arterial administration of histamine evoked a similar increase in respiratory rate, but with a slower onset of action (Fig. 4C). Bilateral vagotomy completely abolished the tachypnea evoked by histamine (Fig. 4B).
Fig. 4.
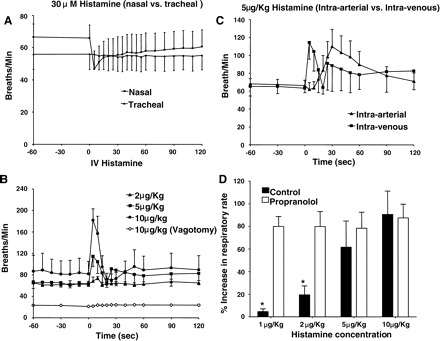
Respiratory reflexes evoked by histamine in anesthetized guinea pigs. Histamine was applied topically to the nasal or tracheal mucosa, (n = 4 or 5) (A) or given intravenously (1–10 μg/kg) (B) or intra-arterially (5 μg/kg) (C) in 100-μl aliquots (n = 4–6). Histamine significantly (P < 0.01) and dose dependently (P < 0.05) increased respiratory rate when administered intravenously or intra-arterially (B and C). Vagotomy reduced basal respiratory rate and prevented entirely the tachypnea evoked by intravenous histamine (B). D: propranolol (1 mg/kg iv; n = 5), at a concentration known to potentiate histamine-evoked bronchospasm, increased the potency of histamine to evoke tachypnea. *Statistically significant difference between the responses in control preparations relative to that evoked following propranolol pretreatment (P < 0.05). None of these histamine challenges evoked coughing.
Histamine is known to activate intrapulmonary rapidly adapting receptors in guinea pigs by virtue of its ability to evoke bronchospasm (3, 4, 9). It is also reported to be without effect on bronchopulmonary C-fibers in guinea pigs (3, 21). We confirmed that histamine evoked bronchospasm at the intravenous doses used in this study, an effect accompanied by a modest fall in mean arterial blood pressure (Table 1). We reasoned that the effects of histamine on respiratory rate were evoked indirectly, secondary to the bronchospasm evoked and the resulting activation of rapidly adapting receptors. Consistent with this hypothesis, we found that propranolol, which potentiates histamine-induced bronchospasm by counteracting the effects of bronchodilating catecholamines released upon histamine infusion (11), greatly potentiated the ability of histamine to induce tachypnea (Fig. 4D). Also consistent with the notion that bronchospasm is the stimulus that accounts for the histamine-evoked tachypnea is the observation that the bronchoconstrictor and neurokinin2 receptor-selective agonist β-Ala8-NKA4-10 (1 nmol/kg) also evoked tachypnea upon intravenous administration (Fig. 5A). But not every stimulus-inducing bronchospasm will mimic exactly the effects of histamine on respiration. Capsaicin, for example, also evokes bronchospasm, but as described above, induces a profound slowing of respiration upon intravenous administration, especially at high doses. By contrast, inhaled or intravenous adenosine induces tachypnea but has essentially no effect on respiratory mechanics in naïve guinea pigs (see Adenosine). Finally, we also studied the effects of the bronchoconstrictor methacholine, which we expected to mimic the effects of histamine on respiratory rate because it stimulates rapidly adapting receptors (9). On the contrary, at doses that evoked bronchospasm comparable to that evoked by histamine, methacholine induced profound but transient respiratory slowing and apneas (Fig. 5B). Unlike that seen in studies with histamine, these effects of methacholine were accompanied by a large, transient fall in blood pressure and heart rate (Table 1) and were completely abolished by the muscarinic receptor antagonist atropine (data not shown).
Fig. 5.
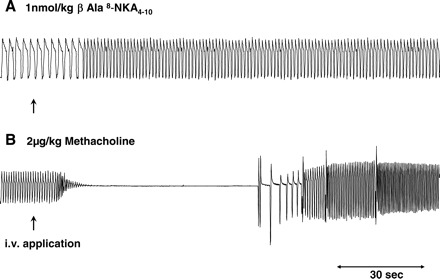
Representative traces showing the respiratory reflex effects of intravenously administered β-Ala8-NKA4-10 (A) and methacholine (B) and in anesthetized guinea pigs. Both of these challenges evoked bronchospasm but no coughing. Traces are representative of five separate experiments.
The effects of sustained positive end-expiratory pressure (PEEP) on respiration were also studied, with the expectation that this would precipitate the Hering Breuer reflex by activating slowly adapting receptors (28, 73, 74). A second air pump connected to our artificial nose with regulated airflows allowed us to control PEEP. As expected, increasing PEEP by 122 ± 14% induced a 14 ± 2% decrease in respiratory rate (n = 10).
Adenosine.
Respiratory rate in anesthetized guinea pigs was unaffected by perfusion of the tracheal lumen with 0.1 μM adenosine (Fig. 6A). Respiratory rate was also unaffected by intra-arterial administration of adenosine. However, upon inhalation, adenosine significantly increased respiratory rate, an effect that persisted for the duration of aerosol challenge (Fig. 6C). Intravenous injection of adenosine (0.01–3 mg/kg) also evoked dose-dependent tachypnea (Fig. 6B), comparable to that evoked by histamine (Fig. 4B). Unlike histamine, however, adenosine was without effect on respiratory mechanics (Table 1). Vagotomy prevented intravenous adenosine-evoked tachypnea and uncovered a modest but significant slowing of respiratory rate (Fig. 6B). These effects of adenosine may be attributable to its effects on C-fibers arising from the nodose ganglia (14, 72). Consistent with this hypothesis, bradykinin (which activates both jugular and nodose C-fibers; Fig. 6D) and 2-methyl-5-HT, which like adenosine, selectively activates nodose C-fibers (15), also evoked tachypnea upon intravenous administration. The onset of action for bradykinin was considerably slower than that of all other stimuli studied.
Fig. 6.
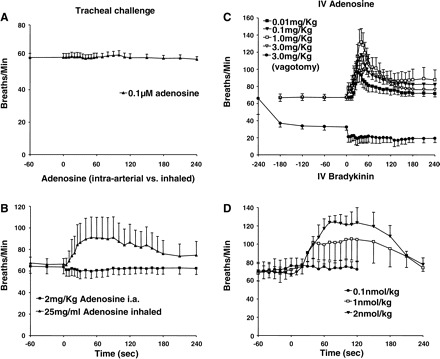
Respiratory reflexes evoked by adenosine and bradykinin in anesthetized guinea pigs. Adenosine was applied topically to the tracheal mucosa (0.1 μM; n = 4) (A) delivered by inhalation (25 mg/ml; n = 5), or intra-arterially (0.01–3 mg/kg; n = 5) (B), or administered intravenously in cumulatively increasing concentrations (0.01–3 mg/kg; n = 5) C: effects of vagotomy on the response to intravenous adenosine were also evaluated (C; n = 3). D: bradykinin was administrated intravenously in 100-μl aliquots at cumulatively increasing concentrations (0.1–5 nmol/kg) and at 5-min intervals (n = 6). Neither bradykinin nor adenosine evoked coughing in anesthetized guinea pigs regardless of dose or route of administration but both significantly (P < 0.01) and dose dependently (P < 0.05) increased respiratory rate upon intravenous administration. Adenosine also significantly increased respiratory rate following inhalation (B; P < 0.01).
Citric acid and hypertonic saline.
Both citric acid and hypertonic saline applied topically to the nasal mucosa induced respiratory slowing comparable to that evoked by capsaicin. The effects of nasal challenge with 6% NaCl on respiration were greater in magnitude than that evoked by nasal 0.1 M citric acid (Fig. 7A). This contrasted with the results following laryngeal (Fig. 7B) and tracheal mucosal (Fig. 7C) application, where the effects of acid matched or exceeded that evoked by hypertonic saline. Moreover, acid, but not hypertonic saline, reliably evoked coughing when applied topically to the laryngeal and tracheal mucosa of anesthetized guinea pigs (Fig. 7D). Inhalation challenges with either 6% hypertonic saline or 0.1 M citric acid had essentially no effects on respiration and did not reliably evoke coughing in anesthetized guinea pigs (data not shown).
Fig. 7.
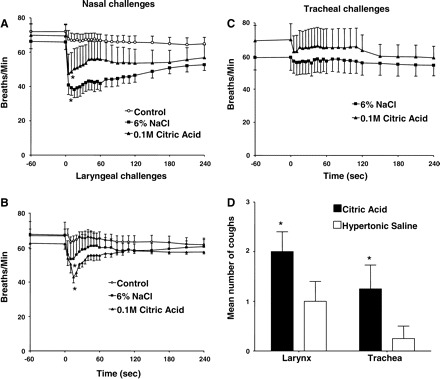
Respiratory reflexes evoked by citric acid and hypertonic saline in anesthetized guinea pigs. Citric acid (0.1 M; n = 6) or 6% NaCl (n = 5 or 6) was applied directly to the nasal (A), laryngeal (B), and tracheal (C) mucosa of anesthetized guinea pigs. Vehicle control experiments (saline) were carried out in parallel (n ≥ 6). Both citric acid and hypertonic saline significantly (*) reduced respiratory rate following nasal or laryngeal challenge relative to vehicle control (P < 0.05). D: unlike all other stimuli studied, both hypertonic saline and citric acid evoked coughing when applied topically to the tracheal or laryngeal mucosa and yet failed to significantly alter respiratory rate when applied topically to the trachea (P > 0.05). All but one animal (tracheal challenge) coughed in response to citric acid applied topically to either the tracheal (n = 8) or laryngeal mucosa (n = 8). By contrast, only 3 out of 4 and 1 out of 4 anesthetized guinea pigs coughed in response to hypertonic saline when applied topically to the larynx (n = 8) or trachea (n = 8), respectively, resulting in significantly (*) more coughing in response to acid than to hypertonic saline (P < 0.05).
Pharmacological modulation of respiratory reflex effects evoked by intravenous challenges.
To further differentiate the reflex effects evoked by capsaicin, histamine, adenosine, and bradykinin challenge, we evaluated the ability of several drug interventions to modulate the tachypnea evoked by each of these stimuli (Fig. 8). As mentioned above (Fig. 3F), ruthenium red (5 mg/kg iv) essentially abolished all respiratory reflex effects evoked by capsaicin (2 μg/kg). But this TRPV1 (and TRPA1) channel blocker had no effect on the respiratory reflexes evoked by intravenous histamine (2 μg/kg), adenosine (1 mg/kg), or bradykinin (1 nmol/kg). Also as mentioned above (Fig. 3F), 300 μg/kg iv albuterol had no effect on reflexes evoked by capsaicin. It also failed to modify the tachypnea evoked by either adenosine or bradykinin but nearly abolished the tachypnea evoked by histamine. Finally, we observed that indomethacin pretreatment (1 mg/kg iv) markedly attenuated the reflex effects evoked by bradykinin but had no effect on the tachypnea evoked by capsaicin, histamine, or adenosine.
Fig. 8.
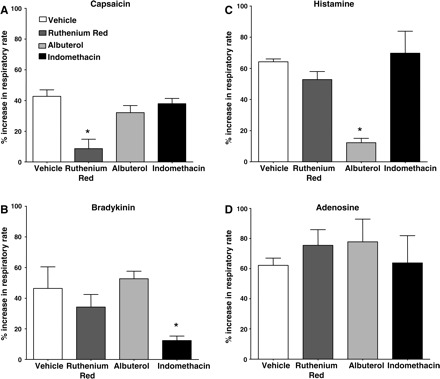
Pharmacological modulation of the tachypnea evoked by intravenous challenges with capsaicin, histamine, bradykinin, and adenosine in anesthetized guinea pigs. Capsaicin (A; 2 μg/ kg), bradykinin (B; 1 nomol/kg), histamine (C; 2 μg/kg), and adenosine (D; 1 mg/ kg) were administered intravenously in 100-μl bolus challenges following pretreatment with vehicle (n = 5), ruthenium red (5 mg/ kg iv; n = 5), albuterol (300 μg/kg iv; n = 5), or indomethacin (1 mg/ kg iv; n = 5). The peak percentage increase in respiratory rate over baseline evoked was assessed in an unpaired experimental design. An asterisk (*) denotes a statistically significant reduction in the reflex response evoked relative to that seen in animals pretreated with vehicle.
DISCUSSION
The results of the present study show that the respiratory reflexes initiated by agents known to activate airway sensory nerve subtypes depend heavily upon the stimuli and the location of challenge within the airways. With respect to cough, the effects of anesthetic on responses to airway irritants must be considered. That said, the preparation used here preserves a robust cough response to mechanical or electrical stimuli delivered to the laryngeal, tracheal, or bronchial mucosa, and to citric acid applied topically to these extrathoracic mucosal surfaces (8, 9). Despite an intact cough reflex to these stimuli, other stimuli selective for afferent nerves implicated in regulating cough, including C-fiber and rapidly adapting receptor stimulants, never evoked cough in these studies regardless of route of administration and despite some profound effects on respiration. We speculate that anesthesia selectively inhibits coughing evoked by activation of some afferent nerve subtypes (e.g., C-fibers), while other afferent nerve subtypes implicated in cough, including intrapulmonary RARs and slowly adapting receptors (SARs), are more modulatory than essential, or play no role at all in cough.
Limitations of the experimental design.
The most significant limitation of this study is that all experiments were carried out following anesthesia. Anesthesia seems to be a more appropriate approach than decerebration, given the subtle effects of anesthesia on respiratory pattern in relation to the profound alterations in respiration associated with decerebration (52). Anesthesia was necessary, as the experiments described are not feasible without some surgical preparation. But anesthesia also modifies the cough reflex. C-fiber-selective stimulants (e.g., capsaicin, resiniferatoxin, bradykinin, citric acid) evoke cough readily in conscious animals and in human subjects but are minimally effective or completely ineffective at evoking cough following anesthesia (8, 9, 18, 19, 22, 35, 36, 68, 69). Other respiratory reflexes are also likely altered following anesthesia. Sneeze, for example, has been reported in conscious guinea pigs (6), but we did not observe sneezing in response to any of the stimuli delivered to the nasal mucosa in this study nor in response to the minor surgical procedures associated with the nasal challenges. Thus, anesthesia, while necessary for mechanistic studies of respiratory reflexes, may complicate their interpretation.
The second limitation of this study is that the stimuli used, while selective for the afferent nerve subtypes targeted, may not be specific. The reflexes evoked by selective activation of one airway afferent nerve subtype are probably accompanied by altered afferent drive from other subtypes, due to the end-organ effects (e.g., bronchospasm, mucus secretion, vascular engorgement), and changes in respiratory pattern that may accompany the initial stimulus, or by sensitizing these afferents to subsequent activation (3, 43). This point leads to the third major limitation of this study, specifically, that we do not know for certain which afferent nerves are activated by the stimuli used. That we did not record from afferent nerves in this study is justified, as this would have necessitated some disruption of the afferent innervation, thus preventing or at least attenuating any reflexes that might have occurred. But the location of the afferents that are activated is not always certain, especially upon intravenous, intra-arterial, or even inhalation challenges. Despite these limitations, we believe that the multiple stimuli and interventions studied, the multiple locations challenged and the extensive published literature on airway afferent nerve excitability in guinea pigs have allowed us to draw several meaningful and occasionally unexpected conclusions from the results.
Reflex effects evoked by C-fibers.
C-fibers are found throughout the airways and lungs of guinea pigs (3, 21, 41, 51, 59, 62–65, 70, 72). Nasal C-fiber subtypes arising from the trigeminal ganglia have been suggested based on their responsiveness to histamine and capsaicin (70). Three C-fiber subtypes have been described in the lower airways. C-fibers arising from the superior vagal (jugular) ganglia innervate all airways from the larynx to the intrapulmonary bronchi and lungs. Two other populations of C-fibers arising from the inferior vagal (nodose) ganglia and from dorsal root ganglia (T1–T4) are found predominantly in the intrapulmonary airways and lungs (41, 51, 72).
Capsaicin activates all airway C-fiber subtypes in the guinea pigs, while having little direct effect on airway mechanoreceptors (3, 51, 70, 72). By contrast, adenosine and 5-HT3 receptor agonists are selective for nodose C-fibers (14, 15, 72). Histamine may activate a subset of nasal C-fibers, but is without effect on C-fibers in the lower airways (3, 21, 63, 70). Our results largely fit what we would have predicted from published data. Capsaicin was active in all locations studied (nasal, laryngeal, tracheal mucosa, inhaled, intravenous, intra-arterial), evoking increases and/or decreases in respiratory rate, depending on the route of administration and to some extent, the dose administered. Histamine had a modest effect when applied topically to the nasal mucosa, but was without effect when applied topically to the trachea. Adenosine and 2-methyl-5-HT had no effect on respiration when applied topically to the tracheal mucosa (where few if any nodose C-fibers terminate), but induced profound tachypnea when delivered intravenously or by inhalation (while having no effect when administered intra-arterially). Comparable results have been reported in human subjects challenged with intravenous adenosine, while in anesthetized rats, adenosine induces only apnea (7, 42). That vagotomy abolished the responses to adenosine and that tachypnea was the only response evoked by adenosine suggests that nodose C-fiber activation is responsible at least, in part, for the tachypnea associated with capsaicin challenge.
It was surprising that the effects of intravenous bradykinin did not mimic the effects of intravenous capsaicin. Rather, intravenous bradykinin produced only tachypnea, whereas intravenous capsaicin evoked tachypnea at low doses but respiratory slowing and even apneas as the doses increased. Both capsaicin and bradykinin activate airway jugular and nodose C-fibers and dorsal root ganglia afferent neurons (3, 51, 72). Perhaps in vivo capsaicin activates a subset of afferents not accessible to or unresponsive to intravenous bradykinin. For example, capsaicin may act on extrapulmonary afferent nerves, which could be largely protected from intravenous bradykinin due to metabolism of the peptide by pulmonary endothelial angiotensin-converting enzyme (13, 24). When inhaled, capsaicin (this study) and bradykinin (48) induce only tachypnea in anesthetized guinea pigs, while intra-arterial capsaicin induces only respiratory slowing (albeit considerably smaller than that evoked by intravenous administration). Perhaps the pattern and kinetics of C-fiber subtype activation induced by bradykinin and capsaicin determine the reflex effects evoked (61).
Afferent nerve-mediated responses to capsaicin and bradykinin are reported to be mediated entirely or partly through activation of the ion channel TRPV1 (1, 37, 67). TRPA1 channel activation is also implicated in bradykinin-evoked signaling (1). We found that ruthenium red nearly abolished capsaicin-evoked reflexes while this TRPV1 and TRPA1 channel blocker was without effect on the reflexes evoked by bradykinin. These data are consistent with the published literature regarding bradykinin-induced effects at afferent nerve endings but inconsistent with some studies of bradykinin-induced activation of afferent nerve cell bodies (12, 38, 44, 58). Also consistent with the published literature was the pronounced effects of indomethacin on bradykinin-evoked reflexes (11, 45, 55).
Another surprising observation was the modest effects of tracheal capsaicin challenge on respiratory rate. A single 100-μl aliquot of capsaicin applied topically to the laryngeal mucosa induced a precipitous fall in respiratory rate that took several minutes to recover. By contrast, continuous (10 min) perfusion (2 ml/min) of nearly the entire length of the extrathoracic trachea with a supramaximal concentration of capsaicin (3 μM) produced only modest and somewhat unpredictable effects on respiration. It seems unlikely that mucosal access was an issue, as we have used this same prep or a slight modification to study the effects of many drugs applied topically to the tracheal mucosa (8, 46). It also seems unlikely that the rate of drug application or the number of afferent nerves activated is any less in the trachea than in the larynx, given the length of the trachea perfused with challenge solution. Rather, it would seem that different populations of afferents are activated in the trachea and larynx, or that the central terminations or neurochemistry of laryngeal and tracheal C-fibers are different (34, 50). We previously found that the jugular ganglia neurons innervating the extrathoracic trachea are primarily C-fibers, whereas the larynx is innervated by jugular C-fibers but also the capsaicin-sensitive A∂-fibers of the jugular ganglia for which no physiological function has been identified (9, 59). It seems unlikely, however, that C-fibers arising from the nodose ganglia contribute to the response to tracheal capsaicin, given the lack of effect of either adenosine or 2-methyl-5-HT when applied topically to the tracheal mucosa.
With respect to cough, we thought it possible that C-fiber stimulation in some location would evoke coughing in anesthetized guinea pigs, despite many studies (including ours) showing that broader acting C-fiber stimuli given by inhalation or intravenously have not evoked cough in anesthetized animals (8, 9, 36, 68, 69). Regardless of the stimulus and route of administration, however, C-fiber activation did not cause cough in anesthetized guinea pigs. These data further highlight the importance of anesthesia in studying cough and the selective effects of anesthesia on C-fiber-dependent cough (10).
Reflex effects evoked by mechanoreceptors.
All airway and lung-afferent nerves, including C-fibers are mechanically sensitive (4, 9, 10, 21, 28, 59, 62, 65, 71). Stimuli such as capsaicin or histamine may thus initiate reflexes secondary to their effects on mucus secretion, the vasculature, or airway smooth muscle. That said, C-fibers are generally less responsive to mechanical stimulation, and most nasal afferent nerves are thought to be C-fibers (3, 21, 28, 59, 72). Similarly, laryngeal and tracheal afferents in guinea pigs are either jugular Aδ or C-fibers, which are mostly unresponsive to mechanical stimulation, and the nodose Aδ-fibers, which are exquisitely sensitive to punctuate mechanical stimuli but insensitive to smooth muscle contraction or extreme changes (increases or decreases) in luminal pressure (9, 21, 59). Other than punctuate mechanical stimuli, then, reflexes attributed to changes in lower airway and/or vascular luminal pressures or smooth muscle contractions are more likely to arise from intrapulmonary airways. Not surprisingly, then, we found that contraction of the trachealis with histamine had no effect on respiratory rate.
Mechanically sensitive RARs and SARs have been localized to the intrapulmonary airways and lungs of guinea pigs and all other mammalian species similarly studied (3, 4, 9, 10, 28, 33, 60, 73). RARs are relatively selectively activated by bronchial smooth muscle contraction or any intervention that decreases lung compliance, whereas SARs are selectively activated by sustained lung stretch, as with PEEP or breath hold. We found that the bronchoconstrictors histamine and β-Ala8-NKA4-10 both evoked tachypnea but not coughing. The effects of histamine on respiration were dose dependent and mirrored by its effects on lung mechanics. Propranolol, which enhances the bronchospasm induced by histamine by blocking the bronchodilating effects of endogenous catecholamines (11), also enhanced the effects of histamine on respiration, while the bronchodilator albuterol inhibited the effects of histamine. It seems unlikely that the effects of histamine are C-fiber dependent, as histamine does not activate C-fibers in guinea pigs (3, 21), and catecholamines would actually increase C-fiber excitability (26). It also seems unlikely that SARs play a prominent role in the response to histamine. Enhanced SAR activity would be expected to slow respiration, as with PEEP. Rather, the effects of histamine are best explained by an effect on RARs.
Bronchoconstrictors, including histamine, substance P, neurokinin A, thromboxane, and leukotriene D4 are very ineffective at inducing cough in awake or anesthetized guinea pigs, or awake human subjects (10). Bronchoconstrictors are, however, very effective stimulants of RARs. Thus, although it is routinely stated that RARs regulate coughing in all species, the evidence in guinea pigs and in humans is, in fact, highly suspect (2, 9, 10, 18). We think the problem is not misinterpretation of published results, but rather, a misuse of the term rapidly adapting receptor. This classification (rapidly adapting) has in our opinion proven useful only in differentiating afferents responsive to lung inflation (RARs and SARs). The tracheal afferents essential for regulating cough in anesthetized guinea pigs are completely unresponsive to airway inflation/distending pressures and yet adapt rapidly to punctuate mechanical stimulation (9, 10). To call these afferents RARs based on their rapid adaptation to a punctuate mechanical stimulation or to acid challenge while ignoring their insensitivity to changes in luminal pressure and other characteristics that differ from intrapulmonary RARs would be misleading. Indeed, intrapulmonary RARs may adapt slowly to airway smooth muscle contraction or lung deflation. The afferent nerves regulating cough in anesthetized guinea pigs seem to be similar to the tracheal “irritant” receptors or “cough receptors” described by Widdicombe and colleagues (66, 73). Whatever they are called, we speculate that such an afferent nerve subtype, distinct from RARs and C-fibers, innervate the airways of all species that cough.
Responses to hypertonic saline and acid.
Inhalation of cold, dry air can be an effective initiator of respiratory reflexes in susceptible patients (23). Cold, dry air, or exercise may induce airway responses is through evaporative water loss and effects on airway surface liquid tonicity. Hypertonic saline is known to be a somewhat selective stimulant of capsaicin-sensitive nerves in guinea pigs (20, 53) but can also activate capsaicin-insensitive nerves [particularly at high (≥6% wt/vol) concentrations]. Water is reported to activate all airway sensory nerves (20). Precisely how changes in tonicity activate airway sensory nerves is unknown. Increases or decreases in airway surface liquid tonicity may prompt volume regulation responses in cells at or near the mucosal surface, including the peripheral terminals of afferent nerves innervating the mucosa. TRP channels (e.g., TRPV2, TRPV4, TRPM7) have been implicated in these responses (54). Whatever the mechanism, we found that hypertonic saline induced robust reflex responses in the nose and larynx, similar to that evoked by capsaicin, and also, although less reliably, evoked coughing when applied topically to the tracheal or laryngeal mucosa. Hypertonic saline may also prompt mediator release from airway epithelial cells and glands, including eicosanoids such as 15-HETE, an endogenous activator of TRPV1 (31, 40, 47), and bradykinin, which may signal, in part, through TRPV1 and TRPA1 activation (1, 12, 76).
Airway pH can also change dramatically in disease and may change acutely and severely with aspiration or microaspiration, as in gastroesophageal reflux (16, 29, 30). We have previously studied the cough reflex evoked by topical application of acid to the tracheal mucosa (8). We extended these observations in the present study, showing that acid applied topically to the nasal or laryngeal mucosa also promotes respiratory reflexes, including cough when applied to the larynx. Acid activates airway sensory nerves by two mechanisms, one involving TRPV1 gating on airway C-fibers and a second TRPV1-independent mechanism, perhaps involving acid sensing ion channels (8, 25, 39). Regarding gastroesophageal reflux disease (GERD) and cough, it is interesting that capsaicin administered selectively to the esophageal lumen did not evoke cough and had no effects on respiration (present study) and yet still sensitized the cough reflex evoked by simultaneous tracheal stimulation in anesthetized guinea pigs (unpublished observations).
Implications for the cough reflex.
The results of this study confirm our previous studies suggesting that only the myelinated, capsaicin-insensitive vagal afferent nerves arising from the nodose ganglia and innervating the laryngeal, tracheal, and bronchial mucosa are both sufficient and necessary for sustaining the cough reflex in anesthetized guinea pigs. We also confirmed that C-fiber activation, regardless of which subpopulation (location, ganglionic origin) is activated, cannot evoke coughing in anesthetized guinea pigs. The evidence implicating C-fibers in cough in conscious guinea pigs and in conscious human subjects is overwhelming. It remains unclear, however, why anesthesia selectively inhibits C-fiber-dependent cough. It is also unclear which C-fiber subtype mediates cough in awake animals and which C-fiber subtype can inhibit cough when activated, an effect well documented in anesthetized animals.
The most common causes of chronic cough in humans not attributable to smoking are asthma, GERD, and upper airway inflammatory diseases (e.g., allergic rhinitis). Although each of these diseases are multifactorial and not appropriately described by a single feature, asthma, GERD, and rhinitis are characterized at least, in part, by reversible lower airways obstruction, acid in the esophagus, and inflammation and mucus accumulation in the sinuses, respectively (32, 49). In the present study, we found that we could not evoke coughing upon intraesophageal capsaicin injection (which, like acid, works through TRPV1), intranasal administration of a variety of stimuli, including histamine, capsaicin, and acid, nor bronchospasm induced by histamine, methacholine, or several other agents. With respect to GERD and upper airway diseases, we speculate that activation of afferents innervating these diseased tissues and organs may sensitize the cough reflex through CNS interactions, lowering the threshold for cough evoked directly from the airways and increasing the urge to cough. We, and others, have published supportive evidence for this concept (27, 47, 48, 56, 57). Regarding airways obstruction and cough, however, the present study provides further evidence that airway obstruction secondary to airway smooth muscle contraction may be in no way related to cough, with these end-organ effects being regulated independently and without apparent consequence on one another (other than the potential effects of airways obstruction on peak airflows during cough). By extension and with the additional evidence discussed above, it has become increasingly difficult to continue to state that the afferent nerves regulating cough are RARs, or more specifically, those well characterized intrapulmonary afferent nerves activated during the dynamic phase of inspiration, that rapidly adapt to sustained lung inflation, and are also activated by bronchial smooth muscle contraction and negative airway luminal pressures (4, 9, 10, 28).
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41: 849–857, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Bergren DR. Chronic tobacco smoke exposure increases cough to capsaicin in awake guinea pigs. Respir Physiol 126: 127–140, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bergren DR. Sensory receptor activation by mediators of defense reflexes in guinea-pig lungs. Respir Physiol 108: 195–204, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Bergren DR, Sampson SR. Characterization of intrapulmonary, rapidly adapting receptors of guinea pigs. Respir Physiol 47: 83–95, 1982 [DOI] [PubMed] [Google Scholar]
- 5.Bolser DC, DeGennaro FC, O'Reilly S, McLeod RL, Hey JA. Central antitussive activity of the NK1 and NK2 tachykinin receptor antagonists, CP-99,994 and SR 48968, in the guinea-pig and cat. Br J Pharmacol 121: 165–170, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brozmanova M, Calkovsky V, Plevkova J, Bartos V, Plank L, Tatar M. Early and late allergic phase related cough response in sensitized guinea pigs with experimental allergic rhinitis. Physiol Res 55: 577–584, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Burki NK, Dale WJ, Lee LY. Intravenous adenosine and dyspnea in humans. J Appl Physiol 98: 180–185, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Canning BJ, Farmer DG, Mori N. Mechanistic studies of acid-evoked coughing in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 291: R454–R463, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 557: 543–558, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol 152: 223–242, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Canning BJ, Reynolds SM, Mazzone SB. Multiple mechanisms of reflex bronchospasm in guinea pigs. J Appl Physiol 91: 2642–2653, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Carr MJ, Kollarik M, Meeker SN, Undem BJ. A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals. J Pharmacol Exp Ther 304: 1275–1279, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Chodimella V, Skidgel RA, Krowiak EJ, Murlas CG. Lung peptidases, including carboxypeptidase, modulate airway reactivity to intravenous bradykinin. Am Rev Respir Dis 144: 869–874, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Chuaychoo B, Lee MG, Kollarik M, Pullmann R Jr, Undem BJ. Evidence for both adenosine A1 and A2A receptors activating single vagal sensory C-fibres in guinea pig lungs. J Physiol 575: 481–490, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuaychoo B, Lee MG, Kollarik M, Undem BJ. Effect of 5-hydroxytryptamine on vagal C-fiber subtypes in guinea pig lungs. Pulm Pharmacol Ther 18: 269–276, 2005 [DOI] [PubMed] [Google Scholar]
- 16.DelGaudio JM. Direct nasopharyngeal reflux of gastric acid is a contributing factor in refractory chronic rhinosinusitis. Laryngoscope 115: 946–957, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Dicpinigaitis PV, Alva RV. Safety of capsaicin cough challenge testing. Chest 128: 196–202, 2005 [DOI] [PubMed] [Google Scholar]
- 18.El-Hashim AZ, Amine SA. The role of substance P and bradykinin in the cough reflex and bronchoconstriction in guinea-pigs. Eur J Pharmacol 513: 125–133, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Forsberg K, Karlsson JA, Theodorsson E, Lundberg JM, Persson CG. Cough and bronchoconstriction mediated by capsaicin-sensitive sensory neurons in the guinea-pig. Pulm Pharmacol 1: 33–39, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Fox AJ, Barnes PJ, Dray A. Stimulation of guinea-pig tracheal afferent fibres by non-isosmotic and low-chloride stimuli and the effect of frusemide. J Physiol 482: 179–187, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox AJ, Barnes PJ, Urban L, Dray A. An in vitro study of the properties of single vagal afferents innervating guinea-pig airways. J Physiol 469: 21–35, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatti R, Andre E, Amadesi S, Dinh TQ, Fischer A, Bunnett NW, Harrison S, Geppetti P, Trevisani M. Protease-activated receptor-2 activation exaggerates TRPV1-mediated cough in guinea pigs. J Appl Physiol 101: 506–511, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Gilbert IA, McFadden ER Jr. Airway cooling and rewarming. The second reaction sequence in exercise-induced asthma. J Clin Invest 90: 699–704, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg R, Osman GH Jr, O'Keefe EH, Antonaccio MJ. The effects of captopril (SQ 14,225) on bradykinin-induced bronchoconstriction in the anesthetized guinea pig. Eur J Pharmacol 57: 287–294, 1979 [DOI] [PubMed] [Google Scholar]
- 25.Gu Q, Lee LY. Characterization of acid signaling in rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 291: L58–L65, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu Q, Lin YS, Lee LY. Epinephrine enhances the sensitivity of rat vagal chemosensitive neurons: role of β3-adrenoceptor. J Appl Physiol 102: 1545–1555, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanacek J, Tatar M, Widdicombe J. Regulation of cough by secondary sensory inputs. Respir Physiol Neurobiol 152: 282–297, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol 127: 113–124, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Horvath I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, Becher G, van Beurden WJ, Corradi M, Dekhuijzen R, Dweik RA, Dwyer T, Effros R, Erzurum S, Gaston B, Gessner C, Greening A, Ho LP, Hohlfeld J, Jobsis Q, Laskowski D, Loukides S, Marlin D, Montuschi P, Olin AC, Redington AE, Reinhold P, van Rensen EL, Rubinstein I, Silkoff P, Toren K, Vass G, Vogelberg C, Wirtz H. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J 26: 523–548, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Hunt J, Yu Y, Burns J, Gaston B, Ngamtrakulpanit L, Bunyan D, Walsh BK, Smith A, Hom S. Identification of acid reflux cough using serial assays of exhaled breath condensate pH (Abstract). Cough 2: 3, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA 97: 6155–6160, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irwin RS, Baumann MH, Bolser DC, Boulet LP, Braman SS, Brightling CE, Brown KK, Canning BJ, Chang AB, Dicpinigaitis PV, Eccles R, Glomb WB, Goldstein LB, Graham LM, Hargreave FE, Kvale PA, Lewis SZ, McCool FD, McCrory DC, Prakash UB, Pratter MR, Rosen MJ, Schulman E, Shannon JJ, Smith Hammond C, Tarlo SM. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 129: 1S–23S, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joad JP, Kott KS, Bonham AC. Nitric oxide contributes to substance P-induced increases in lung rapidly adapting receptor activity in guinea-pigs. J Physiol 503: 635–643, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalia M, Mesulam MM. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol 193: 467–508, 1980 [DOI] [PubMed] [Google Scholar]
- 35.Karlsson JA, Fuller RW. Pharmacological regulation of the cough reflex—from experimental models to antitussive effects in man. Pulm Pharmacol Ther 12: 215–228, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Karlsson JA, Sant'Ambrogio FB, Forsberg K, Palecek F, Mathew OP, Sant'Ambrogio G. Respiratory and cardiovascular effects of inhaled and intravenous bradykinin, PGE2, and PGF2 alpha in dogs. J Appl Physiol 74: 2380–2386, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Kim BM, Lee SH, Shim WS, Oh U. Histamine-induced Ca2+ influx via the PLA(2)/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci Lett 361: 159–162, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Kollarik M, Undem BJ. Activation of bronchopulmonary vagal afferent nerves with bradykinin, acid and vanilloid receptor agonists in wild-type and TRPV1-/- mice. J Physiol 555: 115–123, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol 543: 591–600, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koskela H, Di Sciascio MB, Anderson SD, Andersson M, Chan HK, Gadalla S, Katelaris C. Nasal hyperosmolar challenge with a dry powder of mannitol in patients with allergic rhinitis. Evidence for epithelial cell involvement. Clin Exp Allergy 30: 1627–1636, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Kummer W, Fischer A, Kurkowski R, Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience 49: 715–737, 1992 [DOI] [PubMed] [Google Scholar]
- 42.Kwong K, Hong JL, Morton RF, Lee LY. Role of pulmonary C fibers in adenosine-induced respiratory inhibition in anesthetized rats. J Appl Physiol 84: 417–424, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Lee LY, Morton RF. Histamine enhances vagal pulmonary C-fiber responses to capsaicin and lung inflation. Respir Physiol 93: 83–96, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Lee MG, Macglashan DW Jr, Undem BJ. Role of chloride channels in bradykinin-induced guinea pig airway vagal C-fibre activation. J Physiol 566: 205–212, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayer S, Izydorczyk I, Reeh PW, Grubb BD. Bradykinin-induced nociceptor sensitisation to heat depends on cox-1 and cox-2 in isolated rat skin. Pain 130: 14–24, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Mazzone SB, Canning BJ. An in vivo guinea pig preparation for studying the autonomic regulation of airway smooth muscle tone. Auton Neurosci 99: 91–101, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Mazzone SB, Canning BJ. Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am J Physiol Regul Integr Comp Physiol 283: R86–R98, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol 569: 559–573, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morice AH, Fontana GA, Sovijarvi AR, Pistolesi M, Chung KF, Widdicombe J, O'Connell F, Geppetti P, Gronke L, De Jongste J, Belvisi M, Dicpinigaitis P, Fischer A, McGarvey L, Fokkens WJ, Kastelik J. The diagnosis and management of chronic cough. Eur Respir J 24: 481–492, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Morris JL, Konig P, Shimizu T, Jobling P, Gibbins IL. Most peptide-containing sensory neurons lack proteins for exocytotic release and vesicular transport of glutamate. J Comp Neurol 483: 1–16, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Oh EJ, Mazzone SB, Canning BJ, Weinreich D. Reflex regulation of airway sympathetic nerves in guinea-pigs. J Physiol 573: 549–564, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohi Y, Yamazaki H, Takeda R, Haji A. Phrenic and iliohypogastric nerve discharges during tussigenic stimulation in paralyzed and decerebrate guinea pigs and rats. Brain Res 1021: 119–127, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Pedersen KE, Meeker SN, Riccio MM, Undem BJ. Selective stimulation of jugular ganglion afferent neurons in guinea pig airways by hypertonic saline. J Appl Physiol 84: 499–506, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Pedersen SF, Nilius B. Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymol 428: 183–207, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Petho G, Derow A, Reeh PW. Bradykinin-induced nociceptor sensitization to heat is mediated by cyclooxygenase products in isolated rat skin. Eur J Neurosci 14: 210–218, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Plevkova J, Brozmanova M, Pecova R, Tatar M. Effects of intranasal capsaicin challenge on cough reflex in healthy human volunteers. J Physiol Pharmacol 55 Suppl 3: 101–106, 2004 [PubMed] [Google Scholar]
- 57.Plevkova J, Kollarik M, Brozmanova M, Revallo M, Varechova S, Tatar M. Modulation of experimentally-induced cough by stimulation of nasal mucosa in cats and guinea pigs. Respir Physiol Neurobiol 142: 225–235, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Qin C, Farber JP, Miller KE, Foreman RD. Responses of thoracic spinal neurons to activation and desensitization of cardiac TRPV1-containing afferents in rats. Am J Physiol Regul Integr Comp Physiol 291: R1700–R1707, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Ricco MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol 496: 521–530, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sano M, Tsubone H, Sugano S. Vagal afferent activities and respiratory reflexes during drug-induced bronchoconstriction in the guinea pig. J Vet Med Sci 54: 989–998, 1992 [DOI] [PubMed] [Google Scholar]
- 61.Schertel ER, Adams L, Schneider DA, Smith KS, Green JF. Rapid shallow breathing evoked by capsaicin from isolated pulmonary circulation. J Appl Physiol 61: 1237–1240, 1986 [DOI] [PubMed] [Google Scholar]
- 62.Sekizawa S, Tsubone H. Nasal mechanoreceptors in guinea pigs. Respir Physiol 106: 223–230, 1996 [DOI] [PubMed] [Google Scholar]
- 63.Sekizawa S, Tsubone H, Kuwahara M, Sugano S. Does histamine stimulate trigeminal nasal afferents? Respir Physiol 112: 13–22, 1998 [DOI] [PubMed] [Google Scholar]
- 64.Sekizawa S, Tsubone H, Kuwahara M, Sugano S. Nasal receptors responding to cold and l-menthol airflow in the guinea pig. Respir Physiol 103: 211–219, 1996 [DOI] [PubMed] [Google Scholar]
- 65.Sekizawa SI, Tsubone H. Nasal receptors responding to noxious chemical irritants. Respir Physiol 96: 37–48, 1994 [DOI] [PubMed] [Google Scholar]
- 66.Sellick H, Widdicombe JG. Stimulation of lung irritant receptors by cigarette smoke, carbon dust, and histamine aerosol. J Appl Physiol 31: 15–19, 1971 [DOI] [PubMed] [Google Scholar]
- 67.Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci USA 99: 10150–10155, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tatar M, Sant'Ambrogio G, Sant'Ambrogio FB. Laryngeal and tracheobronchial cough in anesthetized dogs. J Appl Physiol 76: 2672–2679, 1994 [DOI] [PubMed] [Google Scholar]
- 69.Tatar M, Webber SE, Widdicombe JG. Lung C-fibre receptor activation and defensive reflexes in anaesthetized cats. J Physiol 402: 411–420, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor-Clark TE, Kollarik M, MacGlashan DW Jr, Undem BJ. Nasal sensory nerve populations responding to histamine and capsaicin. J Allergy Clin Immunol 116: 1282–1288, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Tsubone H, Sant'Ambrogio G, Anderson JW, Orani GP. Laryngeal afferent activity and reflexes in the guinea pig. Respir Physiol 86: 215–231, 1991 [DOI] [PubMed] [Google Scholar]
- 72.Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol 556: 905–917, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Widdicombe JG. Receptors in the trachea and bronchi of the cat. J Physiol 123: 71–104, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Widdicombe JG. Respiratory reflexes from the trachea and bronchi of the cat. J Physiol 123: 55–70, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiang A, Uchida Y, Nomura A, Iijima H, Sakamoto T, Ishii Y, Morishima Y, Masuyama K, Zhang M, Hirano K, Sekizawa K. Involvement of thromboxane A(2) in airway mucous cells in asthma-related cough. J Appl Physiol 92: 763–770, 2002 [DOI] [PubMed] [Google Scholar]
- 76.Yoshihara S, Geppetti P, Hara M, Linden A, Ricciardolo FL, Chan B, Nadel JA. Cold air-induced bronchoconstriction is mediated by tachykinin and kinin release in guinea pigs. Eur J Pharmacol 296: 291–296, 1996 [DOI] [PubMed] [Google Scholar]


