Abstract
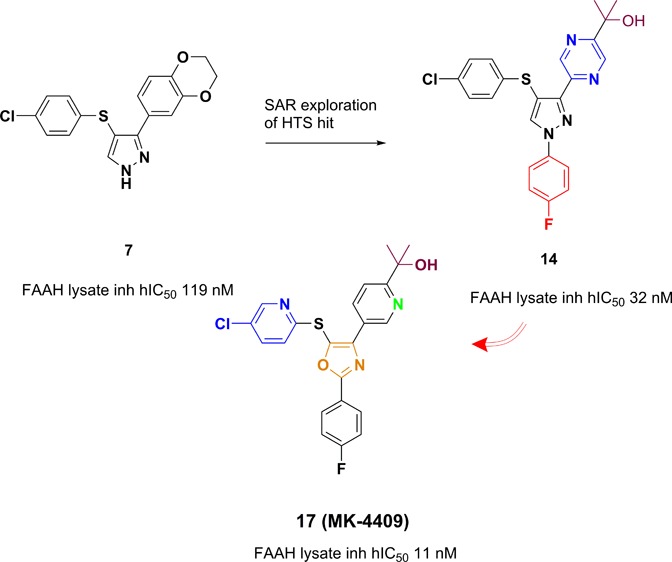
We report herein the identification of MK-4409, a potent and selective fatty acid amide hydrolase (FAAH) inhibitor. Starting from a high throughput screening (HTS) hit, medicinal chemistry efforts focused on optimizing of FAAH inhibition in vitro potency, improving the pharmacokinetic (PK) profile, and increasing in vivo efficacy in rodent inflammatory and neuropathic pain assays.
Keywords: Fatty acid amide hydrolase, FAAH, oxazole, pyrazole, neuropathic pain, inflammatory pain, MK-4409, enzyme, inhibitor, CNS
Fatty acid amide hydrolase (FAAH) is an integral, membrane-bound enzyme responsible for the breakdown of fatty acid ethanolamide (FAE) signaling molecules, such as the endocanabinoid arachidonyl ethanolamide (anandamide, AEA), N-palmitoyl ethanolamide (PEA), and N-oleoyl ethanolamide (OEA). FAAH is a member of the serine hydrolase amidase signature family, which utilizes an unusual serine–serine–lysine catalytic triad.1,2 Inhibition of FAAH leads to elevated levels of these endogenous FAEs, which act on cannabinoid receptors implicated in the suppression of pain transmission.3 Levels of these FAEs were shown to be significantly elevated in FAAH knockout (KO) mice as compared to wild-type controls.4 Both genetic knockout of FAAH and pharmacological modulation of FAAH activity demonstrated reduced sensitivity to pain.5 Thus, FAAH inhibitors are expected to provide therapeutic benefits in the management of inflammatory and neuropathic pain.6−9
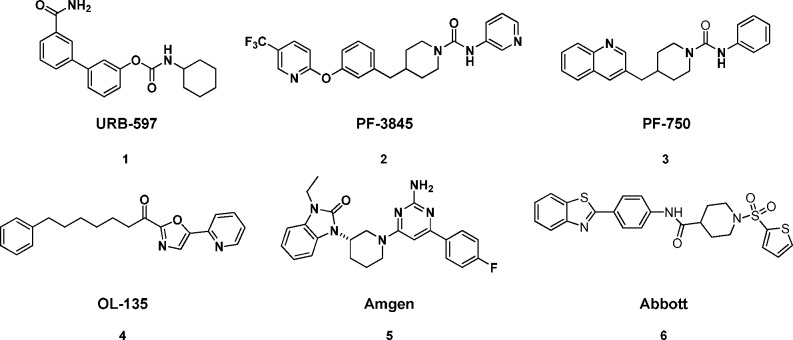
Several classes of covalent and noncovalent FAAH inhibitors have been reported to date (Figure 1). Several covalent FAAH inhibitors that irreversibly inhibit FAAH by carbamylation of Ser241 have been reported and are exemplified by URB-597,10−12 PF-3845, and PF-750.8 A second subclass of FAAH inhibitors are the keto-oxazole class of FAAH inhibitors as exemplified by OL-135,13 which reversibly forms an enzyme-stabilized hemiketal through a particularly reactive electrophilic carbonyl. More recently, however, several scaffolds have been disclosed as reversible noncovalent modifying inhibitors of FAAH. Aminopyrimidine 5(14) and sulfonamide 6(15) are chief among these novel classes of FAAH inhibitors. This approach, in our view, would decrease potential safety concerns over the creation of a long-lived covalent adduct between a compound and the FAAH enzyme.
Figure 1.
Known covalent and noncovalent modifying FAAH inhibitors.
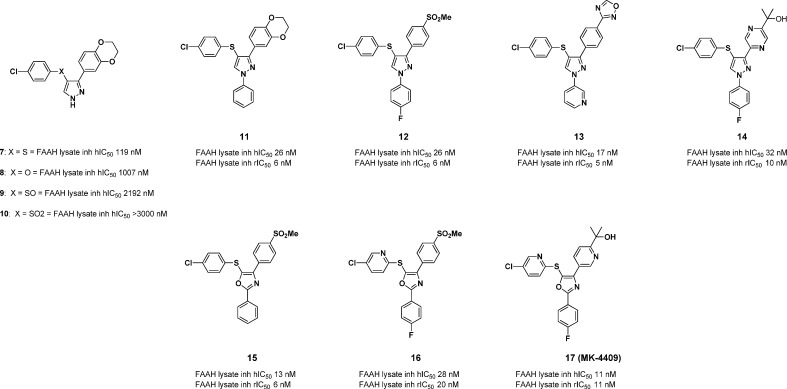
In this letter, we report the identification of a fully reversible, noncovalently modifying FAAH inhibitor derived from a high throughput screening campaign culminating in pyrazole 7 (Figure 2). Pyrazole 7 suffers from moderate potency as well as poor preclinical PK.16 However, when dosed iv, the compound did show moderate efficacy (at 30 mg/kg) in the complete Freund’s adjuvant (CFA) inflammatory pain model, which generated considerable interest within the team.17 In addition, because of a lack of the presence of any obvious covalent modifying site, we directed our studies toward optimization of this pyrazole lead series to identify a selective, potent analogue with desirable pharmacokinetic properties in three preclinical species (rat, dog, and rhesus). Coupled with excellent in vitro potency (<10 nM), we sought to identify a compound that was fully CNS penetrant, maintained enzyme inhibition over a 24 h time frame, displayed selectivity over related enzymes, and possessed desirable efficacy in the CFA pain model at 10 mg/kg or less.
Figure 2.
Key FAAH inhibitor pyrazole/oxazole compounds.
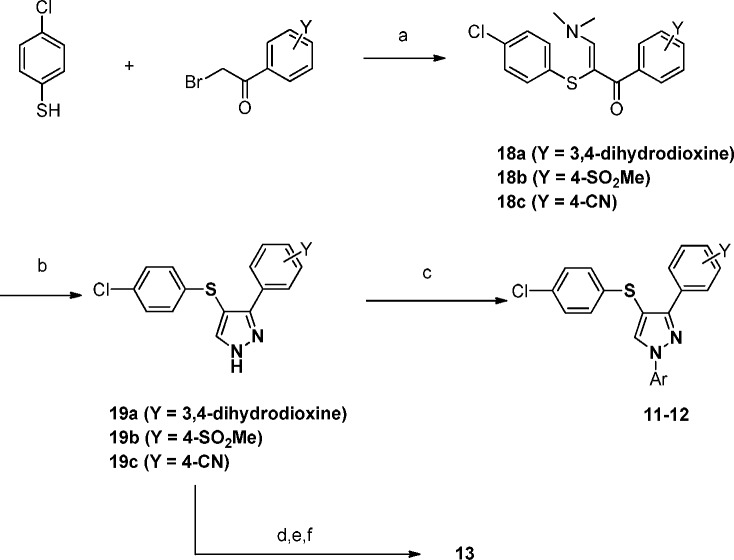
The synthesis of our key analogues was achieved through several different routes. To synthesize pyrazole analogues 11 and 12, 4-chlorothiophenol was condensed with a bromoacetophenone of interest (Scheme 1). The resulting adduct was then treated with DMF-DMA and hydrazine to afford the desired pyrazoles 19a–c. Arylation at the N-1 position was accomplished through a copper mediated coupling to yield analogues 11 and 12. Alternatively for analogue 13, copper mediated C–N bond formation was achieved on the corresponding 4-CN phenyl derivative 18c followed by 1,2,4-oxadiazole formation under standard conditions to afford 13.18
Scheme 1. Synthesis of Analogues 11–13.
Reagents and conditions: (a) Hunig’s base, THF, rt; then DMF-DMA, 100 °C, 59% (18a), 86% (18b), 78% (18c); (b) hydrazine, EtOH, 120 °C, 89% (19a), 86% (19b), 83% (19c); (c) for 11, iodobenzene, K2CO3, K3PO4, CuI, trans-N,N′-dimethyl-1,2-cyclohexane diamine, CH3CN, 77%, for 12, 4-F iodobenzene, K2CO3, K3PO4, CuI, trans-N,N′-dimethyl-1,2-cyclohexane diamine, CH3CN, 59%; (d) 3-iodopyridine, K2CO3, CuI, d,l-proline, DMSO, 80 °C, 96%; (e) HO-NH2 (aq), EtOH, 80 °C, quant; (f) triethylorthoformate, TsOH, 80 °C, 94%.
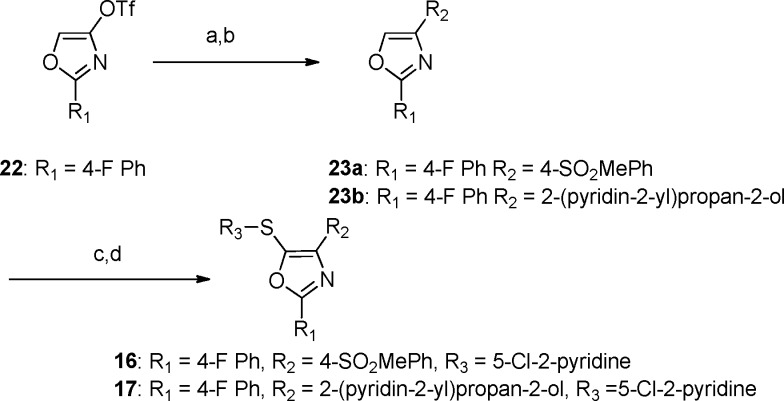
For the oxazole derivatives 16 and 17, the corresponding oxazole triflates as previously described by Panek and co-workers19 were used as the key starting material for each analogue (Scheme 2). One-pot boronate formation and subsequent Suzuki coupling was accomplished for compounds 23a and 23b.20 Halogenation and cross coupling with the desired aryl thiol gave rise to 16 and 17.21
Scheme 2. Synthesis of 16 and 17.
Reagents and conditions: (a) bis-pinacolatodiboron, Pd(dppf)Cl2·CH2Cl2, dppf, KOAc, dioxane, 100 °C; (b) R2X, Pd(PPh3)Cl2, 2.0 M Na2CO3, dioxane, 90 °C, 67% over 2 steps; (c) NBS, TFA, DCM, rt, 60–66%; (d) R3SH, K2CO3, NMP, 120 °C, 69–85%.
Our initial structure–activity relationship (SAR) efforts focused primarily upon improvements in potency and pharmaceutical properties in the pyrazole-containing system 7. Given the low molecular weight and moderate in vitro potency, we believed that numerous modifications could be made to compound 7 to optimize for drug-like properties. We initially sought to eliminate the sulfur-linked aryl ring. Heteroatom modification of the S atom led to a deleterious effect on in vitro potency for FAAH inhibition (S = 119 nM and O = 1007 nM) for compound 8 (Figure 2). Oxidation of the S atom to its various higher oxidation states also led to a reduction in potency (S(O) = 2192 nM, SO2 = >3000 nM) as seen in compounds 9 and 10. This data led us to abandon this strategy and to explore capping of the free pyrazole with various groups. A simple Ph ring directly attached to the N-1 of the pyrazole led to a greater than 4-fold improvement to the intrinsic FAAH potency as exemplified by compound 11.
We next turned our attention to scission of the dioxane ring, which was deemed imperative in order to improve upon the PK properties of this series of compounds. Initial data pointed out clearly that the para position has the greatest potential and flexibility toward structural diversification. Methyl sulfone and 1,2,4-oxadiazole replacements for the dioxane ring were potency-enhancing. The optimized N-subtituent is shown in compound 12 as a para-F phenyl or as a 3-pyridyl group as exemplified in compound 13. Subsequent SAR studies showed importantly that a 4-fold improvement was realized upon introduction of various heterocyclic tertiary alcohol moieties at the 4-position such as that shown in compound 14 while maintaining the para-F phenyl ring, which capped the pyrazole. Compound 7, 11, 12, and 13 were further evaluated for off-target activities as well as pharmacokinetic (PK) studies (Table 1). An off-target activity detected in a Pan Laboratories screen for compounds 12–14 was human vesicular monoamine transporter (VMAT) inhibition. This is of particular concern since this particular transporter is centrally expressed.22
Table 1. In Vitro Data of Key FAAH Inhibitorsa.
| compd | human FAAH lysate IC50 (nM) | RatFAAH lysate IC50 (nM) | MK-499 binding assay, Ki, (nM)b | human VMAT EC50 (nM) |
|---|---|---|---|---|
| 7 | 119 | 125 | 1538 | ND |
| 11 | 27 | 8 | >30000 | ND |
| 12 | 16 | 6 | 637 | 363 |
| 13 | 13 | 4.8 | 5214 | 137 |
| 14 | 32 | 10 | 740 | 1157 |
| 15 | 13 | 6 | 28 540 | ND |
| 16 | 28 | 21 | 2500 | 14000 |
| 17 | 11 | 11 | 1254 | >20000 |
Assay protocols are provided in the Supporting Information. In vitro assay results are the average of at least three duplicates.
Binding displacement of [35S] MK-499.
Additionally, the pyrazole lead series was also plagued by hERG activity. Potent compounds were screened against the hERG K+ channel using a binding assay that measures the displacement of [35S]MK-499, a well characterized hERG K+ channel blocker.23 During this process, we had noticed that upon introduction of an oxazole central ring as a replacement for the pyrazole, a noticeable improvement in MK-499 binding potency was observed. The closely related analogues 12 and 15, which both maintained a phenyl methyl sulfone moiety as the active pharmacophore, had MK-499 binding values of 637 nM and 28 μM, respectively, implying that the oxazole core had quite an advantageous effect on MK-499 over the pyrazole (Table 1). In addition, with the data collected for compounds 16 and 17, it was readily apparent that VMAT inhibition had serendipitously improved upon modification of the central core to the oxazole. Lastly, compound 17 took advantage of the fact that throughout our earlier SAR, we found that optimization of the core thioether ring system to a 2-pyridyl substitutent not only helped with PK, but also improved in vivo efficacy in our current pain models. Several of these compounds were further studied in vivo for rat PK and efficacy based on these improvements in the in vitro profiles.
Compounds 12 and 13 were dosed for rat PK studies at 1 mg/kg iv and 2 mg/kg po (Table 2), and both compounds showed similar half-life, clearance, and volume of distribution. A 3-fold lower bioavailability for compound 13 to compound 12 may be attributed to poor oral absorption despite the improved solubility of compound 13 as compared to compound 12 (FaSSIF solubility for 12 was measured to be 0.003 mg/mL versus 0.027 mg/mL for compound 13). Furthermore, changes of the pharmacophore from the p-methanesulfonyl benzene ring to a pyrazine ring substituted with a 4-tertiary alcohol (14) provided a rather substantial boost in terminal half-life while maintaining the oral exposure observed in compound 12. Lastly, compound 17, which combined optimized substituents by replacing the 4-pyrazinyl tertiary alcohol moiety with a 4-pyridyl tertiary alcohol, along with the thiopyridine substitutent drastically improved the rat PK to levels, which could translate to QD dosing for in vivo efficacy models. To further characterize these compounds, they were dosed in our in vivo efficacy pain models.
Table 2. Rat PK data on FAAH inhibitors 12, 15, 16 and 17.
| compd | AUCNpod ((μM h kg)/mg) | half-life (h) | Cl (mL/min·kg) | Vd (L/kg) | F (%)e |
|---|---|---|---|---|---|
| 12a,b | 1.06 | 1.79 | 34 | 8.4 | 100 |
| 13a,c | 0.39 | 2.57 | 34 | 7.8 | 35 |
| 14a,b | 1.07 | 4.9 | 33.8 | 15.8 | 96 |
| 17a,c | 2.2 | 4.3 | 20 | 6.2 | 120 |
Dosing vehicle DMSO/PEG400/H2O 20/60/20, 1 mg/kg iv.
Dosing vehicle Imwitor/Tween 1/1, 2 mg/kg po.
Dosing vehicle PEG400/Tween80/H2O 40/10/50, 2 mg/kg po.
Dose normalized AUCN.
Bioavailability.
In order to ascertain in vivo efficacy, inflammatory pain was induced by intraplantar injection of complete Freund’s adjuvant (CFA) into the hind paws of rats.17 Mechanical allodynia was assessed at 1 and 3 h post a single oral dose. This study was performed on compounds 13, 14, 16, and 17 (Table 3). A major objective was to try to achieve reasonable efficacy in the CFA at a moderate dose (10 mg/kg) as a criterion for a potential development compound. Compound 13 when dosed at 30 mg/kg showed efficacy at the 1 and 3 h time points (49 and 62% inhibition, respectively). However, the VMAT and MK-499 binding activity led us to focus on other more structurally diverse analogues. The dose responses of compound 14 at both 10 and 30 mg/kg indicated comparable efficacy to compound 13 at both doses, which suggested to us that the heterocyclic tertiary alcohol modification not only improved PK but also provided comparable in vivo efficacy to compound 13 but at a lower dose. More importantly, with both an improved off-target profile and PK profile, compound 17 also showed reduction of inflammatory pain on par with the standard of care, naproxen, at both 10 and 30 mg/kg in a model for inflammatory pain. Taking compound 17 one step further, we decided to evaluate its efficacy in the rat spinal nerve ligation model (SNL) for neuropathic pain.24
Table 3. CFA Data Key FAAH Compounds.
| compd | dosea (mg/kg) | % reversal of allodyniab 1 h time point | % reversal of allodynia 3 h time point |
|---|---|---|---|
| 13 | 30 | 49 | 62 |
| 14 | 30 | 58 | 40 |
| 14 | 10 | 42 | 38 |
| 16 | 10 | 46 | 44 |
| 17 | 30 | 70 | 71 |
| 17 | 10 | 49 | 51 |
Oral dose in mg/kg.
Average percent reversal is calculated as (postdose – predose)/(preinjury – predose) for each rat (n = 6).
Using the SNL model as a measure of efficacy for neuropathic pain, compound 17 was evaluated in vivo at doses of 3, 30, and 100 mg/kg with excellent response observed at all three doses (Figure 3). The efficacy of compound 17 at a level of roughly 50% analgesia when compared to standards of care Pregabalin, Gabapentin, and Duloxetine was shown to be quite favorable. A secondary effect of dosing these standards of care into rodents is loss in cognition due to drowsiness and motor skill impairment. In order to ensure that a FAAH inhibitor would not cause the same type of motor skill impairment seen with other analgesics, we also implemented the rota-rod assay as a secondary assay along with the SNL as a standard counterscreen for these undesirable cognitive effects. Compound 17 not only provided 50% reversal of allodynia at a low dose (3 mg/kg) but also did not show any effect in the concomitant roto-rod assay at doses as high as 100 mg/kg (Figure 4). This was in line with URB-597 (1), a previously described covalent modifying FAAH inhibitor that required a 21 mg/kg dose to achieve comparable efficacy to 17 in our hands.
Figure 3.
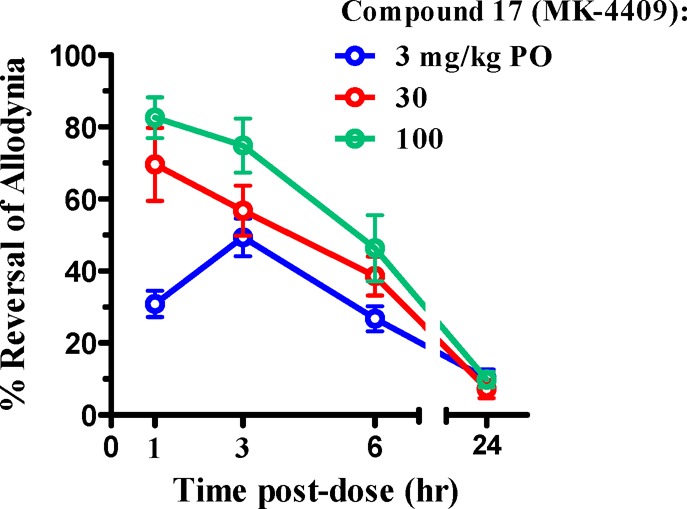
SNL efficacy of compound 17 (MK-4409).
Figure 4.
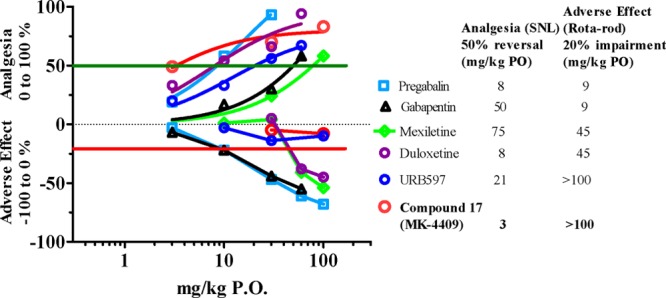
SNL efficacy of compound 17 (MK-4409) at 50% reversal plotted against rota-rod impairment at 20% (adverse effect) compared to current standards of care or positive controls.
On the basis of the promising in vivo and in vitro data generated for compound 17, a full PK profile was obtained across preclinical species.25 The resulting PK parameters supported the development of a QD compound in human. Additionally, free fraction was measured across species (rat, 2.9%; dog, 1.2%; rhesus, 0.8%; human, 1.6%) indicating a high level of plasma protein binding for compound 17. An additional concern we had for this mechanism was tachyphylaxis. To alleviate this concern, we initiated a chronic paradigm in the CFA pain model versus a CB1 and CB2 agonist, WIN 55,212-2 known to produce CB1 desensitization and loss of antinociception under chronic dosing.26
The pan CB agonist WIN 55,212-2 loses its analgesic properties in the CFA assay at roughly day 5. Compound 17, however, continues to show excellent efficacy out to 10 days (Figure 5). Plasma exposures were also obtained at both the 3 and 24 h time points following dosing at 30 mg/kg. One interesting observation is that compound 17 reduced edema observed in the animals over several days, which may contribute to its anti-inflammatory properties.
Figure 5.
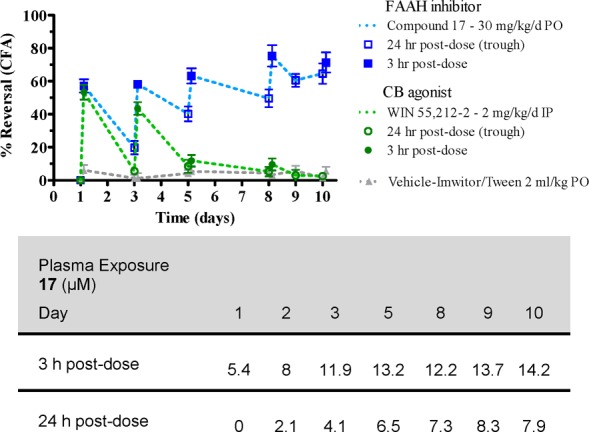
Chronic dosing of compound 17 versus WIN 55,212-2 in CFA pain model along with plasma exposure.
In conclusion, we discovered compound 17 (MK-4409) as a potent and selective reversible noncovalent modifying FAAH inhibitor. MK-4409 showed excellent efficacy in numerous preclinical models including the CFA and SNL pain models. In addition, no cognitive effects were observed for this brain penetrant FAAH inhibitor (measured rat brain/plasma ratio was 2). A chronic dosing paradigm in the CFA assay showed overall anti-inflammatory activity and no tachyphylaxis apparent after 10 days of dosing. MK-4409 (17) was accepted as a development candidate based on the promising preclinical profile. Additional human clinical data will be reported in subsequent publications.
Acknowledgments
The author wishes to thank the department of Laboratory Animal Resources for their assistance in animal dosing and sampling.
Supporting Information Available
Synthetic procedures and analytical data of selected FAAH inhibitors and conditions for all the biological assays. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- Giang D. K.; Cravatt B. F. Molecular characterization of human and mouse fatty acid amide hydrolases. Proc. Natl. Acad. Sci. U.S.A. 1997, 94, 2238–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt B. F.; Lerner R. A.; Boger D. L. Structure determination of an endogenous sleep-inducing lipid, cis-9-octadecenamide (oleamide): A synthetic approach to the chemical analysis of trace quantities of a natural product. J. Am. Chem. Soc. 1996, 118, 580. [Google Scholar]
- Lichtman A. H.; Leung D.; Shelton C. C.; Saghatelian A.; Hardouin C.; Boger D. L.; Cravatt B. F. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J. Pharm. Exp. Ther. 2004, 311, 441–448. [DOI] [PubMed] [Google Scholar]
- Cravatt B. F.; Demarest K.; Patricelli M. P.; Bracey M. H.; Giang D. K.; Martin B. R.; Lichtman A. H. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman A. H.; Shelton C. C.; Advani T.; Cravatt B. F. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor mediated phenotypic hypoalgesia. Pain 2004, 109, 319–327. [DOI] [PubMed] [Google Scholar]
- Gaetani S.; Dipasquale P.; Romano A.; Righetti L.; Cassano T.; Piomelli D.; Cuomo V. The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs. Int. Rev. Neurobiol. 2009, 85, 57–72. [DOI] [PubMed] [Google Scholar]
- Gobbi G.; Bambico F. R.; Mangieri R.; Bortolato M.; Campolongo P.; Solinas M.; Cassano T.; Morgese M. G.; Debonnel G.; Duranti A.; Tontini A.; Tarzia G.; Mor M.; Trezza V.; Goldberg S. R.; Cuomo V.; Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 18620–18625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. S.; Stiff C.; Lazerwith S. E.; Kesten S. R.; Fay L. K.; Morris M.; Beidler D.; Liimatta M. B.; Smith S. E.; Dudley D. T.; Sadagopan N.; Bhattachar S. N.; Kesten S. J.; Nomanbhoy T. K.; Cravatt B. F.; Ahn K. Discovery of PF-04457845: A highly potent, orally bioavailable, and selective urea FAAH inhibitor. ACS Med. Chem. Lett. 2011, 2, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileni M.; Johnson D. S.; Wang Z.; Everdeen D. S.; Liimatta M.; Pabset B.; Bhattacharya K.; Nugent R. A.; Kamtekar S.; Cravatt B. F.; Ahn K.; Stevens R. C. Structure-guided inhibitor design for human FAAH by interspecies active site conversion. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantermet P. G.; Henze D. A. Recent advances toward pain therapeutics. Annu. Rep. Med. Chem. 2011, 46, 19. [Google Scholar]
- Mor M.; Rivara S.; Lodola A.; Plazzi P.; Tarzia G.; Duranti A.; Tontini A.; Piersanti G.; Kathuria S.; Piomelli D. Cyclohexylcarbamic acid 3′- or 4′-substituted biphenyl-3-yl esters as fatty acid amide hydrolase inhibitors: synthesis, quantitative structure–activity relationships, and molecular modeling studies. J. Med. Chem. 2004, 47, 4998. [DOI] [PubMed] [Google Scholar]
- Liu P.; Hamill T. G.; Chioda M.; Chobanian H. R.; Fung S.; Guo Y.; Chang L.; Bakshi R.; Hong Q.; Dellureficio J.; Lin L. S.; Abbadie C.; Alexander J.; Jin H.; Mandala S.; Shiao L.-L.; Li W.; Sanabria S.; Williams D.; Zeng Z.; Hajdu R.; Jochnowitz N.; Rosenbach M.; Karanam B.; Madeira M.; Salituro G.; Powell J.; Xu L.; Terebetski J. L.; Leone J. F.; Miller P.; Cook J.; Holahan M.; Joshi A.; O’Malley S.; Purcell M.; Posavec M.; Chen T.; Riffel K.; Williams M.; Hargreaves R.; Sullivan K. A.; Nargund R. P.; DeVita R. J. Discovery of MK-3168: A PET tracer for imaging brain fatty acid amide hydrolase. ACS Med. Chem. Lett. 2013, 4, 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger D. L.; Miyauchi H.; Du W.; Hardouin C.; Fecik R. A.; Cheng H.; Hwang I.; Hedrick M. P.; Leung D.; Acevado O.; Guimaraes C. R. W.; Jorgensen W. L.; Cravatt B. F. Discovery of a potent, selective, and efficacious class of reversible alpha-ketoheterocycle inhibitors of fatty acid amide hydrolase effective as analgesics. J. Med. Chem. 2005, 48, 1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin D. J.; Ma Z.; Min X.; Li Y.; Hedberg C.; Guimaraes C.; Porter A. C.; Lindstrom M.; Lester-Zeiner D.; Xu G.; Carlson T. J.; Xiao S.; Meleza C.; Connors R.; Wang Z.; Kayser F. Identification of potent, noncovalent fatty acid amide hydrolase (FAAH) inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 2492. [DOI] [PubMed] [Google Scholar]
- Wang X.; Sarris K.; Kage K.; Zhang D.; Brown S. P.; Kolosa T.; Surowy C.; Kouhen O. F.; Muchmore S. W.; Brioni J. D.; Stewart A. O. Synthesis and evaluation of benzothiazole-based analogues as novel, potent, and selective fatty acid amide hydrolase inhibitors. J. Med. Chem. 2009, 52, 170. [DOI] [PubMed] [Google Scholar]
- AUCN (po) = 0.12 μM·h; half-life = 1.9 h; Cl (mL/min·kg) = 50; Vd (L/kg) = 3.6; F(%) = 12.
- Colpaert F. C. Evidence that adjuvant arthritis in the rat is associated with chronic pain. Pain 1987, 28, 201–222. [DOI] [PubMed] [Google Scholar]
- See Supporting Information for synthetic scheme of Compound 14.
- Langille N. F.; Dakin L. A.; Panek J. S. Sonogashira coupling of functionalized trifloyl oxazoles and thiazoles with terminal alkynes: Synthesis of disubstituted heterocycles. Org. Lett. 2002, 4, 2485. [DOI] [PubMed] [Google Scholar]
- Flageau E. F.; Popkin M. E.; Greaney M. F. Suzuki coupling of oxazoles. Org. Lett. 2006, 8, 2495. [DOI] [PubMed] [Google Scholar]
- See Supporting Information for synthetic scheme of Compound 15.
- Rang H. P. Pharmacology 2003, 145. [Google Scholar]
- Karczewski J.; Wang J.; Kane S. A.; Kiss L.; Koblan K. S.; Culberson J. C.; Spencer R. H. Analogs of MK-499 are differentially affected by a mutation in the S6 domain of the hERG K+ channel. Biochem. Pharmacol. 2009, 77, 1602. [DOI] [PubMed] [Google Scholar]
- Kim S. H.; Chung J. M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50, 355. [DOI] [PubMed] [Google Scholar]
- See Supporting Information for Table 4.
- Jin W.; Brown S.; Roche J. P.; Hsieh C.; Celver J. P.; KoVoor A.; Chavki C.; Mackie K. Distinct domains of the CB1 Cannabinoid receptor mediate desensitization and internalization. J. Neurosci. 1999, 19, 3773–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.