Abstract
Superoxide dismutase (SOD) and catalase activities of a drug are of great importance for its effective protection against reactive oxygen species (ROS)-induced injury. Achievement of catalase activity of a synthetic compound remains a challenge. Water-soluble Mn-porphyrins have high SOD and peroxynitrite (ONOO–) reducing activities, but not catalase-like activity. Herein, we are able to retain the fair SOD-like activity of a mononuclear Mn-5-(N-methylpyridinium-4-yl)-10,15,20-triphenyl porphyrin (MnM4PyP3P), while gaining in catalase-like activity with its dinuclear complex, 1,3-di[5-(N-methylene-pyridinium-4-yl)-10,15,20-triphenyl porphynato manganese] benzene tetrachloride (MnPD). Mechanistic study indicates that catalase-like activity of MnPD is due to synergism of two Mn active sites, where hydroxo-Mn(IV) complex is formed as an intermediate. The in vivo experiments demonstrate that MnPD significantly restores the treadmill-running ability of SOD-deficient mouse and thus indicates the therapeutic potential of MnPD. Furthermore, MnPD may serve as a mechanistic tool and indicate the new directions in the synthesis of catalase-like mimics.
Keywords: Reactive oxygen species, hydrogen peroxide, catalase activity, dinuclear Mn-porphyrin, water solubility
Reactive oxygen species (ROS) are involved in pathogenesis of a number of diseases such as atherosclerosis, cancer, and Alzheimer’s diseases as well as aging.1−3 Because of the importance of superoxide and species generated from it, superoxide dismutase (SOD) mimics have been widely studied. Superoxide would react with •NO to form another highly oxidizing species, peroxynitrite (ONOO–). Cationic Mn-porphyrins have been shown to catalyze superoxide dismutation and ONOO–reduction.4,5 We have synthesized cationic Mn-porphyrin derivatives with potential clinical utilities such as neuroprotection and heart protection.6−11
Hydrogen peroxide (H2O2) is generated as a product of superoxide dismutation and is a major signaling species and highly damaging one. It mediates apoptotic cell death or generates highly toxic hydroxy radical (OH•) via reaction with Fe2+, resulting in DNA oxidation and lipid peroxidation.12 Formation of OH• via the reaction of Fe site of Fe-porphyrins with H2O2 under physiological conditions has been previously reported.13 Hence, Mn-porphyrin complexes are more appropriate for antioxidant than Fe-porphyrins.
Several groups indicate in vivo benefits of catalysts of H2O2 dismutation (catalase activity). For example, the extension of life-span and the improvement of insulin resistance were reported by overexpression or administration of catalase.14−17 These reports suggest that not only SOD activity but also catalase activity are essential for artificial antioxidant. Because of the increasing importance of catalase activity, we have focused on the development of water-soluble Mn-porphyrins with catalase activity. Several approaches have been reported for metallo-porphyrins as catalase mimics.18−22 For example, Naruta et al. reported a dinuclear Mn-porphyrin complex as a mimic of dinuclear Mn catalase from Thermus thermophilus.21,22 They attached two Mn-porphyrins to several rigid aromatic backbones to fix the interporphyrin distance to approximately 0.4 nm. Although their catalase activity was very low (ca. 10–4 M–1 min–1), the dinuclear Mn-porphyrin showed the enhanced catalase activity due to synergetic working of two Mn-porphyrins. These previous reports have led us to design water-soluble dinuclear Mn-porphyrin as catalase mimic. As water-soluble nonporphyrin catalase mimic, anionic Fe-corrole has been previously reported.23 However, because of its small size, it may be quickly excreted with urine.
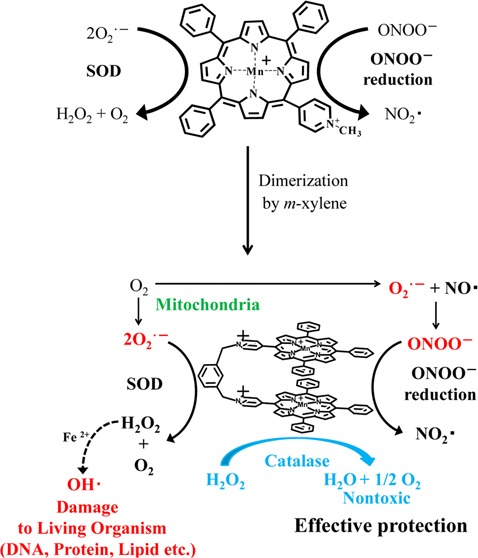
In this study, although the scale-up GMP synthesis is under consideration, we have synthesized novel water-soluble dinuclear Mn-porphyrin complex, 1,3-di[5-(N-methylene-pyridinium-4-yl)-10,15,20-triphenyl porphynato manganese(III)] benzene tetrachloride (MnPD), with multiple antioxidative activities (see experimental section and Figures S1 and S2 of the Supporting Information for the synthesis, characterization, and 3D structure) (Figure 1). We applied m-xylene moiety to bridge scaffold between cationic Mn-porphyrins, which is expected to be less hydrophobic than rigid aromatic backbones.22 Moreover, the flexible fixing by m-xylene allows Mn-porphyrins to take parallel conformation, which is necessary for synergetic working in catalase activity, and m-xylene moiety is considered to maintain Mn–Mn distance approximately 0.4 nm, which is crucial for the dinuclear catalase mimic.21,22 MnPD consists of two Mn-porphyrins cross-linked by m-xylene and has tetracations as a whole, which is essential for increasing water solubility. Thus, MnPD exhibited solubility in 50 mM phosphate buffer (pH 7.4).
Figure 1.
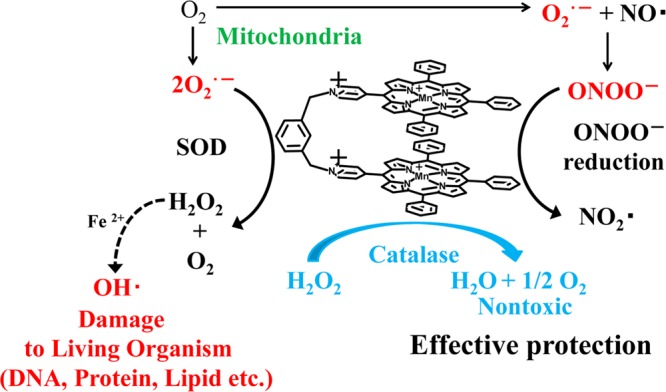
Multiple antioxidative activities of MnPD.
Furthermore, MnPD is expected to exhibit catalase activity as well as SOD and ONOO– reducing activities under almost physiological conditions (Figure 1). Cationic Mn-porphyrins are known to exhibit SOD and ONOO– reducing activities. Therefore, we first checked those activities of MnPD. For comparison, Mn-5-(N-methylpyridinium-4-yl)-10,15,20-triphenyl porphyrin (MnM4PyP3P), a half model for MnPD was also tested for those activities (see Figure S3, Supporting Information, for 1H NMR spectrum of H2M4PyP3P).
SOD activity was measured by stopped-flow kinetic analysis according to the previous method.24 The SOD activity of conventional SOD mimic, Mn-5,10,15,20-tetrakis(N-methylpyridinium-4-yl) porphyrin (MnM4Py4P) was measured as a positive control. The obtained SOD activity (kSOD) for MnM4Py4P was kSOD= (21.0 ± 1.0) × 106 M–1 s–1 (Figure S4, Supporting Information), which is consistent with that of the previous report.24 Under the same conditions, both MnPD and MnM4PyP3P accelerated dismutation of superoxide in initial millisecond time scale (Figures S5a and S6a, Supporting Information). Thus, MnPD and MnM4PyP3P have SOD activity. From the plot of kobs, we determined kSOD (per Mn ion) for MnPD and MnM4PyP3P to be kSOD= (4.7 ± 0.2) × 106 M–1 s–1 and (4.9 ± 0.2) × 106 M–1 s–1, respectively (Figures S5b and S6b, Supporting Information). MnPD and MnM4PyP3P exhibited similar kSOD values.
Next, we measured the ONOO– reducing activity of MnPD. It was measured by the similar procedure to SOD activity.6 The obtained kred value for MnPD was (1.4 ± 0.3) × 106 M–1 s–1 (per Mn ion), while that for MnM4PyP3P was (1.4 ± 0.9) × 106 M–1 s–1 (per Mn ion). The tendency of ONOO– reducting activity is similar to that of SOD activity. This result is also in good agreement with our previous report.7 We have confirmed that SOD and ONOO– reducing activities are unaffected by dimerization of mononuclear Mn-porphyrins.
Finally, we measured the catalase activity of MnPD. It was measured with Clark-type oxygen electrode. The catalase activity (kCAT) was determined as a rate constant of the following reaction:
Time-course of O2 production from 1 mM H2O2 was electrochemically monitored for 180 s at 25 °C in 50 mM phosphate buffer (pH 7.4). The kCAT value was determined from the slope of the plot of the observed rate constant (kobs) as a function of the Mn-porphyrin concentration. The results are shown in Figure 2. Both MnPD and MnM4PyP3P exhibited O2 production in a dose-dependent manner (Figure 2a,c). However, it should be noted that the time-course of O2 production catalyzed by MnPD was obviously different from that catalyzed by MnM4PyP3P as compared at the same concentration range per Mn ions. MnPD exhibited significant O2 production, whereas MnM4PyP3P exhibited little O2 production. From the plot of kobs, we determined kCAT values of (3.3 ± 0.1) × 102 M–1 s–1 for MnPD and 10.9 ± 1.8 M–1 s–1 for MnM4PyP3P, respectively (Figures 2b,d). MnM4PyP3P is considered to be almost catalase-inactive. The obtained rate constants for SOD, ONOO– reducing, and catalase activities are summarized in Table 1.
Figure 2.
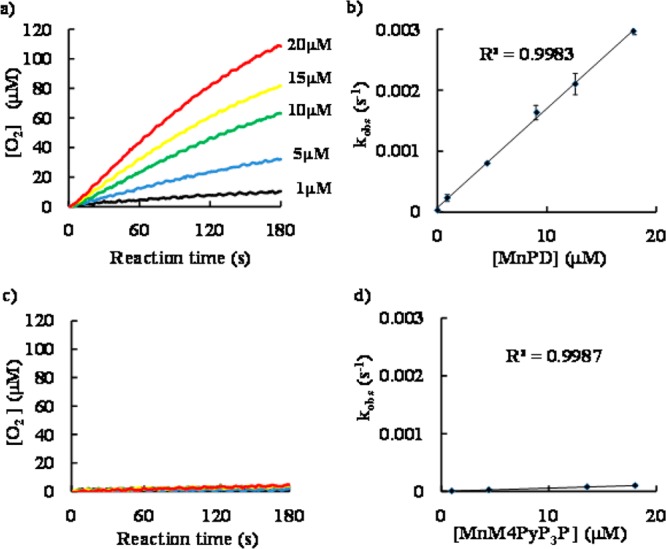
Left: Time-course of O2 production from 1 mM H2O2 (final concentration) catalyzed by (a) MnPD and (c) MnM4PyP3P at 25 °C in 50 mM phosphate buffer (pH 7.4). The concentration of Mn-porphyrin was varied from 1 to 20 μM per Mn ions. Black, 1 μM; blue, 5 μM; green, 10 μM; yellow, 15 μM; red, 20 μM. Amount of O2 production was increased with increasing concentration of Mn-porphyrins. Five curves are almost overlapped in (c) MnM4PyP3P. Right: Plot of the observed rate constant (kobs) of (b) MnPD and (d) MnM4PyP3P as a function of the Mn-porphyrin concentration. kobs for each concentration of Mn-porphyrin was determined as a rate of O2 production in initial 5 s. kCAT value was determined as a mean value obtained from at least three experiments.
Table 1. SOD (kSOD), ONOO– Reducing (kred), and Catalase Activities (kCAT) of Mn-Porphyrin Derivatives.
| compound | kSOD (×106 M–1 s–1)a | kred (×106 M–1 s–1)a | kCAT (×10 M–1 s–1)b |
|---|---|---|---|
| MnPD | 4.7 ± 0.2 | 1.4 ± 0.3 | 33.0 ± 1.0 |
| MnM4PyP3P | 4.9 ± 0.2 | 1.4 ± 0.9 | 1.1 ± 0.2 |
SOD activity was determined by stopped-flow kinetic analysis. The time decay of O2•– was spectrophotometrically monitored at 245 nm (λmax of O2•–) in HEPES buffer (pH 8.1) at 21 °C. The kSOD value was determined from the slope of the plot of observed rate constant (kobs) as a function of Mn-porphyrin concentration. ONOO– reducing activity was determined by the similar procedure to that for SOD activity. Time decay of ONOO– was spectrophotometrically monitored at 302 nm (λmax of ONOO–) in phosphate buffer (pH 7.4) at 36 °C in the presence of 1 mM ascorbic acid. The kred value was determined from the slope of the plot of kobs as a function of the Mn-porphyrin concentration.
Catalase activity was determined with Clark-type oxygen electrode. O2 production from 1 mM H2O2 was electrochemically monitored in phosphate buffer (pH 7.4) at 25 °C. The kobs was calculated as a rate of O2 production in the initial 5 s. The kCAT value was determined from the slope of the plot of kobs as a function of the Mn-porphyrin concentration.
Quite interestingly, there was a remarkable difference in catalase activity between MnPD and MnM4PyP3P, which is a notable point. One reason for this difference is the induction of synergetic working of two Mn-porphyrins cross-linked by m-xylene. Naruta et al. reported a catalytic H2O2 dismutation cycle including synergetic working but not independent working of the two Mn-porphyrins by isotopic experiments and comparative studies with half models.21,22 From their previous reports, we have proposed a catalytic mechanism of catalase activity of dinuclear Mn-porphyrins represented in Figure 3. In the catalytic cycle, the oxidation process from MnIII to MnIV, the formation of the reactive intermediate is a rate-determing step, where high-valent hydroxo-Mn(IV) complex (3) is the likely intermediate.21
Figure 3.
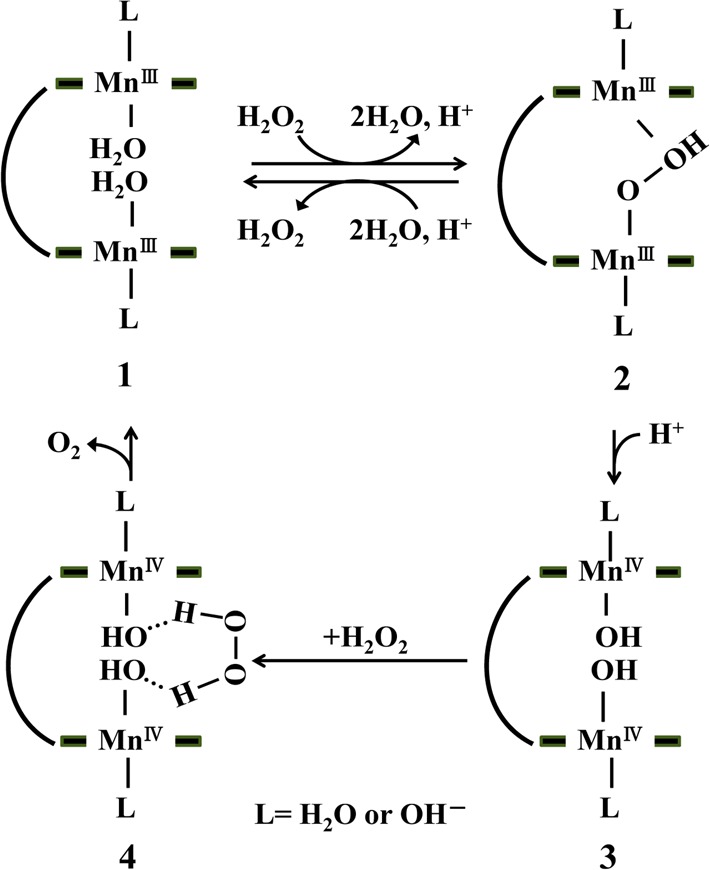
Proposed mechanism of catalase activity of MnPD. L = OH– or H2O.
To gain further information on the mechanism of catalase activity of our MnPD, we first examined pH-dependence of catalase activity. Jin et al. reported that the coordination and dissociation of peroxides (such as H2O2, m-chloroperoxybenzoic acid, HSO5–, and t-BuOOH) on Mn-porphyrins are pH-dependent reactions.25 Moreover, they reported that the coordination (forward reaction) is accelerated at a higher pH region and that the subsequent O–O bond cleavage leading to the formation of high-valent oxo-Mn(V) or oxo-Mn(IV) species is a pH-independent irreversible reaction. These results suggest that the coordination of peroxides is a crucial step for the formation of high-valent Mn species.25 Our proposed mechanism of catalase activity involves the coordination of H2O2 (formation of 2), which is considered to be pH-dependent as well. Therefore, we hypothesized that formation of the reactive intermediate 3 is accelerated at pH 9.4 and catalase activity is increased as compared at pH 7.4. As shown in Figure 4, O2 production of MnPD in 50 mM borate buffer (pH 9.4) was significantly higher than that in phosphate buffer (pH 7.4) (Figure 4a). kCAT value under this condition was determined to be kCAT = (2.3 ± 0.1) × 103 M–1 s–1, which is approximately seven times higher than that at pH 7.4 (Figure 4b). This indicates that the rate-determing step was faster at pH 9.4 than at pH 7.4.
Figure 4.
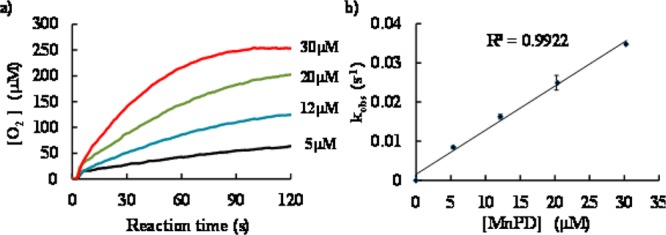
(a) Time-course of O2 production from 1 mM H2O2 (final concentration) catalyzed by MnPD at 25 °C in 50 mM borate buffer (pH 9.4). MnPD concentration was varied from 5 to 30 μM. Black, 5 μM; blue, 12 μM; green, 20 μM; red, 30 μM. (b) Plot of kobs as a function of MnPD concentration. kobs was determined as a rate of O2 production in the initial 5 s. kCAT value was determined as a mean value obtained from at least three experiments.
Furthermore, we attempted to directly detect the reactive intermediate 3 during the catalase reaction. When excess H2O2 was added to the solution of MnPD (1) at pH 9.4, the Soret band at 464 nm decreased and new band at 434 nm appeared (compare Figure S7 and Figure S8, Supporting Information). The new band at 434 nm is different from those of oxo-Mn(V) (MnV(=O)(OH)PD) complex and oxo-Mn(IV) (MnIV(=O)(OH)PD) complex prepared according to the previous report (Figure S9, Supporting Information).26 Consequently, MnIV(=O)(OH)PD complex, another possible intermediate during the dinuclear catalase reaction other than the hydroxo-MnIV complex (MnIV(OH)2PD),22 can be ruled out as the reactive intermediate. Moreover, the increase in O2 production at pH 9.4 is not due to the accelerated four-electron oxidation of water, which involves dinuclear oxo-Mn(V) complex as a reactive intermediate.26 Thus, the new spectrum should be MnIV(OH)2PD complex (3), and 3 would oxidize the second H2O2 molecule to produce O2.
The new band at 434 nm was rapidly decreased in the initial 5 min of the incubation and was almost constant (Figure S10, Supporting Information). Time-course of the absorption spectrum gave a clear shift of λmax from 434 to 464 nm (Figure S7, Supporting Information) with isosbestic points, indicating the reduction reaction from 3 to 1. This is consistent with the time-course of O2 production (reduction from 3 to 1) from H2O2. We did not see any spectral changes of MnPD (1) at pH 7.4 even in the presence of excess H2O2, presumably due to the fast dissociation of H2O2 from Mn center (reverse reaction from 2 to 1) (see Figure S11, Supporting Information, for ground-state absorption spectrum of MnPD at pH 7.4). MnIV(OH)(H2O) PD complex (3) should be the reactive intermediate at pH 7.4. The spectral changes observed for MnPD at pH 9.4 were not observed for MnM4PyP3P. From these results, we tentatively suspect that MnPD dismutes H2O2 via the catalytic cycle as proposed in Figure 3. Acceleration of H2O2 coordination on Mn center may be effective to increase the catalase activity of dinuclear Mn-porphyrins under physiological conditions, although a precise mechanism still needs to be elucidated.
Moreover, as a preliminary study, we examined antioxidative activity of MnPD in vivo. MnM4Py4P was used as control because both MnPD and MnM4Py4P have 4-pyridinium cations in common. As a model of ROS-related disease, skeletal muscle-specific SOD deficient mice show short running time on treadmill due to severe oxidative stress in skeletal muscle.27
The intraperitoneal injection of MnPD significantly restored the running time after 8 and 24 hours, whereas the injection of MnM4Py4P did not (Figure 5). This result demonstrates that MnPD exhibited antioxidative activity in vivo. MnPD as SOD/catalase mimic exhibited antioxidative activity, whereas MnM4Py4P as SOD mimic did not. This may suggest that H2O2 from SOD reaction still needs to be detoxified for effective protection against oxidative stress. However, these differences in in vivo effect may be attributed to several factors such as blood circulation, biodistribution, and metabolism, other than SOD or to catalase activity. Therefore, further investigations such as pharmacokinetic analysis, in vivo ROS quantification, and interactions with biomolecules are now in progress. Besides, a catalase mimic with no SOD activity should be applied to prove that this model is sensitive to H2O2.
Figure 5.
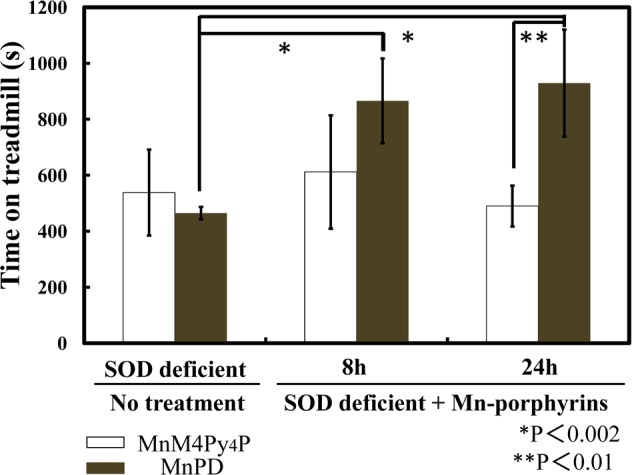
In vivo antioxidative activity of Mn-porphyrin derivatives. MnPD and MnM4Py4P were intraperitoneally injected (36 mg/kg) in SOD deficient (HSA-Sod2–/–) mice. Time on treadmill was recorded 8 and 24 h after the injection. The running time is depicted as a mean value obtained from three mice per group (N = 3, one male and two females). *P and **P means that the results are statistically significant (student’s t test).
In conclusion, we have synthesized dinuclear Mn-porphyrin MnPD cross-linked by m-xylene. MnPD exhibited water solubility and high catalase activity under almost physiological conditions, maintaining its SOD and ONOO– reducing activities. Mechanistic study indicates that catalase-like activity of MnPD is due to synergism of two Mn active sites, where hydroxo-Mn(IV) complex is formed as an intermediate. We have overcome the obstacle of water insolubility of Mn-porphyrins as catalase mimics reported previously. To the best of our knowledge, this is the first report on water-soluble dinuclear Mn-porphyrin bearing high catalase activity. Our results provide a new insight into the development of water-soluble dinuclear Mn-porphyrins with catalase activity. Furthermore, the running test with SOD deficient (HSA-Sod2–/–) mice suggests that MnPD exhibited antioxidative activity in vivo. For effective protection against ROS-induced injury, both SOD and catalase activities are essential. Thus, MnPD would be a new candidate for therapeutic antioxidant.
Acknowledgments
We appreciate Dr. Dai Masui (Tokyo Medical University) for measuring the NOESY NMR spectrum of H2PD.
Glossary
ABBREVIATIONS
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- ONOO–
peroxynitrite
- MnPD
1,3-di[5-(N-methylene-pyridinium-4-yl)-10,15,20-triphenyl porphynato manganese(III)] benzene tetrachloride
- MnM4PyP3P
Mn-5-(N-methylpyridinium-4-yl)-10,15,20-triphenyl porphyrin
- MnM2Py4P
Mn(III)-5,10,15,20-tetrakis(N-methylpyridinium-2-yl) porphyrin
- MnM4Py4P
Mn(III)-5,10,15,20-tetrakis(N-methylpyridinium-4-yl) porphyrin
Supporting Information Available
Synthesis and characterization of MnPD, measurement of catalase activity, SOD activity, in vivo antioxidative activity, and UV/vis spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was partially supported by a Grant-in-Aid (No. 22300166) from Japan Society for the promotion of Science.
The authors declare no competing financial interest.
Supplementary Material
References
- Kunsch C.; Medford R. M. Oxidative Stress as a Regulator of Gene Expression in the Vasculature. Circ. Res. 1999, 85, 753–766. [DOI] [PubMed] [Google Scholar]
- Balaban R. S.; Nemoto S.; Finkel T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [DOI] [PubMed] [Google Scholar]
- Giordano F. J. Oxygen, oxidative stress, hypoxia, and heart failure. J. Clin. Invest. 2005, 115, 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batinic-Haberle I.; Benov L.; Spasojevic I.; Fridovich I. The Ortho Effect Makes Manganese (III) meso-Tetrakis (N-Methylpyridinium-2-yl) Porphyrin a Powerful and Potentially Useful Superoxide Dismutase Mimic. J. Biol. Chem. 1998, 273, 24521–24528. [DOI] [PubMed] [Google Scholar]
- Ferrer-Sueta G.; Batinic-Haberle I.; Spasojevic I.; Fridovich I.; Radi R. Catalytic Scavenging of Peroxynitrite by Isomeric Mn(III) N-Methylpyridylporphyrins in the Presence of Reductants. Chem. Res. Toxicol. 1999, 12, 442–449. [DOI] [PubMed] [Google Scholar]
- Asayama S.; Nakajima T.; Kawakami H. New Water-Soluble Mn-Porphyrin with Catalytic Activity for Superoxide Dismutation and Peroxynitrite Decomposition. Metallomics 2011, 3, 744–748. [DOI] [PubMed] [Google Scholar]
- Haruyama T.; Asayama S.; Kawakami H. Highly Amphiphilic Manganese Porphyrin for Mitochondrial Targeting Antioxidant. J. Biochem. 2010, 147, 153–156. [DOI] [PubMed] [Google Scholar]
- Hayakawa N.; Asayama S.; Noda Y.; Shimizu T.; Kawakami H. Pharmaceutical Effect of Manganese Porphyrins on Manganese Superoxide Dismutase Deficient Mice. Mol. Pharmaceutics 2012, 9, 2956–2959. [DOI] [PubMed] [Google Scholar]
- Asayama S.; Mizushima K.; Nagaoka S.; Kawakami H. Design of Metalloporphyrin-Carbohydrate Conjugates for a New Superoxide Dismutase Mimic with Cellular Recognition. Bioconjugate Chem. 2004, 15, 1360–1363. [DOI] [PubMed] [Google Scholar]
- Asayama S.; Kawamura E.; Nagaoka S.; Kawakami H. Design of Manganese Porphyrin Modified with Mitocondrial Signal Peptide for a New Antioxidant. Mol. Pharmaceutics 2006, 3, 468–470. [DOI] [PubMed] [Google Scholar]
- Kawakami H.; Ohse T.; Kawano M.; Nagaoka S. Superoxide Dismutase Activity of a Novel Macromolecular Manganese Porphyrin. Polym. Adv. Technol. 1999, 10, 270–274. [Google Scholar]
- Halliwell B. Free Radicals, Antioxidants, and Human Disease: Curiosity, Cause or Consequence?. Lancet 1994, 344, 721–724. [DOI] [PubMed] [Google Scholar]
- Kitagishi H.; Tamaki M.; Ueda T.; Hirota S.; Ohta T.; Naruta Y.; Kano K. Oxoferryl Porphyrin/Hydrogen Peroxide System Whose Behaviour is Equivalent to Hydroperoxoferric Porphyrin. J. Am. Chem. Soc. 2010, 132, 16730–16732. [DOI] [PubMed] [Google Scholar]
- Schriner S. E.; Linford N. J.; Martin G. M.; Treuting P.; Obgurn C. E.; Emond M.; Coskun P. E.; Ladiges W.; Wolf N.; Remmen H. V.; Wallace D. C.; Rabinovitch P. Extention of Murine Life Span by Overexpression of Catalase Targeted to Mitochondria. Science 2005, 308, 1909–1911. [DOI] [PubMed] [Google Scholar]
- Orr W. C.; Sohal R. S. Extention of Life-Span by Overexpression of Superoxide Dismutase and Catalase in Drosophila Melanogaster. Science 1994, 263, 1128–1130. [DOI] [PubMed] [Google Scholar]
- Miller R. A. The Anti-Aging Sweepstakes: Catalase Runs for the ROSes. Science 2005, 308, 1875–1876. [DOI] [PubMed] [Google Scholar]
- Ikemura M.; Nishikawa M.; Hyoudou K.; Kobayashi Y.; Yamashita P.; Hashida M. Improvement of Insulin Resistance by Romoval of Systemic Hydrogen Peroxide by PEGylated Catalase in Obese Mice. Mol. Pharmaceutics 2010, 7, 2069–2076. [DOI] [PubMed] [Google Scholar]
- Rosenthal J.; Chng L. L.; Fried S. D.; Nocera D. G. Stereochemical Control of H2O2 Dismutation by Hangman Porphyrins. Chem. Commun. 2007, 2642–2644. [DOI] [PubMed] [Google Scholar]
- Chng L. L.; Chang C. J.; Nocera D. G. Catalytic O–O Activation Chemistry Mediated by Iron Hangman Porphyrins with a Wide Range of Proton-Donating Abilities. Org. Lett. 2003, 5, 2421–2424. [DOI] [PubMed] [Google Scholar]
- Robert A.; Loock B.; Momenteau M.; Meunier B. Catalase Modelling with Metalloporphyrin Complexes Having an Oxygen Ligand in a Proximal Position. Comparison with Complexes Containing a Proximal Nitrogen. Inorg. Chem. 1991, 30, 706–711. [Google Scholar]
- Naruta Y.; Sasayama M.; Ichihara K. Functional Modeling of Manganese-Containing O2 Evolution Enzymes with Manganese Porphyrin Dimers. J. Mol. Catal. A: Chem. 1997, 117, 115–121. [Google Scholar]
- Naruta Y.; Maruyama K. High Oxygen-Evolving Activity of Rigidly Linked Manganese (III) Porphyrin Dimers. A Functional Model of Manganese Catalase. J. Am. Chem. Soc. 1991, 113, 3595–3596. [Google Scholar]
- Mahammed A.; Gross Z. Highly Efficient Catalase Activity of Metalloporphyrins. Chem. Commum. 2010, 46, 7040–7042. [DOI] [PubMed] [Google Scholar]
- Friedel F. C.; Lieb D.; Ivanovic-Burmazovic I. Comparative Studies on Manganese-Based SOD Mimetics, Including the Phosphate Effect, by Using Global Spectral Analysis. J. Inorg. Biochem. 2012, 109, 26–32. [DOI] [PubMed] [Google Scholar]
- Jin N.; Lahyde D. E.; Groves J. T. A “Push–Pull” Mechanism for Heterolytic O–O Bond Cleavage in Hydroperoxo Manganese Porphyrins. Inorg. Chem. 2010, 49, 11516–11524. [DOI] [PubMed] [Google Scholar]
- Shimazaki Y.; Nagano T.; Takesue H.; Tani B.-H.; Ye F.; Naruta Y. Characterization of a Dinuclear MnV=O Complex and Its Efficient Evolution of O2 in the Presence of Water. Angew. Chem., Int. Ed. 2004, 43, 98–100. [DOI] [PubMed] [Google Scholar]
- Kuwahara H.; Horie T.; Ishikawa S.; Tsuda C.; Kawakami S.; Noda Y.; Kaneko T.; Tahara S.; Tachibana T.; Okabe M.; Melki J.; Takano R.; Toda T.; Morikawa D.; Nojiri H.; Kurosawa H.; Shirasawa T.; Shimizu T. Oxidative Stress in Skeletal Muscle Causes Severe Disturbance of Exercise Activity without Muscle Atrophy. Free Radical Biol. Med. 2010, 48, 1252–1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


