Abstract
The 18-kDa translocator protein (TSPO) is overexpressed in many types of cancers and is also abundant in activated microglial cells occurring in inflammatory neurodegenerative diseases. Thus, TSPO has become an extremely attractive subcellular target not only for imaging disease states overexpressing this protein, but also for a selective mitochondrial drug delivery. In this work we report the synthesis, the characterization, and the in vitro evaluation of a new TSPO-selective ligand, 2-(8-(2-(bis(pyridin-2-yl)methyl)amino)acetamido)-2-(4-chlorophenyl)H-imidazo[1,2-a]pyridin-3-yl)-N,N-dipropylacetamide (CB256), which fulfils the requirements for a bifunctional chelate approach. The goal was to provide a new TSPO ligand that could be used further to prepare coordination complexes of a metallo drug to be used in diagnosis and therapy. However, the ligand itself proved to be a potent tumor cell growth inhibitor and DNA double-strand breaker.
Keywords: TSPO, PBR, bifunctional chelate approach, apoptosis, DNA cleavage
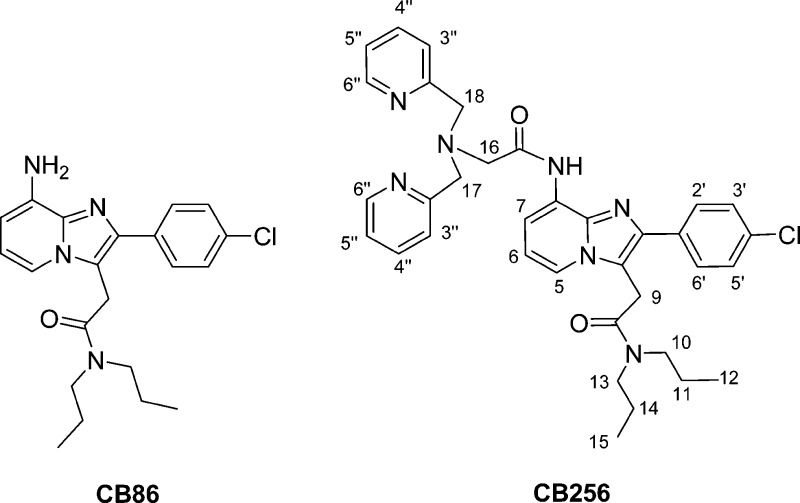
The 18-kDa mitochondrial translocator protein (TSPO),1 also known as peripheral-type benzodiazepine receptor or PBR, has become an attractive target for therapeutic and imaging purposes.2−5 TSPO is poorly expressed in healthy human brain and liver, while it is much more abundant in steroid-synthesizing and rapidly proliferating tissues. In contrast, aberrant TSPO expression has been observed in multiple diseases, including brain, breast, colon, prostate, and ovarian cancers, as well as astrocytomas and hepatocellular and endometrial carcinomas.6,7 Moreover, TSPO expression is also increased in activated microglial cells occurring in inflammatory neurodegenerative diseases such as Alzheimer, Parkinson, Huntington, and multiple sclerosis.8 Thus, TSPO has become an extremely attractive subcellular target not only for an early detection of disease states overexpressing this protein, but also for a selective mitochondrial drug delivery.9−14 Although a wide number of TSPO ligands have been synthesized, only few of them have the ability to deliver metal-based drugs.15 In particular, very recently were reported potent and selective imidazopyridine-based TSPO ligands, which could carry both a cytostatic platinum species and a rhenium complex as a model of 99mTc imaging agent.13,16−19 In the compounds so far investigated, atoms already present in the TSPO ligand were used as donors for anchoring the metal core. A further development could be represented by the use of conjugates in which the TSPO-targeting moiety is covalently linked with an appropriate chelating system, such as di(2-picolyl)amine, forming a strong coordination compound with metal ions in the pertinent oxidation state. The use of an appropriate linker could rule out possible interactions between the metal-core and the targeting moiety so to leave unaltered the pharmacokinetic profile of the tracer. Such a strategy is commonly reported as bifunctional chelate (BFC) approach.20 Hence, starting from the already known potent and selective TSPO ligand CB86 (Chart 1),15 we designed a new TSPO-selective BFC ligand, namely, 2-(8-(2-(bis(pyridin-2-yl)methyl)amino)acetamido)-2-(4-chlorophenyl)H imidazo[1,2-a]pyridin-3-yl)-N,N-dipropylacetamide (CB256 in Chart 1), where, the di(2-picolyl)amine moiety could be used for the synthesis of coordination complexes containing a metallo drug to be used in diagnosis and therapy.
Chart 1. Sketches of the TSPO Ligands CB86 and CB256.
Preliminarily, we performed in vitro studies on the newly synthesized TSPO-selective BFC ligand CB256 in order to assess its affinity toward TSPO. In addition, since it is well-known that TSPO ligands are able to induce apoptosis, we also investigated the cytotoxic activity and the ability to cause morphological changes of the mitochondrion and of the nucleus, the collapse of the mitochondrial membrane potential (ΔΨm), and alterations of the cell cycle progression in C6 glioma cells. CB256 resulted to be highly cytotoxic and able to induce double-strand breaks in DNA.
The TSPO-ligand CB256 was prepared according to the synthetic procedure reported in Scheme 1. Treatment of N,N-di-n-propyl-[2-(8-amino-2-(4-chlorophenyl)imidazo[1,2-a]pyridin-3-yl)]acetamide (CB86 in Scheme 1)17 with bromoacetyl bromide in anhydrous CH2Cl2 affords 2-(8-(2-bromoacetamido)-2-(4-chlorophenyl)imidazo[1,2-a]pyridin-3-yl)-N,N-dipropylacetamide (CB254). Condensation of CB254 with di(2-picolyl)amine in anhydrous THF and in the presence of K2CO3 affords the TSPO ligand CB256.
Scheme 1. Synthetic Pathway of the TSPO Ligand CB256.
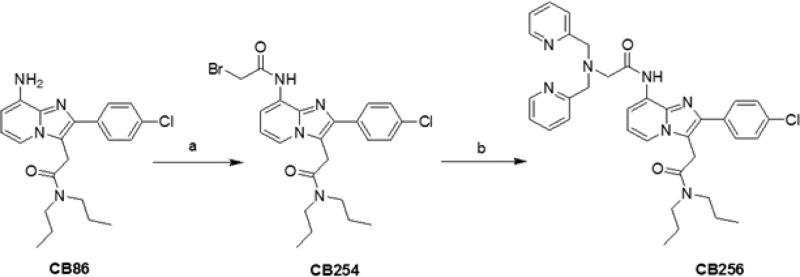
Reagents: (a) Bromoacetyl bromide and TEA in anhydrous CH2Cl2; (b) di(2-picolyl)amine and K2CO3 in anhydrous THF.
The TSPO ligands CB254 and CB256 were obtained in good yield (80 and 65%, respectively) and were fully characterized by elemental analyses, mass spectrometry, and NMR spectroscopy; a detailed description is given in Supporting Information.
The affinity toward TSPO of the CB256 ligand was tested by measuring its ability to displace the reference compound [3H]-PK11195 from binding to TSPO on membrane extracts of C6 glioma cells.21 The results, reported in Table 1, show that CB256 has still an appreciable affinity for TSPO, which, however, is considerably lower than that of the reference compound PK11195 and of its precursor CB86.
Table 1. Affinities (Ki, nM) for TSPO on Membrane Extracts of C6 Glioma Cells.
| compd | Ki (nM) |
|---|---|
| PK11195 | 0.40 ± 0.05 |
| CB256 | 239 ± 43 |
| CB86 | 1.6a |
According to ref (16).
TSPO ligands are known to exert a proapoptotic activity by acting on the mitochondria.22 Hence, we deemed appropriate to test the ability of CB256 to interfere with the vitality of tumor cells. Table 2 summarizes the cytotoxicity of CB256 against rat C6 glioma cells selected for their high level of TSPO expression. Cisplatin was used as a reference cytostatic compound. CB256 proved to be extremely effective, as shown by its IC50 value, which is in the submicromolar range (IC50 values of 0.27 and 0.73 μM for CB256 and cisplatin, respectively). The cytotoxic activities of CB256 and cisplatin were also determined against the cisplatin sensitive A2780 and the cisplatin resistant A2780cisR human ovarian carcinoma cell lines.23 A2780cisR cells are resistant to cisplatin due to a combination of decreased uptake, enhanced DNA repair/tolerance, and enhanced GSH levels.23 Interestingly, unlike cisplatin, CB256 is equally active toward the sensitive and the resistant cell lines with a resistance factor, defined as IC50(resistant)/IC50(sensitive), close to 1 (Table 2).
Table 2. Cytotoxicity of CB256 and Cisplatin toward C6, A2780, and A2780cisR Cancer Cell Lines.
| IC50 (μM) |
|||
|---|---|---|---|
| compd | C6a | A2780a | A2780cisRa |
| CB256 | 0.27 ± 0.01 | 9.02 ± 0.36 | 9.21 ± 0.37 (1.02)b |
| CB86c | 19.71 ± 1.16 | ||
| cisplatinc | 0.73 ± 0.51 | 2.94 ± 0.43 | 9.17 ± 0.43 (3.11)b |
Cells were treated with a range of drug concentrations (from 0.05 to 100 μM) and incubated at 37 °C for a period of 72 h.
Resistance factor.
According to ref (16).
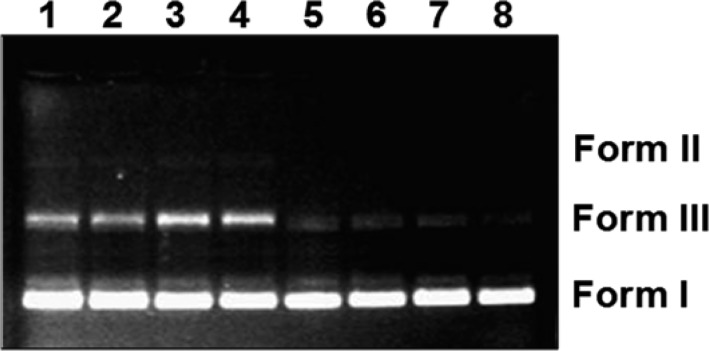
In order to explain the unexpected cytotoxicity of CB256 (ca. 75 times more cytotoxic than analogous CB86), we decided to explore the ability of this TSPO ligand to produce single- and double-strand DNA lesions. We noticed a certain analogy between the di(2-picolyl)amine moiety present in CB256 and synthetic models of bleomycin, such as (3-Clip-Phen) and others, which, when associated with copper(I), can cause single- and double-strand DNA lesions.24 Hence, pET plasmid DNA was used to monitor the DNA cleavage ability of CB256, previously treated (2 h) with Cu(II) (5 equiv) in the presence of a reducing agent (GSH, 50 equiv). The assay is based on the different electrophoretic mobility on agarose gel of the three major forms of a plasmid DNA: supercoiled (Form I, intact double strand, faster migration), open circular (Form II, single strand nick, slower migration), and linear (Form III, double strand nick, intermediate migration). Untreated pET plasmid DNA (control) is mostly present in the supercoiled form (Figure 1, lane 5). At the lowest concentration of CB256 used in our experimental setting (0.5 μM), Form III is already visible in good amount if compared with untreated DNA. Upon rising the CB256 concentration, (1, 2, and 10 μM), Form III increases correspondingly (Figure 1, lanes 2–4), while Form II remains always very low, indicating that CB256 performs mainly double-strand cuts in supercoiled plasmid DNA. As negative controls, we tested the cleaving activity of CuCl2 incubated with GSH (lane 6), of CB256 without CuCl2 (lane 7), and of CB86 incubated with CuCl2 and GSH in the same conditions of CB256 (lane 8). CB86, lacking the metal-coordinating moiety, was used at the highest concentration assayed for CB256 (10 μM). In all the controls, there was no appreciable formation of Form III DNA, hence indicating absence of DNA cleavage activity. We also proved that the presence of copper(I) is essential for CB256 to be able to cleave DNA. Such a DNA cleaving ability could be at the basis of the remarkable cytotoxic activity of CB256 administered to cultured cells.
Figure 1.
Cleavage assay of pET plasmid DNA. CB256, preincubated for 2 h with CuCl2 (5 equiv) and GSH (50 equiv), was reacted with pET plasmid DNA for 1 h at 37 °C. CB256 concentration: 0.5 μM, Lane 1; 1 μM, Lane 2; 2 μM, Lane 3; 10 μM, Lane 4. Untreated DNA, Lane 5. Control with CuCl2 and GSH, without CB256, Lane 6. Control with 10 μM CB256, without CuCl2, Lane 7. Control with 10 μM CB86 preincubated for 2 h with CuCl2 (5 equiv) and GSH (50 equiv), Lane 8. 1% Agarose gel stained with ethidium bromide.
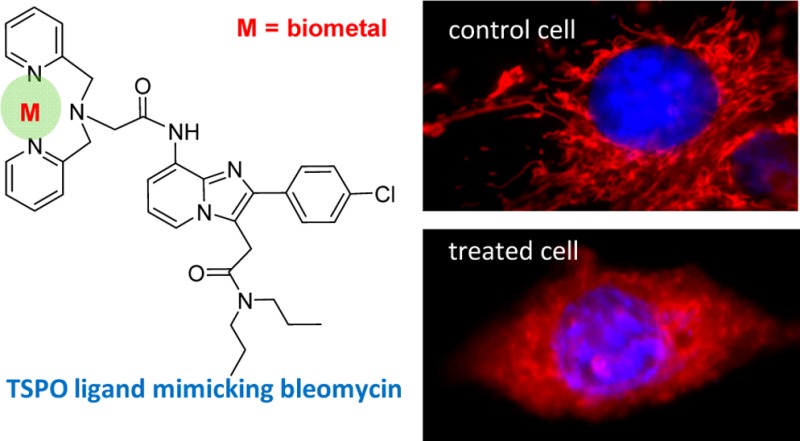
Moreover, since TSPO ligands act on mitochondria, we analyzed the structure of these organelles and of the nuclei in cells treated with CB256 or cisplatin. MitoTracker Red and DAPI dyes were used as mitochondrial and nuclear specific markers, respectively. The C6 glioma cells were incubated with cisplatin or CB256 at a concentration of 10 μM for 24 h, and the mitochondrial and nuclear structure modifications were evaluated using a digital imaging system. Figure 2, top lane, shows representative images of control C6 glioma cells where the typical nucleus and the tubular interconnected mitochondrial network are evident. In contrast, cells treated with cisplatin and CB256 (Figure 2, medium and bottom lanes, respectively) exhibit marked morphological alteration of the organelles and of the nucleus. In particular, cells exhibit fragmented nuclei and mitochondria, with MitoTracker Red diffused into the cytosol. Although the CB256 action on C6 cells requires further careful investigation, it seems likely that the TSPO ligand induces cell death by targeting mitochondria.
Figure 2.

Mitochondrial and nuclear modifications of C6 glioma cells exposed to cisplatin (10 μM) or CB256 (10 μM) for 24 h. Untreated cells were used as control.
To highlight the antiproliferative activity of CB256, the mitochondrial membrane potential (ΔΨm) was also evaluated by using the mitochondria-specific fluorescent probe 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide, also named JC-1. Cytofluorimetric analysis of JC-1-loaded C6 cells showed that only a small percentage of control cells had depolarized mitochondria (0.21%) (Figure 3, left panel). On the contrary, an increased number of cells with depolarized mitochondria was observed in C6 cells exposed to CB256 (10 μM) or cisplatin (10 μM) for 24 h (2.47 and 18.21%, respectively) (Figure 3, right and middle panels, respectively). In particular, CB256 induces ΔΨm dissipation, an event that is known to precede the cell death by apoptosis. These data are in good agreement with the morphological analysis evidenced by fluorescence microscopy.
Figure 3.
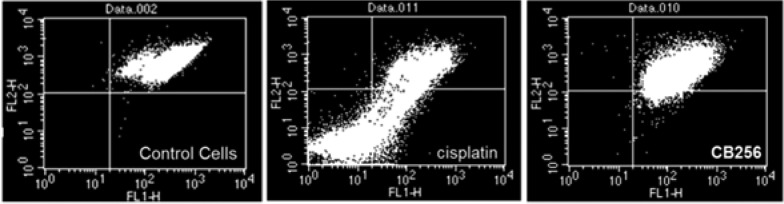
Effect of ΔΨm dissipation on C6 glioma cells exposed to CB256 (10 μM) or cisplatin (10 μM) for 24 h. Untreated cells were used as control.
To deeper explore the antiproliferative activity of CB256, the ability to interfere with the C6 glioma cell cycle progression was evaluated by flow cytometry. Cells were exposed to CB256 or cisplatin at a concentration of 10 μM for 24 h. As shown in Figure 4, only cisplatin showed a marked accumulation of cells in the G2/M phase suggesting an arrest of the cell cycle. Unlike cisplatin, cells treated with CB256 showed quite normal cell cycle histograms suggesting that, after 24 h of incubation, the residual cells begin to cycle (Figure 4). C6 glioma cells were also incubated for a longer time (72 h) with IC50 concentrations of CB256 (0.3 μM). Sub-G1 hypoploid cell population, indicating apoptosis, was observed in the cells treated with CB256 as compared to the control (Table 3).
Figure 4.
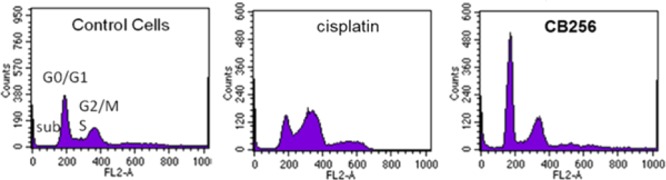
Flow cytometric analysis of C6 glioma cells following exposure to CB256 or cisplatin (10 μM) for 24 h. Untreated cells were used as control.
Table 3. Percentages of C6 Glioma Cells in Sub-G1, G0/G1, S, and G2/M Cell Cycle Phases after Treatment with Compound CB256.
| % of cells
in cell cycle phasesa |
||||
|---|---|---|---|---|
| sub-G1 | G0/G1 | S | G2/M | |
| control cells | 5.89 | 54.94 | 11.15 | 28.02 |
| CB256 | 9.06 | 49.07 | 13.18 | 28.69 |
Cells were treated with CB256 (0.3 μM) and incubated for 72 h. Untreated cells were used as control.
In conclusion, the aim of this work was to synthesize a new 2-phenyl-imidazopyridin-dipropylacetamide ligand (CB256), which could act as a smart carrier of metallo drugs to TSPO-overexpressing cells. The affinity of CB256 for TSPO, evaluated in vitro on membrane extracts from C6 rat glioma cells, proved to be in the submicromolar range. A rather unexpected result was the high cytotoxicity of CB256, which was ca. 75 times greater than that of the precursor ligand CB86, which lacks the metal-coordinating moiety. We argued that such a high toxicity could be due to the ability of the dipicolylaminic moiety to react with an essential metal ion, such as copper, and cause mostly double-strand DNA lesions. Experiments performed on pET plasmid DNA have shown that, indeed, this is the case. It is also noteworthy that CB256 is equally cytotoxic toward A2780 and A2780cisR tumor cell lines. The ability of CB256 to cause collapse of the mitochondrial membrane potential (ΔΨm), to interfere with the cell-cycle progression of glioma C6 cells, and to cause mitochondrial and nuclear morphology modifications was also investigated suggesting that CB256, like cisplatin, is able to induce apoptosis in cancer cells. All data indicate that CB256 can be thus considered a synthetic mimic of bleomycin with affinity for TSPO receptor, a feature worth further investigation.
Acknowledgments
We thank Mr. Giovanni Dipinto and Mr. Antonio Palermo for their skillful technical assistance.
Glossary
ABBREVIATIONS
- TSPO
18-kDa translocator protein
- PBR
peripheral-type benzodiazepine receptor
- BFC
bifunctional chelate approach
Supporting Information Available
Detailed information about synthetic procedures, characterization, and in vitro biological assays. This material is available free of charge via the Internet at http://pubs.acs.org.
Funding was provided by the University of Bari (Italy), the Italian Ministero dell’Università e della Ricerca (MIUR), the Fondo per gli investimenti della Ricerca di Base (FIRB RINAME RBAP114AMK), and the European Union (COST CM1105, Metallo-Drug Design and Action).
The authors declare no competing financial interest.
Supplementary Material
References
- Papadopoulos V.; Baraldi M.; Guilarte T. R.; Knudsen T. B.; Lacapère J.-J.; Lindemann P.; Norenberg M. D.; Nutt D.; Weizman A.; Zhang M.-R.; Gavish M. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [DOI] [PubMed] [Google Scholar]
- Rupprecht R.; Papadopoulos V.; Rammes G.; Baghai T. C.; Fan J.; Akula N.; Groyer G.; Adams D.; Schumacher M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discovery 2010, 9, 971–988. [DOI] [PubMed] [Google Scholar]
- Trapani G.; Denora N.; Trapani A.; Laquintana V. Recent advances in ligand targeted therapy. J. Drug Targeting 2012, 20, 1–22. [DOI] [PubMed] [Google Scholar]
- Denora N.; Cassano T.; Laquintana V.; Lopalco A.; Trapani A.; Cimmino C. S.; Laconca L.; Giuffrida A.; Trapani G. Novel codrugs with GABAergic activity for dopamine delivery in the brain. Int. J. Pharm. 2012, 437, 221–231. [DOI] [PubMed] [Google Scholar]
- Galiegue S.; Tinel N.; Casellas P. The peripheral benzodiazepine receptor: a promising therapeutic drug target. Curr. Med. Chem. 2003, 10, 1563–1572. [DOI] [PubMed] [Google Scholar]
- Maaser K.; Grabowski P.; Sutter A. P.; Höpfner M.; Foss H. D.; Stein H.; Berger G.; Gavish M.; Zeitz M.; Scherübl H. Overexpression of the peripheral benzodiazepine receptor is a relevant prognostic factor in stage III colorectal cancer. Clin. Cancer Res. 2002, 8, 3205–3209. [PubMed] [Google Scholar]
- Veenman L.; Levin E.; Weisinger G.; Leschiner S.; Spanier I.; Snyder S. H.; Weizman A.; Gavish M. Peripheral-type benzodiazepine receptor density and in vitro tumorigenicity of glioma cell lines. Biochem. Pharmacol. 2004, 68, 689–698. [DOI] [PubMed] [Google Scholar]
- Scarf A. M.; Ittner L. M.; Kassiou M. The translocator protein (18 kDa): central nervous system disease and drug design. J. Med. Chem. 2009, 52, 581–592. [DOI] [PubMed] [Google Scholar]
- Laquintana V.; Denora N.; Musacchio T.; Lasorsa M.; Latrofa A.; Trapani G. Peripheral benzodiazepine receptor ligand-PLGA polymer conjugates potentially useful as delivery systems of apoptotic agents. J. Controlled Release 2009, 137, 185–195. [DOI] [PubMed] [Google Scholar]
- Denora N.; Laquintana V.; Trapani A.; Suzuki H.; Sawada M.; Trapani G. New fluorescent probes targeting the mitochondrial-located translocator protein 18 kDa (TSPO) as activated microglia imaging agents. Pharm. Res. 2011, 28, 2820–2832. [DOI] [PubMed] [Google Scholar]
- Denora N.; Laquintana V.; Trapani A.; Lopedota A.; Latrofa A.; Gallo J. M.; Trapani G. Translocator protein (TSPO) ligand-Ara-C (cytarabine) conjugates as a strategy to deliver antineoplastic drugs and to enhance drug clinical potential. Mol. Pharmaceutics 2010, 7, 2255–2269. [DOI] [PubMed] [Google Scholar]
- Denora N.; Laquintana V.; Lopalco A.; Iacobazzi R. M.; Lopedota A.; Cutrignelli A.; Iacobellis G.; Annese C.; Cascione M.; Leporatti S.; Franco M. In vitro targeting and imaging the translocator protein TSPO 18-kDa through G(4)-PAMAM-FITC labeled dendrimer. J. Controlled Release 2013, 172, 1111–1125. [DOI] [PubMed] [Google Scholar]
- Margiotta N.; Ostuni R.; Ranaldo R.; Denora N.; Laquintana V.; Trapani G.; Liso G.; Natile G. Synthesis and characterization of a platinum(II) complex tethered to a ligand of the peripheral benzodiazepine receptor. J. Med. Chem. 2007, 50, 1019–1027. [DOI] [PubMed] [Google Scholar]
- Laquintana V.; Denora N.; Lopalco A.; Lopedota A.; Cutrignelli A.; Lasorsa F. M.; Agostino G.; Franco M. Translocator protein ligand–PLGA conjugated nanoparticles for 5-fluorouracil delivery to glioma cancer cells. Mol. Pharmaceutics 2014, 11, 859–871. [DOI] [PubMed] [Google Scholar]
- Cappelli A.; Mancini A.; Sudati F.; Valenti S.; Anzini M.; Belloli S.; Moresco R. M.; Matarrese M.; Vaghi M.; Fabro A.; Fazio F.; Vomero S. Synthesis and biological characterization of novel 2-quinolinecarboxamide ligands of the peripheral benzodiazepine receptors bearing technetium-99m or rhenium. Bioconjugate Chem. 2008, 19, 1143–1153. [DOI] [PubMed] [Google Scholar]
- Margiotta N.; Denora N.; Ostuni R.; Laquintana V.; Anderson A.; Johnson S. W.; Trapani G.; Natile G. Platinum(II) complexes with bioactive carrier ligands having high affinity for the translocator protein. J. Med. Chem. 2010, 53, 5144–5154. [DOI] [PubMed] [Google Scholar]
- Denora N.; Laquintana V.; Pisu M. G.; Dore R.; Murru L.; Latrofa A.; Trapani G.; Sanna E. 2-Phenyl-imidazo[1,2-a]pyridine compounds containing hydrophilic groups as potent and selective ligands for peripheral benzodiazepine receptors: synthesis, binding affinity and electrophysiological studies. J. Med. Chem. 2008, 51, 6876–6888. [DOI] [PubMed] [Google Scholar]
- Piccinonna S.; Margiotta N.; Denora N.; Iacobazzi R. M.; Pacifico C.; Trapani G.; Natile G. A model radiopharmaceutical agent targeted to translocator protein 18 kDa (TSPO). Dalton Trans. 2013, 42, 10112–11015. [DOI] [PubMed] [Google Scholar]
- Piccinonna S.; Denora N.; Margiotta N.; Laquintana V.; Trapani G.; Natile G. Synthesis, characterization, and binding to the translocator protein (18 kDa, TSPO) of a new rhenium complex as a model of radiopharmaceutical agents. Z. Anorg. Allg. Chem. 2013, 639, 1606–1612. [Google Scholar]
- Liu S. The role of coordination chemistry in the development of target-specific radiopharmaceuticals. Chem. Soc. Rev. 2004, 33, 445–461. [DOI] [PubMed] [Google Scholar]
- Miettinen H.; Kononen J.; Haapasalo H.; Helén P.; Sallinen P.; Harjuntausta T.; Helin H.; Alho H. Expression of peripheral-type benzodiazepine receptor and diazepam binding inhibitor in human astrocytomas: relationship to cell proliferation. Cancer Res. 1995, 55, 2691–2695. [PubMed] [Google Scholar]
- Shoukrun R.; Veenman L.; Shandalov Y.; Leschiner S.; Spanier I.; Karry R.; Katz Y.; Weisinger G.; Weizman A.; Gavish M. The 18-kDa translocator protein, formerly known as the peripheral-type benzodiazepine receptor, confers proapoptotic and antineoplastic effects in a human colorectal cancer cell line. Pharmacogenet. Genomics 2008, 1811977–88. [DOI] [PubMed] [Google Scholar]
- Kelland L. R.; Abel G.; McKeage M. J.; Jones M.; Goddard P. M.; Valenti M.; Murrer B. A.; Harrap K. R. Preclinical antitumor evaluation of bis-acetato-ammine-dichloro-cyclohexylamine platinum(IV): an orally active platinum drug. Cancer Res. 1993, 53, 2581–2586. [PubMed] [Google Scholar]
- Özalp-Yaman Ş.; de Hoog P.; Amadei G.; Pitié M.; Gamez P.; Dewelle J.; Mijatovic T.; Meunier B.; Kiss R.; Reedijk J. Platinated copper(3-Clip-Phen) complexes as effective DNA-cleaving and cytotoxic agents. Chem.—Eur. J. 2008, 14, 3418–3426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.