Abstract
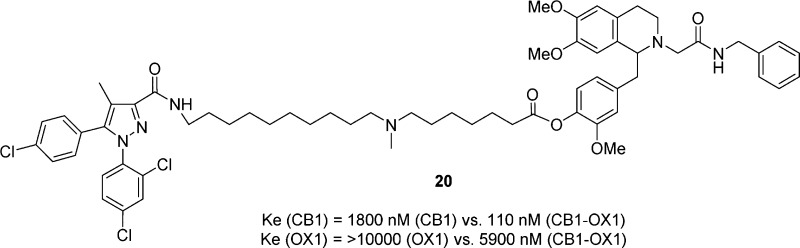
Cannabinoid CB1 and orexin OX1 receptors have been suggested to form heterodimers and oligomers. Aimed at studying these complexes, a series of bivalent CB1 and OX1 ligands combining SR141716 and ACT-078573 pharmacophores were designed, synthesized, and tested for activity against CB1 and OX1 individually and in cell lines that coexpress both receptors. Compound 20 showed a robust enhancement in potency at both receptors when coexpressed as compared to individually expressed, suggesting possible interaction with CB1-OX1 dimers. Bivalent ligands targeting CB1-OX1 receptor dimers could be potentially useful as a tool for further exploring the roles of such heterodimers in vitro and in vivo.
Keywords: Cannabinoid, orexin, heterodimer, bivalent ligands
A growing list of biochemical and functional evidence suggests that G protein-coupled receptors (GPCRs), historically considered monomers, form and function as homo- and heterodimers, or even higher-ordered oligo-mers.1−5 These receptor dimers/oligomers often display unique ligand binding, distinct phenotypic trafficking, and altered signaling properties compared to their individual monomers.3,6,7 A wide variety of GPCRs, including cannabinoid,8−11 opioid,12−16 dopamine,17−19 and serotonin receptors,20 have been demonstrated to form dimers or oligomers both within and across receptor types. In particular, the CB1 and OX1 receptors were shown to dimerize in cotransfected cells by coexpression, coimmunoprecipitation, and resonance energy transfer studies.21,22 Moreover, these two receptors have overlapping distribution in certain brain areas such as the lateral hippocampus, and hypothalamic CB1 mRNA was found to be coexpressed with prepro-orexin.23,24 Finally, the in vivo functional cross-talk between these two receptors has been demonstrated in the finding that pretreatment with subeffective doses of the CB1 antagonist SR141716 attenuated the orexigenic actions of orexin A in rats.25
GPCR dimerization has been widely reported in primary cultures and, more recently, has also been demonstrated in native tissues.26,27 However, their in vivo existence and functional significance have yet to be fully understood.4 One approach to study these complexes is to develop bivalent ligands that preferentially interact with the receptor heterodimers to probe receptor heterodimerization in vivo.28 It is anticipated that these bivalent ligands, provided that they have the appropriate linker length, will bind to the receptor with high affinity due to the small containment volume for the second pharmacophore, resulting in thermodynamically more favorable binding interactions than the monovalent binding of two molecules. Such an approach may result in enhanced selectivity and efficacy and improved safety relative to drugs that address only a single target.28 For example, Portoghese et al. have reported a range of homo- and heterodimeric opioid ligands with varying linker length, some of which displayed significantly greater potency and selectivity compared to their monomeric congeners.29 In addition, bivalent ligands are capable of inducing the formation of heterodimers, thereby shifting the equilibrium between monomers, homomers, and heteromers as demonstrated in recent studies on MOP and CCK2 receptors.30
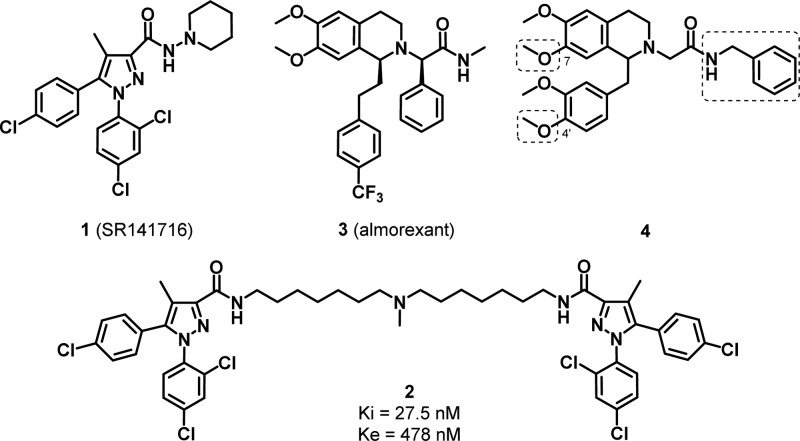
In this study we present our efforts in developing bivalent ligands targeting CB1 and OX1 heterodimers. The key elements of ligand design are the selection of the individual ligands, the identification of the point of attachment for the linker, and the nature and length of the linker. The CB1 pharmacophore selected is 1,5-diarylpyrazole, based on the CB1 antagonist/inverse agonist SR141716 (1). We have previously demonstrated the efficacy of symmetric CB1 bivalent ligands based on this scaffold targeting the homodimeric CB1 receptor (2),31 in which linkers of 15 atoms seemed to be optimal within the series tested. The 1,2,3,4-tetrahydroisoquinoline scaffold derived from the orexin antagonist almorexant (ACT-078573, 3) was selected as the OX1 pharmacophore. Compound 4, a lead compound in the discovery of 3, showed reasonable potency at and selectivity for the OX1 receptor.32,33 There is limited structure–activity relationship (SAR) information on this tetrahydroisoquinoline scaffold, and determining a suitable point of attachment was required. Therefore, we performed focused SAR studies on compound 4 at three locations (Figure 1).
Figure 1.
CB1 and OX1 ligands and a CB1 bivalent ligand.
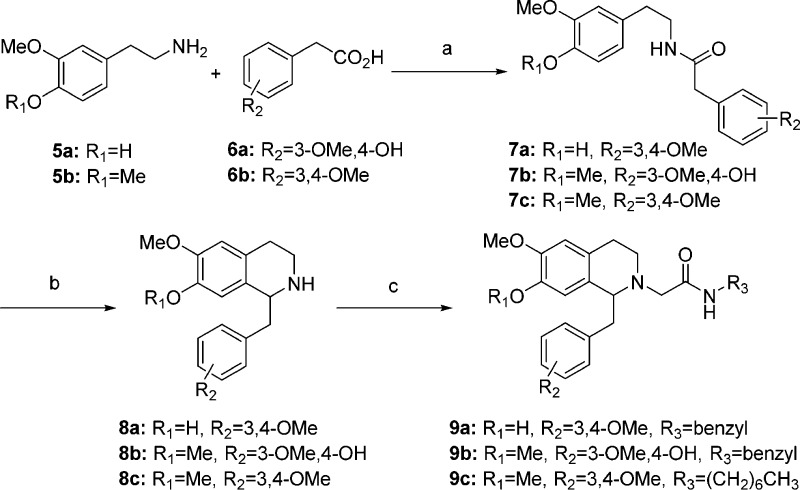
The tetrahydroisoquinoline-based orexin antagonists were prepared following procedures in the literature and developed in our laboratory (Scheme 1).32,33 Thus, the phenethylamines 5a–b and phenylacetic acid derivatives 6a–b were coupled using HBTU to give the amides 7a–c. Bischler–Napieralski reaction using phosphorus oxychloride gave the cyclization to the dihydroisoquinoline, which could be readily reduced to the tetrahydroisoquinoline 8a–c using sodium borohydride. The amine was then alkylated with the α-bromoacetamide, prepared by coupling of bromoacetylbromide and the corresponding amine,33 to give 9a–c.
Scheme 1. Synthesis of OX1 Antagonists.
Reagents and conditions: (a) HBTU, iPr2EtN, DMF; (b) (i) POCl3, toluene; (ii) NaBH4, MeOH; (c) R3NHCOCH2Br, iPr2EtN, Bu4NI, DMF.
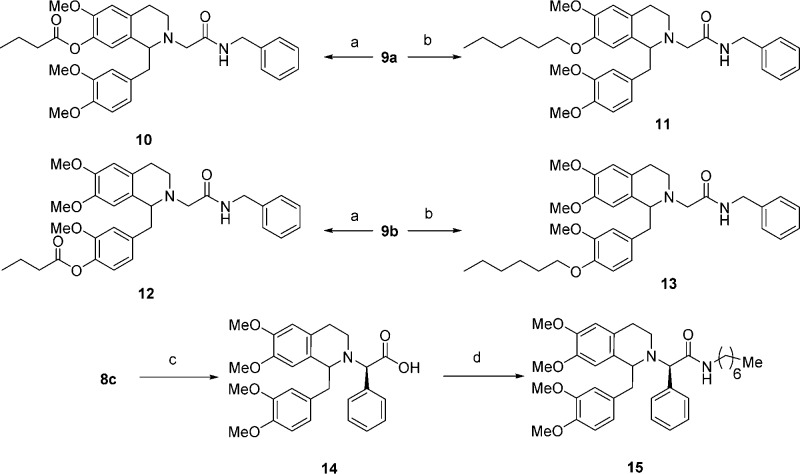
Alterations at the three proposed locations (9a–c) were achieved as shown in Scheme 2. Thus, acylation with butyric acid of the 7-hydroxyl of the tetrahydroisoquinoline (9a) or the hydroxyl of the 1-benzyl group (9b) gave the esters 10 and 12, respectively. Similarly, alkylation of 9a or 9b with 1-bromohexane provided respectively the ethers 11 and 13. For the α-phenyl analogue (15), a slightly different route than that of 9c was followed. N-Alkylation of 8c with the α-bromoester followed by hydrolysis of resulting ester provided the acid 14. A final amide coupling gave 15.
Scheme 2. Modifications at Three Locations.
Reagents and conditions: (a) butyric acid, BOP, iPr2EtN, CH2Cl2; (b) 1-bromohexane, K2CO3, Bu4NI, DMF; (c) (i) ethyl bromophenylacetate, iPr2EtN, Bu4NI, DMF; (ii) 2 N NaOH, EtOH; (d) 1-aminoheptane, BOP, iPr2EtN, CH2Cl2.
Activity of these substituted tetrahydroisoquinolines at OX1 was determined in a calcium mobilization based functional assay using RD-HGA16 cell lines overexpressing the OX1 receptor, as previously described.33,34 The apparent dissociation constant Ke was calculated, and the values are the mean of at least three independent experiments performed in duplicate (Table 1). The results were then used to determine the optimal linker attachment positions to construct bivalent ligands.
Table 1. OX1 Antagonist Activity.
| number | Ke (nM) ± SEM |
|---|---|
| 4 | 200 ± 50 |
| 9c | 590 ± 230 |
| 10 | 78 ± 24 |
| 11 | 310 ± 80 |
| 12 | 43 ± 4 |
| 13 | 120 ± 20 |
| 15 | 22 ± 10 |
The 7-position of the tetrahydroisoquinoline has been shown to favor substitutions with more steric demanding groups.32,33 Indeed, replacement of the methoxyl group with a butyl ester gave 10, which displayed increased potency over 4. The 7-hexylether 11 was slightly less potent than 4. These results confirm tolerance of steric bulk at this position. For the 4-position on the 1-benzyl group, both ester 12 and the 4′-hexylether 13 were more potent than the parent compound, suggesting this position as a viable point for linker attachment. Finally, the N-heptyl derivative 9c was lower in potency, but in general, the change was tolerated. Further encouraging results came from the α-phenyl compound 15, which showed the greatest potency of the series despite a large heptyl group. Together, the results showed that all three positions could potentially be suitable for ligand attachment.
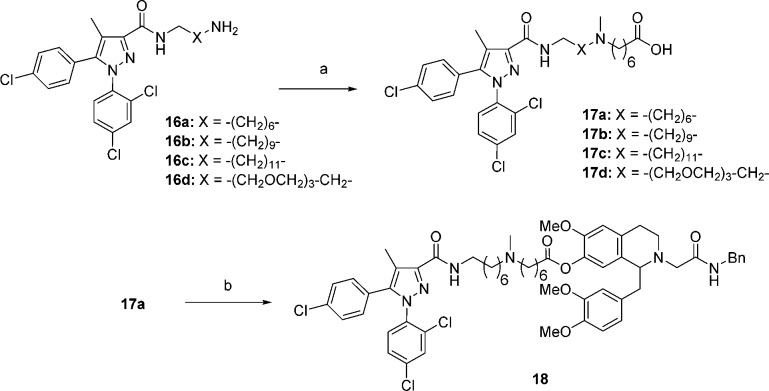
Synthesis of the bivalent ligands with linkers at the three respective positions was accomplished in an analogous fashion to the monomer synthesis (Schemes 3–5). For bivalent ligands with the 7-position ester linker (18, Scheme 3), synthesis began with amines 16a–d, which were readily synthesized following procedures previously described by us.31 Alkylation of 16a–d with ethyl 7-bromoheptanoate, followed by N-methylation via reductive amination and ester hydrolysis gave the acids 17a–d. Finally, reaction of 17a with intermediate 9a via a BOP mediated coupling gave ester-linked bivalent ligand 18.
Scheme 3. Synthesis of Bivalent Ligand 18.
Reagents and conditions: (a) (i) Br-(CH2)6-CO2Et, Et3N, toluene; (ii) HCHO, Na(AcO)3BH, 1,2-DCE; (iii) 2 N NaOH, EtOH; (b) 9a, BOP, iPr2EtN, CH2Cl2.
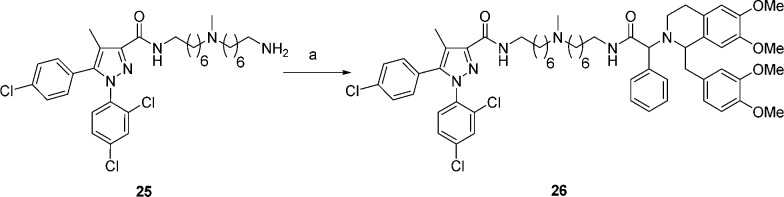
Scheme 5. Synthesis of Bivalent Ligand 26.
Reagents and conditions: (a) 14, BOP, iPr2EtN, DMF.
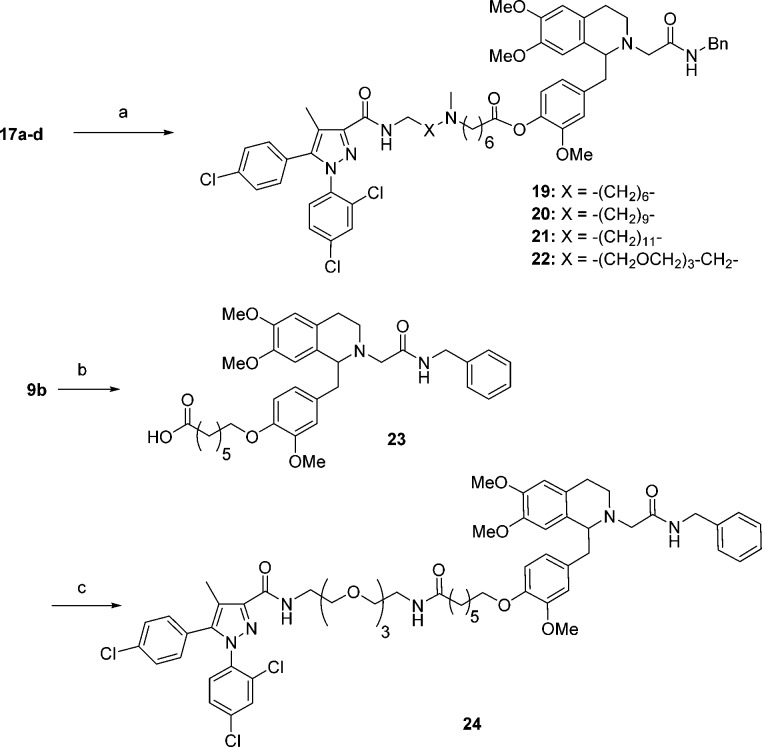
Bivalent ligands linked at the 1-benzyl position (19–22 and 24) were prepared as shown in Scheme 4. Similar to the preparation of 18, bivalent ligands 19–22 with the ester linkage were synthesized by amide coupling of 17a–d to 9b. The synthesis of 24 started with alkylation of phenol 9b with ethyl 7-bromoheptanoate in the presence of potassium carbonate in DMF, followed by the hydrolysis of the resulting ester to give acid 23. Coupling between 23 and 16d using BOP as coupling agent provided bivalent ligand 24.
Scheme 4. Synthesis of Bivalent Ligands 19–22 and 24.
Reagents and conditions: (a) 9b, BOP, iPr2EtN, CH2Cl2; (b) (i) Br-(CH2)6-CO2Et, K2CO3, DMF; (ii) 2 N NaOH, EtOH; (c) 16d, BOP, iPr2EtN, DMF.
Finally, for bivalent ligand with the α-phenylacetamide linkage (26), previously prepared amine 25(31) was coupled to the acid 14 with BOP as the coupling agent to give bivalent ligand 26 (Scheme 5).
The synthesized bivalent ligands were first evaluated for their activity at the CB1 and OX1 receptors, respectively, in calcium functional assays in RD-HGA16 cell lines stably expressing either CB1 or OX1 receptors (Table 2). CP55,940 was employed as the agonist for activity at the CB1 receptor and orexin A at the OX1 receptor. The bivalent ligands showed a significant drop in activity over the individual components at either CB1 or OX1 receptors. In the CB1 cell lines, all the bivalent ligands were active but showed reduced activity at the CB1 receptor. Whereas SR141716 shows a Ke of 1 nM, the best activity shown in this series is 1100 nM (21). Surprisingly, they were mostly inactive at the OX1 receptor in cells singly expressing the OX1 receptor.
Table 2. Activity of Bivalent Ligands against CB1, OX1, and Coexpressed Receptors.
|
Ke (nM) ± SEM |
|||||
|---|---|---|---|---|---|
| No. | linker length (atoms) | CB1 vs CP55,940 | CB1-OX1 vs CP55,940 | CB1-OX1 vs Orexin-A | OX1 vs Orexin-A |
| 1 | 1.1 ± 0.1 | 1.0 ± 0.6 | >10000 | >10000 | |
| SB334867 | >10000 | >10000 | 32 ± 20 | 45 ± 12 | |
| 18 | 15 | 2100 ± 140 | 1200 ± 270 | >10000 | 8800 ± 2300 |
| 19 | 15 | 1800 ± 710 | 610 ± 16 | >10000 | 8460 ± 680 |
| 20 | 18 | 1800 ± 1300 | 110 ± 45 | 5900 ± 1300 | >10000 |
| 21 | 20 | 1100 ± 500 | 410 ± 46 | >10000 | >10000 |
| 22 | 19 | 1800 ± 680 | 420 ± 280 | 4500 ± 2200 | 3500 ± 1300 |
| 24 | 19 | 8000 ± 2800 | 580 ± 260 | >10000 | 8600 ± 1200 |
| 26 | 15 | 3000 ± 1100 | 230 ± 11 | >10000 | 5500 ± 1300 |
Cells coexpressing both CB1 and OX1 receptors were obtained by stably expressing OX1 receptors in RD-HGA16 cells that already stably express CB1 receptors. The expression level of the newly transfected OX1 receptors was determined in radioligand binding studies using [125I]-orexin A, which demonstrated 293.6 pmol OX1 receptors/106 cells. These results are in accordance with other dual-expressing systems that have been shown to readily form CB1-OX1 receptor heterodimers, where Hilairet et al. reported Bmax of 338 fmol/106 cells for the OX1 receptor in their cells coexpressing CB1 and OX1 receptors.22
In these dual-expressing cells, SR141716 showed CB1 potency that is comparable to that in the cells singly expressing the CB1 receptor (Table 2). Similarly, SB334867, an OX1 selective antagonist, showed almost identical potency in these cells and cells singly expressing the OX1 receptors. As a control, SR141716 did not block orexin A activity at the OX1 receptor, and conversely, SB334867 did not block CP55940 activity at up to 10 μM in these cells (Table 2). These data confirm that both the CB1 and OX1 receptors function properly in the dual-transfected cells and that there is no change in the receptor intrinsic pharmacological properties.
The bivalent ligands were then evaluated in the dual-expressing cells for activity at the CB1 or OX1 receptors. In general, the bivalent ligands were active at the CB1 receptor, but little or no activity was observed at the OX1 receptor, showing a trend similar to that seen in cells singly expressing these receptors. This suggests that the OX1 receptor, unlike the CB1 receptor, may have limited tolerance of steric bulk. Alternatively, since almorexant has been shown to be a noncompetitive antagonist at the OX2 receptor,35 an allosteric binding site could also be present at the OX1 receptor with these bivalent ligands that negatively impact receptor binding.
Interestingly, an enhancement of CB1 activity was observed with all bivalent ligands compared to cells singly expressing the CB1 receptor. Specifically, the CB1 potency is enhanced in all cases by approximately 2–5-fold, which is similar to the responses seen in our CB1 homodimer study.31 On the contrary, compound 20 was the only compound that showed a slight potency enhancement at the OX1 receptor.
During bivalent ligand studies, normally a decrease and then increase in affinity/potency with the elongation of the linker is present when these bivalent ligands simultaneously bind to both receptors. Such a trend was present in our CB1 homobivalent ligands and other studies.29,31,36,37 The effect of linker length on activity was investigated in the 4′-ester series (19–21). Interestingly, compound 20 with an 18-atom linker was more potent than 19 (15 atoms) and 21 (20 atoms) at the CB1 receptor. At the OX1 receptor, 20 was the only compound that showed activity, though in the micromolar range. Moreover, 20 in fact showed potency enhancement at both the CB1 and the OX1 receptor, though the effect is minimal at the OX1 receptor. While these results may suggest binding of 20 to both receptors, more potent bivalent ligands are clearly needed before a more definitive answer can be obtained.
The nature and position of the linker should also be considered. We have previously observed in homodimers of CB1 antagonists that hydrophobic linkers are generally preferred and peptide-based linkers show poor activity. In this case, the glycol linkers (22 and 24) showed comparable activity to those with hydrophobic linkers at the CB1 receptor. The 4-position of the pyrazole on SR141716 was successfully employed in building homobivalent ligands. These CB1-OX1 bivalent ligands were linked via this position and again showed reasonable activity at the CB1 receptor. On the orexin pharmacophore, three different positions of attachment for the linker were identified that tolerated steric bulk prior to the synthesis of the bivalent ligands. However, the fact that all the bivalent ligands have significantly reduced or abolished activity at the OX1 receptor indicates that all of these positions only tolerate limited size. While the α-phenyl acetamide (15) was the most active monomer at the OX1 receptor, the resulting bivalent ligand 26 showed diminished activity in coexpressing CB1-OX1 cells. In general, the measured activity was low so it is difficult to see any appreciable potency enhancement.
In summary, we have explored the tetrahydroisoquinoline scaffold with regard to finding potential sites for linkers and, based on the findings, developed a series of bivalent CB1-OX1 agents. While all the bivalent ligands showed reasonable potency at the CB1 receptor, most of them were inactive up to 10 μM at the OX1 receptor. One compound (20) with an 18-atom linker showed enhancement in activity against both CB1 and OX1 in a cell line coexpressing both receptors suggesting possible interaction with both receptors. Clearly, bivalent ligands with improved potency, particularly at the OX1 binding site, are needed for further studies. Once available, these bivalent ligands can potentially be exploited to further probe the interaction of these two receptors and may lead to novel therapeutic approaches to neurological disorders mediated by these receptors.
Acknowledgments
We thank Tiffany Langston, Keith Warner, Dr. Ann Decker, and Dr. Elaine Gay for their valuable technical assistance.
Glossary
ABBREVIATIONS
- BOP
(benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate
- CB1
cannabinoid receptor type 1
- HBTU
O-benzotriazole-N,N,N′,N′-tetramethyl-uronium-hexafluoro-phosphate
- HPLC
high performance liquid chromatography
- OX1
orexin 1 receptor
- OX2
orexin 2 receptor
- SAR
structure–activity relationship
Supporting Information Available
Synthesis and characterization of target compounds and radioligand binding studies. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was supported by National Institute on Drug Abuse, National Institutes of Health, USA (grant nos DA026582 and DA032837).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Milligan G. G protein-coupled receptor dimerization: function and ligand pharmacology. Mol. Pharmacol. 2004, 66, 1–7. [DOI] [PubMed] [Google Scholar]
- Bouvier M. Oligomerization of G-protein-coupled transmitter receptors. Nat. Rev. Neurosci. 2001, 2, 274–286. [DOI] [PubMed] [Google Scholar]
- Milligan G. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br. J. Pharmacol. 2009, 158, 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller C.; Kuhhorn J.; Gmeiner P.; Class A. G-protein-coupled receptor (GPCR) dimers and bivalent ligands. J. Med. Chem. 2013, 56, 6542–6559. [DOI] [PubMed] [Google Scholar]
- George S. R.; O’Dowd B. F.; Lee S. P. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat. Rev. Drug. Discovery 2002, 1, 808–820. [DOI] [PubMed] [Google Scholar]
- Minneman K. P. Heterodimerization and surface localization of G protein coupled receptors. Biochem. Pharmacol. 2007, 73, 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. G-protein-coupled receptor heterodimers: pharmacology, function and relevance to drug discovery. Drug. Discovery Today 2006, 11, 541–549. [DOI] [PubMed] [Google Scholar]
- Kearn C. S.; Blake-Palmer K.; Daniel E.; Mackie K.; Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk?. Mol. Pharmacol. 2005, 67, 1697–1704. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptor homo- and heterodimerization. Life Sci. 2005, 77, 1667–1673. [DOI] [PubMed] [Google Scholar]
- Wager-Miller J.; Westenbroek R.; Mackie K. Dimerization of G protein-coupled receptors: CB1 cannabinoid receptors as an example. Chem. Phys. Lipids 2002, 121, 83–89. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer A. N.; Hogenboom F.; Wardeh G.; De Vries T. J. Interactions between CB1 cannabinoid and mu opioid receptors mediating inhibition of neurotransmitter release in rat nucleus accumbens core. Neuropharmacology 2006, 51, 773–781. [DOI] [PubMed] [Google Scholar]
- Cvejic S.; Devi L. A. Dimerization of the delta opioid receptor: implication for a role in receptor internalization. J. Biol. Chem. 1997, 272, 26959–26964. [DOI] [PubMed] [Google Scholar]
- Filizola M.; Weinstein H. Structural models for dimerization of G-protein coupled receptors: the opioid receptor homodimers. Biopolymers 2002, 66, 317–325. [DOI] [PubMed] [Google Scholar]
- Garzon J.; Juarros J. L.; Castro M. A.; Sanchez-Blazquez P. Antibodies to the cloned mu-opioid receptor detect various molecular weight forms in areas of mouse brain. Mol. Pharmacol. 1995, 47, 738–744. [PubMed] [Google Scholar]
- Bhushan R. G.; Sharma S. K.; Xie Z.; Daniels D. J.; Portoghese P. S. A bivalent ligand (KDN-21) reveals spinal delta and kappa opioid receptors are organized as heterodimers that give rise to delta(1) and kappa(2) phenotypes. Selective targeting of delta-kappa heterodimers. J. Med. Chem. 2004, 47, 2969–2972. [DOI] [PubMed] [Google Scholar]
- Gomes I.; Gupta A.; Filipovska J.; Szeto H. H.; Pintar J. E.; Devi L. A. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc. Natl. Acad. Sci. U.S.A. 2004, 101, 5135–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearn C. S.; Blake-Palmer K.; Daniel E.; Mackie K.; Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk?. Mol. Pharmacol. 2005, 67, 1697–1704. [DOI] [PubMed] [Google Scholar]
- Ng G. Y.; O’Dowd B. F.; Lee S. P.; Chung H. T.; Brann M. R.; Seeman P.; George S. R. Dopamine D2 receptor dimers and receptor-blocking peptides. Biochem. Biophys. Res. Commun. 1996, 227, 200–204. [DOI] [PubMed] [Google Scholar]
- Guo W.; Shi L.; Javitch J. A. The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J. Biol. Chem. 2003, 278, 4385–4388. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K. Functional significance of serotonin receptor dimerization. Exp. Brain Res. 2013, 230, 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J.; Pediani J. D.; Canals M.; Milasta S.; Milligan G. Orexin-1 receptor-cannabinoid CB1 receptor heterodimerization results in both ligand-dependent and -independent coordinated alterations of receptor localization and function. J. Biol. Chem. 2006, 281, 38812–38824. [DOI] [PubMed] [Google Scholar]
- Hilairet S.; Bouaboula M.; Carriere D.; Le Fur G.; Casellas P. Hypersensitization of the orexin 1 receptor by the CB1 receptor: evidence for cross-talk blocked by the specific CB1 antagonist, SR141716. J. Biol. Chem. 2003, 278, 23731–23737. [DOI] [PubMed] [Google Scholar]
- Hervieu G. J.; Cluderay J. E.; Harrison D. C.; Roberts J. C.; Leslie R. A. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience 2001, 103, 777–797. [DOI] [PubMed] [Google Scholar]
- Cota D.; Marsicano G.; Tschop M.; Grubler Y.; Flachskamm C.; Schubert M.; Auer D.; Yassouridis A.; Thone-Reineke C.; Ortmann S.; Tomassoni F.; Cervino C.; Nisoli E.; Linthorst A. C.; Pasquali R.; Lutz B.; Stalla G. K.; Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Invest. 2003, 112, 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo I.; Gomez de Heras R.; Rodriguez de Fonseca F.; Navarro M. Pretreatment with subeffective doses of rimonabant attenuates orexigenic actions of orexin A-hypocretin 1. Neuropharmacology 2008, 54, 219–225. [DOI] [PubMed] [Google Scholar]
- Gupta A.; Mulder J.; Gomes I.; Rozenfeld R.; Bushlin I.; Ong E.; Lim M.; Maillet E.; Junek M.; Cahill C. M.; Harkany T.; Devi L. A. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci. Signaling 2010, 3, ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albizu L.; Cottet M.; Kralikova M.; Stoev S.; Seyer R.; Brabet I.; Roux T.; Bazin H.; Bourrier E.; Lamarque L.; Breton C.; Rives M. L.; Newman A.; Javitch J.; Trinquet E.; Manning M.; Pin J. P.; Mouillac B.; Durroux T. Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat. Chem. Biol. 2010, 6, 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morphy R.; Rankovic Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005, 48, 6523–6543. [DOI] [PubMed] [Google Scholar]
- Portoghese P. S. From models to molecules: opioid receptor dimers, bivalent ligands, and selective opioid receptor probes. J. Med. Chem. 2001, 44, 2259–2269. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; Akgun E.; Harikumar K. G.; Hopson J.; Powers M. D.; Lunzer M. M.; Miller L. J.; Portoghese P. S. Induced association of mu opioid (MOP) and type 2 cholecystokinin (CCK2) receptors by novel bivalent ligands. J. Med. Chem. 2009, 52, 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Gilliam A.; Maitra R.; Damaj M. I.; Tajuba J. M.; Seltzman H. H.; Thomas B. F. Synthesis and biological evaluation of bivalent ligands for the cannabinoid 1 receptor. J. Med. Chem. 2010, 53, 7048–7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koberstein R.; Aissaoui H.; Bur D.; Clozel M.; Fischli W.; Jenck F.; Mueller C.; Nayler O.; Sifferlen T.; Treiber A.; Weller T. Tetrahydroisoquinolines as orexin receptor antagonists: strategies for lead optimization by solution-phase chemistry. Chimia 2003, 57, 270–275. [Google Scholar]
- Perrey D. A.; German N. A.; Gilmour B. P.; Li J. X.; Harris D. L.; Thomas B. F.; Zhang Y. Substituted tetrahydroisoquinolines as selective antagonists for the orexin 1 receptor. J. Med. Chem. 2013, 56, 6901–6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrey D. A.; Gilmour B. P.; Runyon S. P.; Thomas B. F.; Zhang Y. Diaryl urea analogues of SB-334867 as orexin-1 receptor antagonists. Bioorg. Med. Chem. Lett. 2011, 21, 2980–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe P.; Borroni E.; Pinard E.; Wettstein J. G.; Knoflach F. Biochemical and electrophysiological characterization of almorexant, a dual orexin 1 receptor (OX1)/orexin 2 receptor (OX2) antagonist: comparison with selective OX1 and OX2 antagonists. Mol. Pharmacol. 2009, 76, 618–631. [DOI] [PubMed] [Google Scholar]
- Tanaka T.; Nomura W.; Narumi T.; Masuda A.; Tamamura H. Bivalent ligands of CXCR4 with rigid linkers for elucidation of the dimerization state in cells. J. Am. Chem. Soc. 2010, 132, 15899–15901. [DOI] [PubMed] [Google Scholar]
- Shonberg J.; Scammells P. J.; Capuano B. Design strategies for bivalent ligands targeting GPCRs. ChemMedChem 2011, 6, 963–974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.