Abstract
Parkinson’s disease (PD) is a major health problem affecting millions of people worldwide. Recent studies provide compelling evidence that altered Ca2+ homeostasis may underlie disease pathomechanism and be an inherent feature of all vulnerable neurons. The downstream effects of altered Ca2+ handling in the distinct subcellular organelles for proper cellular function are beginning to be elucidated. Here, we summarize the evidence that vulnerable neurons may be exposed to homeostatic Ca2+ stress which may determine their selective vulnerability, and suggest how abnormal Ca2+ handling in the distinct intracellular compartments may compromise neuronal health in the context of aging, environmental, and genetic stress. Gaining a better understanding of the varied effects of Ca2+ dyshomeostasis may allow novel combinatorial therapeutic strategies to slow PD progression.
Keywords: Parkinson’s disease, dopamine, calcium, mitochondria, endoplasmic reticulum, lysosomes, Golgi
INTRODUCTION – WHICH NEURONS DIE IN PD?
Parkinson’s disease (PD) is an incurable late-onset neurodegenerative disorder which is strongly associated with aging, as evidenced by the exponential increase in incidence above the age of 65 (de Rijk et al., 1997; de Lau et al., 2004). Due to extended life expectancy, the prevalence of PD is estimated to double by 2030. Therefore, deciphering the molecular mechanisms underlying the disease, with the aim of developing novel disease-modifying therapies, has become an urgent and crucial task in PD-related research. Whilst PD is a disease of neurons, not all neurons are affected. The motor symptoms of PD, such as resting tremor, bradykinesia, and rigidity are clearly linked to the death of dopamine (DA) neurons in the substantia nigra pars compacta (SNc). Similarly, the clinical gold-standard treatment of L-DOPA (3,4-dihydroxy-L-phenylalanine), a DA precursor, indicates that DA neurons are crucial to the disease. However, the neuropathological hallmarks of PD, which are the presence of proteinaceous intracellular deposits called Lewy bodies or Lewy neurites in surviving neurons, are more distributed and not exclusive to DA neurons. Non-DA neurons which show pathology in PD include cholinergic neurons in the dorsal motor nucleus of the vagus (DMV) and basal forebrain (BF), noradrenergic neurons in the locus ceruleus (LC), and serotonergic neurons in the raphe nuclei (RN; Braak et al., 2004). Neurodegeneration is also not evident in all dopaminergic neuronal populations. For example, DA neurons in the ventral tegmental area (VTA) are relatively unaffected (Matzuk and Saper, 1985; Kish et al., 1988; Ito et al., 1992; Damier et al., 1999). Thus, elucidating why the diverse neurons are at risk for degeneration is essential if we want to formulate testable hypotheses as to the cause(s) underlying PD.
WHY DO NEURONS DIE IN PD – FROM DOPAMINE TO MITOCHONDRIA
Distinct mechanisms have been proposed to account for the preferential loss of DA neurons in PD. One hypothesis proposed that DA itself may be the culprit, as oxidation of cytosolic DA and its metabolites can lead to the production of cytotoxic free radicals and oxidative stress (Greenamyre and Hastings, 2004). However, since not all dopaminergic neurons are at risk in PD, and since elevating DA levels in PD patients by L-DOPA administration does not accelerate the progression of PD (Fahn, 2005), DA unlikely is the principal culprit, even though its effects may further worsen the cellular deficits related to oxidant stress and/or protein aggregation triggered by other means (see below).
Another hypothesis has linked PD to mitochondrial dysfunction (Henchcliffe and Beal, 2008; Schapira, 2008; Vila et al., 2008). Mitochondria are crucial organelles for cellular energy production. The transport of electrons down the electron transport chain (ETC) releases energy which is used by complex I, III, and IV to pump protons from the mitochondrial matrix to the mitochondrial intermembrane space, creating a proton gradient and an electrochemical gradient across the mitochondrial inner membrane, the latter of which is being used by ATP synthase to convert ADP to ATP. Mitochondria comprise one of the major cellular producers of reactive oxygen species (ROS), as electrons in the ETC are occasionally captured by oxygen to produce superoxide anion radicals, with complex I and III being the major culprits for production of these radicals (Cali et al., 2011).
There is extensive evidence for mitochondrial involvement in both sporadic and genetic PD. Toxins such as MPTP, rotenone, and paraquat, which inhibit complex I, can cause a Parkinsonian phenotype (Betarbet et al., 2000; Przedborski et al., 2004). In addition, postmortem tissue samples derived from the SNc from sporadic PD patients display a drastic decrease in the activity of complex I (Mann et al., 1994). A deficit in ETC can cause mitochondria-derived oxidative stress in the form of ROS and other radicals. Indeed, the decreased activity of complex I in PD patients seems due to oxidative damage (Keeney et al., 2006) and also affects other cellular components such as lipids and DNA (Zhang et al., 1999). Oxidative damage may also be responsible for the high levels of somatic mitochondrial DNA (mtDNA) deletions in SNc DA neurons (Bender et al., 2006; Kraytsberg et al., 2006), and the physical proximity of mtDNA to the site of ROS generation may indeed make them a vulnerable target. Since seven proteins involved in the formation of complex I are encoded by the mitochondrial genome, this may give rise to further ETC dysfunction and oxidative stress, leading to accelerated loss of SNc DA neurons.
However, the observed decrease in complex I deficiency in homogenates from nigral tissue from PD patients is too big to be restricted to SNc DA neurons, and only a proportion of PD patients show complex I inhibition in the SNc (Jenner, 2001). In addition, whilst toxins such as the herbicide rotenone cause ubiquitous complex I inhibition, dopaminergic degeneration is observed in the SNc, but not in the VTA area (Betarbet et al., 2000). Thus, inhibition of mitochondrial complex I activity per se cannot explain the selective vulnerability of neurons which die in PD.
WHY DO NEURONS DIE IN PD – PACEMAKING, Ca2+ DYSHOMEOSTASIS, AND OXIDANT STRESS
A hypothesis, put forward by Surmeier’s group, suggests that specific and shared physiological features are responsible for the risk of a subset of neurons to degenerate in PD (Guzman et al., 2010; Surmeier et al., 2011; Goldberg et al., 2012), and comprises probably the best working model to explain disease pathomechanism to date (Figure 1).
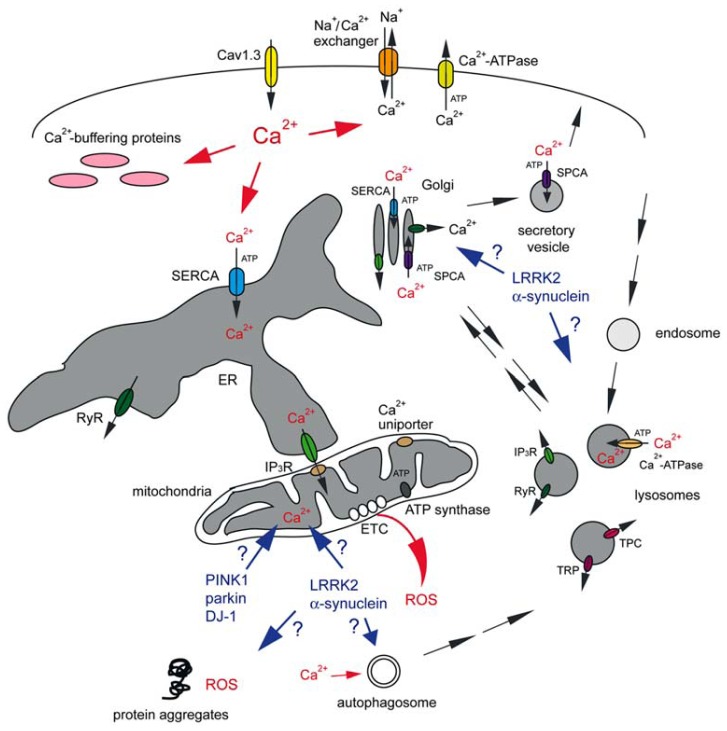
FIGURE 1.
Abnormal Ca2+ signaling in SNc DA neurons may cause mitochondrial oxidant stress, proteostasis deficits and eventual cell death. Vulnerable neuronal populations display spontaneous slow pacemaking activity employing Cav1.3 L-type Ca2+ channels, prominent Ca2+ currents and low intrinsic Ca2+ buffering capacities. Ca2+ inside the neuron can be transported back across the plasma membrane either via plasma membrane Ca2+-ATPase at the cost of ATP consumption, or through the Na+/Ca2+ exchanger which uses the Na+ gradient across the plasma membrane. Ca2+ is rapidly sequestered by interactions with Ca2+ buffering proteins or taken up into a variety of intracellular organelles. The ER uses a high-affinity Ca2+-ATPase [the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA)] to pump Ca2+ into the ER lumen at the cost of ATP consumption. This pump is also present on cis and medial Golgi membranes, whilst secretory vesicles employ a secretory pathway Ca2+-ATPase (SPCA) which is also be present on the trans Golgi complex. Ca2+ uptake into acidic organelles is mediated by a molecularly unidentified Ca2+-ATPase. Ca2+ flows back into the cytosol from the ER lumen through IP3 receptors (IP3R) or ryanodine receptors (RyR). IP3R are also present on cis and medial Golgi membranes, RyR on trans Golgi membranes, and RyR, TRP and TPC channels are present on acidic organelles. Mitochondria, often in close apposition to the ER or plasma membrane, can take up Ca2+ into the matrix through a mitochondrial Ca2+ uniporter. Ca2+ transfer between ER and mitochondria involves the IP3R on the ER membrane. Ca2+ within mitochondria is necessary for proper ETC function to generate ATP by ATP synthase, but mitochondrial Ca2+ overload can cause mitochondrial oxidant stress (ROS). Toxins as well as familial mutations in PINK1, parkin and DJ-1 affect mitochondrial ATP production and Ca2+ handling, even though the molecular details remain to be determined. The effects of familial mutations in LRRK2 and α-synuclein on mitochondrial functioning are even less clear, but those mutant proteins may cause additional deficits in proteostasis through mechanisms involving Ca2+-regulated events such as autophagy. This may also include alterations in the trafficking of Golgi-derived vesicles to the plasma membrane, resulting in changes in vesicle secretion and in the steady-state levels of surface receptors. Golgi deficits may cause altered trafficking of enzymes destined to lysosomes, with concomitant deficits in lysosomal degradative capacity, or alterations in retromer-mediated retrieval from endolysosomes back to the Golgi. Finally, changes in acidic store Ca2+ levels may affect various endo-lysosomal trafficking steps or the degradative capacity of acidic organelles per se. For further details see text.
Neurons are electrically excitable, using steep electrochemical gradients (mainly Na+ and K+ gradients) across their plasma membrane to integrate incoming chemical signals, and pass them on to other neurons. Voltage-dependent Ca2+ channels in most neurons are only opened by strong depolarization during an action potential. These channels close relatively slowly during membrane repolarization, such that the total Ca2+ influx during a spike is very sensitive to spike duration. To minimize global increases in Ca2+, neurons which need to spike at high frequencies tend to restrict Ca2+ entry by keeping spikes very brief, and tend to express Ca2+ buffering proteins to help manage intracellular Ca2+ levels (Augustine et al., 2003).
In contrast to many other neurons, SNc DA neurons are autonomously active in the absence of synaptic input (Grace and Bunney, 1983). Such pacemaking activity is necessary to maintain a basal DA tone in the striatum; without it, movement ceases (Surmeier and Schumacker, 2013). Whilst most neurons rely on Na+ to drive this pacemaking activity, SNc DA neurons also engage L-type Ca2+ channels with a Cav1.3 pore-forming subunit (Bonci et al., 1998; Puopolo et al., 2007). Although not strictly necessary for pacemaking, L-type Ca2+ channels help support pacemaking (Guzman et al., 2009). SNc DA neurons exhibit slow, broad spikes, causing a significant increase in intracellular Ca2+ levels, and they lack relevant intrinsic Ca2+ buffering capacity (Foehring et al., 2009; Guzman et al., 2009). The combination of these features, namely spontaneous activity that can be intrinsically generated, broad action potentials, prominent Ca2+ currents and low intrinsic Ca2+ buffering capacities are common to all neurons at risk for neurodegeneration in PD, irrespective of their neurotransmitter content (Surmeier and Schumacker, 2013). In contrast, relatively non-affected VTA DA neurons, whilst also slow pacemaking neurons, have low L-type Ca2+ channel densities and express high levels of the Ca2+ buffering protein calbindin (German et al., 1992; Khaliq and Bean, 2010).
GETTING RID OF Ca2+ – AN ENERGETICALLY COSTLY PROCESS
The shared physiological phenotype of at-risk neurons means that they will have a larger burden to handle increased intracellular Ca2+ levels. As Ca2+ is a universal second messenger, controlling a wide variety of cellular events ranging from regulation of enzyme activity to programmed cell death, it is under tight homeostatic control (Petersen et al., 2005). Pumping Ca2+ out of the cytosol is an energy-consuming process. Cytosolic Ca2+ levels are set to around 100 nM, which is 20,000-fold lower than the Ca2+ concentration in the extracellular space. This contrasts with the concentration differences of Na+ and K+ ions across the plasma membrane, which is in the range of 10–30-fold. Thus, thermodynamic considerations dictate that it will be energetically much more expensive to move Ca2+ ions across the plasma membrane as compared to Na+ or K+ ions (Surmeier and Schumacker, 2013).
Ca2+ ions are removed from the cytosol by either exchangers or pumps. Exchangers, such as the Na+/Ca2+ exchanger use the Na+ gradient to move Ca2+ ions out of the cytosol. Pumps, such as the plasma membrane Ca2+-ATPase, use ATP to drive the movement of ions against a concentration gradient. Ca2+ buffering proteins further help to decrease the free Ca2+ concentration. Importantly, Ca2+ which is not rapidly pumped out of the neuron is sequestered into intracellular organelles including the endoplasmic reticulum (ER), mitochondria, Golgi, and lysosomes (Figure 1; Berridge et al., 2000; Rizzuto, 2001; Pinton et al., 2008; Lloyd-Evans and Platt, 2011; Kaufman and Malhotra, 2014).
How the increased demand for Ca2+ handling causes increased risk for degeneration of the vulnerable neuronal populations remains to be fully elucidated. One hypothesis proposes that due to their high basal ATP consumption rates related to Ca2+ handling, vulnerable neurons will have a lesser bioenergetic or respiratory reserve, which is defined as the difference between the maximum capacity for ATP generation by oxidative phosphorylation and the basal ATP consumption rate (Nicholls, 2008). A smaller respiratory reserve may put these neurons at risk when their metabolic demands increase, such as during bursts of spiking or upon toxin exposure. Indeed, when ATP levels are not sufficient to meet demands, a deterioration of the membrane potential would be followed by massive Ca2+ influx and cell death.
The increased metabolic demand of SNc neurons may also give rise to an increase in the basal level of mitochondrial oxidant stress, as high rates of metabolic activity cause increased ROS production (Figure 1; Lee et al., 2001). In support of this, pacemaking in SNc neurons was shown to generate mitochondrial oxidant stress, which was not apparent in neighboring VTA DA neurons (Guzman et al., 2010). Such oxidant stress was largely prevented in the presence of L-type Ca2+ channel antagonists, clearly implicating those channels and the resultant increase in intracellular Ca2+ as culprits for downstream oxidant stress generated by high demands for mitochondrial ATP production.
Mitochondrial oxidant stress causes mild mitochondrial depolarization or uncoupling (Guzman et al., 2010), which leads to a decline in energy production and generation of ROS, causing damage to proteins, lipid, and DNA. In accordance with this, mtDNA deletions are significantly greater in SNc DA neurons from older as compared to younger subjects, and from neurons from PD patients as compared to unaffected individuals (Bender et al., 2006; Kraytsberg et al., 2006), with no changes observed in other brain areas. The accumulation of mtDNA deletions, with effects on mitochondrial respiratory chain function, will thus lead to further bioenergetic deficiency that manifests over time.
GETTING RID OF Ca2+ – NOT JUST A PROBLEM OF ENERGY
It is clear that cytosolic Ca2+ levels have to be maintained within a small range of concentrations for optimal survival of SNc DA neurons (Michel et al., 2013). However, apart from the extra bioenergetic burden to control intracellular Ca2+ levels, altered Ca2+ handling by various intracellular organelles may threaten neuronal viability as well. Indeed, mitochondrial oxidant stress in SNc DA neurons can be diminished when limiting mitochondrial Ca2+ uptake, without affecting pacemaking (Guzman et al., 2010). This is important, as it suggests that mitochondrial oxidant stress may be the consequence of increased mitochondrial Ca2+ load, rather than a mere reflection of the need for increased ATP production.
Ca2+ is well-known to modulate mitochondrial function. The Ca2+ uniporter uses the mitochondrial membrane potential to take Ca2+ up into the mitochondrial matrix (Kirichok et al., 2004; Santo-Domingo and Demaurex, 2010), where it increases ATP production by stimulating enzymes of the tricarboxylic acid (TCA) cycle, and thus helps to maintain increased metabolic demands associated with electrical activity and influx of Ca2+ (McCormack and Denton, 1990). However, too much Ca2+ in mitochondria compromises mitochondrial function by causing a transient collapse of the mitochondrial membrane potential (McCormack and Denton, 1990), which thus transiently halts the production of ATP.
The mitochondrial Ca2+ uniporter drives rapid and massive Ca2+ entry at high cytosolic Ca2+ concentrations only thought to be reached in microdomains near plasma membrane Ca2+ channels and Ca2+ release channels on the ER. Indeed, the primary intracellular organelle dealing with Ca2+ homeostasis is thought to be the ER (Berridge, 2002; Verkhratsky, 2005). The ER is responsible for the coordinated production, delivery, and degradation of proteins in a process called proteostasis. It forms a continuous intracellular network which extends throughout the somatodendritic tree (Choi et al., 2006), and contains high-affinity ATP-dependent transporters [(sarco-ER Ca2+-ATPase (SERCA)] to move Ca2+ from the cytoplasm into the ER lumen. Ca2+ sequestered in the ER can be released at sites where it can be pumped back across the plasma membrane, or can be used locally to modulate cellular function (Verkhratsky, 2005). The Ca2+ store in the ER is highly interconnected with other intracellular Ca2+ stores, such that ER Ca2+ dyshomeostasis will affect Ca2+ handling in other organelles as well. For example, inositol 1,4,5-trisphosphate (IP3) receptors which reside at direct ER-mitochondrial contacts termed MAMs (mitochondria-associated ER membranes) allow for direct flux of Ca2+ from ER into mitochondria (Csordas et al., 2006; Rizzuto and Pozzan, 2006; Kaufman and Malhotra, 2014), which may then lead to the mitochondrial Ca2+ overload described above (Figure 1). Indeed, stimulation of Ca2+ release from the ER by ryanodine, accompanied by an increase in cytosolic Ca2+ levels, was found to protect DA neurons from spontaneous or induced neurodegeneration (Guerreiro et al., 2008). Thus, relieving the Ca2+ load in the ER, without significantly causing Ca2+ transfer from ER to mitochondria through IP3 receptors, may prove beneficial to the survival of DA neurons, possibly via preventing ER-mediated mitochondrial Ca2+ overload. Altered ER Ca2+ concentrations are also associated with altered changes in cytosolic Ca2+ concentration upon ER release, and thus can affect the downstream signaling functions of this organelle (Morikawa et al., 2000; LaFerla, 2002).
Apart from its signaling function, Ca2+ plays an inherently important role for the functioning of the ER by acting as an allosteric regulator of protein processing and folding. Depletion of ER Ca2+ stores induces ER stress and the unfolded protein response (Paschen and Mengesdorf, 2005). Too much intraluminal ER Ca2+ may compromise proteostasis as well. For example, L-type Ca2+ channel blockers have been shown to restore folding and lysosomal delivery of mutant lysosomal enzymes responsible for a variety of lysosomal storage diseases (Mu et al., 2008). Similarly, decreasing ER Ca2+ levels by SERCA inhibitors seems to enhance the folding and plasma membrane trafficking of mutant cystic fibrosis transmembrane conductance regulator (CFTR; Egan et al., 2002, 2004). Precise Ca2+ imaging experiments will be required to determine the intraluminal ER Ca2+ levels upon such treatments. Nevertheless, these data indicate that altering ER Ca2+ homeostasis can have profound effects on folding and trafficking of proteins destined to other subcellular locations including lysosomes and the plasma membrane (Figure 1), with obvious downstream effects both on plasma membrane functioning/signaling and lysosomal degradative capacity.
INTRACELLULAR Ca2+ STORES AND Ca2+ HANDLING: THE NEGLECTED PLAYERS
In addition to the ER and mitochondria, two other compartments deserve attention as significant intracellular Ca2+ store. The first is the Golgi apparatus, which shares some functions and biochemical markers with the ER. The Golgi complex is a highly dynamic intracellular organelle which processes and sorts membrane proteins derived from the ER to the cell surface, secretory vesicles or lysosomes, and which also receives retrograde transport input. Thus, damage to neuronal Golgi structure can have important functional consequences for protein and vesicular trafficking (Fan et al., 2008). Interestingly, Golgi fragmentation has been observed in nigral neurons from PD patients (Fujita et al., 2006), and recent studies indicate that increased neuronal activity causes reversible Golgi fragmentation in a manner dependent on Ca2+-calmodulin-dependent protein kinase (Thayer et al., 2013). It will be interesting to determine whether Golgi fragmentation is a shared phenotype of vulnerable neurons in PD, and if it can be modulated by L-type Ca2+ channel antagonists. In addition, it remains to be seen whether neuronal activity-dependent Golgi fragmentation causes Golgi-derived Ca2+ release which may alter the spatio-temporal complexity of cellular Ca2+ signaling.
The Golgi complex serves as a bona fide Ca2+ store, containing Ca2+-ATPases, Ca2+ release channels and Ca2+-binding proteins (Figure 1; Scherer et al., 1996; Pinton et al., 1998; Lin et al., 1999). The Golgi seems to handle Ca2+ differently dependent on its sub-compartments. Whilst cis- and medial Golgi compartments contain the SERCA ATPase and IP3 receptors, the trans Golgi takes Ca2+ up exclusively via SPCA1 (secretory pathway Ca2+-ATPase isoform 1), and at least in some cells contains ryanodine receptors (Lissandron et al., 2010). Thus, the Golgi can serve as a Ca2+ store responding to local Ca2+-induced Ca2+ release or to second messengers such as cyclic ADP ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) which have been shown to activate ryanodine receptors (Fliegert et al., 2007). Decreasing Ca2+ in the trans-Golgi complex alters the structure of the entire Golgi apparatus, with effects on sorting of proteins to the plasma membrane through the secretory pathway (Lissandron et al., 2010; Micaroni, 2012). For example, depletion of SPCA1 has been shown to disrupt polarized trafficking, impairing neuronal differentiation, and the generation of functional neurites (Sepúlveda et al., 2009). The mechanism by which intraluminal Ca2+ in the Golgi may regulate sorting is starting to emerge. For example, sorting of some secretory proteins has been shown to require actin remodeling by ADF/cofilin, SPCA1, and a soluble Golgi-resident Ca2+-binding protein (von Blume et al., 2011, 2012). Sorting may depend on a transient influx of Ca2+ into the trans Golgi induced by the binding of ADF/cofilin to SPCA1, which may facilitate the association of secretory proteins with the Golgi-resident Ca2+-binding protein, acting as a soluble receptor to segregate a subset of secretory proteins (Kienzle and von Blume, 2014). In sum, alterations in intraluminal Ca2+ concentrations can impact both on cellular Ca2+ signaling as well as on Golgi structure and secreted protein cargo sorting (Micaroni, 2012), and it will be interesting to determine whether this may cause cell-autonomous deficits for example by altering the formation and trafficking of small dense-core DA-containing vesicles (Bauerfeind et al., 1995), or non-cell-autonomous events such as altering the secretion of neurotrophic factors with downstream effects on dopaminergic cell survival (Kordower and Bjorklund, 2013).
Ca2+ is also stored in a variety of acidic organelles (Patel and Docampo, 2010). Acidic organelles containing Ca2+ include endosomes, lysosomes, lysosome-related organelles and secretory granules. Amongst acidic organelles, lysosomes probably comprise the most prominent Ca2+ stores, and may contain an average free Ca2+ concentration in the range of 500 μM, similar to the Ca2+ concentration within the ER (Lloyd-Evans et al., 2008). Ca2+ uptake into lysosomes is thought to be mediated by pumps. Indeed, purified lysosomes from neutrophils, fibroblasts, and rat liver have been shown to take up Ca2+ in an ATP-dependent manner (Klempner, 1985; Lemons and Thoene, 1991; Ezaki et al., 1992; Adachi et al., 1996). The molecular nature of the lysosomal Ca2+-ATPase remains to be determined, even though some data indicate that it may be driven by SERCA3 (López et al., 2005). Alternatively, Ca2+ loading into lysosomes has been suggested to involve ER Ca2+ leak, such that small fluctuations in ER Ca2+ levels may cause large effects on lysosomal Ca2+ load (Bezprozvanny, 2012). Acidic stores also possess Ca2+-permeable channels such as IP3/ryanodine receptors, TRP channels (transient receptor potential channel superfamily), and TPCs (two-pore channels), which are members of the TRP channel superfamily as well (Figure 1). TPC channels located on endosomes and lysosomes have been reported to be targets for NAADP, the most potent Ca2+ mobilizing messenger (Churchill et al., 2002; Guse and Lee, 2008). However, they do not directly bind to NAADP (Lin-Moshier et al., 2012; Walseth et al., 2012), and their gating properties and ion selectivity have recently been questioned (Wang et al., 2012; Cang et al., 2013). This may be due to the fact that they can heterodimerize in-between themselves as well as with a subset of TRP channels, which are gated by NAADP as well (Patel and Docampo, 2010), and further work will be necessary to elucidate how second messengers such as NAADP may trigger Ca2+ release from acidic organelles, and the precise channels involved.
Lysosomal impairments seem intricately linked to PD pathogenesis. Lysosomes are the primary degradative organelle in all cell types, and their function is particularly important in non-dividing cells such as neurons. Several diseases associated with lysosomal dysfunction (lysosomal storage diseases) have been identified, and many of them affect brain function. Conversely, many neurodegenerative diseases also exhibit lysosomal dysfunction (Schultz et al., 2011). Lysosomal impairments are observed in sporadic PD brain and toxic as well as genetic rodent models of PD-related neurodegeneration (Dehay et al., 2013). The mechanisms involved may be varied, including defects in the lysosomal delivery of enzymes required for degradation, defects in lysosomal acidification or altered intralysosomal Ca2+ handling. Importantly, the lysosomal degradative system is characterized by many vesicular fusion events along the endocytic pathway which depend on intraluminal Ca2+, and lysosomal Ca2+ is also required for luminal content condensation (Pryor et al., 2000; Luzio et al., 2007). Whilst precise Ca2+ imaging experiments will be required to determine whether SNc neurons display alterations in intralysosomal Ca2+ levels, such lysosomal Ca2+ dyshomeostasis is expected to cause impaired turnover of dysfunctional mitochondria, which would further aggravate mitochondria-derived oxidant stress in vulnerable neurons.
In the context of proteostasis, it is also worthy considering effects of altered intracellular Ca2+ levels on autophagy, a process employed by cells to get rid of protein aggregates and defunct organelles, and deficits of which are also clearly implicated in PD (Lynch-Day et al., 2012). There is some controversy as to whether increases in Ca2+ promote or inhibit autophagy. This may be due to the subcellular localization of the source of the Ca2+ signal and may also depend on cellular state (Decuypere et al., 2011). Under normal conditions, the IP3 receptor-mediated Ca2+ transfer from the ER to mitochondria, which maintains mitochondrial ATP production, seems to inhibit autophagy. In contrast, an increase in cytosolic Ca2+ concentrations can stimulate autophagy (Figure 1; Decuypere et al., 2011). In both cases, this may involve the activity of AMPK, which is activated when cellular ATP levels drop and/or when cytosolic Ca2+ levels increase. Activation of autophagy, combined with a decrease in lysosomal degradative capacity, may then lead to the observed accumulation of autolysosomal structures observed in PD brains (Anglade et al., 1997).
PD, AGING, RISK FACTORS, AND GENETICS
Age is clearly the single strongest risk factor for PD. The physiological properties of SNc DA neurons indicate that they will be at a higher risk of age-related cell death due to their enhanced burden of Ca2+ handling. Indeed, these neurons seem to be lost at a higher rate (5–10% every 10 years) than many other neurons in the brain, some of which do not display significant loss over 60–70 years (Stark and Pakkenberg, 2004). Environmental factors may further alter intracellular Ca2+ handling, or may impact upon downstream cellular events triggered by Ca2+ dyshomeostasis, playing either protective or damaging roles. As mentioned above, for example toxins known to cause PD increase mitochondrial oxidant stress, thus impacting upon the same pathway already affected in vulnerable neurons.
Similarly, genetic forms of PD would be expected to converge on pathways affected by altered intracellular Ca2+ handling. Familial mutations in a variety of genes, with either autosomal-recessive (parkin, PINK1, DJ-1) or autosomal-dominant [(α-synuclein, leucine-rich repeat kinase (2LRRK2)] inheritance account for approximately 10% of PD cases (Trinh and Farrer, 2013). Of those, parkin, PINK1, and DJ-1 are clearly implicated in mitochondrial homeostasis and Ca2+ handling (Scarffe et al., 2014). For example, DJ-1 seems to protect against mitochondrial oxidant stress evoked by pacemaking in dopaminergic neurons by interfering with mitochondrial uncoupling in response to calcium-induced stress (Guzman et al., 2010). Depletion of DJ-1 seems to decrease expression of certain mitochondrial uncoupling proteins, even though the underlying mechanism(s) remain to be determined. PINK1 has been proposed to contribute to maintaining bioenergetic function of mitochondria by regulating Ca2+ efflux via the Na+/Ca2+ exchanger, and PINK1 deficiency was reported to cause mitochondrial Ca2+ overload, resulting in mitochondrial oxidant stress (Gandhi et al., 2009). Other studies indicate that PINK1 deficiency is associated with mitochondrial fragmentation, decreased membrane potential and decreased agonist-stimulated Ca2+ entry, thus pinpointing to a role for PINK1 in mitochondrial Ca2+ uptake rather than Ca2+ extrusion, and concomitant decreased ATP production (Heeman et al., 2011). Similarly, parkin deficiency has been reported to cause mitochondrial fragmentation and ER-mitochondria Ca2+ crosstalk, thus affecting cellular bioenergetics (Cali et al., 2013). Both parkin and PINK1 cooperate to regulate mitochondrial quality control events such as fission and fusion, degradation of defunct mitochondria by autophagy (mitophagy), mitochondrial transport, and biogenesis (Scarffe et al., 2014). Whilst the molecular mechanism(s) at present remain sketchy, these three proteins seem to be implicated in the same Ca2+-mediated pathway which is already compromised in sporadic PD (Figure 1).
Other proteins implicated in familial PD such as α-synuclein and LRRK2 have been consistently shown to cause dysfunction of the autophagy/lysosomal degradation system (Figure 1; Manzoni and Lewis, 2013), but how they may impact upon ER-mitochondrial Ca2+ handling and mitochondrial oxidant stress is less clear. Autosomal-dominant mutations in LRRK2 have been shown to cause deficits in Ca2+ homeostasis, leading to mitochondrial depolarization and enhanced mitophagy, which can be prevented by L-type Ca2+ channel inhibitors (Papkovskaia et al., 2012; Cherra et al., 2013). Greater levels of mtDNA damage can be observed in LRRK2 mutant patient cells as compared to healthy subjects (Sanders et al., 2014), but whether this is due to altered mitochondrial Ca2+ handling remains to be determined.
Apart from directly affecting mitochondrial Ca2+ handling, gene products involved in familial PD may also affect Ca2+ homeostasis in other intracellular organelles such as ER, Golgi, or lysosomes, with downstream effects on proteostasis and protein aggregation. Precise Ca2+ imaging experiments in the context of both sporadic and familial PD models will be required to reveal possible alterations in intracellular Ca2+ handling by these distinct organelles. For example, altered lysosomal Ca2+ levels may be responsible for the observed changes in lysosomal morphology, clustering, and degradative capacity described for mutant LRRK2-expressing cells (MacLeod et al., 2006; Tong et al., 2010; Dodson et al., 2012; Gómez-Suaga et al., 2012; Orenstein et al., 2013). Such changes, concomitant with an increase in cytosolic Ca2+ levels (Gómez-Suaga et al., 2012), may lead to aberrations in autophagic clearance, followed by a deficit in proteostasis. Impaired proteostasis in the presence of mutant α-synuclein has recently been shown to indirectly increase mitochondrial oxidant stress, suggesting that proteostatic extra-mitochondrial stress may be additive with mitochondrial oxidant stress observed in SNc DA neurons (Figure 1; Dryanovski et al., 2013). Whilst the mechanism(s) by which this occurs requires further investigation, it seems to involve NADPH oxidase activity. These data indicate that extramitochondrial oxidant stress may significantly contribute to PD, such that reverting proteostasis deficits may also be therapeutically beneficial in slowing down PD progression. In this context, Golgi-derived proteostasis effects may be worth considering as well, and may underlie altered risk for sporadic (Beilina et al., 2014) as well as familial PD, where Golgi phenotypes have been observed upon mutant α-synuclein and LRRK2 expression (Lin et al., 2009), even though whether this is related to altered Ca2+ handling in the Golgi remains to be determined. In sum, Ca2+ dyshomeostasis seems to be central towards our understanding of both sporadic and familial PD, and can affect a plethora of cellular events related to mitochondrial bioenergetics and oxidant stress as well as proteostasis (at the level of the ER, Golgi, and lysosomes) which may in turn increase extramitochondrial-derived oxidant stress to further threaten the viability of affected neurons.
NOVEL HOPES FOR TREATMENT OPTIONS?
The above-mentioned findings indicate that L-type Ca2+ channel antagonists may be viable therapeutic targets in the early stages of PD. There are oral antagonists [dihydropyridines (DHP)] available, with good blood–brain barrier permeability and a long record of safe use in humans. Adult SNc DA neurons can compensate for L-type Ca2+ channel antagonism and continue pacemaking (Chan et al., 2007), and mice do not show obvious motor, learning, or cognitive deficits when treated with L-type Ca2+ channel antagonists (Bonci et al., 1998), suggesting that these compounds do not alter the functional activity of SNc DA neurons. Indeed, several studies in humans indicate that these compounds diminish the risk of developing PD (Becker et al., 2008; Ritz et al., 2010; Pasternak et al., 2012). However, they do not seem to slow progression of PD (Marras et al., 2012), maybe because of their relatively poor potency against Cav1.3 L-type Ca2+ channels, or because other factors may become more prominent during disease manifestation. Such factors may in part derive from alterations in intracellular Ca2+ stores, with the resultant varied downstream effects on cellular proteostasis.
Much work remains to be done before gaining a clearer understanding of the role of Ca2+ dysregulation in the pathogenesis of PD. It is becoming increasingly clear that abnormal Ca2+ handling may have pleiotropic effects on a variety of intracellular events resulting in mitochondrial oxidant stress, deficits in ER proteostasis, endolysosomal/autophagic trafficking and alterations in Golgi function which require further investigation. Thus, whilst L-type Ca2+ channel antagonists may attack the source of the problem, improving the deteriorated cellular functions of mitochondria, ER, lysosomes, or Golgi may be an efficient complementary strategy to attack the varied downstream effects of the increased burden of handling intracellular Ca2+ in vulnerable neurons. Maybe a feasible future therapeutic strategy should not involve a “hit-hard” principle employed for example to treat cancer patients, but rather a “hit-softly, continue hitting, and hit at multiple places at a time” principle aimed at correcting a combination of cellular deficits derived from improper Ca2+ handling employing combination-type therapies.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful for the financial support of our work by the Spanish Ministry of Economy and Competitiveness (BFU2011-29899), the Junta de Andalucia (grant number CTS 6816), and the Michael J. Fox Foundation. Pilar Rivero-Ríos was supported by CEI Biotic Granada (CAEP2-13).
REFERENCES
- Adachi T., Arai K., Ohkuma S. (1996). A comparative study of (Ca2+–Mg2+)-ATPase on the lysosomal membrane and ecto-ATPase on the plasma membrane from rat liver. Biol. Pharm. Bull. 19 1291–1297 10.1248/bpb.19.1291 [DOI] [PubMed] [Google Scholar]
- Anglade P., Vyas S., Javoy-Agid F., Herrero M. T., Michel P. P., Marquez J., et al. (1997). Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol. Histopathol. 12 25–31 [PubMed] [Google Scholar]
- Augustine G. J., Santamaria F., Tanaka K. (2003). Local calcium signaling in neurons. Neuron 40 331–346 10.1016/S0896-6273(03)00639-1 [DOI] [PubMed] [Google Scholar]
- Bauerfeind R., Jelinek R., Hellwig A., Huttner W. B. (1995). Neurosecretory vesicles can be hybrids of synaptic vesicles and secretory granules. Proc. Natl. Acad. Sci. U.S.A. 92 7342–7346 10.1073/pnas.92.16.7342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C., Jick S. S., Meier C. R. (2008). Use of antihypertensives and the risk of Parkinson disease. Neurology 70 1438–1444 10.1212/01.wnl.0000303818.38960.44 [DOI] [PubMed] [Google Scholar]
- Beilina A., Rudenko I. N., Kaganovich A., Civiero L., Chau H., Kalia S. K., et al. (2014). Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc. Natl. Acad. Sci. U.S.A. 111 2626–2631 10.1073/pnas.1318306111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A., Krishnan K. J., Morris C. M., Taylor G. A., Reeve A. K., Perry R. H., et al. (2006). High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 38 515–517 10.1038/ng1769 [DOI] [PubMed] [Google Scholar]
- Berridge M. J. (2002). The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium 32 235–249 10.1016/S0143416002001823 [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Lipp P., Bootman M. D. (2000). The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1 11–21 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Betarbet R., Sherer T. B., MacKenzie G., Garcia-Osuna M., Panov A. V., Greenamyre J. T. (2000). Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 3 1301–1306 10.1038/81834 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I. (2012). Presenilins: a novel link between intracellular calcium signaling and lysosomal function? J. Cell Biol. 198 7–10 10.1083/jcb.201206003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A., Grillner P., Mercuri N. B., Bernardi G. (1998). L-type calcium channels mediate a slow excitatory synaptic transmission in rat midbrain dopaminergic neurons. J. Neurosci. 18 6693–6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Ghebremedhin E., Rueb U., Bratzke H, Del Tredici K. (2004). Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 318 121–134 10.1007/s00441-004-0956-9 [DOI] [PubMed] [Google Scholar]
- Cali T., Ottolini D., Brini M. (2011). Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson’s disease. Biofactors 37 228–240 10.1002/biof.159 [DOI] [PubMed] [Google Scholar]
- Cali T., Ottolini D., Negro A., Brini M. (2013). Enhanced parkin levels favor ER-mitochondria crosstalk and guarantee Ca2+ transfer to sustain cell bioenergetics. Biochim. Biophys. Acta 1832 495–508 10.1016/j.bbadis.2013.01.004 [DOI] [PubMed] [Google Scholar]
- Cang C., Zhou Y., Navarro B., Seo Y. J., Aranda K., Shi L., et al. (2013). mTOR regulates lysosomal ATP-sensitive two-pore Na+ channels to adapt to metabolic state. Cell 152 778–790 10.1016/j.cell.2013.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. S., Guzman J. N., Ilijic E., Mercer J. N., Rick C., Thatch T., et al. (2007). ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature 447 1081–1086 10.1038/nature05865 [DOI] [PubMed] [Google Scholar]
- Cherra S. J., III, Steer E., Gusdon A. M., Kiselyov K., Chu C. T. (2013). Mutant LRRK2 elicits calcium imbalance and depletion of dendritic mitochondria in neurons. Am. J. Pathol. 182 474–484 10.1016/j.ajpath.2012.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. M., Kim S. H., Chung S., Uhm D. Y., Park M. K. (2006). Regional interaction of endoplasmic reticulum Ca2+ signals between soma and dendrites through rapid luminal Ca2+ diffusion. J. Neurosci. 26 12137–12136 10.1523/JNEUROSCI.3158-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill G. C., Okada Y., Thomas J. M., Genazzani A. A., Patel S., Galione A. (2002). NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 111 703–708 10.1016/S0092-8674(02)01082-6 [DOI] [PubMed] [Google Scholar]
- Csordas G., Renken C., Varnai P., Walter L., Weaver D., Buttle K. F., et al. (2006). Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 174 915–921 10.1083/jcb.200604016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier P., Hirsch E. C., Agid Y., Graybiel A. M. (1999). The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122 1437–1448 10.1093/brain/122.8.1437 [DOI] [PubMed] [Google Scholar]
- Decuypere J.-P., Bultynck G., Parys J. B. (2011). A dual role for Ca2+ in autophagy regulation. Cell Calcium 50 242–250 10.1016/j.ceca.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Dehay B., Martinez-Vicente M., Caldwell G. A., Caldwell K. A., Yue Z., Cookson M. R., et al. (2013). Lysosomal impairment in Parkinson’s disease. Mov. Disord. 28 725–732 10.1002/mds.25462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau L. M., Giesbergen P. C., de Rijk M. C., Hofman A., Koudstaal P. J., Breteler M. M. (2004). Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology 63 1240–1244 10.1212/01.WNL.0000140706.52798.BE [DOI] [PubMed] [Google Scholar]
- de Rijk M. C., Tzourio C., Breteler M., Dartigues J. F., Amaducci L., Lopez-Pousa S., et al. (1997). Prevalence of parkinsonism in Parkinson’s disease in Europe: the EUROPARKINSON collaborative study. European community concerted action on the epidemiology of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 62 10–15 10.1136/jnnp.62.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson M. W., Zhang T., Jiang C., Chen S., Guo M. (2012). Roles of the Drosophila LRRK2 homolog in rab7-dependent lysosomal positioning. Hum. Mol. Genet. 21 1350–1363 10.1093/hmg/ddr573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryanovski D. I., Guzman J. N., Xie Z., Galteri D. J., Volpicelli-Daley L. A., Lee V. M., et al. (2013). Calcium entry and α-synuclein inclusions elevate dendritic mitochondrial oxidant stress in dopaminergic neurons. J. Neurosci. 33 10154–10164 10.1523/JNEUROSCI.5311-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M. E., Glockner-Pagel J., Ambrose C. A., Cahill P. A., Pappoe L., Balamuth N., et al. (2002). Calcium-pump inhibitors induce functional surface expression of Delta F508-CFTR protein in cystic fibrosis epithelial cells. Nat. Med. 8 485–492 10.1038/nm0502-485 [DOI] [PubMed] [Google Scholar]
- Egan M. E., Pearson M., Weiner S. A., Rajendran V., Rubin D., Glöckner-Pagel J., et al. (2004). Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 304 600–602 10.1126/science.1093941 [DOI] [PubMed] [Google Scholar]
- Ezaki J., Himeno M., Kato K. (1992). Purification and characterization of (Ca2+–Mg2+)-ATPase in rat liver lysosomal membranes. J. Biochem. 112 33–39 [DOI] [PubMed] [Google Scholar]
- Fan J., Hu Z., Zeng L., Lu W., Tang X., Zhang J., et al. (2008). Golgi apparatus and neurodegenerative diseases. Int. J. Dev. Neurosci. 26 523–534 10.1016/j.ijdevneu.2008.05.006 [DOI] [PubMed] [Google Scholar]
- Fahn S. (2005). Does levodopa slow or hasten the rate of progression of Parkinson’s disease? J. Neurol. 252(Suppl. 4) IV37–IV42 10.1007/s00415-005-4008-5 [DOI] [PubMed] [Google Scholar]
- Fliegert R., Gasser A., Guse A. H. (2007). Regulation of calcium signalling by adenine-based second messengers. Biochem. Soc. Trans. 35 109–114 10.1042/BST0350109 [DOI] [PubMed] [Google Scholar]
- Foehring R. C., Zhang X. F., Lee J. C., Callaway J. C. (2009). Endogenous calcium buffering capacity of substantia nigral dopamine neurons. J. Neurophysiol. 102 2326–2333 10.1152/jn.00038.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Ohama E., Takatama M., Al-Sarraj S., Okamoto K. (2006). Fragmentation of Golgi apparatus of nigral neurons with alpha-synuclein-positive inclusions in patients with Parkinson’s disease. Acta Neuropathol. 112 261–265 10.1007/s00401-006-0114-4 [DOI] [PubMed] [Google Scholar]
- Gandhi S., Wood-Kaczmar A., Yao Z., Plun-Favreau H., Deas E., Klupsch K., et al. (2009). PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol. Cell 33 627–638 10.1016/j.molcel.2009.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- German D. C., Manaye K. F., Sonsalla P. K., Brooks B. A. (1992). Midbrain dopaminergic cell loss in Parkinson’s disease and MPTP-induced parkinsonism: sparing of calbindin-D28k-containing cells. Ann. N.Y. Acad. Sci. 648 42–62 10.1111/j.1749-6632.1992.tb24523.x [DOI] [PubMed] [Google Scholar]
- Goldberg J. A., Guzman J. N., Estep C. M., Ilijic E., Kondapalli J., Sanchez-Padilla J., et al. (2012). Calcium entry induces mitochondrial oxidant stress in vagal neurons at risk in Parkinson’s disease. Nat. Neurosci. 15 1414–1421 10.1038/nn.3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Suaga P., Luzón-Toro B., Churamani D., Zhang L., Bloor-Young D., Patel S., et al. (2012). Leucine-rich repeat kinase 2 regulates autophagy through a calcium-dependent pathway involving NAADP. Hum. Mol. Genet. 21 511–525 10.1093/hmg/ddr481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A. A., Bunney B. S. (1983). Intracellular and extracellular electrophysiology of nigral dopaminergic neurons–2. Action potential generating mechanisms and morphological correlates. Neuroscience 10 317–331 10.1016/0306-4522(83)90136-7 [DOI] [PubMed] [Google Scholar]
- Greenamyre J. T., Hastings T. G. (2004). Parkinson’s–divergent causes, convergent mechanisms. Science 304 1120–1122 10.1126/science.1098966 [DOI] [PubMed] [Google Scholar]
- Guerreiro S., Toulorge D., Hirsch E., Marien M., Sokoloff P., Michel P. P. (2008). Paraxanthine, the primary metabolite of caffeine, provides protection against dopaminergic cell death via stimulation of ryanodine receptor channels. Mol. Pharmacol. 74 980–989 10.1124/mol.108.048207 [DOI] [PubMed] [Google Scholar]
- Guse A. H., Lee H. C. (2008). NAADP: a universal Ca2+ trigger. Sci. Signal. 1:re10 10.1126/scisignal.144re10 [DOI] [PubMed] [Google Scholar]
- Guzman J. N., Sanchez-Padilla J., Chan C. S., Surmeier D. J. (2009). Robust pacemaking in substantia nigra dopaminergic neurons. J. Neurosci. 29 11011–11019 10.1523/JNEUROSCI.2519-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman J. N., Sanchez-Padilla J., Wokosin D., Kondapalli J., Ilijic E., Schumacker P. T., et al. (2010). Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 468 696–700 10.1038/nature09536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeman B., Van den Haute C., Aelvoet S. A., Valsecchi F., Rodenburg R. J., Reumers V., et al. (2011). Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J. Cell Sci. 124 1115–1125 10.1242/jcs.078303 [DOI] [PubMed] [Google Scholar]
- Henchcliffe C., Beal M. F. (2008). Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat. Clin. Pract. Neurol. 4 600–609 10.1038/ncpneuro0924 [DOI] [PubMed] [Google Scholar]
- Ito H., Goto S., Sakamoto S., Hirano A. (1992). Calbindin-D28k in the basal ganglia of patients with parkinsonism. Ann. Neurol. 32 543–550 10.1002/ana.410320410 [DOI] [PubMed] [Google Scholar]
- Jenner P. (2001). Parkinson’s disease, pesticides and mitochondrial dysfunction. Trends Neurosci. 24 245–247 10.1016/S0166-2236(00)01789-6 [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Malhotra J. D. (2014). Calcium trafficking integrates endoplasmic reticulum function with mitochondrial bioenergetics. Biochim. Biophys. Acta 10.1016/j.bbamcr.2014.03.022 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney P. M., Xie J., Capaldi R. A., Bennett J. P. , Jr (2006) Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J. Neurosci. 26 5256–5264 10.1523/JNEUROSCI.0984-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq Z. M., Bean B. P. (2010). Pacemaking in dopaminergic ventral tegmental area neurons: depolarizing drive from background and voltage-dependent sodium conductances. J. Neurosci. 30 7401–7413 10.1523/JNEUROSCI.0143-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienzle C, von Blume J. (2014). Secretory cargo sorting at the trans-Golgi network. Trends Cell Biol. 10.1016/j.tcb.2014.04.007 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kirichok Y., Krapivinsky G., Clapham D. E. (2004). The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427 360–364 10.1038/nature02246 [DOI] [PubMed] [Google Scholar]
- Kish S.J., Shannak K., Hornykiewicz O. (1988). Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N. Engl. J. Med. 318 876–880 10.1056/NEJM198804073181402 [DOI] [PubMed] [Google Scholar]
- Klempner M. S. (1985). An adenosine triphosphate-dependent calcium uptake pump in human neutrophil lysosomes. J. Clin. Invest. 76 303–310 10.1172/JCI111961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower J. H., Bjorklund A. (2013). Trophic factor gene therapy for Parkinson’s disease. Mov. Disord. 28 96–109 10.1002/mds.25344 [DOI] [PubMed] [Google Scholar]
- Kraytsberg Y., Kudryavtseva E., McKee A. C., Geula C., Kowall N. W., Khrapko K. (2006). Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat. Genet. 38 518–520 10.1038/ng1778 [DOI] [PubMed] [Google Scholar]
- LaFerla F. M. (2002). Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat. Rev. Neurosci. 3 862–872 10.1038/nrn960 [DOI] [PubMed] [Google Scholar]
- Lee I., Bender E., Arnold S., Kadenbach B. (2001). New control of mitochondrial membrane potential and ROS formation–a hypothesis. Biol. Chem. 382 1629–1636 10.1515/BC.2001.198 [DOI] [PubMed] [Google Scholar]
- Lemons R. M., Thoene J. G. (1991). Mediated calcium transport by isolated human fibroblast lysosomes. J. Biol. Chem. 266 14378–14382 [PubMed] [Google Scholar]
- Lin P., Yao Y., Hofmeister R., Tsien R. Y., Farquhar M. G. (1999). Overexpression of CALNUC (nucleobindin) increases agonist and thapsigargin releasable Ca2+ storage in the Golgi. J. Cell Biol. 145 279–289 10.1083/jcb.145.2.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Parisiadou L., Gu X. L., Wang L., Shim H., Sun L., et al. (2009). Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s disease-related mutant alpha-synuclein. Neuron 64 807–827 10.1016/j.neuron.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Moshier Y., Walseth T. F., Churamani D., Davidson S. M., Slama J. T., Hooper R., et al. (2012). Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J. Biol. Chem. 287 2296–2307 10.1074/jbc.M111.305813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissandron V., Podini P., Pizzo P., Pozzan T. (2010). Unique characteristics of Ca2+ homeostasis of the trans-Golgi compartment. Proc. Natl. Acad. Sci. U.S.A. 107 9188–9203 10.1073/pnas.1004702107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Evans E., Morgan A. J., He X., Smith D. A., Elliot-Smith E., Sillence D. J., et al. (2008) Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 14 1247–1255 10.1038/nm.1876 [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E., Platt F. M. (2011). Lysosomal Ca2+ homeostasis: role in pathogenesis of lysosomal storage diseases. Cell Calcium 50 200–205 10.1016/j.ceca.2011.03.010 [DOI] [PubMed] [Google Scholar]
- López J. J., Camello-Almaraz C., Pariente J. A., Salido G. M., Rosado J. A. (2005). Ca2+ accumulation into acidic organelles mediated by Ca2+ and vacuolar H+-ATPases in human platelets. Biochem. J. 390 243–252 10.1042/BJ20050168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio J. P., Bright N. A., Pryor P. R. (2007). The role of calcium and other ions in sorting and delivery in the late endocytic pathway. Biochem. Soc. Trans. 35 1088–1091 10.1042/BST0351088 [DOI] [PubMed] [Google Scholar]
- Lynch-Day M. A., Mao K., Wang K., Zhao M., Klionsky D. J. (2012) The role of autophagy in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2 a009357 10.1101/cshperspect.a009357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod D., Dowman J., Hammond R., Leete T., Inoue K., Abeliovich A. (2006). The familial Parkinsonism gene LRRK2 regulates neurite process morphology. Neuron 52 587–593 10.1016/j.neuron.2006.10.008 [DOI] [PubMed] [Google Scholar]
- Mann V. M., Cooper J. M., Daniel S. E., Srai K., Jenner P., Marsden C. D., et al. (1994). Complex I, iron, and ferritin in Parkinson’s disease substantia nigra. Ann. Neurol. 36 876–881 10.1002/ana.410360612 [DOI] [PubMed] [Google Scholar]
- Manzoni C., Lewis P. A. (2013). Dysfunction of the autophagy/lysosomal degradation pathway is a shared feature of the genetic synucleinopathies. FASEB J. 27 3424–3429 10.1096/fj.12-223842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marras C., Gruneir A., Rochon P., Wang X., Anderson G., Brotchie J., et al. (2012). Dihydropyridine calcium channel blockers and the progression of parkinsonism. Ann. Neurol. 71 362–369 10.1002/ana.22616 [DOI] [PubMed] [Google Scholar]
- Matzuk M. M., Saper C. B. (1985). Preservation of hypothalamic dopaminergic neurons in Parkinson’s disease. Ann. Neurol. 18 552–555 10.1002/ana.410180507 [DOI] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. (1990). The role of mitochondrial Ca2+ transport and matrix Ca2+ in signal transduction in mammalian tissues. Biochim. Biophys. Acta 1018 287–291 10.1016/0005-2728(90)90269-A [DOI] [PubMed] [Google Scholar]
- Micaroni M. (2012). Calcium around the Golgi apparatus: implications for intracellular membrane trafficking. Adv. Exp. Med. Biol. 740 439–460 10.1007/978-94-007-2888-2_18 [DOI] [PubMed] [Google Scholar]
- Michel P. P., Toulorge D., Guerreiro S., Hirsch E. C. (2013). Specific needs of dopamine neurons for stimulation in order to survive: implication for Parkinson disease. FASEB J. 27 3414–3423 10.1096/fj.12-220418 [DOI] [PubMed] [Google Scholar]
- Morikawa H., Imani F., Khodakhah K., Williams J. T. (2000). Inositol 1,4,5-triphosphate-evoked responses in midbrain dopamine neurons. J. Neurosci. 20 RC103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu T. W., Fowler D. M., Kelly J. W. (2008). Partial restoration of mutant enzyme homeostasis in three distinct lysosomal storage disease cell lines by altering calcium homeostasis. PLoS Biol. 6:e26 10.1371/journal.pbio.0060026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G. (2008). Oxidative stress and energy crises in neuronal dysfunction. Ann. N.Y. Acad. Sci. 1147 53–60 10.1196/annals.1427.002 [DOI] [PubMed] [Google Scholar]
- Orenstein S. J., Kuo S. H., Tasset I., Arias E., Koga H., Fernandez-Carasa I., et al. (2013). Interplay of LRRK2 with chaperone-mediated autophagy. Nat. Neurosci. 16 394–406 10.1038/nn.3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkovskaia T. D., Chau K. Y., Inesta-Vaquera F., Papkovsky D. B., Healy D. G., Nishio K., et al. (2012). G2019S leucine-rich repeat kinase 2 causes uncoupling protein-mediated mitochondrial depolarization. Hum. Mol. Genet. 21 4201–4213 10.1093/hmg/dds244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W., Mengesdorf T. (2005). Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium 38 409–415 10.1016/j.ceca.2005.06.019 [DOI] [PubMed] [Google Scholar]
- Pasternak B., Svanstrom H., Nielsen N. M., Fugger L., Melbye M., Hviid A. (2012). Use of calcium channel blockers and Parkinson’s disease. Am. J. Epidemiol. 175 627–635 10.1093/aje/kwr362 [DOI] [PubMed] [Google Scholar]
- Patel S., Docampo R. (2010). Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 20 277–286 10.1016/j.tcb.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Michalak M., Verkhratsky A. (2005). Calcium signalling: past, present and future. Cell Calcium 38 161–169 10.1016/j.ceca.2005.06.023 [DOI] [PubMed] [Google Scholar]
- Pinton P., Giorgi C., Siviero R., Zecchini E., Rizzuto R. (2008). Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27 6407–6418 10.1038/onc.2008.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P., Pozzan T., Rizzuto R. (1998). The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J. 17 5298–5308 10.1093/emboj/17.18.5298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor P. R., Mullock B. M., Bright N. A., Gray S. R., Luzio J. P. (2000). The role of intraorganellar Ca2+ in late endosome–lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol. 149 1053–1062 10.1083/jcb.149.5.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S., Tieu K., Perier C., Vila M. (2004). MPTP as a mitochondrial neurotoxic model of Parkinson’s disease. J. Bioenerg. Biomembr. 36 375–379 10.1023/B:JOBB.0000041771.66775.d5 [DOI] [PubMed] [Google Scholar]
- Puopolo M., Raviola E., Bean B. P. (2007). Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. J. Neurosci. 27 645–656 10.1523/JNEUROSCI.4341-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B., Rhodes S. L., Qian L., Schernhammer E., Olsen J. H., Friis S. (2010). L-type calcium channel blockers and Parkinson disease in Denmark. Ann. Neurol. 67 600–606 10.1002/ana.21937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R. (2001). Intracellular Ca2+ pools in neuronal signalling. Curr. Opin. Neurobiol. 11 306–311 10.1016/S0959-4388(00)00212-9 [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Pozzan T. (2006). Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 86 369–408 10.1152/physrev.00004.2005 [DOI] [PubMed] [Google Scholar]
- Sanders L. H., Laganiere J., Cooper O., Mak S. K., Vu B. J., Huang Y. A., et al. (2014). LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson’s disease patients: reversal by gene correction. Neurobiol. Dis. 62 381–386 10.1016/j.nbd.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo-Domingo J., Demaurex N. (2010). Calcium uptake mechanisms of mitochondria. Biochim. Biophys. Acta 1797 907–912 10.1016/j.bbabio.2010.01.005 [DOI] [PubMed] [Google Scholar]
- Scarffe L. A., Stevens D. A., Dawson V. L., Dawson T. M. (2014). Parkin and PINK1: much more than mitophagy. Trends Neurosci. 10.1016/j.tins.2014.03.004 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira A. H. (2008). Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 7 97–109 10.1016/S1474-4422(07)70327-7 [DOI] [PubMed] [Google Scholar]
- Scherer P. E., Lederkremer G. Z., Williams S., Fogliano M., Baldini G., Lodish H. F. (1996). Cab45, a novel Ca2+-binding protein localized to the Golgi lumen. J. Cell Biol. 133 257–268 10.1083/jcb.133.2.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz M. L., Tecedor L., Chang M., Davidson B. L. (2011). Clarifying lysosomal storage diseases. Trends Neurosci. 34 401–410 10.1016/j.tins.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda M. R., Vanoevelen J., Raeymaekers L., Mata A. M., Wuytack F. (2009). Silencing the SPCA1 (secretory pathway Ca2+-ATPase isoform 1) impairs Ca2+ homeostasis in the Golgi and disturbs neural polarity. J. Neurosci. 29 12174–12182 10.1523/JNEUROSCI.2014-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark A. K., Pakkenberg B. (2004). Histological changes of the dopaminergic nigrostriatal system in aging. Cell Tissue Res. 318 81–92 10.1007/s00441-004-0972-9 [DOI] [PubMed] [Google Scholar]
- Sulzer D. (2007). Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 30 244–250 10.1016/j.tins.2007.03.009 [DOI] [PubMed] [Google Scholar]
- Surmeier D. J., Guzman J. N., Sanchez-Padilla J., Goldberg J. A. (2011). The origins of oxidant stress in Parkinson’s disease and therapeutic strategies. Antioxid. Redox Signal. 14 1289–1301 10.1089/ars.2010.3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier D. J., Schumacker P. T. (2013). Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease. J. Biol. Chem. 288 10736–10741 10.1074/jbc.R112.410530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer D. A., Jan Y. N., Jan L. Y. (2013). Increased neuronal activity fragments the Golgi complex. Proc. Natl. Acad. Sci. U.S.A. 110 1482–1487 10.1073/pnas.1220978110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y., Yamaguchi H., Giaime E., Boyle S., Kopan R., Kelleher R. J., III, et al. (2010). Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc. Natl. Acad. Sci. U.S.A. 107 9879–9884 10.1073/pnas.1004676107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh J., Farrer M. (2013). Advances in the genetics of Parkinson disease. Nat. Rev. Neurol. 9 445–454 10.1038/nrneurol.2013.132 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. (2005). Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol. Rev. 85 201–279 10.1152/physrev.00004.2004 [DOI] [PubMed] [Google Scholar]
- Vila M., Ramonet D., Perier C. (2008). Mitochondrial alterations in Parkinson’s disease: new clues. J. Neurochem. 107 317–328 10.1111/j.1471-4159.2008.05604.x [DOI] [PubMed] [Google Scholar]
- von Blume J., Alleaume A. M., Cantero-Recasens G., Curwin A., Carreras-Sureda A., Zimmermann T., et al. (2011). ADF/cofilin regulates secretory cargo sorting at the TGN via the Ca2+ ATPase SPCA1. Dev. Cell 20 652–662 10.1016/j.devcel.2011.03.014 [DOI] [PubMed] [Google Scholar]
- von Blume J., Alleaume A. M., Kienzle C., Carreras-Sureda A., Valverde M., Malhotra V. (2012). Cab45 is required for Ca2+-dependent secretory cargo sorting at the trans-Golgi network. J. Cell Biol. 199 1057–1066 10.1083/jcb.201207180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseth T. F., Lin-Moshier Y., Jain P., Ruas M., Parrington J. (2012). Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide phosphate (NAADP)-binding proteins in sea urchin egg. J. Biol. Chem. 287 2308–2315 10.1074/jbc.M111.306563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang X., Dong X. P., Samie M., Li X., Cheng X., et al. (2012). TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell 151 372–383 10.1016/j.cell.2012.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Perry G., Smith M. A., Robertson D., Olson S. J., Graham D. G., et al. (1999) Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am. J. Pathol. 154 1423–1429 10.1016/S0002-9440(10)65396-5 [DOI] [PMC free article] [PubMed] [Google Scholar]