Abstract
Macromolecular Gd(III) based contrast agents are effective for contrast enhanced blood pool and cancer MRI in preclinical studies. However, their clinical applications are impeded by potential safety concerns associated with slow excretion and prolonged retention of these agents in the body. To minimize the safety concerns of macromolecular Gd contrast agents, we have recently designed and developed biodegradable macromolecular Gd contrast agents based on polydisulfide Gd(III) complexes. In this study, we designed and synthesized a new generation of the polydisulfide Gd(III) complexes containing macrocyclic Gd(III) chelate, Gd-DOTA monoamide, to further improve the in vivo kinetic stability of the Gd(III) chelates of the contrast agents. (N6-Lysyl)lysine Gd-DOTA monoamide and 3-(2-carboxyethyldisulfanyl)propanoic acid copolymers (GODC) was synthesized by copolymerization of (N6-lysyl)lysine DOTA monoamide and dithiobis (succinimidylpropionate), followed by complexation with Gd(OAc)3. The GODC had an apparent molecular weight of 26.4 kDa and T1 relaxivity of 8.25 mM−1s−1 per Gd at 1.5 T. The polymer chains of GODC were readily cleaved by L-cysteine and the chelates had high kinetic stability against transmetallation in the presence of an endogenous metal ion Zn2+. In vivo MR study showed that GODC produced strong and prolonged contrast enhancement in the vasculature and tumor periphery of mice with breast tumor xenografts. GODC is a promising biodegradable macromolecular MRI contrast agent with high kinetic stability for MR blood pool imaging.
Keywords: Gd, biodegradable macromolecular MRI contrast agent, blood pool imaging, cancer imaging, kinetic stability
INTRODUCTION
Magnetic resonance imaging (MRI) is a clinical diagnostic modality without ionization radiation and provides both morphological and functional information (1,2). In comparison with other clinical imaging modalities, MRI is advantageous in visualizing soft tissue with high spatial resolution. In order to provide more accurate diagnostic imaging, contrast agents are often used to enhance the image contrast between the tissue of interest and surrounding tissues. Over the past 30 years, various paramagnetic compounds have been investigated as MRI contrast agents (3–6). Among these compounds, stable Gd(III) chelates are the most commonly used contrast agents in clinical practice (7). The small molecular Gd(III)-based clinical agents with short blood half-lives have relatively low relaxivities, rapidly extravasate from vasculature and excrete through renal glomerular filtration (8,9). These drawbacks limit their applications in clinical blood pool MRI, including both MR angiography and cancer imaging (10,11).
Two types of blood pool imaging agents, the small Gd(III) chelates that reversibly bind to plasma proteins such as MS-325 and macromolecular Gd(III) complexes, have been developed to address the limitations of clinical agents (12–16). Although MS-325 is effective for MR angiography, it is not as effective as polymeric Gd(III) chelates for evaluating tumor vascularity (11,17–19). Macromolecular Gd agents, such as polylysine Gd(III) chelate conjugates, polysaccharide Gd(III) chelate conjugates, Gadomer 17, PAMAM dendrimer Gd(III) chelate conjugates (20–23), are generally prepared by conjugating stable Gd(III) chelates to biocompatible polymers (24,25). These macromolecular agents have high relaxivities (in the range of 7~25 mM−1s−1 at 1.5T) (15,20–23), about 2–4 folds higher than their small molecular counterparts such as Gd-DTPA and Gd-DOTA (generally in the range of 3~5 mM−1s−1 at 1.5T) (3,26) due to slower molecular rotational correlation time. They also exhibit prolonged blood circulation and limited extravasation (25,27). Preclinical studies have shown that these macromolecular agents are effective for MR angiography, cancer imaging and characterization of tumor vascularity (28,29). Although macromolecular Gd(III) complexes have demonstrated some advantageous features over small molecular Gd(III) chelates, including both protein binding and non-binding agents, in preclinical studies, the clinical application of macromolecular Gd(III) based contrast agents has been impeded by the safety concerns associated with their slow excretion, which can lead to significantly higher tissue deposition of Gd(III) complexes than the small molecular agents (8), and consequently increase the probability of toxic Gd(III) ion release, causing chronic adverse effects such as the recently reported nephrogenic systemic fibrosis (8,30,31).
In order to alleviate the safety concerns of macromolecular contrast agents, we have recently developed extracellular degradable polydisulfides based polymeric MR contrast agents to accelerate the clearance of Gd chelates and to minimize the tissue accumulation of Gd after the MRI examinations (29,32,33). These agents were prepared by copolymerization of DTPA anhydride with disulfide-containing monomers, followed by complexation with Gd(III). The disulfide bonds in the polymeric backbone was readily cleaved by in vivo plasma free thiols through disulfide-thiol exchange reaction. Pharmacokinetic studies revealed that the polydisulfide Gd chelates act as macromolecular contrast agents initially after administration, and are gradually reduced into excretive oligomeric and small Gd chelates in plasma, which were readily excreted via renal filtration with a Gd(III) tissue retention comparable to clinical Gd(III) based MRI contrast agents. Preclinical studies showed that the polydisulfide Gd-DTPA complexes had better efficacy for contrast enhanced vascular and tumor imaging and characterization of tumor vascularity than small molecular Gd(III) based contrast agents (34,35). However, linear chelates, Gd-DTPA and its derivatives, exhibit relatively low kinetic stability, which may result in the release of free Gd(III) ions in vivo via transmetallation with endogenous metal ions (30,31,36,37).
Chelate structure plays an important role in the overall stability of Gd complexes, including thermodynamic stability and kinetic stability (8,36–38). The current clinically available Gd-based contrast agents are prepared from the derivatives of two types of ligands, the linear ligands, diethylenetriaminepentaacetic acid (DTPA) and its derivatives, and the macrocyclic ligand, 1,4,7,10-tetraazacyclododecome–1,4,7,10-tetraacetic acid (DOTA) and its derivatives. The macrocyclic Gd(III) complexes exhibit much higher kinetic stability than the linear Gd(III) chelates and no transmetallation in the presence of endogenous metal ions (39–41). The polydisulfide Gd-DTPA complexes had similar kinetic stability as the linear agent MultiHance®, higher than that of Omniscan®, but lower than that of ProHance®, a macrocyclic chelate contrast agent (42). Previously, we investigated two biodegradable macromolecular contrast agents based on the more stable macrocyclic chelates, poly(L-glutamic acid)-cystamine-(Gd-DOTA monoamide) conjugate and the PAMAM-cystamine-(Gd-DOTA monoamide) conjugate (43,44). Both systems showed good tumor diagnosis capacity as well as improved elimination in comparison to their non-degradable counterparts. However, limitations were also observed for these agents. The poly(L-glutamic acid) conjugate eliminated much slower than the polydisulfide GDCC due to the steric hindrance of randomly coiled structure of the polymer backbone on the reduction of the disulfide spacer (43,45). The PAMAM conjugate had efficient degradation, but dendrimer became highly toxicity after the disulfide cleavage (44). Thereore, we hypothesize that the combination of macrocyclic chelates Gd(III) and polydisulfides can produce safe and effective biodegradable macromolecular contrast agents with a clinical potential.
In this study, we have designed and synthesized a new generation of biodegradable macromolecular contrast agents based on macrocyclic Gd chelates to improve their kinetic stability. Herein, we report the synthesis of a polydisulfide with Gd-DOTA monoamide side chains, the copolymers of (N6-lysyl)lysine Gd-DOTA monoamide and 3-(2-carboxyethyldisulfanyl)propanoic acid (GODC), as a new biodegradable macromolecular MRI contrast agent. We characterized the kinetics stability of G(III) chelates in the agents and in vitro and in vivo degradation of the polymers. We evaluated the effectiveness of GODC for contrast enhanced MR vascular imaging and tumor imaging in a mouse beast cancer model.
EXPERIMENTAL SECTION
Materials
The Fmoc-lys(ivDDe)-OH was purchased from Chem-Impex International (Wood Dale, IL). The 2-chlorotrityl chloride resin, 1-hydroxybenzotriazole (HOBT), 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) and benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP) were purchased from Nova Biochem (Darmstadt, Germany). 1,4,7,10-Tetraazacyclododecane-1, 4,7-tris-tert-butyl acetate-10-acetic acid [DOTA-tris(t-Bu)] was purchased from TCI America (Portland, OR). N,N-Diisopropylethylamine (DIPEA), piperidine, hydrazine and trifluoroacetic acid (TFA) were purchased from Alfa Aesar (Ward Hill, MA). N,N-Dimethylformamide anhydrous (DMF), dichloromethane (DCM), methanol, ethyl ether and phosphate buffer saline (PBS) was purchased from Fisher Scientific (Pittsburgh, PA). Fmoc-lys(Fmoc)-OH, the Kaiser test kit, ethylenediamine dihydrochloride, 1,2-ethanedithiol (EDT), triisopropylsilane (TIS), Xylenol orange, dithiobis (succinimidylpropioniate) (DSP) and Gd(OAc)3 were purchased from Sigma-Aldrich, Inc. (Louis, MO). All reagents were used without further purification unless otherwise stated. The synthetic product was verified and characterized using high performance chromatography (HPLC, Agilent), the matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectra (Bruker Autoflex III), 1H-NMR (300 MHz Varian Gemini NMR spectrometer) and Fourier transform infrared (FT-IR, Varian Inc.). The MALDI-TOF mass spectrum was acquired on a Voyager DE-STR spectrometer (PerSpeptive BioSystems) in linear mode with R-cyano-4-hydroxycinnamic acid as a matrix. The polymer conjugates were purified by ultrafiltration with Millipore’s Amicon Ultra-15 centrifugal filter of 3 kDa molecular weight cut-offs against de-ionized water. The Gd(III) and Zn(II) content was measured by inductively coupled plasma-optical emission spectroscopy (ICP-OES Optima 3100XL, Perkin-Elmer, Norwalk, CT).
Synthesis of (N6-lysyl)lysine tetraazacyclododecanetetraacetic acid (DOTA) monoamide
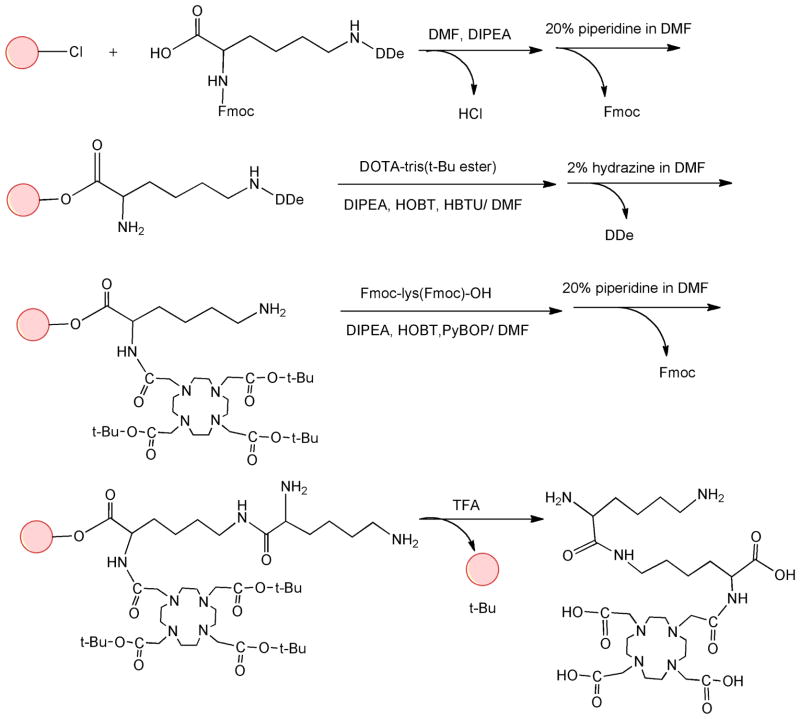
(N6-Lysyl)lysine DOTA monoamide was synthesized using a solid phase peptide chemistry approach (Figure 1). Fmoc-lys(ivDDe)-OH (0.52 g, 0.91 mmol) was first loaded to the 2-chlorotrityl chloride resin (1.15 g, 1.50 mmol, swelled in dichloromethane) with 800 μl DIPEA in 10 ml DMF and shaken for 2 hrs. The reaction media was then filtered out, and 10 ml methanol with 400 μl DIPEA was added to block the un-reacted active sites on the resin (20 min × 2). The resin was washed with DCM and DMF. A Kaiser test was carried out with a small amount of resin sample, and showed negative result (yellow color), indicating no detection of primary amine. The resin was then reacted with 10 ml 20% piperidine/DMF to remove the Fmoc group. DCM and DMF were used to wash out the remaining piperidine. After the reaction, Kaiser test of the resin showed positive result (blue color), indicating the removal of the Fmoc group. DOTA-tris(t-Bu) ester (1.04 g, 0.91 mmol) was then reacted to the resin with HOBT (0.37 g, 0.91 mmol), HBTU (1.03 g, 0.91 mmol) and 300 μl DIPEA in 10 ml DMF. The reaction media was shaken for 2 hrs, washed with DCM and DMF. The next step was carried out when Kaiser test showed negative results, indicating complete reaction of the primary amine. The resin was then reacted with 10 ml 2% hydrazine/DMF to remove the ivDDe group. After removal of the ivDDe group (positive Kaiser test), Fmoc-lys (Fmoc)-OH (1.60 g, 0.91 mmol) was loaded to the resin with PyBOP (1.41 g, 0.91 mmol), HOBT (0.37 g, 0.91 mmol) and 500 μl DIPEA in 10 ml DMF. The reaction was carried out for 50 minutes. The Fmoc group was then removed using 10 ml 20% piperidine/DMF. After removal of the Fmoc, the resin was washed with DCM, dried. A mixture of trifluoroacetic acid (TFA), 1,2-ethanedithiol (EDT), water, TIS (95:2.5:2.5:2, 15 ml) was used to remove the tert-butyl groups and separate the final product from the resin. The reaction media was filtered out, and the raw product of (N6-lysyl)lysine DOTA monoamide was precipitated in ethyl ether. The precipitate was recrystallized in DMSO and DIPEA (5:1). DIPEA also served as a base to remove TFA from the (N6-lysyl)lysine DOTA monoamide. HPLC was used to verify the purity of the final product and showed good purity of the monomer after the recrystallization. (N6-Lysyl)lysine DOTA monoamide was then characterized by 1H-NMR and MALDI-TOF mass spectrometry. The yield of the monomer was 62%. 1H-NMR (300 MHz, D2O, 25°C): 1.20–1.35 (m, 4H), 1.40–1.50 (m, 2H), 1.55–1.85 (m, 6H), 2.85–2.95 (t, 2H), 2.98–3.50 (m, 19H), 3.60–4.05 (m, 8H), 4.15–4.25 (t, 1H). MALDI-TOF MS: 661.02 (M+H+), 683.19 (M+Na+) (measured); 660.76 (calculated).
Figure 1.
Synthetic scheme of (N6-lysyl)lysine DOTA monoamide.
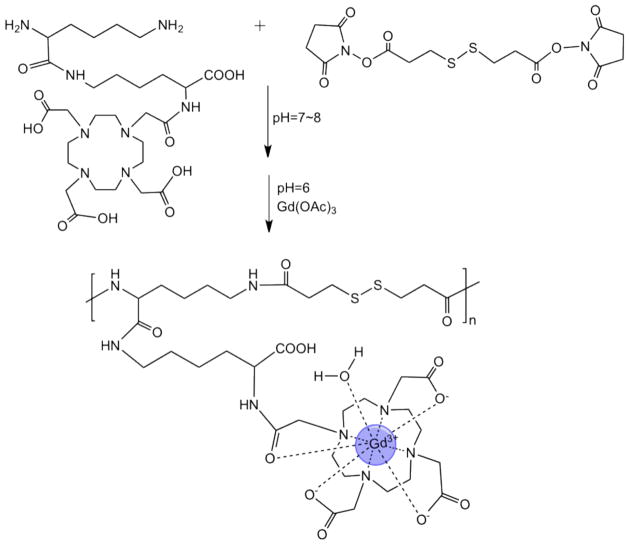
Synthesis of (N6-lysyl)lysine DOTA monoamide and 3-(2-carboxyethyldisulfanyl) propanoic acid copolymers (GODC)
The polymerization was performed by slowly adding the DMF solution of dithiobis (succinimidylpropionate) (DSP) portion by portion to the concentrated aqueous solution of (N6-lysyl)lysine DOTA monoamide while stirring. The reaction was stirred at room temperature for 4 hours after adding the DSP solution. The product was purified through ultrafiltration using a filter with a molecular weight cut-off of 3,000 Da to remove low molecular weight oligomers, and then lyophilized to give the colorless polymeric ligand. The yield of the purified product was 10%. The number- and weight-average molecular weights of the copolymers were determined using size exclusion chromatography on an AKTA FPLC system with a Superose™ 12 column (GE Healthcare Life Sciences), calibrated with water soluble poly[N-(2-hydroxylpropyl)methacrylamide] standards. The polymeric ligand was characterized by 1H-NMR and FT-IR. 1H-NMR (300 MHz, D2O, 25°C): 1.05–1.20 (m, 4nH), 1.20–1.40 (s, 4nH), 1.40–1.68 (m, 4nH), 2.25–2.65 (m, 8nH), 2.70–3.14 (m, 16nH), 3.15–3.45 (s, 8nH), 3.50–3.75 (d, 4nH), 3.76–4.20 (s, 2nH). FT-IR: 2800–3680 cm−1 (νO-H), 1440–1800 cm−1 (νC=O), 1100–1240 cm−1 (δO-H, νC-O and νC-N).
Complexation with Gd(III)
The polymeric ligand was complexed with two folds excess of Gd(OAc)3 at pH 6 for 48 hrs at room temperature. The resulting solution was purified via dialysis against water using a membrane with molecular weight cut-off of 8,000 Da, and lyophilized to give a final white product, (N6-lysyl)lysine Gd-DOTA monoamide 3-(2-carboxyethyldisulfanyl) propanoic acid copolymer (GODC). Size exclusion chromatography was used to determine the apparent molecular weights of the complexed polymer. Xylenol orange was used to detect the presence of free Gd(III) ions. No color change was observed with Xylenol orange in the polymer solution after dialysis, indicating complete removal of the excess of Gd(OAc)3. The Gd(III) content of the polymer was determined using inductive coupled atomic emission spectrometry (ICP-OES, PerkinElmers®).
Relaxivity measurement
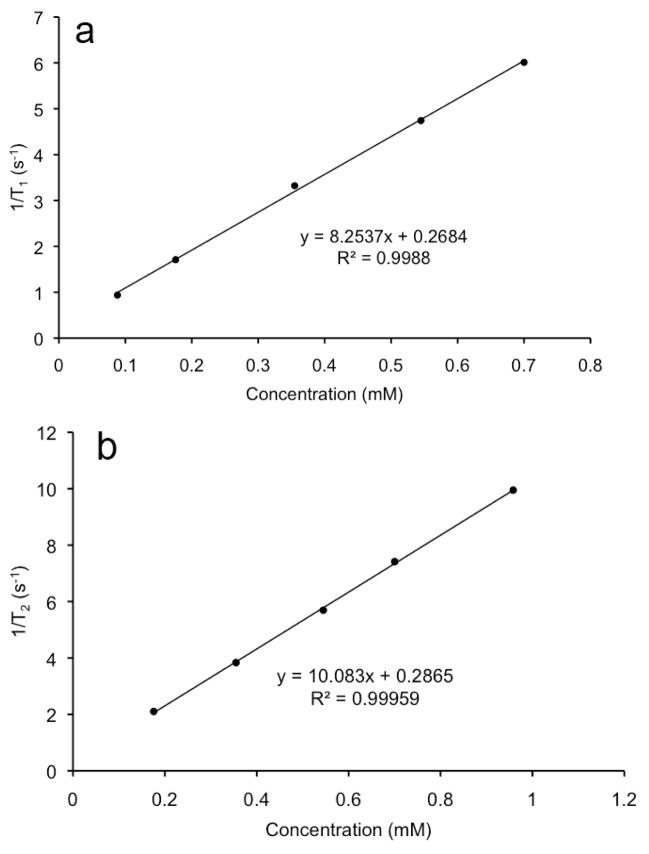
The T1 relaxivity of GODC was determined using a Bruker Minispec® Relaxometer (1.5T, 60 Hz) at 37°C. Different concentrations of GODC (0.2, 0.4, 0.6 and 0.8 mM of Gd) were prepared, and their T1 values were obtained using a inversion-recovery pulse sequence from the relaxometer. The longitudinal relaxivity (r1) was calculated as the slope of the plot of 1/T1 versus the concentration of Gd(III). Similarly, the T2 relaxivity was determined using a Carr-Purcell-Meiboom-Gill (CPMG) spin echo method. The Gd(III) content of each sample was confirmed by ICP-OES after the MR measurement.
Degradation of the polydisulfide
In vitro degradation study of GODC polymer was carried out by incubating GODC (0. 42 mM-Gd) with cysteine at plasma thiol concentration (15 μM) (46,47) to mimic the in vivo conditions. The polydisulfide with 0.42 mM of Gd(III) were incubated with 15 μM L-cysteine in PBS buffer at pH 7.4 and 37°C for 24 hours. The change of apparent molecular weight of GODC during the incubation was determined using size exclusion chromatography (Superose® 6 column, AKTA® FPLC).
The degradation mechanism of the polydisulfide was also investigated both in vitro and in vivo. For in vitro experiment, GODC was incubated with an excess of L-cysteine (1.5 mM) in PBS solution at 37°C for 60 minutes. The reaction mixture was analyzed by MALDI-TOF mass spectrometry. For in vivo experiment, GODC was administered to mice at a dose of 0.1 mmol-Gd/kg via a tail vein, and urine samples were collected after the injection and analyzed by MALDI-TOF mass spectrometry.
Kinetic Stability
The kinetic stability of Gd-DOTA monoamide in the polydisulfide against transmetallation was evaluated in vitro in comparison with our previously reported Gd-DTPA cystamine copolymers (GDCC). GODC (0.42 mM-Gd) or GDCC (0.42 mM-Gd) was incubated with ZnCl2 at the plasma Zn2+ concentration (50 μM) in PBS buffer at pH=7.4 and 37°C for 2 hrs. Samples were collected before and at 30, 60 and 120 minutes of incubation. The polymer bound Gd(III) and released Gd(III) ions were separated using a PD-10 column (GE Health Science). The concentration of polymer bound Gd(III) was measured by ICP-OES (Perkin-Elmers). The kinetic stability was determined as the percentage of bound Gd(III) in polymers post incubation to that before incubation. GODC and GDCC incubated with Zn(II) free PBS buffer were used as negative controls.
Mouse Tumor Model
Female BALB/c mice weighted 18–22 g were purchased from Charles River (Wilmington, MA, USA). The animals were cared following an approved protocol and the guidelines of the Animal Resource Center of Case Western Reserve University. Mouse breast cancer 4T1 cells (5 × 104 cells suspended in 50 μl Matrigel) were injected into the inguinal mammary fat pads of mice. The MRI study was performed when the tumor size reached 0.5–1.0 cm in diameter in 2 weeks.
In vivo MRI study
The contrast enhancement of GODC was preliminarily evaluated in mice using the clinical agent Magnevist® as a control. The tumor bearing mice were anaesthetized using 2.5% ~ 3.5% of isoflurane in oxygen initially, and maintained at 1.5%–2.5% during the experiment. The temperature, respiration and electrocardiograms (ECGs) of the mice were monitored during image acquisitions. The animals were kept warm at 34°C by blowing warm air to the mouse cradle monitored by a temperature control module. The contrast agent was intravenously administered to anesthetized mouse via a tail vein at a dose of 0.1 mmol-Gd/kg. MR images were acquired before injection and at different time points post injection for up to 30 minutes on a Siemens 1.5T clinical scanner with a 3D FLASH pulse sequence (211 FOV, 1.1 mm slice thickness, 14.0 ms TR, 3.09 ms TE, 25° flip angle, 2 averages, 64×256 matrix size, 32 slices per slab) and a 2D spin-echo sequence (19.1 ms TE, 465 ms TR, 25° flip angle, 0.8 mm slice thickness, 100×256 matrix size, 211 mm FOV, 2 averages). Three tumor-bearing mice were used for each contrast agent. Signal intensity of the regions of interest (ROI) was measured using the Osirix software. Signal increase of the tumor periphery was calculated using the ratio of the signal post-contrast to that of pre-contrast within the ROI at different time points post injection. Statistical analysis was performed using a two-way ANOVA with Bonferroni’s, assuming statistical significance at p < 0.05.
RESULTS
Synthesis of (N6-lysyl)lysine Gd-DOTA monoamide and 3-(2-carboxyethyldisulfanyl) propanoic acid copolymers (GODC)
The monomer containing Gd-DOTA, (N6-lysyl)lysine Gd-DOTA monoamide, was first synthesized using solid phase chemistry, Figure 1. The new biodegradable macromolecular MRI contrast agent, (N6-lysyl)lysine Gd-DOTA monoamide and 3-(2-carboxyethyldisulfanyl) propanoic acid copolymers (GODC), was synthesized by condensation polymerization of (N6-lysyl)lysine Gd-DOTA monoamide and dithiobis(succinimidyl-propionate), Figure 2. The polymeric ligand, (N6-lysyl)lysine DOTA monoamide and 3-(2-carboxyethyldisulfanyl) propanoic acid copolymers, was characterized 1H-NMR and FT-IR. The number average and weight average molecular weights of GODC were 24.2 and 26.4 kDa. The Gd(III) content in the agent was 15.08% (w/w) as determined by ICP-OES. The calculated Gd content was 15.63% (w/w). There were approximately 25 Gd(III) chelates per polymer chain on average. The r1 and r2 relaxivities of GODC were 8.25 mM−1s−1 and 10.08 mM−1s−1 at 1.5 T (Figure 3). The T1 relaxivity (r1) was approximatly 2 folds of that of Gd(DO3A-HP) (ProHance®, 4.1 mM−1s−1 at 1.5T, 20°C) (26).
Figure 2.
Synthetic scheme of (N6-lysyl)lysine-(Gd-DOTA) monoamide 3-(2-carboxyethyldisulfanyl) propanoic acid copolymer (GODC).
Figure 3.
The relaxation rate (1/T1 and 1/T2) versus concentration plot of (N6-lysyl)lysine-(Gd-DOTA) monoamide dithiobispropionic acid copolymer (GODC).
Degradability of GODC
The polydisulfide structure of GODC was designed so that the polymers could be readily degraded and excreted via renal filtration in vivo via the disulfide-thiol exchange reaction (32). The degradability of GODC was demonstrated in the incubation study with L-cysteine, the most abundant free thiols in plasma (46). Figure 4 shows that the number average molecular weight of GODC decreased from 24.2 kDa before incubation to 23 kDa, 22.1 kDa, 19.7 kDa, 18.6 and 13.1 kDa at 30, 60, 120, 240 min, and 24 hrs in the incubation. In terms of polymerization degree, the polymers were reduced from 25 chelates per chain to about 12 chelates per chain after 24 hrs of in vitro incubation.
Figure 4.
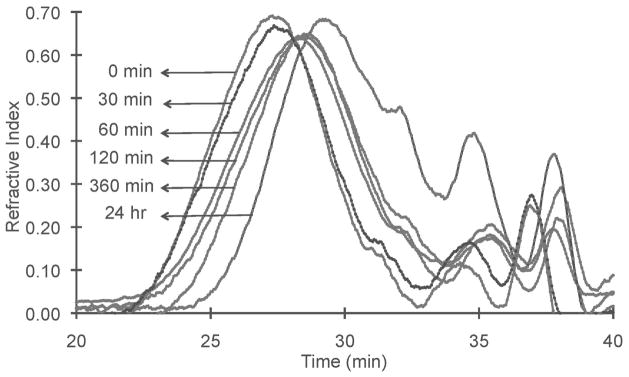
The apparent molecular weight distribution before (0 min) and in the incubation of GODC against cysteine (15 μM) at plasma concentration for 30, 60, 120, 360 min and 24 hrs at 37°C.
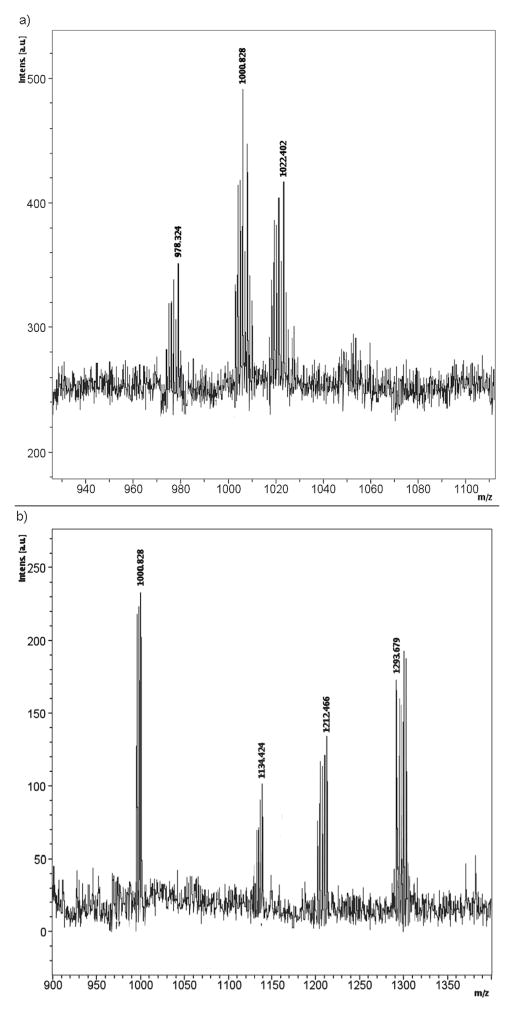
The polydisulfide was reduced to the smallest repeat units by disulfide-thiol exchange reaction when a sufficient amount of free thiols was present, Figure 5. Figure 6a shows the MALDI-TOF mass spectrum of a mixture of GODC with an excess of L-cysteine incubated for 60 minutes. The degradation product peaks were compound I (m/z=1000.8, M+Na+; m/z=1022.4, M+2Na+) and compound II (m/z=1212.5, M+H+; m/z=1234.1, M+Na+), the smallest repeat units of the polymer. The degradation products [978.3 (I+H+), 1000 (I+Na+), 1212 (II+H+)] were also identifed by MALDI-TOF mass spectrometry in urine samples collected after intravenous injection of GODC, Figure 6b. The major metabolites in the urine samples had the mass of 998.3 (I+H+), 1000.8 (I+Na+), 1117.9 (unkown), 1134.4 (compound I-homocysteine+Na+), 1212.5 (II+H+) and 1293.7 (unkown).
Figure 5.
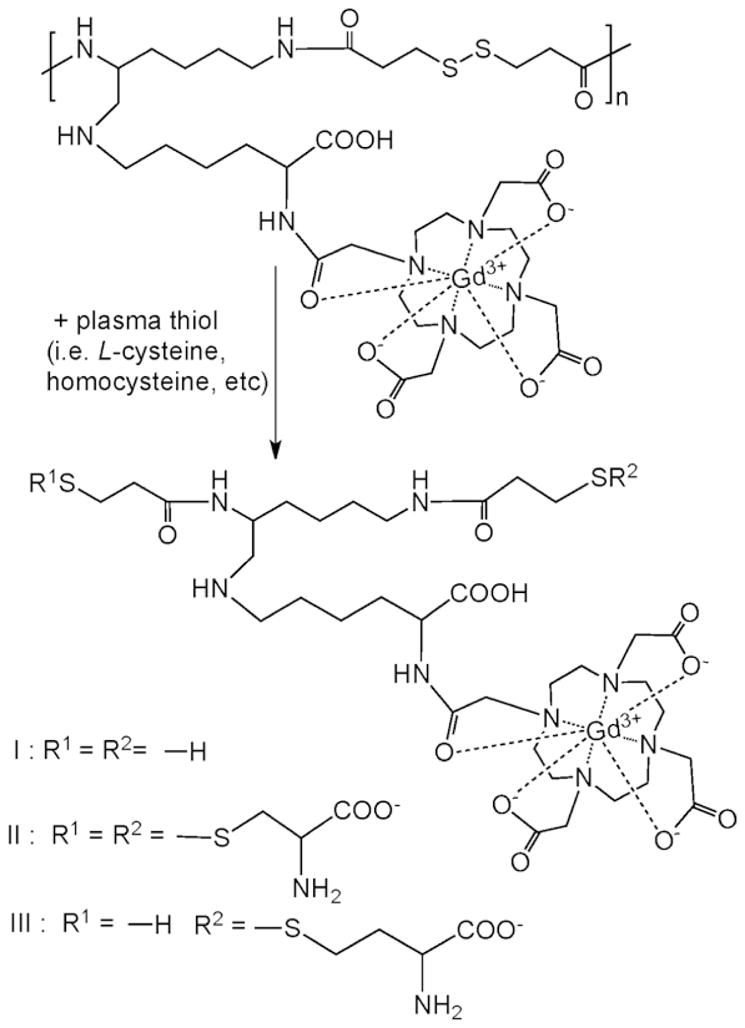
Complete degradation products of GODC with L-cysteine.
Figure 6.
The MALDI-TOF mass spectrum of degradation products (a) collected 60 minutes post incubation of GODC with an excess of L-cysteine (1.5 mM), and mouse urine samples (b) collected 30 minutes after the injection of GODC.
Kinetic stability of GODC
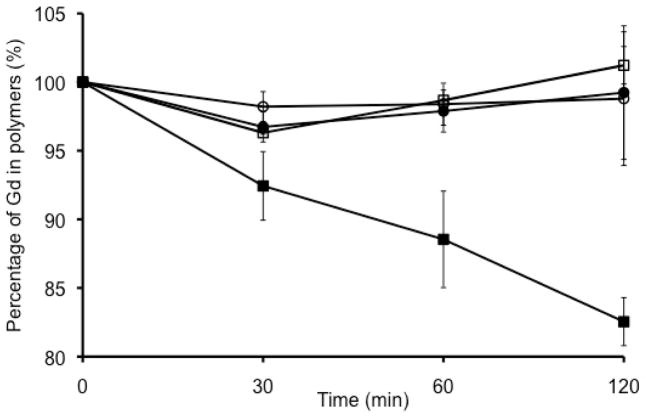
The new polydisulfide macrocyclic Gd chelate, GODC, demonstrated much higher kinetic stability against transmetallation than a polydisulfide of linear Gd chelates, GDCC. Figure 7 shows that a gradual loss of Gd(III) was observed in GDCC, from initially 100 % to 92% at 30 min, 88 % at 90 min and 82 % at 120 min in the incubation with Zn2+. In comparison, GODC incubated with Zn2+ had comparable Gd(III) content as GODC and GDCC incubated with PBS, indicating no transmetallation observed for GODC.
Figure 7.
The Gd(III) content in GDCC (square) and GODC (circle) before and in the incubation in PBS buffer with ZnCl2 (50 μM, filled) and without ZnCl2 (open) for 30, 60, and 120 min.
In vivo MR imaging
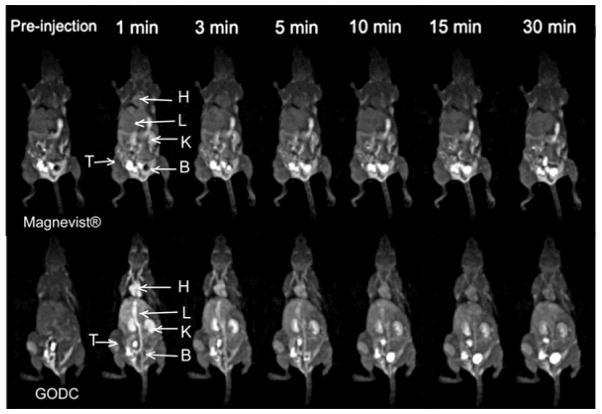
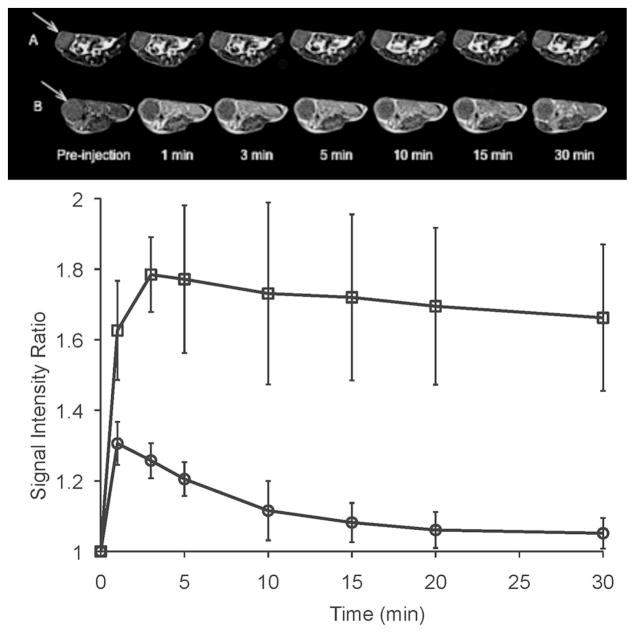
Figure 8 showed the T1-weighted 3D maximum intensity projection MR images of mice bearing orthotopic 4T1 mouse malignant tumor before and after intravenous injection of GODC and Magnevist®. Significant and prolonged blood pool contrast enhancement was observed with GODC in comparison with Magnevist®, indicating the relatively prolonged blood circulation of GODC. Enhancement in the blood pool gradually descreased, but was still visible at 30 minutes post-injection. Contrast enhancement inside the urinary bladder was observed at 3 minutes postinjection for Magnevist®, and at 10 minutes postinjection for GODC. This indicated rapid excretion of the Gd(III) chelate of GODC. Figure 9a shows the axial T1-weighted 2D spin-echo MR images and signal intensity ratio of the tumor before and at different time points after injection of the agents. GODC resulted in more significant enhancement in the tumor periphery of mice than that of Magnevist®. Quantitative analysis of the signal intensity in the tumor periphery showed that GODC produced stronger signal enhancement in the tumor than Magnevist® for at least 30 minutes (Figure 9b).
Figure 8.
Three-dimensional maximum intensity projection images (coronal view) of mice bearing 4T1 breast tumor xenografts before and at 1, 3, 5, 10, 15 and 30 minutes post injection of Magnevist® and GODC at a dose of 0.1 mmol-Gd/kg. The major organs were pointed out as H (heart), L (liver), K (kidney), B (bladder) and T (tumor).
Figure 9.
a) Axial 2D spin echo images of mice bearing 4T1 breast tumor xenografts before and at 1, 3, 5, 10, 15 and 30 minutes post injection of Magnevist® (A) and GODC (B) at a dose of 0.1 mmol-Gd/kg. b) The region of interest (ROI) of tumor periphery on mice with Magnevist® (A) and GODC (B). c) Signal intensity ratio of tumor periphery of mice bearing malignant breast cancer before and at different time points after administration of Magnevist® (circle) and GODC (square).
DISCUSSION
A polydisulfide with macrocyclic Gd(III) chelate side chains, (N6-lysyl)lysine Gd-DOTA monoamide and 3-(2-carboxyethyldisulfanyl) propanoic acid copolymers (GODC), was synthesized as a biodegradable macromolecular MRI contrast agent with improved kinetic stability against the previously reported polydisulfide DTPA based agents (32,35). The monomer (N6-lysyl)lysine DOTA monoamide had an unsymmetrical structure. Consequently, the condensation polymerization of the monomers could result in polymer chains with random arrangement of (N6-lysyl)lysine Gd-DOTA monoamide. The final product GODC could have three possible arrangements of (N6-lysyl)lysine Gd-DOTA monoamide in the polymer chains: two α-amines of two (N6-lysyl)lysine Gd-DOTA monoamide molecules connected to the same DSP molecule, two ε-amines connected to the same DSP, and one α-amine and one ε-amine connected to DSP. The polymerization might also result in cyclized products. High concentration of monomers were used in the polymerization reaction to minimize the cyclization. The arrangement of the monomers in the polymer chains should not affect degradability and magnetic properties of GODC as a biodegradable macromolecular MRI contrast agent. GODC were readily reduced into oliogomers in the presence of a low concentration of cysteine (15 μM) and the smallest repeat units in the presence of sufficient cysteine, the most abundant species of small molecular free thiols in human plasma. Blood plasma also contains other thiols, including glutathione, homocysteine, cysteinylglycine and serum albumin (46). Cysyeine was used to represent the total small molecular free thiols. Serum albumine is of high concentration in plasma, but the exchange reation between the thiol in serum albumin and the polydisulfide is prevented by the steric hindrance of the protein and polymers. This was proved in our previous study with the Gd-DTPA based polydisulfides, which showed no reaction with serum albumin (48). Mass spectrometric analysis of the urine sample after the injection of GODC validated the in vivo degradation of the polymers by plasma thiols. The metabolites of the smallest repeat unit of the polymer and thiol exchange products with cysteine and homocysteine (m/z=1134.4) were identified in the urine samples. It appeared that the degradation rate of GODC was slower than our previous reported Gd-DTPA cystamine copolymers (GDCC) (49). This was because the former was negatively charged and the latter was neutral. Similar results have also been observed for other negatively charged polydisulfides, e.g. Gd-DTPA cystine copolymers (49). Negative charges around disulfide in GODC inhibited the disulfide-thiol exchange reaction between the polymers and cysteine, which was also negatively charged at pH 7.4 in PBS buffer, due to charge repulsion. The in vitro degradation of GODC at 15 μM cysteine appears slower than its clearance revealed by MRI. This might be attributed to the fixed amount of cysteine used in the in vitro study, while free thiols in the plasma was constantly replenished by the liver. Nevertheless, the polydisulfide could be reduced into the smallest repeat units in the presence of sufficient free thiols.
Similar as our previously reported Gd-DTPA based polydisulfides, the newly developed Gd-DOTA based polydisulfides, GODC, had much higher relaxivities than Gd-DOTA and Gd(DO3A-HP) (26,29,35). T1 relaxivity of GODC was approximately two times higher than that of Gd-DOTA and Gd(DO3A-HP) at 1.5 T. The significant increase of T1 relaxivity of GODC might be attributed to its relatively large molecular size and hydrodynamic volume. The relaxivity of GODC was lower than that of dendrimer based macromolecular contrast agents such as Gadomer 17 or other PAMAM dendrimer Gd chelate conjugates (15,22,23). This might be due to the relatively flexible hydrophilic backbone of linear GODC polymer chains.
As expected, the macrocyclic chelates in the polydisulfide GODC showed higher kinetic stability than the linear DTPA diamide chelates in GDCC. As reported in the literature, endogenous Zn2+ ions were the main cause of in vivo transmetallation of Gd(III) based contrast agents with linear igands. No obvious release of free Gd(III) ions was detected for GODC in the presence of Zn2+ ions, consistent to other macrocyclic Gd(III) chelates (8,31,42). High kinetic stability of the new Gd(III) based MRI contrast agent was critical to allow complete excretion of the agent from the body and minimize toxic side effects associated with incompleted excretion of Gd(III) based agents. The mass spectrometric analysis showed that the agent was excreted in the urine as the intact Gd(III) chelates.
GODC was effective for contrast enhanced blood pool imaging, resulting in strong contrast enhancement in the blood of the heart and vasculature, and in tumor periphery. As compared to GDCC with similar weight (23 kDa) reported in our previous study (34,35), it appears that GODC produced more prolonged blood pool enhancement than GDCC, possibly due to the relatively slow degradation rate of GODC. Nevertheless, GODC could be readily excreted via renal filtration after in vivo degradation. Strong bladder enhancement was observed as earlier as 10 minutes post injection, indicating the excretion of degraded Gd(III) chelates. In comparison, the clinical agent Magnevist® generate short and less enhancement in the blood pool. The prolonged blood pool enhancement of GODC is largely due to its large size, which result in limited extravasation and prolonged circulation. Moreover, the significant enhancement in signal intensity can also be attributed to the increased relaxivity of GODC. Significantly stronger tumor enhancement was observed with GODC, but was limited in the periphery area of tumor. Little differences of enhancement was observed in the tumor core between GODC and Magnevist®. High pressure in the necrotic tumor core may hinder the penetration of of the agents. The new Gd(III) macrocyclic chelate based biodegradable macromolecular MRI contrast agent GODC is more effective for contrast enhanced blood pool and tumor MRI than the small molecular clinical agent.
CONCLUSION
A new polydisulfide Gd-DOTA complexes, GODC, was synthesized and evaluated as a biodegradable macromolecular MRI contrast agent. GODC demonstrated high kinetic stability against transmetallation with endogenous ions. It was readily reduced by endogenous thiols into smaller Gd(III) chelates or oligomers that could be readily excreted via renal filtration. GODC is a promising safe and effective biodegradable macromolecular MRI contrast agent for further development. It has a potential for MR cardiovascular imaging and cancer imaging.
Acknowledgments
The research work is supported in part by the NIH grant R01 EB00489.
References
- 1.Barrett T, Brechbiel M, Bernardo M, Choyke PL. MRI of tumor angiogenesis. J Magn Reson Imaging. 2007;26(2):235–249. doi: 10.1002/jmri.20991. [DOI] [PubMed] [Google Scholar]
- 2.Padhani AR. Dynamic contrast-enhanced MRI in clinical oncology: current status and future directions. J Magn Reson Imaging. 2002;16(4):407–422. doi: 10.1002/jmri.10176. [DOI] [PubMed] [Google Scholar]
- 3.Lauffer RB. Paramagnetic Metal Complexes as Water Proton Relaxation Agents for NMR Imaging: Theory and Design. Chem Rev. 1987;87(5):901–927. [Google Scholar]
- 4.Koretsky AP, Silva AC. Manganese-enhanced magnetic resonance imaging (MEMRI) NMR Biomed. 2004;17(8):527–531. doi: 10.1002/nbm.940. [DOI] [PubMed] [Google Scholar]
- 5.Hermann P, Kotek J, Kubicek V, Lukes I. Gadolinium(III) complexes as MRI contrast agents: ligand design and properties of the complexes. Dalton Trans. 2008;(23):3027–3047. doi: 10.1039/b719704g. [DOI] [PubMed] [Google Scholar]
- 6.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem Rev. 1999;99(9):2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 7.Aime S, Cabella C, Colombatto S, Geninatti Crich S, Gianolio E, Maggioni F. Insights into the use of paramagnetic Gd(III) complexes in MR-molecular imaging investigations. J Magn Reson Imaging. 2002;16(4):394–406. doi: 10.1002/jmri.10180. [DOI] [PubMed] [Google Scholar]
- 8.Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging. 2009;30(6):1259–1267. doi: 10.1002/jmri.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellin MF, Vasile M, Morel-Precetti S. Currently used non-specific extracellular MR contrast media. Eur Radiol. 2003;13(12):2688–2698. doi: 10.1007/s00330-003-1912-x. [DOI] [PubMed] [Google Scholar]
- 10.Schalla S, Higgins CB, Saeed M. Contrast agents for cardiovascular magnetic resonance imaging. Current status and future directions. Drugs R D. 2002;3(5):285–302. doi: 10.2165/00126839-200203050-00001. [DOI] [PubMed] [Google Scholar]
- 11.Turetschek K, Floyd E, Helbich T, Roberts TP, Shames DM, Wendland MF, Carter WO, Brasch RC. MRI assessment of microvascular characteristics in experimental breast tumors using a new blood pool contrast agent (MS-325) with correlations to histopathology. J Magn Reson Imaging. 2001;14(3):237–242. doi: 10.1002/jmri.1179. [DOI] [PubMed] [Google Scholar]
- 12.Brasch RC. Rationale and applications for macromolecular Gd-based contrast agents. Magn Reson Med. 1991;22(2):282–287. doi: 10.1002/mrm.1910220225. [DOI] [PubMed] [Google Scholar]
- 13.Goyen M, Shamsi K, Schoenberg SO. Vasovist-enhanced MR angiography. Eur Radiol. 2006;16 (Suppl 2):B9–14. doi: 10.1007/s10406-006-0162-9. [DOI] [PubMed] [Google Scholar]
- 14.Brasch R, Turetschek K. MRI characterization of tumors and grading angiogenesis using macromolecular contrast media: status report. Eur J Radiol. 2000;34(3):148–155. doi: 10.1016/s0720-048x(00)00195-9. [DOI] [PubMed] [Google Scholar]
- 15.Bumb A, Brechbiel MW, Choyke P. Macromolecular and dendrimer-based magnetic resonance contrast agents. Acta Radiol. 2010;51(7):751–767. doi: 10.3109/02841851.2010.491091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauffer RB, Parmelee DJ, Ouellet HS, Dolan RP, Sajiki H, Scott DM, Bernard PJ, Buchanan EM, Ong KY, Tyeklar Z, Midelfort KS, McMurry TJ, Walovitch RC. MS-325: a small-molecule vascular imaging agent for magnetic resonance imaging. Acad Radiol. 1996;3 (Suppl 2):S356–358. doi: 10.1016/s1076-6332(96)80583-6. [DOI] [PubMed] [Google Scholar]
- 17.Perreault P, Edelman MA, Baum RA, Yucel EK, Weisskoff RM, Shamsi K, Mohler ER., 3rd MR angiography with gadofosveset trisodium for peripheral vascular disease: phase II trial. Radiology. 2003;229(3):811–820. doi: 10.1148/radiol.2293021180. [DOI] [PubMed] [Google Scholar]
- 18.Shamsi K, Yucel EK, Chamberlin P. A summary of safety of gadofosveset (MS-325) at 0. 03 mmol/kg body weight dose: Phase II and Phase III clinical trials data. Invest Radiol. 2006;41(11):822–830. doi: 10.1097/01.rli.0000242836.25299.8f. [DOI] [PubMed] [Google Scholar]
- 19.Barrett T, Kobayashi H, Brechbiel M, Choyke PL. Macromolecular MRI contrast agents for imaging tumor angiogenesis. Eur J Radiol. 2006;60(3):353–366. doi: 10.1016/j.ejrad.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Schuhmann-Giampieri G, Schmitt-Willich H, Frenzel T, Press WR, Weinmann HJ. In vivo and in vitro evaluation of Gd-DTPA-polylysine as a macromolecular contrast agent for magnetic resonance imaging. Invest Radiol. 1991;26(11):969–974. doi: 10.1097/00004424-199111000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Helbich TH, Gossman A, Mareski PA, Raduchel B, Roberts TP, Shames DM, Muhler M, Turetschek K, Brasch RC. A new polysaccharide macromolecular contrast agent for MR imaging: biodistribution and imaging characteristics. J Magn Reson Imaging. 2000;11(6):694–701. doi: 10.1002/1522-2586(200006)11:6<694::aid-jmri17>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Bryant LH, Jr, Brechbiel MW, Wu C, Bulte JW, Herynek V, Frank JA. Synthesis and relaxometry of high-generation (G = 5, 7, 9, and 10) PAMAM dendrimer-DOTA-gadolinium chelates. J Magn Reson Imaging. 1999;9(2):348–352. doi: 10.1002/(sici)1522-2586(199902)9:2<348::aid-jmri30>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Misselwitz B, Schmitt-Willich H, Ebert W, Frenzel T, Weinmann HJ. Pharmacokinetics of Gadomer-17, a new dendritic magnetic resonance contrast agent. MAGMA. 2001;12(2–3):128–134. doi: 10.1007/BF02668094. [DOI] [PubMed] [Google Scholar]
- 24.Ladd DL, Hollister R, Peng X, Wei D, Wu G, Delecki D, Snow RA, Toner JL, Kellar K, Eck J, Desai VC, Raymond G, Kinter LB, Desser TS, Rubin DL. Polymeric gadolinium chelate magnetic resonance imaging contrast agents: design, synthesis, and properties. Bioconjug Chem. 1999;10(3):361–370. doi: 10.1021/bc980086+. [DOI] [PubMed] [Google Scholar]
- 25.Caravan P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev. 2006;35(6):512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 26.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40(11):715–724. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 27.Aime S, Castelli DD, Crich SG, Gianolio E, Terreno E. Pushing the sensitivity envelope of lanthanide-based magnetic resonance imaging (MRI) contrast agents for molecular imaging applications. Acc Chem Res. 2009;42(7):822–831. doi: 10.1021/ar800192p. [DOI] [PubMed] [Google Scholar]
- 28.Brasch R, Pham C, Shames D, Roberts T, van Dijke K, van Bruggen N, Mann J, Ostrowitzki S, Melnyk O. Assessing tumor angiogenesis using macromolecular MR imaging contrast media. J Magn Reson Imaging. 1997;7(1):68–74. doi: 10.1002/jmri.1880070110. [DOI] [PubMed] [Google Scholar]
- 29.Lu ZR, Ye F, Vaidya A. Polymer platforms for drug delivery and biomedical imaging. J Control Release. 2007;122(3):269–277. doi: 10.1016/j.jconrel.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sieber MA, Lengsfeld P, Frenzel T, Golfier S, Schmitt-Willich H, Siegmund F, Walter J, Weinmann HJ, Pietsch H. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol. 2008;18(10):2164–2173. doi: 10.1007/s00330-008-0977-y. [DOI] [PubMed] [Google Scholar]
- 31.Thomsen HS, Morcos SK. Nephrogenic systemic fibrosis and nonionic linear chelates. AJR Am J Roentgenol. 2007;188(6):W580. doi: 10.2214/AJR.06.1651. author reply W581. [DOI] [PubMed] [Google Scholar]
- 32.Lu ZR, Mohs AM, Zong Y, Feng Y. Polydisulfide Gd(III) chelates as biodegradable macromolecular magnetic resonance imaging contrast agents. Int J Nanomedicine. 2006;1(1):31–40. doi: 10.2147/nano.2006.1.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zong Y, Wang X, Goodrich KC, Mohs AM, Parker DL, Lu ZR. Contrast-enhanced MRI with new biodegradable macromolecular Gd(III) complexes in tumor-bearing mice. Magn Reson Med. 2005;53(4):835–842. doi: 10.1002/mrm.20402. [DOI] [PubMed] [Google Scholar]
- 34.Zong Y, Guo J, Ke T, Mohs AM, Parker DL, Lu ZR. Effect of size and charge on pharmacokinetics and in vivo MRI contrast enhancement of biodegradable polydisulfide Gd(III) complexes. J Control Release. 2006;112(3):350–356. doi: 10.1016/j.jconrel.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Lu ZR, Wu X. Polydisulfide Based Biodegradable Macromolecular Magnetic Resonance Imaging Contrast Agents. Isr J Chem. 2010;50(2):220–232. doi: 10.1002/ijch.201000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ersoy H, Rybicki FJ. Biochemical safety profiles of gadolinium-based extracellular contrast agents and nephrogenic systemic fibrosis. J Magn Reson Imaging. 2007;26(5):1190–1197. doi: 10.1002/jmri.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morcos SK. Extracellular gadolinium contrast agents: differences in stability. Eur J Radiol. 2008;66(2):175–179. doi: 10.1016/j.ejrad.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 38.Cacheris WP, Quay SC, Rocklage SM. The relationship between thermodynamics and the toxicity of gadolinium complexes. Magn Reson Imaging. 1990;8(4):467–481. doi: 10.1016/0730-725x(90)90055-7. [DOI] [PubMed] [Google Scholar]
- 39.Laurent S, Elst LV, Copoix F, Muller RN. Stability of MRI paramagnetic contrast media: a proton relaxometric protocol for transmetallation assessment. Invest Radiol. 2001;36(2):115–122. doi: 10.1097/00004424-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Puttagunta NR, Gibby WA, Smith GT. Human in vivo comparative study of zinc and copper transmetallation after administration of magnetic resonance imaging contrast agents. Invest Radiol. 1996;31(12):739–742. doi: 10.1097/00004424-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 41.White GW, Gibby WA, Tweedle MF. Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Invest Radiol. 2006;41(3):272–278. doi: 10.1097/01.rli.0000186569.32408.95. [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Zong Y, Ye Z, Lu ZR. Stability and biodistribution of a biodegradable macromolecular MRI contrast agent Gd-DTPA cystamine copolymers (GDCC) in rats. Pharm Res. 2010;27(7):1390–1397. doi: 10.1007/s11095-010-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu ZR, Wang X, Parker DL, Goodrich KC, Buswell HR. Poly(l-glutamic acid) Gd(III)-DOTA conjugate with a degradable spacer for magnetic resonance imaging. Bioconjug Chem. 2003;14(4):715–719. doi: 10.1021/bc0340464. [DOI] [PubMed] [Google Scholar]
- 44.Xu R, Wang Y, Wang X, Jeong EK, Parker DL, Lu ZR. In Vivo evaluation of a PAMAM-cystamine-(Gd-DO3A) conjugate as a biodegradable macromolecular MRI contrast agent. Exp Biol Med (Maywood) 2007;232(8):1081–1089. doi: 10.3181/0702-RM-33. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Feng Y, Ke T, Schabel M, Lu ZR. Pharmacokinetics and tissue retention of (Gd-DTPA)-cystamine copolymers, a biodegradable macromolecular magnetic resonance imaging contrast agent. Pharm Res. 2005;22(4):596–602. doi: 10.1007/s11095-005-2489-7. [DOI] [PubMed] [Google Scholar]
- 46.Andersson A, Lindgren A, Hultberg B. Effect of thiol oxidation and thiol export from erythrocytes on determination of redox status of homocysteine and other thiols in plasma from healthy subjects and patients with cerebral infarction. Clin Chem. 1995;41(3):361–366. [PubMed] [Google Scholar]
- 47.Deneke SM. Thiol-based antioxidants. Curr Top Cell Regul. 2000;36:151–180. doi: 10.1016/s0070-2137(01)80007-8. [DOI] [PubMed] [Google Scholar]
- 48.Lu ZR, Parker DL, Goodrich KC, Wang X, Dalle JG, Buswell HR. Extracellular biodegradable macromolecular gadolinium(III) complexes for MRI. Magn Reson Med. 2004;51(1):27–34. doi: 10.1002/mrm.10656. [DOI] [PubMed] [Google Scholar]
- 49.Zong Y, Wang X, Jeong EK, Parker DL, Lu ZR. Structural effect on degradability and in vivo contrast enhancement of polydisulfide Gd(III) complexes as biodegradable macromolecular MRI contrast agents. Magn Reson Imaging. 2009;27(4):503–511. doi: 10.1016/j.mri.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]