Abstract
Fourteen saxicolous lichens from trans-Himalayan Ladakh region were identified by morpho-anatomical and chemical characteristics. The n-hexane, methanol and water extracts of the lichens were evaluated for their antioxidant capacities. The lichen extracts showing high antioxidant capacities and rich phenolic content were further investigated to determine their cytotoxic activity on human HepG2 and RKO carcinoma cell lines. The ferric reducing antioxidant power (FRAP), 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH) and nitric oxide (NO) radical scavenging capacities and β-carotene-linoleic acid bleaching property exhibited analogous results where the lichen extracts showed high antioxidant action. The lichen extracts were also found to possess good amount of total proanthocyanidin, flavonoid and polyphenol. The methanolic extract of Lobothallia alphoplaca exhibited highest FRAP value. Methanolic extract of Xanthoparmelia stenophylla showed the highest ABTS radical scavenging capacity. The n-hexane extract of Rhizoplaca chrysoleuca exhibited highest DPPH radical scavenging capacity. Highest antioxidant capacity in terms of β-carotene linoleic acid bleaching property was observed in the water extract of Xanthoria elegans. Similarly, Melanelia disjuncta water extract showed highest NO scavenging capacity. Among n-hexane, methanol and water extracts of all lichens, the methanolic extract of Xanthoparmelia mexicana showed highest total proanthocyanidin, flavonoid and polyphenol content. From cytotoxic assay, it was observed that the methanolic extracts of L. alphoplaca and M. disjuncta were exhibiting high cytotoxic effects against cancer cell growth. Similarly, the water extract of Dermatocarpon vellereum, Umbilicaria vellea, X. elegans and M. disjuncta and the methanolic extract of M. disjuncta and X. stenophylla were found to possess high antioxidant capacities and were non-toxic and may be used as natural antioxidants for stress related problems. Our studies go on to prove that the unique trans-Himalayan lichens are a hitherto untapped bioresource with immense potential for discovery of new chemical entities, and this biodiversity needs to be tapped sustainably.
Introduction
Free radicals having one or more unpaired electrons are generated as a by-product in normal or pathological cell metabolism. Reactive oxygen species (ROS) react swiftly with free radicals to become radicals themselves thereby starting free radical chain reaction. Superoxide anion (O2–), hydrogen peroxide (H2O2), hydroxyl radical (HO•) and singlet oxygen (1O2) are the various forms of reactive oxygen species [1], [2]. Excess ROS in our body damages biological molecules leading to the development of degenerative diseases such as premature aging, heart diseases, cancer, inflammation, diabetes, genotoxicity, arthritis and many more [3], [4]. Exogenous sources of free radicals viz. tobacco smoke, ionizing radiation, certain pollutants, organic solvents, pesticides etc. trigger the process of generation of ROS within the body [5], [6]. The most efficient way to exterminate free radicals that cause the oxidative stress is through antioxidant supplementation.
Antioxidants are compounds which can impede the oxidation process by reacting with free radicals, chelating catalytic metals and scavenging oxygen in biological systems [7]. Hence, antioxidants are of prime importance in preventing various pathophysiological dysfunctions and diseases [8]–[10]. However, the synthetic antioxidants viz. butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), tertbutylhydroquinone (TBHQ) and propyl gallate (PG) have been reported to exert toxic effects [11]. Consequently, there is a growing interest towards finding natural antioxidants of plant resources without any undesirable effect [1], [6]. Ethnopharmacological and in vitro studies on medicinal plants and vegetables strongly supports the fact that phytoconstituents with antioxidant capacity are capable of exerting protective effects against oxidative stress in biological systems [12], [13]. Therefore, it is of prime importance to utilize natural antioxidants for their protective effect against oxidative stress and physiological dysfunctions [14], [15]. Natural antioxidants obtained from various resources such as plants, micro- and macro-algae, macromycetes and lichens have become one of the major research areas in recent times. In the quest for novel natural antioxidant sources, our prime interest has focused on lichens.
Lichens, the unanimously disseminated organisms occurring in the most adverse and varied geo-climatic circumstances ranging from the ice-capped poles to the tropics and from the plains to the highest mountains on earth and substrates, encompass the most inimitable group of organisms in nature, growing simultaneously in a close successful symbiotic alliance of two distinct organisms, an exhabitant fungus (the mycobiont) and the photoautotrophic partner algae (the phycobiont) [16], [17]. Lichenology remains rather neglected throughout the world, though collectively with mosses they form the omnipresent organisms in ecosystem casing over 10% of the earths’ terrestrial habitats predominantly at higher elevations [18], [19]. Lichens are known to have therapeutic effects on various diseases in traditional system of medicine of many countries. A number of factors such as specific and extreme habitat, slow growth and long life are the basis for the production of diverse bioactive compounds having protective functions against several physical and biological influences [20], [21]. Various scientific reports suggest that the lichens have antimicrobial, antiviral, antitumor, antiinflammatory, analgesic and antipyretic, antiproliferative and antiprotozoal potentials [22], [23]. The antioxidant properties of lichens and their secondary metabolites are poorly known and in recent time the potential of lichens as resources of natural antioxidants have been investigated by researchers [24]–[29] and only few of them have been reported to have promising antioxidative potential [25], [30]. In the world, India is a rich centre of lichens diversity, contributing nearly 15% of the 13500 species of lichens so far recorded [31]. Therefore, it is necessary to explore the remaining unreported lichen species of this country.
The Indian Himalayan region is well known for its varied characteristic ecosystems endowed with rich floristic and faunal wealth. Plants of the Himalayan region are widely used in traditional medical systems both as prophylactics and therapeutics for high altitude maladies and a number of natural products and botanical supplements have been developed from our institutes, which are useful to combat these problems. These products have been reported to possess high nutritional and antioxidant properties. Extensive research work was carried out by the previous investigators to explore the medicinal and aromatic plants of trans-Himalaya [32]–[34]. However, research reports on lichen flora of this megadiversity hotspot of the world are very limited. In our recent study, we have reported the diversity of lichens along altitudinal and land use gradients from trans-Himalyan region. The harsh climatic condition of trans-Himalayan Ladakh, along with its fragile ecosystem, desert soil and rocky habitat constitute ecological niche for lichen species [35], [36]. In the present investigation, we first aimed to study the antioxidant capacity and phenolic profile of lichens from this region. Out of all lichen families under investigation, lichen species of families viz. Umbilicariaceae (Umbilicaria esculenta, U. vellea, U. muhlenbergii and other Umbilicaria spp.), Teloschistaceae (Xanthoria parietina, X. elegans) and Parmeliaceae (Xanthoparmelia chlorochroa, X. conspersa, Parmelia tinctorum, P. nilgherrensis, P. reticulata and P. sancti-algelia) are used as food ingredients and for medicinal purposes in different parts of the world [37]–[43].
However, the medicinal and therapeutic effects with respect to antioxidant capacities, cytotoxic activity and phenolic composition of different extracts of saxicolous lichens viz. Dermatocarpon vellereum, Umbilicaria vellea, Rhizoplaca chrysoleuca, Rhizoplaca melanophthalma, Pleopsidium flavum, Xanthoparmelia mexicana, Acarospora badiofusca, Xanthoria elegans, Lecanora frustulosa, Lobothallia alphoplaca, Physconia muscigena, Melanelia disjuncta, Xanthoparmelia stenophylla and Peccania coralloides from trans-Himalayan cold desert of Ladakh have not been reported till date. For this rationale, the present investigation was desinged to estimate the in vitro antioxidant capacities and phenolic profile of n-hexane, methanol and water extracts of the aforementioned fourteen saxicolous lichens from trans-Himalaya. The lichen extracts showing high antioxidant capacities and rich phenolic content were further investigated to determine their cytotoxic activity on human hepatocellular carcinoma HepG2 and colon carcinoma RKO cells.
Materials and Methods
Chemicals and reagents
1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,4,6-tripyridyl-s-triazine (TPTZ), ferrous sulfate (FeSO4.7H2O), aluminium chloride (AlCl3), sodium acetate (C2H3NaO2), sodium carbonate (Na2CO3), potassium persulfate (K2S2O8), potassium chloride (KCl), ferric chloride (FeCl3), sodium nitroprusside {Na2[Fe(CN)5NO].2H2O}, sulfanilic acid (C6H7NO3S), butylated hydroxytoluene (BHT), ascorbic acid, gallic acid, quercetin and catechin were purchased from Sigma-Aldrich (St. Louis, MO, USA). RPMI medium 1640, penicillin, streptomycin, trypsin-EDTA, sulphorhodamine B, trichloroacetic acid, acetic acid, Tris base and all other chemicals used for the cytotoxicity assay were of analytical grade and also purchased from Sigma-Aldrich (St. Louis, MO, USA). Folin Ciocalteu's phenol reagent, vanillin, silica gel precoated aluminium TLC plates (20×20 cm), hydrochloric acid, sulphuric acid, methanol, n-hexane, chloroform, ethanol, toluene, dioxane, acetic acid, diethyl ether, formic acid and sodium carbonate were purchased from Merck Chemical Supplies (Merck KGaA, Darmstadt, Germany). All the other chemicals used including solvents were of analytical grade.
Ethics statement
All necessary permits were obtained for the described field studies and lichen collection. The permit was issued by Dr. B. Balaji (IFS), Divisional Forest Officer, Leh Forest Division, Jammu & Kashmir, India.
Sample collection
Amongst the fourteen lichen specimens thirteen lichens viz. Dermatocarpon vellereum (Zschacke), Umbilicaria vellea (L.) Ach. em. Frey, Rhizoplaca chrysoleuca (Sm.) Zopf, Rhizoplaca melanophthalma (DC) Leuck. & Poelt, Pleopsidium flavum (Bell.) Korb., Xanthoparmelia mexicana (Gyeln.) Hale, Acarospora badiofusca (Nyl.) Th. Fr., Xanthoria elegans (Links.) Th. Fr., Lecanora frustulosa (Dick.) Ach., Lobothallia alphoplaca (Wahlenb. ex Ach.), Physconia muscigena (Ach.) Poelt., Melanelia disjuncta (Essl.), Xanthoparmelia stenophylla (Ach.) Ahti & Hawksw. and Peccania coralloides (Massal.) Massal. were collected from the northward slope of hill [native primary scrubland located 34°08′08.86"N, 77° 34′36.0"E and 3530 m altitude above sea level (ASL)], Indus valley, Leh-Ladakh, J&K, India, in the peak winter period (minimum temperature −28°C, maximum temperature −3°C) of January, 2012. Umbilicaria vellea was collected from the location near Chang-La Top (barren cold desert located 34°02′36.05"N, 77°56′26.6"E and 5189 m altitude ASL), Changthang valley, Leh-Ladakh, J&K, India in May, 2012. Lichens were carefully collected using scalpel and forceps following standard procedure. Then dust, soil, and rock debris were removed by using brushes, forceps and needles, shade dried to a constant weight (dry weight, dw) and lichen samples were kept at room temperature until extraction in the sterile petriplate (Tarsons 10.0 cm TPX, 461030, Tarsons Products Pvt. Ltd., Kolkata, India).
Identification, morpho-anatomical and colorimetric characterisation of lichen species
Lichens were identified morpho-anatomically using a stereomicroscope, light microscope, and chemically with the help of color reactions, UV light and standardized thin-layer chromatography (TLC) [44], [45]. The precise identification of lichen species on the basis of occurrence of chemical compounds was performed by spot test and thin layer chromatography (TLC). Silica gel was used as stationary phase while different solvent systems were used as mobile phase. Three solvent systems viz. solvent system A (toluene: dioxane: acetic acid:: 180: 60: 8), solvent system B (hexane: diethyl ether: formic acid:: 130: 100: 20) and solvent system C (toluene: acetic acid:: 200: 30) were used as mobile phase. TLC was performed in accordance with the previous reports for lichen identification [44], [45]. Identification was done using relevant key and monographs [46], [47]. The voucher specimens are preserved in the Lichen Herbarium (LWG), CSIR–National Botanical Research Institute (NBRI), Lucknow, India.
Sample preparation and extraction
Lichen specimens were air dried at room temperature and ground separately to powder. Finely ground dry thalli of all lichen species (170.4 mg to 330 mg depending upon their abundance) were sequentially extracted in 1 ml of solvents viz. n-hexane, methanol and water by vortexing for 10 min, and then centrifugation at 10,000 rpm for 15 min. This cycle of extraction for each lichen sample was at 10°C and repeated two times as each solvent added freshly to ensure complete extraction [48]. The extracts were filtered and transferred in new micro centrifuge tubes until debris was completely removed. The extracts was then concentrated by keeping in water bath at 20–40°C. These concentrated extracts were kept at −20°C for further use.
Antioxidant capacities
Ferric reducing antioxidant power (FRAP) assay
Ferric reducing antioxidant power (FRAP) assay was performed as suggested by previous investigators [49]. A total of 75 µl of extract and 225 µl of distilled water were added to 2.25 ml of freshly prepared FRAP reagent [10 parts of 300 mM sodium acetate buffer at pH 3.6, 1 part of 10 mM 2,4,6-tri (2-pyridyl)-s-triazine (TPTZ) solution and 1 part of 20 mM FeCl3.6H2O]. The content was incubated in the dark for 30 min. The increase in absorbance with the formation of colored product (ferrous tripyridyltriazine complex) was measured at 593 nm. The antioxidant capacity of the lichen extract was determined based on a calibration curve plotted using FeSO4.7H2O at a concentration ranging between 0.125 and 2 mM. The calibration equation for FeSO4.7H2O was y = 0.464×−0.0114, R2 = 0.9996, where x is the concentration of FeSO4.7H2O mM/l and y is the absorbance at 593 nm. Results were expressed in mM Fe (II)/g of extract.
ABTS radical scavenging activity
The ABTS assay was performed according to the previously established protocol [50]. The stock solution was prepared by mixing equal volumes of 7 mM ABTS solution and 2.45 mM potassium persulfate solution followed by incubation for 12 h at room temperature in the dark to yield a dark-colored solution containing ABTS•+ radicals. Working solution was prepared freshly before each assay by diluting the stock solution by mixing of stock solution to 50% methanol for an initial absorbance of about 0.700±0.02 at 745 nm, with temperature control set at 30°C. Free radical scavenging activity was assessed by mixing 30 µl of different fractions (different concentration in respective solvents) with 300 µl of ABTS working standard. The decrease in absorbance was measured after 6 min at 30°C. Quercetin and ascorbic acid were used as positive control. The scavenging activity was estimated based on the percentage of ABTS radicals scavenged by the following formula:
Where, A0 was absorption of control, AS was absorption of tested extracts and standards.
The half maximal inhibitory concentration (IC50) for scavengers (radical scavenging concentration50 or RSa50) was calculated [34], [51]. The RSa50 value was determined by plotting the scavenging capacity against the logarithm of sample concentration.
Inhibitory effect on 1,1-diphenyl-2-picrylhydrazyl radical (DPPH)
We followed the method suggested by previous investigators [52], [53] for estimating the DPPH radical scavenging capacity of lichen extracts. The 0.1 mM solution of DPPH in methanol was prepared and 500 µl of the solution was reacted with 25 µl of the extracted lichen sample. A control was reacted with 25 µl of solvent instead of the extract. The mixture was incubated at room temperature for 30 min before the shrink in absorbance at 517 nm was recorded. Quercetin and ascorbic acid were used as positive control. The percentage inhibition was calculated as follows:
Where, A0 was absorption of control, AS was absorption of tested extracts and standards.
The IC50 values were also calculated as described in the previous section.
β-carotene-linoleic acid bleaching assay
For the β-carotene-linoleic acid bleaching assay, the previously depicted method [54] was used. A stock solution of β-carotene and linoleic acid was prepared as follows: 0.5 mg of β-carotene was dissolved in 1 ml of chloroform, 25 µl of linoleic acid and 200 mg of Tween 20 were added. After chloroform evaporation under vacuum, 100 ml of distilled water was added to the residue. An aliquot of 500 µl of each extract or BHT (in different concentration) was pipetted into separated test tubes and 5 ml of the previous mixture was added. The test tubes were incubated for 2 h (120 min) at 50°C together with the control sample. The absorbance was measured at 470 nm at the beginning (t = 0 min) and after the experiment (t = 120 min). BHT was used as positive control. The antioxidant capacity was calculated as percentage inhibition of oxidation using the following equation:
where AA% is percent of antioxidant activity, A0 is the absorbance at beginning of reaction, At is the absorbance after 2 h with sample extract, A00 is the absorbance at beginning of reaction without sample extract and A0t is the absorbance after 2 h without sample extract. The IC50 or RSa50 values were also calculated as described in the previous section.
Nitric oxide (NO) scavenging capacity
The method of Wang et al. (2009) [55] was used to assay the scavenging activity of extracts on nitric oxide. The reaction solution (100 µl) containing 10 mM sodium nitroprusside in PBS (pH 7.0) was reacted with 10 µl extracts of different concentrations. The reaction mixture was then incubated at 37°C for 1 h. Thereafter 50 µl aliquot was mixed with 50 µl of Griess reagent [1.0 ml of sulfanilic acid reagent {0.33% prepared in 20% glacial acetic acid at room temperature for 5 min with 1 ml of naphthyethylenediamine dihydrochloride (0.1%, w/v)}] and the absorbance at 540 nm was measured. Percent inhibition of nitric oxide produced was calculated by comparing with the absorbance value of the negative control (10 mM sodium nitroprusside and PBS). BHT was used as positive control. The IC50 or RSa50 values were also calculated as described in the previous section.
Phytochemical compositions
Determination of total proanthocyanidin content (TPAC)
Determination of proanthocyanidin was performed according to the method of Sun et al. (1998) [56]. A volume of 0.5 ml extract solution was allowed to react with 3 ml of 4% vanillin-methanol solution and 1.5 ml hydrochloric acid. Then the mixture was allowed to stand for 15 min. The absorbance was measured at 500 nm at 30°C. Total proanthocyanidin content was calculated as catechin equivalent (CAE) in mg/g of extract using the following equation based on the calibration curve: y = 0.0015×−0.0209, R2 = 0.9975, where x was the absorbance and y was the CAE. Extract samples were evaluated at a final concentration of 100 µg/ml.
Determination of total flavonoid content (TFC)
Total flavonoid was analyzed with the method of Ordonz et al. (2006) [57]. Briefly, 100 µl of sample and 2% AlCl3 in ethanol solution were allowed to react. The reaction mixture was incubated for 1 h at room temperature and the absorbance was measured at 420 nm. Total flavonoid content was calculated as quercetin equivalent (QAE) in mg/g of extract by using the following equation based on the calibration curve: y = 0.0235x−0.1803, R2 = 0.9994, where x was the absorbance and y was the QAE at a final concentration of 100 µg/ml.
Determination of total polyphenol content (TPC)
Total polyphenol content (TPC) in extract was determined by Folin Ciocalteu's colorimetric method as described by Gao et al. (2000) [58]. Extract solution (10 µl) was mixed with 20 µl of 10% Folin Ciocalteu's reagent and 200 µl of H2O, and incubated at room temperature for 3 min. After that 100 µl of sodium carbonate (20%, w/v) was added to the reaction mixture. The content was incubated at room temperature in the dark for 30 min and absorbance was measured at 765 nm. Estimation of total polyphenol content was calculated as gallic acid equivalent (GAE) in mg/g of extract on the basis of calibration curve of gallic acid, y = 0.0104× −0.0589, R2 = 0.9978, where x was absorbance and y was GAE at a final concentration of 100 µg/ml.
Cytotoxic effects of lichen extracts
Cell cultures
Human hepatocellular carcinoma (HepG2) and colon carcinoma (RKO) cell lines acquired in liquid nitrogen (−180°C) were procured from American Type Culture Collection (ATCC), USA. Cells were maintained in RPMI medium 1640 (supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml of penicillin and 100 µg/ml of streptomycin, pH 7.4) at 37°C in 5% CO2 and 95–100% humidified air atmosphere.
Morphological determination of cell toxicity
Logarithmically growing cells were seeded in 96 flat-well plate, (HepG2 cells 3,000/well and RKO cells 3,000/well) and incubated for 24 h to have a partial monolayer. The extracts of lichens were dissolved in RPMI medium 1640 and cells were treated with different concentration (29.26, 43.90, 65.84, 98.77, 148.15, 222.22 and 333.33 µg/ml) of extracts. Thereafter, cells were incubated at 37°C in a 5% CO2 and 95–100% humidified air atmosphere (Touch 190S, LEEC, Nottingham, UK). Microscopic examination was performed (10X eyepiece and 10X objective lenses) using inverted phase contrast microscope (Dewinter Optical, Inc., Delhi, India). The changes in cellular morphology following different treatments were acquired at different time intervals (24, 48 and 72 h).
Sulphorhodamine B (SRB) assay for cytotoxicity screening
The Sulphorhodamine B (SRB assay) was performed for evaluating cellular growth and colorimetric determination of cytotoxicity of lichen extracts was performed [59], [60]. The cells were harvested with 0.5% trypsin-EDTA. At zero time after extract inoculation in the cell culture and after 72 h of incubation at 37°C in 5% CO2, in humidified incubator (95–100% humidity), the SRB assay was performed. At both points of time, the medium was removed and the cells were fixed with trichloroacetic acid (20%, w/v) at 4°C for 1 h, stained for 30 min with SRB (0.4%, w/v) dissolved in 1% acetic acid for 30 min and washed four times with 1% acetic acid. The protein-bound dye was made solubilised with 10 mmol/L tris base, pH 10.5 and the absorbance was recorded at 565 nm using spectrophotometer (Spectramax M2e, Molecular Devices, Germany).
The percentage growth (PG) at each extract concentration level was calculated as:
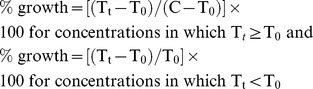 |
The 50% growth inhibition concentration (GI50, µg/ml) was the concentration where {(Tt−T0)/(C−T0)} ×100 = 50. Total growth inhibition concentration (TGI, µg/ml) was the concentration where {(Tt −T0)/(C−T0)} ×100 = 0 = PG. Lethal concentration that kills 50% of the cells (LC50, µg/ml) was the concentration level where {(Tt−T0)/T0} ×100 = −50. These parameters were determined following established reports [61]–[63] and expressed by plotting graph of cell growth against different concentration of each lichen extract. Where, T0 = absorbance of the extract treated cells just after time zero, and Tt = absorbance of the extract treated cells after 72 h, C = absorbance of control (cells untreated with extract) after 72 h.
Statistical Analysis
All the experimental results were expressed as mean ± standard deviation (SD) using statistical analysis with SPSS 17.0 (Statistical Program for Social Sciences, SPSS Corporation, Chicago, IL) version. Analysis of variance (ANOVA) in a completely randomised design, Duncan's multiple range test and Pearson's correlation coefficients were performed to compare the data. Post hoc analysis was performed using Neuman Keuls Test, and values with p<0.05 were considered significant.
Results
Morpho-anatomical features of identified lichen species
In the present study lichen specimens were collected from Chang-La Top of Changthang valley and northward slope of hill of Indus valley, Ladakh, India. The collected lichens specimens were identified using standardized analytical techniques, significant lichen identification keys and monographs [44]–[47]. The thallus structure of all lichen species has been illustrated in Fig. 1. The data on morphological, anatomical, colorimetric and taxonomic charateristics recorded during the identification for fourteen lichen species have been depicted in Table 1 and Table 2. Wide variations in the parameters were observed among the investigated lichen species. Three lichen species additions viz. Lobothallia alphoplaca, Rhizoplaca melanophthalma and Xanthoparmelia mexicana were reported to the area near the northward slope of hill of Indus valley for the first time as per our previously reported work [35].
Figure 1. Thallus of lichen species studied in the present investigation.
a: Dermatocarpon vellereum; b: Umbilicaria vellea; c: Rhizoplaca chrysoleuca; d: Rhizoplaca melanophthalma; e: Pleopsidium flavum; f: Xanthoparmelia mexicana; g: Acarospora badiofusca; h: Xanthoria elegans; i: Lecanora frustulosa; j: Lobothallia alphoplaca; k: Physconia muscigena; l: Melanelia disjuncta; m: Xanthoparmelia stenophylla; n: Peccania coralloides.
Table 1. Taxonomic description of lichen species.
| Dermatocarpon vellereum Zschacke (Family: Verrucariaceae) |
| Thallus saxicolous, foliose, umbilicate, monophyllous, leathery, upper side light brownish to brownish red, white to dark pruinose, lower side black, with dense, thick, stumpy, coralloid rhizinomorphs. Perithecia pale red; ascospores ellipsoid, 9-12×(5) 6–9 µm. |
| Umbilicaria vellea (L.) Ach. em. Frey (Family: Umbilicariaceae) |
| Thallus saxicolous, monophyllous, umbilicate, grey to blackish grey, smooth. Areolate, pruinose, lower side black, rhizomorphs dimorphic. Apothecia black, gyrodiscus, ascospores simple, colourless 8.5–13×6.8–10 µm. |
| Rhizoplaca chrysoleuca (Sm.) Zopf (Family: Lecanoraceae) |
| Thallus saxicolous, foliose, umbilicate, monophyllous or polyphyllous, monophyllous upto 3 cm across, polyphyllous with thick lobes united by a stalk at centre; lower side brown at centre, bluish black in outer part. Apothecia to 5 mm in diameter, disc orange- red to red, pruinose; ascospores 8.5–12×3.5–6 µm. Medulla K−, Pd+ yellowish. Placodialic acid was present. |
| Rhizoplaca melanophthalma (DC) Leuck. & Poelt (Family: Lecanoraceae) |
| Thallus saxicolous, peltate, monophyllous, up to 3 cm across, lobes round to crenate, lower side reddish brown. Apothecia to 3 mm in diameter, sessile, disc bluish brown to black, spores 9–11.5×5–5.5. Medulla P+ yellow, Placodialic and rarely psoromic acid were present. |
| Pleopsidium flavum (Bell.) Korb. (Family: Acarosporaceae) |
| Thallus saxicolous, marginally lobate, yellow, effigurate, ± areolate, marginal lobes of thallus rough, subconvex, scabrid and 1.5–2 mm long. Apothecia plane, solitary, to 0.1–1 mm in diameter, immersed in areolae, disc plane brown, margin thick, persistent, spores simple, hyaline, ellipsoid, 4–5×1.7–2 µm. |
| Xanthoparmelia mexicana (Gyeln.) Hale (Family: Parmeliaceae) |
| Thallus saxicolous, foliose; lobes 1.5–3 mm wide, black rimmed; upper side yellow- green, isidiate; isidia subglobose to cylindrical, simple to coralloid branched, black tipped; lower side brownish, rhizinate; medulla white. |
| Acarospora badiofusca (Nyl.) Th. Fr. (Family: Acarosporaceae) |
| Thallus wide spreading, areolate, areoles rather small, 0.5–1.5 mm wide, ± rounded, numerous, at times wavy, irregular, rarely imbricate, flat or ± convex, reddish brown, algal layer continuous. Apothecia 0.4–2 mm diameter, mostly single, rarely 2 to 4 contiguous per areoles, rounded or gyrose-contorted, sessile, thalline exiple usually distinct, ± elevated, entire, concolorous with disc, hymenium 60–75(−90) µm tall, disc flat-convex, oftened roughened, usually red brown to brown black, always darker than thallus, paraphyses 2.5–3 µm wide at base, 4–5 µm at tips, asci 200 spored, spores 3–6×1.5–2.5 µm, ellipsoid. |
| Xanthoria elegans (Links.) Th. Fr. (Family: Teloschistaceae) |
| Thallus saxicolous, foliose, suborbicular, lobes radiating, compact, convex, nodulose with densely crowded apothecia in central part, upper side orange-red to reddish brown, lower side grey, medulla white, ± hollow. Apothecia up to 1 mm in diammeter, spores 12–16 (−18)×6–8 (−10) µm with 4–5 µm thick transverse septum. Upper surface K+ purple, Pd−, C−, I−. Parietin, fallacinal, emodin, teloschistin, parietinic acid found to be present. Chemosyndrome A was also detected. |
| Lecanora frustulosa (Dick.) Ach. (Family: Lecanoracea) |
| Thallus placodioid, closely adpressed, greenish yellow to yellow brown, margin pale or whitish above, 0.3–0.7 mm wide,1.0–3.8 mm long lower surface pale brown to black. Apothecia sessile, densely aggregate in centre of the thallus, 0.4–1.8 mm diameter, spores 8 per ascus, ellipsoidal, 9.0–16.0×4.5–7.0 um. Thallus and apothecial margin K−, C−, KC+ yellowish, PD−. Usnic acid and zeorin was reported in the species. |
| Lobothallia alphoplaca (Wahlenb. ex Ach.) Hafellner (Family: Megasporaceae) |
| Thallus saxicolous, crustose, whitish- grey, verrucose areolate centrally, radiating laciniae marginally, hollow. Apothecia rounded, 0.5–2.0 (−2.5) mm diameter, disc plane to slightly convex, dark brown to brown black, margin whitish, smooth, ascospores 8 per ascus, 8–12×4–8 µm. Thallus K+ yellow then red, Pd+ yellow-orange, C−, KC−. Norstictic and salazininc acids were detected. |
| Physconia muscigena (Ach.) Poelt. (Family: Physciaceae) |
| Thallus tericolous or muscicolous, to 10 cm across, lobes 1–1.5 (apically 3) mm wide; upper side brownish, lacking isidia or soredia; lower side black; rihizines squarrosely branched. Apothecia to 5 mm in diameter, ascospores 23–32 (−35)×12–16 µm. |
| Melanelia disjuncta Essl. (Family: Parmeliacea) |
| Thallus appressed, lobes flat to slightly convex or concave, contiguous, upper surface usually dark olive brown, dark brown or blackish, pseudocyphellae small, submarginal, sorediate, lower surface, moderately rhizinate. Apothecia infrequent, sessile, margin sorediate, rugose, pseudocyphellate, spores ellipsoid, 9–12.5×5–7 µm. Perlatolic and stenosporic acid were present. |
| Xanthoparmelia stenophylla (Ach.) Ahti & Hawksw. (Family: Parmeliaceae) |
| Thallus saxicolous, foliose, pulvinate, lobes sublinear, 1.2–5 mm wide, brownish at apices, secondary lobules developing at centre, often erhizinate, upper side yellow-green, lacking isidia and soredia, lower side brownish, rhizinate. |
| Peccania coralloides (Massal.) Massal. (Family: Lichinaceae) |
| Thallus fruticose, dry black, lobes erect surface smooth, cylindrical, central hyphal strand compact, photobiont a cyanobacterium. Apothecia terminal, emerging long, 0.5–1 mm in diameter, urceolate, disc concave to flat, black or dark brown, hymenium tainted, 100–120 µm high, IKI (+) blue, asci cylindrical clavate, 8 spored, from 50–70×15–22 µm, spores spherical or ellipsoidal, hyaline, 8–15×6–10 µm. |
Table 2. Morpho-anatomical measurements and colorimetric characteristics of identified lichens.
| Lichen species | Thallus size (cm) | Lobe width (mm) | Thallus upper surface color | Apothecia/Perithecia (mm) | Ascospore size (µm) |
| Dermatocarpon vellereum | 5.5 | 55 | Light brown | 0.3 | 10.5×7.5 |
| Umbilicaria vellea | 3.5 | 35 | Grey | - | - |
| Rhizoplaca chrysoleuca | 3 | 30 | Yellowish green | 4 | 10.2×4.7 |
| Rhizoplaca melanophthalma | 2.7 | 27 | Yellowish to brownish green | 2.5 | 10.1×5.2 |
| Pleopsidium flavum | 4.5 | 0.8 | Bright yellow | 1 | 4.2×1.8 |
| Xanthoparmelia mexicana | 3.9 | 3 | Light yellow green | - | - |
| Acarospora badiofusca | 4.5 | 1 | Reddish brown | 0.9 | 4.5×2 |
| Xanthoria elegans | 6 | 0.9 | Orange-red | 1 | 14.5×8.2 |
| Lecanora frustulosa | 4.8 | 0.5 | Greenish yellow | 1.2 | 12.5×5.8 |
| Lobothallia alphoplaca | 8.2 | 1.2 | Ashy grey | 1.8 | 10.5×7.2 |
| Physconia muscigena | 7.8 | 1.2 | Brownish white | 3.6 | 27.5×14.1 |
| Melanelia disjuncta | 2.9 | 1.5 | Dark brown | 1.8 | 10.5×5.9 |
| Xanthoparmelia stenophylla | 10.6 | 3.5 | Yellowish green | 4.6 | 8.8×5.9 |
| Peccania coralloides | 2.5 | 1.4 | Black | 0.7 | 11.5×8.5 |
Antioxidant capacities
Ferric reducing antioxidant power (FRAP)
The capacity of the lichen extracts to reduce ferric ions was evaluated by performing FRAP assay. In the present study, the extraction of antioxidants was done sequentially with n-hexane, methanol and water. All the lichen extracts exhibited high FRAP antioxidant capacity (Table 3). However, the rate of FRAP activity was found to vary with the lichen species and the extracting solvent. It was observed that the water extracts retained moderate FRAP values, while methanol extracts retained maximum FRAP and the n-hexane extracts retained least amount of FRAP for all lichen species. The methanol extracts of all lichens were found to possess significantly higher (p<0.05) FRAP values in comparison with the water and n-hexane extracts. Among all extracts, methanol extract of L. alphoplaca exhibited highest FRAP value (1673.32±94.83 µM Fe (II)/g extract). The maximum FRAP values in n-hexane and water extracts were observed in X. elegans (136.55±12.91 µM Fe (II)/g extract) and P. coralloides (335.19±12.92 µM Fe (II)/g extract), respectively. The positive standards viz., ascorbic acid (9851.60±752.15 µM Fe (II)/g extract) and BHT (3160.14±251.72 µM Fe (II)/g extract) were found to exhibit significantly higher FRAP values than the lichen extracts (Table 3).
Table 3. Ferric reducing antioxidant power (FRAP) of high altitude cold desert saxicolous lichensa.
| Lichen species | µM Fe (II)/g extract | ||
| Water | Methanol | n-Hexane | |
| Dermatocarpon vellereum | 178.15±8.83 | 1222.30±82.24* | 89.45±7.81*# |
| Umbilicaria vellea | 42.27±2.87 | 603.81±62.14* | 61.43±5.88*# |
| Rhizoplaca chrysoleuca | 95.57±6.02 | 1112.16±74.21* | 108.87±10.56# |
| Rhizoplaca melanophthalma | 133.31±8.62 | 648.08±67.82* | 33.66±4.81*# |
| Pleopsidium flavum | 70.58±4.86 | 618.56±59.98* | 96.93±9.14*# |
| Xanthoparmelia mexicana | 77.49±5.23 | 1479.63±85.12* | 115.23±10.23*# |
| Acarospora badiofusca | 123.02±8.26 | 1394.79±92.61* | 114.16±12.66# |
| Xanthoria elegans | 105.34±7.91 | 191.86±18.01* | 136.55±12.91*# |
| Lecanora frustulosa | 76.32±4.63 | 338.68±34.81* | 74.02±6.86# |
| Lobothallia alphoplaca | 47.25±2.39 | 1673.32±94.83* | 52.40±6.11*# |
| Physconia muscigena | 72.85±5.04 | 740.90±67.86* | 127.64±10.86*# |
| Melanelia disjuncta | 61.76±4.49 | 395.33±44.21* | 98.12±8.68*# |
| Xanthoparmelia stenophylla | 133.65±7.31 | 646.72±67.11* | 37.38±4.45*# |
| Peccania coralloides | 335.19±12.92 | 1353.46±84.18* | 109.82±11.66*# |
| Ascorbic acid | 9851.60±752.15 | ||
| BHTb | 3160.14±251.72 | ||
Mean ±SD of three replicates;
Butylated hydroxytoluene.
p<0.05: *compared with water extract; #compared with methanol extract.
ABTS radical scavenging capacity
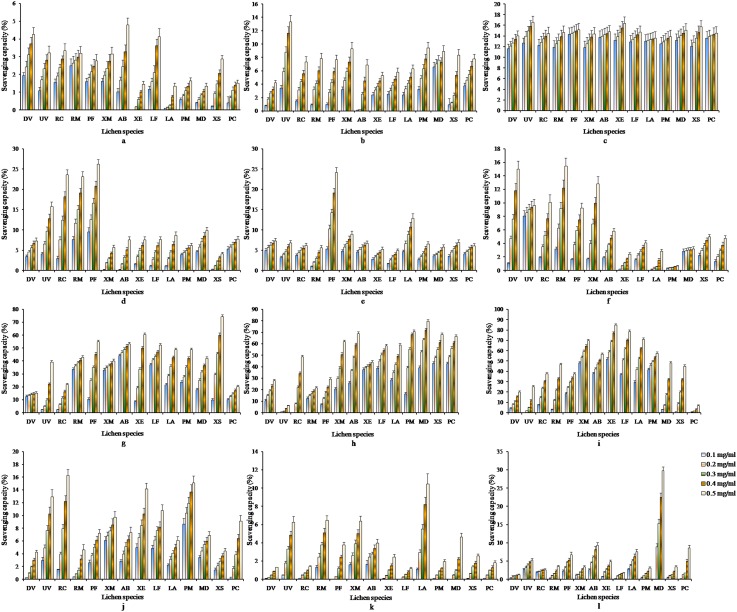
The ABTS radical scavenging capacity (%) of the fourteen lichen species in different solvent extracts has been depicted in Fig. 2. The extracts scavenged the ABTS radical in a dose dependent manner at concentration of 0.1–0.5 mg/ml. The positive controls viz. quercetin and ascorbic acid at concentration of 0.1–0.5 mg/ml were also found to produce dose dependent inhibition of ABTS radical. The IC50 or RSa50 values of different solvent extracts and positive controls viz. quercetin and ascorbic acid were calculated in the present study and have been depicted in Table 4. The methanol extract of X. stenophylla showed the highest ABTS radical scavenging capacity (IC50 1.88±0.09 mg/ml). The maximum ABTS radical scavenging capacity in n-hexane and water extracts was rendered by A. badiofusca (IC50 6.55±0.10 mg/ml) and U. vellea (IC50 3.45±0.11 mg/ml). ABTS radical scavenging capacity of methanol extracts was significantly higher (p<0.05) in comparison with water and n-hexane extracts for all the lichen species. Although X. elegans water extract (IC50 5.08±0.10 mg/ml) showed significantly higher (p<0.05) radical scavenging capacity than the methanol and n-hexane extracts. The n-hexane extract of most of the lichen species under investigation exhibited significantly lower (p<0.05) antioxidant capacity when compared with the methanol and water extracts. However, the n-hexane extracts of A. badiofusca (IC50 6.55±0.10 mg/ml), L. alphoplaca (IC50 9.52±0.13 mg/ml) and P. coralloides (IC50 14.84±0.09 mg/ml) showed significantly higher (p<0.05) antioxidant capacity in comparison with the corresponding water extracts. Quercetin (IC50 0.33±0.03 mg/ml) and ascorbic acid (IC50 0.18±0.01 mg/ml) were found to produce significantly higher (p<0.05) radical scavenging potential compared to the lichen extracts (Table 4).
Figure 2. Effect of lichen extracts on various radical-scavenging capacities.
a: ABTS radical scavenging capacity of n-hexane extracts; b: ABTS radical scavenging capacity of methanol extracts; c: ABTS radical scavenging capacity of water extracts; d: DPPH radical scavenging capacity of n-hexane extracts; e: DPPH radical scavenging capacity of methanol extracts; f: DPPH radical scavenging capacity of water extracts; g: Scavenging effect of n-hexane extracts on β-carotene-linoleic acid bleaching assay; h: Scavenging effect of methanol extracts on β-carotene-linoleic acid bleaching assay; i: Scavenging effect of water extracts on β-carotene-linoleic acid bleaching assay; j: Nitric oxide radical scavenging capacity of n-hexane extracts; k: Nitric oxide radical scavenging capacity of methanol extracts; l: Nitric oxide radical scavenging capacity of water extracts. DV: Dermatocarpon vellereum; UV: Umbilicaria vellea; RC: Rhizoplaca chrysoleuca; RM: Rhizoplaca melanophthalma; PF: Pleopsidium flavum; XM: Xanthoparmelia mexicana; AB: Acarospora badiofusca; XE: Xanthoria elegans; LF: Lecanora frustulosa; LA: Lobothallia alphoplaca; PM: Physconia muscigena; MD: Melanelia disjuncta; XS: Xanthoparmelia stenophylla; PC: Peccania coralloides.
Table 4. Scavenging effect of high altitude cold desert saxicolous lichens on ABTS and DPPH radicalsa.
| Lichen species | ABTS (IC50/RSa50), mg/ml | DPPH (IC50/RSa50), mg/ml | ||||
| Water | Methanol | n-Hexane | Water | Methanol | n-Hexane | |
| Dermatocarpon vellereum | 6.39±0.10 | 6.24±0.10 | 8.12±0.10*# | 1.59±0.11 | 7.85±0.10* | 1.24±0.10*# |
| Umbilicaria vellea | 3.45±0.11 | 1.92±0.11* | 9.31±0.09*# | 10.33±0.09 | 5.25±0.10* | 1.23±0.10*# |
| Rhizoplaca chrysoleuca | 7.61±0.10 | 3.45±0.09* | 10.67±0.15*# | 2.44±0.11 | 8.48±0.10* | 1.02±0.02*# |
| Rhizoplaca melanophthalma | 5.34±0.11 | 2.99±0.10* | 26.50±0.10*# | 1.65±0.09 | 3.91±0.02* | 1.74±0.09# |
| Pleopsidium flavum | 8.74±0.10 | 3.08±0.11* | 16.47±0.09*# | 2.54±0.10 | 1.09±0.01* | 5.09±0.02*# |
| Xanthoparmelia mexicana | 6.75±0.09 | 3.12±0.10* | 12.52±0.10*# | 1.97±0.08 | 4.58±0.11* | 4.15±0.10*# |
| Acarospora badiofusca | 14.60±0.10 | 2.45±0.09* | 6.55±0.10*# | 5.07±0.04 | 8.21±0.10j* | 2.48±0.10*# |
| Xanthoria elegans | 5.08±0.10 | 7.09±0.10* | 11.78±0.10*# | 7.71±0.11 | 8.10±0.10* | 3.37±0.10*# |
| Lecanora frustulosa | 8.72±0.10 | 5.71±0.11* | 10.49±0.10*# | 7.90±0.09 | 6.61±0.10* | 3.04±0.03*# |
| Lobothallia alphoplaca | 26.47±0.10 | 5.02±0.10* | 9.52±0.13*# | 4.13±0.11 | 2.29±0.01* | 2.87±0.09*# |
| Physconia muscigena | 11.10±0.11 | 3.15±0.08* | 19.11±0.10*# | 62.18±0.12 | 5.59±0.10* | 8.62±0.10*# |
| Melanelia disjuncta | 8.45±0.08 | 7.58±0.08* | 21.67±0.10*# | 67.44±0.10 | 8.40±0.10* | 3.65±0.10*# |
| Xanthoparmelia stenophylla | 4.42±0.10 | 1.88±0.09* | 7.99±0.09*# | 7.11±0.09 | 5.38±0.11* | 5.08±0.02*# |
| Peccania coralloides | 15.91±0.10 | 4.87±0.10* | 14.28±0.09*# | 5.95±0.05 | 10.25±0.09* | 8.53±0.12*# |
| Quercetin | 0.33±0.03$ | 0.08±0.02$ | ||||
| Ascorbic acid | 0.18±0.01$ | 0.05±0.01$ | ||||
Mean ±SD of three replicates
p<0.05: *compared with water extract; #compared with methanol extract; $compared with all lichen extracts.
DPPH radical scavenging capacity
The free radical scavenging capacity of the fourteen lichen species in different extracts and the two positive controls viz. quercetin and ascorbic acid were compared through their ability to scavenge DPPH radical. The DPPH radical scavenging capacity of the lichen extracts and the positive controls increased in a dose dependent manner at concentration of 0.1–0.5 mg/ml. The half maximal inhibitory/scavenging concentration (IC50 or RSa50) values were determined and have been depicted in Table 4. Lower value of IC50 indicates higher DPPH radical scavenging capacity. The rate of DPPH radical scavenging capacity was found to depend on lichen species and extracting solvent. For most of the lichen species, DPPH radical scavenging capacity of n-hexane extract was significantly higher (p<0.05) in comparison with methanol and water extract. The radical scavenging capacity of n-hexane extracts of D. vellereum (IC50 1.24±0.10 mg/ml), U. vellea (IC50 1.23±0.10 mg/ml), R. chrysoleuca (IC50 1.02±0.02 mg/ml), A. badiofusca (IC50 2.48±0.10 mg/ml), X. elegans (IC50 3.37±0.10 mg/ml), L. frustulosa (IC50 3.04±0.03 mg/ml), M. disjuncta (IC50 3.65±0.10 mg/ml) and X. stenophylla (IC50 5.08±0.02 mg/ml) increased significantly (p<0.05) when compared with the methanol and water extracts. However, the water extract of R. melanophthalma (IC50 1.65±0.09 mg/ml), X. mexicana (IC50 1.97±0.08 mg/ml) and P. coralloides (IC50 5.95±0.05 mg/ml) showed significant increase (p<0.05) in radical scavenging ability than n-hexane and methanol extract. In addition, the methanol extract of L. alphoplaca (IC50 2.29±0.01 mg/ml), P. muscigena (IC50 5.59±0.10 mg/ml) and P. flavum (IC50 1.09±0.01 mg/ml) were found to produce significantly higher (p<0.05) radical scavenging capacity when compared with the corresponding n-hexane and water extracts. The n-hexane extract of R. chrysoleuca exhibited the highest radical scavenging capacity (IC50 1.02±0.02 mg/ml) among all lichen extracts under our study. The maximum DPPH radical scavenging capacity in methanol and water extracts was retained by P. flavum (IC50 1.09±0.01 mg/ml) and D. vellereum (IC50 1.59±0.11 mg/ml), respectively. Quercetin (IC50 0.08±0.02 mg/ml) and ascorbic acid (IC50 0.05±0.01 mg/ml) were found to produce significantly higher (p<0.05) DPPH radical scavenging effect compared to the lichen extracts (Table 4).
β-carotene-linoleic acid bleaching assay
The β-carotene-linoleic acid bleaching capacity of different solvent extracts of fourteen lichen species was expressed as scavenging capacity (%). All the lichen extracts and positive control BHT showed β-carotene-linoleic bleaching activity in a dose dependent manner at concentration of 0.1-0.5 mg/ml and it was found to vary with extracting solvent and lichen species (Fig. 2). The IC50 or RSa50 values were determined for each lichen extract and BHT (Table 5). In the present study, the highest antioxidant capacity was observed in water extract of X. elegans (IC50 0.08±0.01 mg/ml). The highest antioxidant capacity for n-hexane and methanol extracts was determined in X. stenophylla (IC50 0.33±0.11 mg/ml) and M. disjuncta (IC50 0.18±0.08 mg/ml) respectively. The methanol and water extracts of majority of the lichen species showed significantly higher (p<0.05) antioxidant capacity in comparison with the n-hexane extract. The methanol extracts of D. vellereum (IC50 1.01±0.11 mg/ml), R. chrysoleuca (IC50 0.52±0.11 mg/ml), P. muscigena (IC50 0.22±0.08 mg/ml), M. disjuncta (IC50 0.18±0.08 mg/ml), P. coralloides (IC50 0.22±0.11 mg/ml), X. stenophylla (IC50 0.22±0.10 mg/ml) and A. badiofusca (IC50 0.32±0.10 mg/ml) were found to produce significantly higher (p<0.05) antioxidant capacity when compared with the corresponding n-hexane and water extracts. In addition, the water extracts of X. mexicana (IC50 0.14±0.08 mg/ml), L. frustulosa (IC50 0.20±0.10 mg/ml), L. alphoplaca (IC50 0.28±0.09 mg/ml), U. vellea (IC50 0.50±0.10 mg/ml), R. melanophthalma (IC50 0.53±0.10 mg/ml) and X. elegans (IC50 0.08±0.01 mg/ml) showed significantly higher (p<0.05) antioxidant capacity in comparison with the corresponding methanol and n-hexane extracts. However, P. flavum n-hexane extract (IC50 0.45±0.10 mg/ml) showed significantly higher (p<0.05) antioxidant capacity than the corresponding methanol and water extracts. BHT was used as positive control and it showed significantly higher (p<0.05) antioxidant capacity (IC50 0.04±0.01 mg/ml) in comparison with all lichen extracts (Table 5).
Table 5. Scavenging effect of high altitude cold desert saxicolous lichens on β-carotene-linoleic acid bleaching assay and nitric oxide radicalsa.
| Lichen species | β-carotene-linoleic acid bleaching assay (IC50/RSa50), mg/ml | Nitric oxide (IC50/RSa50), mg/ml | ||||
| Water | Methanol | n-Hexane | Water | Methanol | n-Hexane | |
| Dermatocarpon vellereum | 1.33±0.10 | 1.01±0.11* | 5.74±0.10*# | 18.56±0.10 | 14.06±0.10* | 6.29±0.10*# |
| Umbilicaria vellea | 0.50±0.10 | 2.41±0.11* | 0.57±0.08# | 8.11±0.11 | 3.55±0.09* | 2.11±0.11*# |
| Rhizoplaca chrysoleuca | 0.56±0.09 | 0.52±0.11 | 1.14±0.10*# | 28.33±0.09 | 17.91±0.11* | 1.39±0.11*# |
| Rhizoplaca melanophthalma | 0.53±0.10 | 1.85±0.09* | 0.76±0.10*# | 4.15±0.09 | 4.06±0.11 | 3.31±0.10*# |
| Pleopsidium flavum | 0.76±0.09 | 0.89±0.10 | 0.45±0.10*# | 3.98±0.09 | 4.37±0.07* | 4.12±0.11# |
| Xanthoparmelia mexicana | 0.14±0.08 | 0.38±0.05* | 1.12±0.10*# | 7.25±0.08 | 4.14±0.09* | 5.09±0.10*# |
| Acarospora badiofusca | 0.37±0.08 | 0.32±0.10 | 0.34±0.11 | 2.82±0.10 | 8.71±0.10* | 4.27±0.07*# |
| Xanthoria elegans | 0.08±0.01 | 0.88±0.10* | 0.42±0.10*# | 4.97±0.10 | 6.24±0.10* | 2.75±0.08*# |
| Lecanora frustulosa | 0.20±0.10 | 0.31±0.11 | 0.49±0.10* | 7.21±0.11 | 18.61±0.10* | 3.33±0.09*# |
| Lobothallia alphoplaca | 0.28±0.09 | 0.39±0.10 | 0.50±0.10* | 4.27±0.09 | 1.75±0.09* | 5.24±0.11*# |
| Physconia muscigena | 0.32±0.10 | 0.27±0.08 | 0.52±0.10# | 4.62±0.10 | 13.59±0.10* | 2.61±0.09*# |
| Melanelia disjuncta | 0.54±0.08 | 0.18±0.08* | 0.62±0.10# | 0.75±0.09 | 11.20±0.10* | 5.96±0.09*# |
| Xanthoparmelia stenophylla | 0.62±0.11 | 0.22±0.11* | 0.33±0.11* | 3.26±0.07 | 8.69±0.11* | 7.13±0.11*# |
| Peccania coralloides | 1.71±0.10 | 0.22±0.10* | 1.64±0.11# | 1.46±0.13 | 12.56±0.10* | 2.26±0.10*# |
| BHTb | 0.04±0.01$ | 0.15±0.04$ | ||||
Mean ±SD of three replicates; bButylated hydroxytoluene
p<0.05: *compared with water extract; #compared with methanol extract; $compared with all lichen extracts.
Nitric oxide scavenging assay
The scavenging capacity (%) of the different solvent extracts of fourteen lichen species against nitric oxide released by sodium nitroprusside was studied and the result has been depicted in Fig. 2. The percentage radical scavenging capacity of the extracts and the reference standard BHT against nitric oxide radical was increased in a dose dependent mode at concentration of 0.1–0.5 mg/ml. The IC50 or RSa50 values of lichen extracts and BHT were calculated and have been depicted in Table 5. M. disjuncta (IC50 0.75±0.09 mg/ml) water extract showed the highest nitric oxide radical scavenging capacity among all lichen extracts under investigation. For n-hexane, methanol and water extracts, maximum radical scavenging capacity was detected in R. chrysoleuca (IC50 1.39±0.11 mg/ml), L. alphoplaca (IC50 1.75±0.09 mg/ml) and M. disjuncta (IC50 0.75±0.09 mg/ml) respectively. Majority of the lichen species were found to produce significantly higher (p<0.05) nitric oxide radical scavenging capacity in their n-hexane and and water extracts in comparison with corresponding methanol extracts. The n-hexane extracts of D. vellereum (IC50 6.29±0.10 mg/ml), U. vellea (IC50 2.11±0.11 mg/ml), R. chrysoleuca (IC50 1.39±0.11 mg/ml), R. melanophthalma (IC50 3.31±0.10 mg/ml), X. elegans (IC50 2.75±0.08 mg/ml), L. frustulosa (IC50 3.33±0.09 mg/ml) and P. muscigena (IC50 2.61±0.09 mg/ml) were found to exhibit significantly higher (p<0.05) radical scavenging capacity in comparison with the corresponding water and methanol extracts. In addition, the water extracts of P. coralloides (IC50 1.46±0.13 mg/ml), X. stenophylla (IC50 3.26±0.07 mg/ml), M. disjuncta (IC50 0.75±0.09 mg/ml), A. badiofusca (IC50 2.82±0.10 mg/ml) and P. flavum (IC50 1.01±0.11 mg/ml) showed significantly increased (p<0.05) radical scavenging capacity in comparison with the corresponding n-hexane and methanol extracts. However, the methanol extracts of X. mexicana (IC50 4.14±0.09 mg/ml) and L. alphoplaca (IC50 1.75±0.09 mg/ml) were found to produce significantly higher (p<0.05) radical scavenging capacity in comparison with the corresponding n-hexane and water extracts. BHT showed significantly higher (p<0.05) nitric oxide radical scavenging capacity (IC50 0.15±0.04 mg/ml) in comparison with all lichen extracts (Table 5).
Phytochemical compositions
Total proanthocyanidin content (TPAC)
The TPAC of the different lichen extracts was expressed as mg CAE/100 g dry weight (dw) of extract. The methanol extracts of all lichen species were found to contain significantly higher (p<0.05) TPAC in comparison with the corresponding n-hexane and water extracts. Methanol extract of X. mexicana showed highest TPAC (4218.59±5.21 mg CAE/100 g) among all lichen extracts. The maximum TPAC in the n-hexane and water extracts was found in M. disjuncta (178.52±1.56 mg CAE/100 g) and P. coralloides (1071.82±0.64 mg CAE/100 g) respectively (Table 6).
Table 6. Total proanthocyanidins (TPAC), flavanoids (TFC), and phenolic contents (TPC) in high altitude cold desert saxicolous lichensa.
| Lichen species | TPAC (mg CAE/100 g dw) | TFC (mg QAE/100 g dw) | TPC (mg GAE/100 g dw) | ||||||
| Water | Methanol | n-Hexane | Water | Methanol | n-Hexane | Water | Methanol | n-Hexane | |
| Dermatocarpon vellereum | 181.04±23.03 | 1506.34±142.65* | 120.24±14.93*# | 104.82±9.44 | 296.11±26.35* | 77.00±6.82*# | 471.19±20.46 | 2423.12±71.61* | 158.32±7.18*# |
| Umbilicaria vellea | 191.24±30.22 | 1356.98±132.75* | 143.41±12.72*# | 43.15±6.26 | 812.07±77.60* | 80.20±9.89*# | 240.80±14.14 | 2812.92±78.56* | 151.69±8.87*# |
| Rhizoplaca chrysoleuca | 152.82±18.62 | 709.93±78.83* | 144.11±15.93*# | 74.74±8.59 | 532.01±61.51* | 138.03±12.43*# | 228.07±12.62 | 2786.48±79.82* | 255.7±10.58*# |
| Rhizoplaca melanophthalma | 138.87±15.68 | 1202.16±148.54* | 70.25±8.53*# | 84.54±9.70 | 647.38±68.48* | 62.68±8.54*# | 272.30±11.68 | 2148.85±66.12* | 96.37±6.55*# |
| Pleopsidium flavum | 146.88±16.62 | 521.57±46.25* | 135.49±16.09*# | 66.45±7.52 | 272.54±26.97* | 162.90±31.58*# | 184.35±12.50 | 2096.01±68.20* | 174.78±9.27*# |
| Xanthoparmelia Mexicana | 195.24±25.51 | 4218.59±454.21* | 151.01±16.93*# | 142.68±15.50 | 3607.56±319.18* | 154.70±14.71*# | 169.87±10.52 | 5439.93±61.45* | 226.31±11.40*# |
| Acarospora badiofusca | 184.25±24.67 | 1303.54±152.62* | 127.68±13.52*# | 113.02±12.83 | 663.63±71.33* | 122.74±10.40*# | 324.95±14.73 | 2816.60±88.59* | 171.91±10.56*# |
| Xanthoria elegans | 176.01±22.01 | 1228.56±124.09* | 162.53±14.38*# | 117.06±11.01 | 256.85±20.21* | 158.25±23.28*# | 241.78±12.08 | 1974.04±51.98* | 203.42±10.11*# |
| Lecanora frustulosa | 156.27±14.59 | 638.56±66.78* | 144.98±15.03*# | 63.28±8.70 | 357.52±30.73* | 202.19±30.97*# | 192.56±8.56 | 1422.16±46.76* | 212.31±11.43*# |
| Lobothallia alphoplaca | 121.64±15.28 | 1409.38±144.93* | 69.09±9.74*# | 33.49±4.18 | 522.38±61.43* | 64.18±5.91*# | 157.89±8.17 | 2284.40±63.81* | 81.62±6.82*# |
| Physconia muscigena | 135.25±12.60 | 1241.10±138.07* | 154.03±18.97*# | 58.32±7.57 | 363.15±40.23* | 150.28±15.12*# | 196.27±9.58 | 2496.12±69.56* | 196.36±12.86*# |
| Melanelia disjuncta | 167.47±20.40 | 844.53±83.43* | 178.52±24.56*# | 88.15±9.44 | 528.29±48.35* | 140.64±16.44*# | 261.22±11.39 | 2522.67±64.54* | 198.03±12.51*# |
| Xanthoparmelia stenophylla | 302.18±36.65 | 1521.79±158.46* | 64.85±8.57*# | 97.81±10.60 | 1456.46±131.17* | 95.98±11.57*# | 332.46±19.49 | 1891.28±38.04* | 162.78±10.60*# |
| Peccania coralloides | 509.82±64.64 | 1648.33±162.32* | 143.18±15.67*# | 122.97±14.54 | 1444.84±152.48* | 142.03±12.22*# | 604.07±21.67 | 2745.55±81.41* | 180.28±12.86*# |
Mean ±SD of three replicates
p<0.05: *compared with water extract; #compared with methanol extract.
Total flavonoid content (TFC)
The TFC was expressed as mg QAE/100 g dw of extract. In our present investigation, the methanol extracts of all lichen species were found to have significantly higher (p<0.05) TFC in comparison with the corresponding n-hexane and water extracts. The methanol extract of X. mexicana showed highest TFC (3607.56±4.18 mg QAE/100 g) among all lichen extracts. The maximum TFC in n-hexane and water extracts was determined in L. frustulosa (202.19±30.97 mg QAE/100 g) and X. mexicana (142.68±15.50, mg QAE/100 g) respectively (Table 6).
Total polyphenol content (TPC)
The TPC of the lichen extracts was expressed as mg GAE/100 g dw of extract. In our current investigation, the methanol extracts of all lichen species were found to have significantly higher (p<0.05) TPC in comparison with the corresponding n-hexane and water extracts. The methanol extract of X. mexicana showed highest TPC (5439.93±61.45 mg GAE/100 g) among all lichen extracts. The maximum TPC in n-hexane and water extracts was determined in R. chrysoleuca (255.70±10.58 mg GAE/100 g) and P. coralloides (604.07±21.67 mg GAE/100 g) respectively (Table 6).
Cytotoxic effects of lichen extracts
Depending on high antioxidant capacities, we have screened eight lichen extracts [LW1: water extract of D. vellereum, LW2: water extract of U. vellea, LW8: water extract of X. elegans, LW12: water extract of M. disjuncta, LM5: methanol extract of M. disjuncta, L10M: methanol extract of L. alphoplaca, L12M: methanol extract of M. disjuncta, L13M: methanol extract of X. stenophylla] out of the total fourty two lichen extracts to investigate the cytotoxicity and antiproliferative activities in cellular model.
HepG2 cells
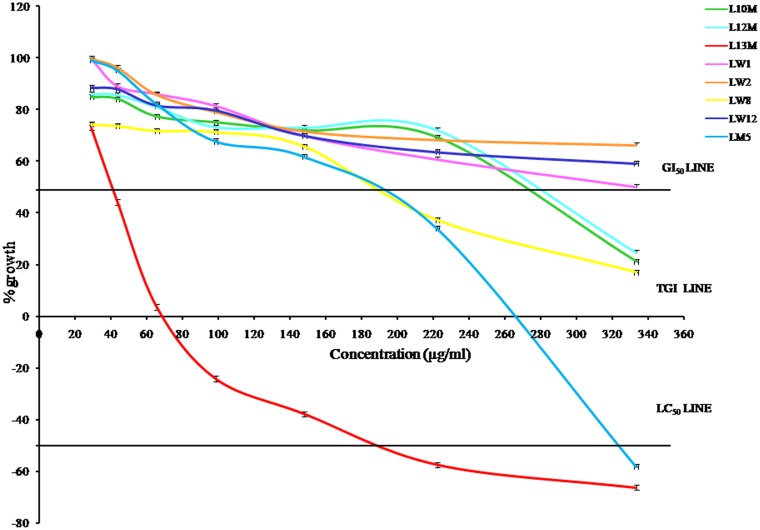
In HepG2 cells, extract LW2 and LW12 showed no toxicity as they remained far above from GI50. The % growth with treatment of LW2 and LW12 at highest concentration level 333.33 µg/ml was 66.22±3.88 and 59.171±4.33 respectively (Fig. 3). LW1 was found to be less toxic as it approached the GI50 at 333.33 µg/ml (% growth 49.967±3.81) and all concentrations were non-toxic below this level. L10M and L12M crossed the GI50 at 275 µg/ml (% growth 21.28±1.94) and 281 µg/ml (% growth 24.61±1.87) and were found to be toxic. LW1, L10M and L12M were non-toxic upto 222.22 µg/ml. LW8 was very toxic at 333.33 µg/ml where it approached almost near to GI90 and crossed GI50 at 189 µg/ml. L13M and LM5 were extremely toxic for HepG2 cells as they crossed the GI50 at 41 µg/ml and 191 µg/ml, TGI at 69 µg/ml and 266 µg/ml, and LC50 at 190 µg/ml and 324 µg/ml respectively. They showed −66.31±4.52% and −58.19±4.54% growth at 333.33 µg/ml. All these results have been depicted in Fig. 3.
Figure 3. Cytotoxic effect of lichen extracts on HepG2 cells.
LW1: water extract of D. vellereum, LW2: water extract of U. vellea, LW8: water extract of X. elegans, LW12: water extract of M. disjuncta, LM5: methanol extract of M. disjuncta, L10M: methanol extract of L. alphoplaca, L12M: methanol extract of M. disjuncta, L13M: methanol extract of X. stenophylla.
RKO cells
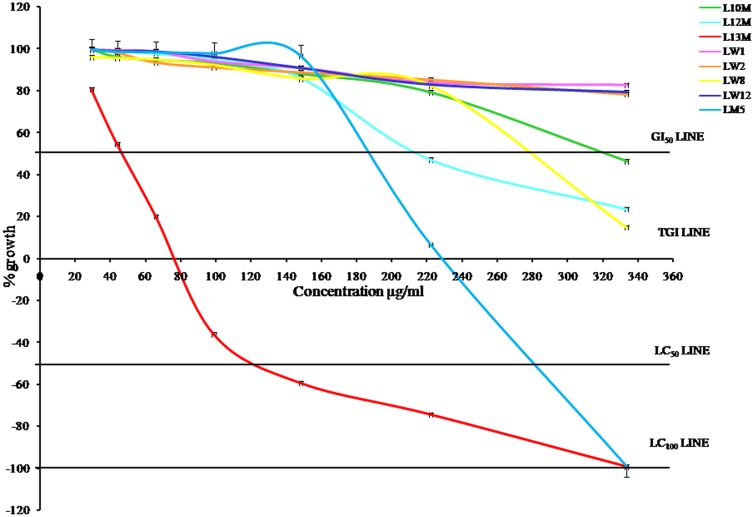
In case of RKO cells, LW1, LW2 and LW12 were non-toxic at all concentration levels. However, LW8 and L10M crossed GI50 at 289 and 330 µg/ml. The percent growth recorded with the treatment of LW8 and L10M extracts at highest concentration 333.33 µg/ml was 14.77±1.87 and 46.25±3.53 respectively. L12M was toxic as it crossed GI50 at 213 µg/ml, cells showed 23.58±2.15% growth at 333.33 µg/ml and it was non-toxic upto148.15 µg/ml concentration. LM5 and L13M were toxic as they approached the LC99 at 333.33 µg/ml and showed −99.21±0.88% and −99.28±0.38% growth respectively. LM5 crossed the LC50, TGI and GI50 at 282, 229 and 187 µg/ml respectively. L13M crossed the LC50, TGI and GI50 at 121, 76 and 46 µg/ml respectively. All these results have been depicted in Fig. 4.
Figure 4. Cytotoxic effect of lichen extracts on RKO cells.
LW1: water extract of D. vellereum, LW2: water extract of U. vellea, LW8: water extract of X. elegans, LW12: water extract of M. disjuncta, LM5: methanol extract of M. disjuncta, L10M: methanol extract of L. alphoplaca, L12M: methanol extract of M. disjuncta, L13M: methanol extract of X. stenophylla.
Morphology of treated cells by microscopic analysis
In morphological visualization after 24, 48 and 72 h, extract LW1, LW2 and LW12 were non toxic at all concentration levels whereas L13M was highly toxic as it showed toxicity up to 98.77 µg/ml for HepG2 cell line and up to 65.84 µg/ml for RKO cell line. Extract LW8, L5M and L12M were non toxic up to 222.22, 148.15 and 148.45 µg/ml, respectively, for both cell lines. L10M was found to be non toxic up to 222.22 µg/ml for HepG2 cell line and up to 148.15 µg/ml for RKO cell line. In HepG2 cell line, extract LM5 showed dead cells at 333.33 µg/ml after 24, 48 and 72 h; and 50% dead cells at 222.22 µg/ml after 24 h. L12M showed 50% cell death at 333.33 µg/ml after 24 and 48 h. L13M showed dead cells at 222.22 and 333.33 µg/ml after 24 h, at 98.77 to 333.33 µg/ml after 48 h and at 148.15 to 333.33 µg/ml after 72 h. In RKO cells, L13M showed dead cells at 222.22 and 333.33 µg/ml after 24 h, at 98.77 to 333.33 µg/ml after 48 h and at 148.15 to 222.22 µg/ml after 72 h. LM5 showed dead cells at 333.33 µg/ml after 48 and 72 h. All results related to the morphological analysis have been depicted in Table 7.
Table 7. Cytotoxicity of lichen extract examined by microscopic visualization (10X eyepiece and 10X objective lenses) to investigate cell density and cell health.
| HepG2 carcinoma cells | ||||||||||||||||||||||||||||
| S. No | Conc. (µg/ml) | C | LW1 | LW2 | LW8 | LW12 | LM5 | L10M | L12M | L13M | ||||||||||||||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||
| 1 | 333.33 | nt | nt | nt | nt | nt | nt | nt | nt | nt | gafp | nt | ga | nt | nt | nt | d | d | d | gafp | nt | ga | dfp | dfp | ga | d | d | d |
| 2 | 222.22 | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | dfp | nt | nt | nt | nt | nt | ga | nt | nt | d | d | d |
| 3 | 148.15 | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | ga | d | d |
| 4 | 98.77 | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | ga | dfp | ga |
| 5 | 65.84 | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt |
| 6 | 43.90 | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt |
| 7 | 29.26 | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt |
c: control, nt: non-toxic, ga: growth arrested, gafp: growth arrested 50%, dfp: death 50%, dsp: death 70%, dep: death 80%, d: dead.
Discussion
Lichens, one of the prominent life-form in the trans-Himalayan Ladakh region contain natural antioxidants which provide protection from the damage caused by the climatic conditions of high altitude environments and support their adaptability in extreme environmental conditions like extreme habitats (rocks in high altitude cold desert), low temperature, drought, prolonged winter, high solar irradiance and ultraviolet radiations. Numerous extracellular deposits in lichen thallus may contribute to the activation of antioxidant defence system in these conditions. In addition, diverse secondary metabolites are synthesized by lichens that are unique and found rarely in other plants [63]. In recent time, much attention has been paid on the biological roles of lichen secondary substances that have been found to have a lot of positive biological activities viz. antitumour, antibacterial, antifungal, antiviral, antiinflammatory and antioxidant capacities [64]. Therefore the secondary metabolites of high altitude cold desert lichens might be a potential resource of natural antioxidants and deserved to be tested for their antioxidant capacities.
The antioxidant capacities of plant extracts have been ascribed to various mechanisms of actions such as deterrence of chain initiation, radical scavenging activity, binding of transition metal ion catalysts, increasing endogenous status of antioxidant enzymes etc to prevent oxidative damage [65]. In order to understand the different mechanism of action of antioxidants, several antioxidant assays have been developed because the use of more than one assay would give a better insight into the true antioxidant potential of the test samples. Hence, it is important to use different assays for evaluating the antioxidant capacities of plant extracts that may have different mechanisms of antioxidant actions [66]. Various mechanisms of antioxidant action along with a range of methods of initiation, detection and measurement of oxidative processes in vitro and in vivo suggest probable explanations for structure-activity relationships. In general, the antioxidant capacity is known to be associated with the species, extraction solvent, method of extraction, temperature, conditions of the test systems, composition of the extract and hydrophobic or hydrophilic nature of the antioxidants. In our result, a wide range of antioxidant properties in different lichen extracts were observed that may be attributed to presence of diverse antioxidant compounds with different polarity in these lichen species. The bioactive antioxidants in the lichen extracts showed their diverse antioxidative effects in different antioxidant assays depending upon their individual redox reactions that may differ from each other by different magnitude of electrostatic interactions. In agreement with previous findings, the present study also revealed that the lichen extracts are the complex mixture of different compounds with distinct antioxidant capacities [67], [68]. In our result, different trends of antioxidant and radical scavenging capacities of different lichen extracts were observed which may be due to the presence of different types of compounds in these lichen species. The resulting antioxidant action depends on the individual redox reactions of bioactive compounds present in the lichens and for this reason it may differ from each other by different magnitude of molecular interactions. The antioxidant capacities of the studied lichen extracts were found to be dependent on species type and polarity of extracting solvent.
Previous reports on lichen diversity in the Antarctic regions revealed that lichens are well adapted for extreme climatic conditions and they can tolerate long periods of drought, severe cold and arid atmosphere. They have developed protective mechanisms to evade damage caused by high radiation and freezing temperatures [69]–[71] and all these adaptations have positive influence for their growth and survival in response to climate change and anthropogenic activities. There is dearth of information about the lichen species of the trans-Himalayan cold desert and it is therefore necessary to access the diversity, occurrence of potential bioactive compounds and antioxidant capacity of the trans-Himalayan lichens with considerable details. Lichens are the prominent life-form in the trans-Himalayan cold desert that produce diverse natural antioxidants having protective action against stressful environments of high altitude and also account for their adaptability in extreme climatic conditions. The numerous extracellular deposits in lichens may potentially contribute to the overall antioxidant protection [72]. The bioactive antioxidants produced by lichens also provide protection from ultra violet B (UV-B) radiation [70]. In Antarctica, the antioxidant activity in lichens is used as a measure of UV-B radiation that reaches the surface of the terrestrial environment. In the present study, we also found strong antioxidant capacity in the lichen extracts of trans-Himalayan high altitude that is in concordance with the previous reports on antioxidant content in Antarctic lichens [27], [70].
Researchers pay keen attention on the biological roles of lichen secondary substances that are reported to have biological activities such as antitumour, antibacterial, antifungal, antiviral, antiinflammatory and antioxidant activities [73], [74]. Interestingly, we observed higher antioxidant capacity in some lichen extracts in comparison with the antioxidant capacities of native plants of trans-Himalayan cold desert [48], [75], that signifies the importance of lichens as therapeutic agents. Therefore, the trans-Himalayan saxicolous lichens might be a potential resource of natural antioxidants for their high antioxidant capacities that may be attributed to the elevated content of diverse secondary metabolites.
Biologically active phytochemicals like polyphenols, flavonoids, flavonols, proanthocyanidins, alkaloids, terpenoids, carotenoids etc. are derived from plant sources and natural products which have promising health promoting properties and protective effects against chronic diseases while acting in combination [76]. Phenolics are the major secondary metabolites from plant sources that play crucial function in the regulation of plant growth and development in stressful and unfavourable climatic conditions. Lichens of the trans-Himalayan cold desert grow in extreme environmental conditions with severe cold, arid and water scarcity which in turn could cause up-regulated pathways of secondary metabolite synthesis and increased production of polyphenolics and other unique secondary metabolites that have protective effects against oxidative stress owing to their antioxidant capacities [34], [72], [77], [78].
It is well established that the antioxidant capacity of plant is associated with the phenolic compounds [79]. On the other hand, few studies revealed no correlation between antioxidant activity and TFC and TPC content of the extracts [80]–[83]. Therefore, it is important to investigate the effect of total phenolic content on the antioxidant capacity of the studied lichen extracts. Strong positive correlations were found among the antioxidant capacity assays (ABTS, DPPH, β-carotene-linoleic acid bleaching, nitric oxide, FRAP) and phenolic contents (TPAC, TFC, TPC) in all lichen extracts. Therefore, in agreement with previous reports, the results on correlations of different antioxidant assays and total phenolic content have demonstrated that the antioxidant capacity of the lichen extracts was positively associated with total phenolic content [33], [34], [78], [79], [84]. Thus, the present study highlights the antioxidant capacities rendered by the lichen extracts under oxidative stress.
In recent time a good number of research investigations were carried out to establish the positive biological properties of lichen extracts and their metabolites in different assays like antimicrobial [85]–[90], antioxidant [87]–[93], cytotoxic and antiproliferative [85], [86], [88], [94]–[100], antiviral [101]–[103], genotoxic and anticancer [91], [96], [97], antimycotic, antiinflammatory, analgesic, antipyretic, antiradiation, allelochemical, antiherbivore etc. [104]–[106]. Hence, lichens can be utilized as a natural bioresource for antioxidant and cytotoxic agent due to their promising biological activity and protective antistress function. At the same time, the in vitro antioxidant capacity assays obtained by the available chemical tests are not sufficient to prove the medicinal and therapeutic potential of these natural resources unless they are supported by the scientific data with the cytoprotective effects of these compounds in cell culture system. Therefore, the antioxidant capacity and cytotoxic effect of a given compound should be studied directly in a cellular model as it provides the optimal environment for possible interaction of the intracellular components with the compound of interest that would be missed in a chemical assay system. Moreover, we must be well aware of the safety of these lichen extracts because of the fact that many natural antioxidant may act as pro-oxidant based on the dose and on the ambient redox conditions [107], they may have different means for exerting their action, including interactions with intracellular signal transduction machineries and/or inducing the expression of antioxidant and detoxification enzymes [108] and could act as an anticancer agent [109]–[111]. In this milieu, we extend our present study towards the evaluation of cytotoxicity of eight lichen extracts out of the total fourty two extracts of fourteen lichen species those were taken for the initial screening based on antioxidant capacities and bioactive phytochemical constituents (the lichen extracts possessing high antioxidant capacities and potential phytocompounds were selected for cell line study) on human hepatocellular carcinoma HepG2 and colon carcinoma RKO cells.
In comparison to RKO cells, HepG2 cells were more susceptible to LW1, LW2, LW8 and LW12 and the RKO cells were mostly susceptible to the remaining extracts (Table 7, Fig. 3–5). LW1 was toxic to HepG2 cells as it approached GI50 at 333.33 µg/ml, whereas it was non-toxic to RKO cells as there was 82.68±3.78% growth at the same concentration. The 222.22 µg/ml concentration of LW8 was toxic for HepG2 cells as there was 37.24±3.55% growth at this level whereas RKO cells was non-toxic at the same concentration level with 82.2±4.21% growth. The lichen X. elegans (LW8) are edible as medicine [37]–[43] and from this result, it was apparent that extract LW8 with 222.22 µg/ml concentration level could be used as anticancer agent for HepG2 cells along with gamma radiation in order to reduce the dose of radiation to avoid damage to normal cells. Moreover, extract LM5 and L13M can be used for further trial in search of potent sensitizer depending upon their LC50 values against both types of carcinoma cells, whereas LW1, LW2, LW8 and LW12 can be used for further trial in search of potent radioprotector at the safe and non-toxic concentration levels considered under the present study. Hence, from the results of cytotoxic effects of lichen extracts in carcinoma cells we observed that the methanol extract of L. alphoplaca and M. disjuncta were exhibiting high cytotoxic effects against the cancer cell growth and these extracts hold immense potential for use as anticancer agents. However identification of potential bioactive compound needs to be done in different extracts. Similarly, the water extract of D. vellereum, U. vellea, X. elegans and M. disjuncta and the methanol extract of M. disjuncta and X. stenophylla showed high antioxidant capacities and were found to be non-toxic and may be used as natural antioxidants for stress related problems.
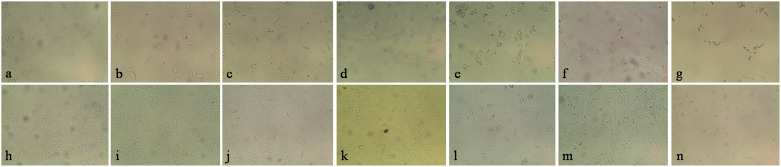
Figure 5. Microscopic images (10X eyepiece and 10X objective lenses) showing cell growth and morphology after 72 h treatment of lichen extracts in HepG2 and RKO carcinoma cells. a.
HepG2 cells with 90% growth, b. HepG2 cells with 70% arrested growth, c. HepG2 cells with 80% arrested growth, d. HepG2 cells with 99% arrested growth, e. HepG2 cells under stress, f. HepG2 cells under stress near death, g. HepG2 dead cells, h. RKO cells with 90% growth, i. RKO cells with 50% arrested growth, j. RKO cells with 70% arrested growth, k. RKO cells with 80% arrested growth, l. RKO cells with 99% arrested growth, m. RKO cells under stress, n. RKO dead cells.
The results of cytotoxicity assay revealed that the selected lichen extracts used in the present study exert cytotoxicity and antiproliferative action on HepG2 and RKO cell lines (Fig. 3–5, Table 7). The loss of viability of the dying cells as evidenced by the morphological changes was scrutinized by microscopy. Depending on the concentration, the lichen extracts exhibited different levels of cytotoxicity like cell shrinkage, aggregation and cell death (Fig. 5). In accordance with National Cancer Institute (NCI) guideline, the crude extract is considered active if the GI50 value <100 µg/ml [112], [113]. From our result in both cell lines, it was clear that GI50 of L13M was well below 100 µg/ml and that of LM5 was above 100 µg/ml, but at 333.33 µg/ml concentration both crude extracts showed almost equal percent of deaths of cells. All selected lichen extracts exhibited cytotoxic effect with increased cell death whereas reduction of cytotoxicity was observed with reduced extract concentration which finds support to the previous investigations [114].
Our results corroborate the previous investigations where cytotoxic and anticancer action of lichen extracts were explored in different lichen species from different parts of the world [91], [96], [98], [99], [106]. In agreement with our results, protective effects of U. vellea and X. elegans extracts against cellular damage by environmental stressors and survival potential of these lichens under extreme environmental condition was reported previously [100], [115]–[123]. However, the biological properties of other lichens remain to be investigated and to the best of our knowledge, this is the first study to explore the medicinal and therapeutic activities of trans-Himalayan lichen species.
Conclusions
In the present study, fourteen saxicolous lichens from trans-Himalayan cold desert of Ladakh have been identified with their morpho-anatomical and chemical compositions and the antioxidant and free radical scavenging capacities of these lichen extratcts were reported for the first time. This study revealed that these lichen species have broad spectrum free radical scavenging effect and high antioxidant capacity. Moreover, the antioxidant capacity was found to be associated with the species and the polarity of solvent used for extraction. Our studies clearly showed that the high altitude saxicolous lichens can be an interesting source of new antioxidative substrates with the potential to be used for scavenging various types of free radicals by different scavenging mechanisms. The lichen extracts were found to contain considerable amount of phenolic compounds which were responsible for their high antioxidant and free radical scavenging ability and could be used as natural source of antioxidants to ameliorate oxidative stress related disorders. Among all extracts, eight lichen extracts having high antioxidant capacity and phenolic compounds were further investigated for cytotoxic action on human carcinoma cell lines and these extracts were found to exhibit anticancer and radioprotective effects that would be of great interest for further chemical investigations and could lead to the discovery of novel cytotoxic anticancer molecules from lichens of the trans-Himalayan region. With these present primary findings, further studies are also required to ascertain the biological activities of the lichen extracts in animal models.
Acknowledgments
The authors express their sincere gratitude to Dr. Manas K. Mandal, Director General-Life Sciences, DRDO Headquarters for his wholehearted support. The authors acknowledge the contribution of Debasmita Ghosh Dhar, Maharaja Manindra Chandra College, Kolkata and Ritendra Mishra, DIHAR, DRDO who helped in copyediting and proofreading of the manuscript.
Funding Statement
The study was entirely supported by Defence Research & Development Organisation (DRDO), Ministry of Defence, Government of India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Halliwell B (1995) How to characterize an antioxidant: an update. Bioch Soc Sym 61: 85–91. [DOI] [PubMed] [Google Scholar]
- 2. Halliwell B (2005) The antioxidant paradox. Lancet 355: 1179–1180. [DOI] [PubMed] [Google Scholar]
- 3. Halliwell B, Gutteridge JM (1990) Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 186: 1–85. [DOI] [PubMed] [Google Scholar]
- 4. Gulcin I, Buyukokuroglu ME, Oktay M, Kufrevioglu IO (2003) Antioxidant and analgesic activities of turpentine of Pinus nigra Arn. Subsp. Pallsiana (Lamb.) Holmboe. J Ethnopharmacol 86: 51–58. [DOI] [PubMed] [Google Scholar]
- 5. Robinson FE, Maxwell SRJ, Thorpe GHG (1997) An investigation of the antioxidant activity of black tea using enhanced chemiluminescence. Free Radic Res 26: 291–302. [DOI] [PubMed] [Google Scholar]
- 6. Buyukokuroglu ME, Gulcin I, Oktay M, Kufrevioglu OI (2001) In vitro antioxidant properties of dantrolene sodium. Pharmacolog Res 44: 1199–2000. [DOI] [PubMed] [Google Scholar]
- 7. Halliwell B, Gutteridge JMC (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 219: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao G, Sofic E, Prior RL (1997) Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic Biol Med 22: 749–760. [DOI] [PubMed] [Google Scholar]
- 9. Arouma OI (1998) Free radicals, oxidative stress and antioxidants in human health and disease. J Am Oil Chem Soc 75: 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Souri E, Amin G, Farsam H, Jalalizadeh H, Barezi S (2008) Screening of thirteen medicinal plant extracts for antioxidant activity. Iran J Pharm Res 7: 149–154. [Google Scholar]
- 11. Zhang WM, Li B, Han L, Zhang HD (2009) Antioxidant activities of extracts from areca (Areca catectu L.) flower, husk and seed. Afr J Biotechnol 8: 3887–3892. [Google Scholar]
- 12. Cao G, Sofic ER, Prior RL (1996) Antioxidant capacity of tea and common vegetables. J Agric Food Chem 44: 3426–3431. [Google Scholar]
- 13. Cesquini M, Torsoni MA, Stoppa GR, Oqo SH (2003) t-BuOH-induced oxidative damage in sickle red blood cells and the role of flavonoids. Biomed Pharmacother 57: 124–129. [DOI] [PubMed] [Google Scholar]
- 14. Gulcin I, Kufrevioglu OI, Oktay M, Buyukokuroglu ME (2004) Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J Ethnopharmacol 90: 205–215. [DOI] [PubMed] [Google Scholar]
- 16.Hawksworth DL, Honegger R (1994) The lichen thallus: a symbiotic phenotype of nutritionally specialized fungi and its response to gall producers. In: Williams MAJ, editor.Plant Galls. The Systematics Association, Special Vol. 49 , Oxford: Clarendon Press. [Google Scholar]
- 17. Paudel B, Datta Bhattarai H, Prasad Pandey D, Seoun Hur J, Gyu Hong S, et al. (2012) Antioxidant, antibacterial activity and brine shrimp toxicity test of some mountainous lichens from Nepal. Biol Res 45: 387–391. [DOI] [PubMed] [Google Scholar]
- 18. Nash III TH, Egan RS (1988) The Biodiversity of Lichens and Bryophytes. Bibliotheca Lichenologica 30: 11–22. [Google Scholar]
- 19. Ahmadjian V (1995) Lichens are more important than you think. BioScience 45: 124. [Google Scholar]
- 20. Molnár K, Farkas E (2010) Current results on biological activities of lichen secondary metabolites: a review. Z Naturforsch C 65: 157–173. [DOI] [PubMed] [Google Scholar]
- 21. Mitrovic T, Stamenkovic S, Cvetkovic V, Nikoli M, Togie S, et al. (2011) Lichens as source of versatile bioactive compounds. Biologica Nyssana 2: 1–6. [Google Scholar]
- 22. Halama P, van Haluwin C (2004) Antifungal activity of lichen extracts and lichenic acids. BioControl 49: 95–107. [Google Scholar]
- 23. Ranković B, Mišić M, Sukdolak S (2007) Antimicrobial activity of the lichens Cladonia furcata, Parmelia caperata, Parmelia pertusa, Hypogymnia physodes and Umbilicaria polyphylla . Br J Biomed Sci 64: 143–148. [DOI] [PubMed] [Google Scholar]
- 24. Behera BC, Verma N, Sonone A, Makhija U (2006) Determination of antioxidative potential of lichen Usnea ghattensis in vitro. . LWT-Food Sci Technol 39: 80–85. [Google Scholar]
- 25. Gulluce M, Aslan A, Sokmen M, Sahin F, Adiguzel A, et al. (2006) Screening the antioxidant and antimicrobial properties of the lichens Parmelia saxatilis, Platismatia glauca, Ramalina pollinaria, Ramalina polymorpha and Umbilicaria nylanderiana . Phytomedicine 13: 515–521. [DOI] [PubMed] [Google Scholar]
- 26. Bhattarai HD, Paudel B, Hong SG, Lee HK, Yim JH (2008) Thin layer chromatography analysis of antioxidant constituents of lichens from Antarctica. J Nat Med 62: 481–484. [DOI] [PubMed] [Google Scholar]
- 27. Paudel B, Bhattarai HD, Lee JS, Hong SG, Shin HW, et al. (2008) Antioxidant activity of polar lichens from King George Island (Antarctica). Polar Biol 31: 605–608. [Google Scholar]
- 28. Luo H, Yamamoto Y, Kim JA, Jung JS, Koh YJ, et al. (2009) Lecanoric acid, a secondary lichen substance with antioxidant properties from Umbilicaria antarctica in maritime Antarctica (King George Island). Polar Biol 32: 1033–1040. [Google Scholar]
- 29. Kinoshita K, Togawa T, Hiraishi A, Nakajima Y, Koyama K, et al. (2010) Antioxidant activity of red pigments from the lichens Lethariella sernanderi, L. cashmeriana and L. sinensis . J Nat Med 64: 85–88. [DOI] [PubMed] [Google Scholar]
- 30. Ranković B, Ranković D, Kosanić M, Marić D (2010) Antioxidant and antimicrobial properties of the lichens Anaptychya ciliaris, Nephroma parile, Ochrolechia tartarea and Parmelia centrifuga. Cent. Eur J Biol 5: 649–655. [Google Scholar]
- 31. Negi HR (2000) On the patterns of abundance and diversity of macrolichens of Chopta-Tunganath in the Garhwal Himalaya. J Biosci 25: 367–378. [DOI] [PubMed] [Google Scholar]
- 32. Ballabh B, Chaurasia OP, Ahmed Z (2007) Herbal products from high altitude plants of Ladakh Himalaya. Curr Sci 92: 1664–1665. [Google Scholar]
- 33. Dhar P, Tayade AB, Bajpai PK, Sharma VK, Das SK, et al. (2012) Antioxidant capacities and total polyphenol contents of hydro-ethanolic extract of phytococktail from Trans-Himalaya. J Food Sci 77: 156–161. [DOI] [PubMed] [Google Scholar]
- 34. Dhar P, Bajpai PK, Tayade AB, Chaurasia OP, Srivastava RB, et al. (2013) Chemical composition and antioxidant capacities of phytococktail extracts from trans-Himalayan cold desert. BMC Complement Altern Med 13: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kumar J, Khare R, Rai H, Upreti DK, Tayade AB, et al. (2012) Diversity of lichens along altitudinal and land use gradients in the Trans Himalayan cold desert of Ladakh. Nature and Science 10: 1–9. [Google Scholar]
- 36. Negi HR, Upreti DK (2000) Species diversity and relative abundance of lichens in Rumbak catchment of Hemis National Park in Ladakh. Curr Sci 78: 1105–1112. [Google Scholar]
- 37. Ingólfsdóttir K, Kook Lee S, Bhat KP, Lee K, Chai HB, et al. (2000) Evaluation of selected lichens from Iceland for cancer chemopreventive and cytotoxic activity. Pharm Biol 38: 313–317. [DOI] [PubMed] [Google Scholar]
- 38. Karagoz A, Aslan A (2005) Antiviral and cytotoxic activity of some lichen extracts. Biologia 60: 281–286. [Google Scholar]
- 39.Richardson DHS (1991) Lichens and man. In: Frontiers in Mycology. Hawksworth DL, editor.Wallingford: CAB International. pp. 187–210.
- 40.Chopra RN, Chopra IC, Handa KL, Kapur LD (1958) Indigenous Drugs of India. Calcutta & New Delhi: Academic Publishers. pp. 643.
- 41.Richardson DHS (1988) Medicinal and other Economic aspects of lichens. In: CRC handbook of lichenology. Volume III. Galun M, editor.Boca Raton Florida: CRC press, UK. pp. 93–108.
- 42.Hu SY, Kong YC, But PPH (1980) An Enumeration of the Chinese Materia Medica. Hong Kong: The Chinese University Press. p. 101.
- 43. Gonzalez-Tejero MR, Martinez-Lirola MJ, Casares-Porcel M, Molero-Mesa J (1995) Three lichens used in popular medicine in eastern Andalucia (Spain). Econc Bot 49: 96–98. [Google Scholar]
- 44.Elix JE, Ernst-Russel KD (1993) A Catalogue of Standardized Thin Layer Chromatographic Data and Biosynthetic Relationships for Lichen Substances. 2nd ed. Australian National University, Canberra, Australia.
- 45.Orange A, James PW, White FJ (2001) Microchemical Methods for the Identification of Lichens. British Lichen Society, London, UK. p. 101.
- 46.Divakar PK, Upreti DK (2005) Parmelioid Lichens in India: A Revisionary Study. Bishen Singh Mahendra Pal Singh. Dehradun. p. 488.
- 47.Awasthi DD (2007) A compendium of the Macrolichens from India, Nepal and Sri Lanka. Bishen Singh Mahendra Pal Singh, Dehradun, India. p. 580
- 48. Korekar G, Stobdan T, Singh H, Chaurasia OP, Singh SB (2011) Phenolic content and antioxidant capacity of various solvent extracts from seabuckthorn (Hippophae rhamnoides L.) fruit pulp, seeds, leaves and stem bark. Acta Alimentaria 40: 449–458. [Google Scholar]
- 49. Ikram EHK, Eng KH, Jalil AMM, Ismail A, Idris S, et al. (2009) Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. J Food Composit Anal 22: 388–393. [Google Scholar]
- 50. Pellegrini N, Re R, Yang M, Rice-Evans CA (1999) Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2, 2′- azinobis (3-ethylbenzothiazolyne-6-sulfonic acid) radical cation decolorization assay. Methods Enzymol (Oxidants and Antioxidants Part A) 299: 379–389. [Google Scholar]
- 51. Teke GN, Lunga PK, Wabo HK, Kuiate JR, Vilarem G, et al. (2011) Antimicrobial and antioxidant properties of methanol extract, fractions and compounds from the stem bark of Entada abyssinica Stend ex A. Satabie. BMC Complement Altern Med 11: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT 28: 25–30. [Google Scholar]
- 53. Zhang D, Hamauzu Y (2004) Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chem 88: 503–509. [Google Scholar]
- 54. Dapkevicius A, Venskutonis R, van Beek TA, Linssen JPH (1998) Antioxidant activity of extracts obtained different isolation procedures from some aromatic herbs grown in Lithuania. J Sci Food Agric 77: 140–146. [Google Scholar]
- 55. Wang ZH, Hsu CC, Yin MC (2009) Antioxidative characteristics of aqueous and ethanol extracts of glossy privet fruit. Food Chem 112: 914–918. [Google Scholar]
- 56. Sun JS, Tsuang YW, Chen IJ, Huang WC, Hang YS, et al. (1998) An ultra-weak chemiluminescence study on oxidative stress in rabbits following acute thermal injury. Burns 24: 225–231. [DOI] [PubMed] [Google Scholar]
- 57. Ordonez AAL, Gomez JD, Vattuone MA, Isla MI (2006) Antioxidant activities of Sechium edule (Jacq.) Swart extracts. Food Chem 97: 452–458. [Google Scholar]
- 58. Gao X, Bjork L, Trajkovski V, Uggla M (2000) Evaluation of antioxidant activities of rosehip ethanol extracts in different test systems. J Sci Food Agric 80: 2021–2027. [Google Scholar]
- 59. Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, et al. (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82: 1107–1112. [DOI] [PubMed] [Google Scholar]
- 60. Rubinstein LV, Shoemaker RH, Paull KD, Simon RM, Tosini S, et al. (1990) Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst 82: 1113–1118. [DOI] [PubMed] [Google Scholar]
- 61. Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, et al. (1991) Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83: 757–766. [DOI] [PubMed] [Google Scholar]
- 62.Boyd MR, Paull KD, Rubinstein L (1992) Data display and analysis strategies for the NCI disease-oriented in vitro drug screen. In: Antitumor drug discovery and development. Valeriote FA, Corbett T, Baker L, editors.Amsterdam, The Netherlands: Kluwer Academic Publishers. pp. 11–34.
- 63. Stocker-Wörgötter E (2008) Metabolic diversity of lichen-forming ascomycetous fungi: culturing, polyketide and shikimate metabolite production, and PKS gene. Nat Prod Rep 25: 188–200. [DOI] [PubMed] [Google Scholar]
- 64. Oksanen I (2006) Ecological and biotechnological aspects of lichens. Appl Microbiol Biotechnol 73: 723–734. [DOI] [PubMed] [Google Scholar]
- 65. Zheng W, Wang SY (2001) Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 49: 5165–5170. [DOI] [PubMed] [Google Scholar]
- 66. Wong C, Li H, Cheng K, Chen F (2006) A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem 97: 705–711. [Google Scholar]
- 67. Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, et al. (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15: 127–130. [DOI] [PubMed] [Google Scholar]
- 68. Hou WC, Lin RD, Cheng KT, Hung YT, Cho CH, et al. (2003) Free radical scavenging activity of Taiwanese native plants. Phytomedicine 10: 170–175. [DOI] [PubMed] [Google Scholar]
- 69. Kappen L (2000) Some aspects of the great success of lichens in Antarctica. Antarctic Science 12: 314–324. [Google Scholar]
- 70. Robinson S, Wasley J, Tobin A (2003) Living on the Edge - plants and global change in continental and maritime Antarctica. Glob Change Biol 9: 1681–1717. [Google Scholar]
- 71. Bartak M, Vaczi P, Hajek J, Smykla J (2007) Low temperature limitation of primary photosynthetic processes in Antarctic lichens Umbilicaria antarctica and Xanthoria elegans . Polar Biol 31: 47–51. [Google Scholar]
- 72. Stocker-Wörgötter E (2008) Metabolic diversity of lichen-forming ascomycetous fungi: culturing, polyketide and shikimate metabolite production, and PKS genes. Nat Prod Rep 25: 188–200. [DOI] [PubMed] [Google Scholar]
- 73. Oksanen I (2006) Ecological and biotechnological aspects of lichens. Appl Microbiol Biotechnol 73: 723–734. [DOI] [PubMed] [Google Scholar]
- 74. Hidalgo ME, Quilhot FW, Lissi E (1994) Antioxidant activity of depsides and depsidones. Phytochem 37: 1585–1587. [DOI] [PubMed] [Google Scholar]
- 75. Korekar G, Stobdan T, Arora R, Yadav A, Singh SB (2011) Antioxidant capacity and phenolics content of apricot (Prunus armeniaca L.) kernel as a function of genotype. Plant Foods Hum Nutr 66: 376–383. [DOI] [PubMed] [Google Scholar]
- 76. Jeong WY, Jin JS, Cho YA, Lee JH, Park S, et al. (2011) Determination of polyphenols in three Capsicum annuum L. (bell pepper) varieties using high-performance liquid chromatography–tandem mass spectrometry: Their contribution to overall antioxidant and anticancer activity. J Sep Sci 34: 2967–2974. [DOI] [PubMed] [Google Scholar]
- 77. Chandrasekhar MJN, Praveen B, Nanjan MJ, Suresh B (2006) Chemoprotective effect of Phyllanthus maderaspatensis in modulating cisplatin-induced nephrotoxicity and enotoxicity. Pharm Biol 44: 100–106. [Google Scholar]
- 78. Tayade AB, Dhar P, Sharma M, Chauhan RS, Chaurasia OP, et al. (2013) Antioxidant capacities, phenolic contents, and GC/MS analysis of Rhodiola imbricata Edgew. root extracts from Trans-Himalaya. J Food Sci 78: 402–410. [DOI] [PubMed] [Google Scholar]
- 79. Velioglu YS, Mazza G, Gao L, Oomah BD (1998) Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J Agric Food Chem 46: 4113–4117. [Google Scholar]
- 80. Heinonen IM, Lehtonen PJ, Hopia AI (1998) Antioxidant activity of berry and fruit wines and liquors. J Agric Food Chem 46: 25–31. [DOI] [PubMed] [Google Scholar]
- 81. Anagnostopoulou MA, Kefalas P, Papageorgiou VP, Assimopoulou AN, Boskou D (2006) Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem 94: 19–25. [Google Scholar]
- 82. Nickavar B, Kamalinejad M, Izadpanah H (2007) In vitro free radical scavenging activity of five salvia species. Pak J Pharm Sci 20: 291–294. [PubMed] [Google Scholar]
- 83. Odabasoglu F, Aslan A, Cakir A, Suleyman H, Karagoz Y, et al. (2005) Antioxidant activity, reducing power and total phenolic content of some lichen species. Fitoterapia 76: 216–219. [DOI] [PubMed] [Google Scholar]
- 84. Hong Y, Lin S, Jiang Y, Ashraf M (2008) Variation in contents of total phenolics and flavonoids and antioxidant activities in the leaves of 11 Eriobotrya species. Plant Foods Hum Nutr 63: 200–204. [DOI] [PubMed] [Google Scholar]
- 85. Açikgöz B, Karalti I, Ersöz M, Coşkun ZM, Cobanoğlu G, et al. (2013) Screening of antimicrobial activity and cytotoxic effects of two Cladonia species. Z Naturforsch C 68: 191–197. [PubMed] [Google Scholar]
- 86. Manojlović N, Ranković B, Kosanić M, Vasiljević P, Stanojković T (2012) Chemical composition of three Parmelia lichens and antioxidant, antimicrobial and cytotoxic activities of some their major metabolites. Phytomedicine 19: 1166–1172. [DOI] [PubMed] [Google Scholar]
- 87. Kosanić M, Ranković B, Stanojković T (2012) Antioxidant, antimicrobial, and anticancer activity of 3 Umbilicaria species. J Food Sci 77: 20–25. [DOI] [PubMed] [Google Scholar]
- 88. Mitrović T, Stamenković S, Cvetković V, Tošić S, Stanković M, et al. (2011) Antioxidant, antimicrobial and antiproliferative activities of five lichen species. Int J Mol Sci 12: 5428–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ranković BR, Kosanić MM, Stanojković TP (2011) Antioxidant, antimicrobial and anticancer activity of the lichens Cladonia furcata, Lecanora atra and Lecanora muralis . BMC Complement Altern Med 11: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gulluce M, Aslan A, Sokmen M, Sahin F, Adiguzel A, et al. (2006) Screening the antioxidant and antimicrobial properties of the lichens Parmelia saxatilis, Platismatia glauca, Ramalina pollinaria, Ramalina polymorpha and Umbilicaria nylanderiana . Phytomedicine 13: 515–521. [DOI] [PubMed] [Google Scholar]
- 91. Kosanić M, Manojlović N, Janković S, Stanojković T, Ranković B (2013) Evernia prunastri and Pseudoevernia furfuraceae lichens and their major metabolites as antioxidant, antimicrobial and anticancer agents. Food Chem Toxicol 53: 112–118. [DOI] [PubMed] [Google Scholar]
- 92. Melo MG, dos Santos JP, Serafini MR, Caregnato FF, Pasquali MA, et al. (2011) Redox properties and cytoprotective actions of atranorin, a lichen secondary metabolite. Toxicol In Vitro 25: 462–468. [DOI] [PubMed] [Google Scholar]
- 93. Tanas S, Odabasoglu F, Halici Z, Cakir A, Aygun H, et al. (2010) Evaluation of anti-inflammatory and antioxidant activities of Peltigera rufescens lichen species in acute and chronic inflammation models. J Nat Med 64: 42–49. [DOI] [PubMed] [Google Scholar]
- 94.Ari F, Celikler S, Oran S, Balikci N, Ozturk S, et al. (2012) Genotoxic, cytotoxic, and apoptotic effects of Hypogymnia physodes (L.) Nyl. on breast cancer cells. Environ Toxicol doi:10.1002/tox.21809. [DOI] [PubMed]
- 95. Burlando B, Ranzato E, Volante A, Appendino G, Pollastro F, et al. (2009) Antiproliferative effects on tumour cells and promotion of keratinocyte wound healing by different lichen compounds. Planta Med 75: 607–613. [DOI] [PubMed] [Google Scholar]
- 96. Zeytinoglu H, Incesu Z, Tuylu BA, Turk AO, Barutca B (2008) Determination of genotoxic, antigenotoxic and cytotoxic potential of the extract from lichen Cetraria aculeata (Schreb.) Fr. in vitro . Phytother Res 22: 118–123. [DOI] [PubMed] [Google Scholar]
- 97.Grujičić D, Stošić I, Kosanić M, Stanojković T, Ranković B, et al. (2014) Evaluation of in vitro antioxidant, antimicrobial, genotoxic and anticancer activities of lichen Cetraria islandica. Cytotechnology [In Press]. [DOI] [PMC free article] [PubMed]
- 98. Bézivin C, Tomasi S, Lohézic-Le Dévéhat F, Boustie J (2003) Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 10: 499–503. [DOI] [PubMed] [Google Scholar]
- 99. Bézivin C, Tomasi S, Rouaud I, Delcros JG, Boustie J (2004) Cytotoxic activity of compounds from the lichen: Cladonia convoluta . Planta Med 70: 874–877. [DOI] [PubMed] [Google Scholar]
- 100. Ingólfsdóttir K, Kook Lee S, Bhat KP, Lee K, Chai HB, et al. (2000) Evaluation of selected lichens from iceland for cancer chemopreventive and cytotoxic activity. Pharm Biol 38: 313–317. [DOI] [PubMed] [Google Scholar]
- 101. Neamati N, Hong H, Mazumder A, Wang S, Sunder S, et al. (1997) Depsides and depsidones as inhibitors of HIV-1 integrase: discovery of novel inhibitors through 3D database searching. J Med Chem 40: 942–951. [DOI] [PubMed] [Google Scholar]
- 102. Esimone CO, Grunwald T, Wildner O, Nchinda G, Tippler B, et al. (2005) In vitro pharmacodynamic evaluation of antiviral medicinal plants using a vector-based assay technique. J Appl Microbiol 99: 1346–1355. [DOI] [PubMed] [Google Scholar]
- 103. Esimone CO, Ofokansi KC, Adikwu MU, Ibezim EC, Abonyi DO, et al. (2007) In vitro evaluation of the antiviral activity of extracts from the lichen Parmelia perlata (L.) Ach. against three RNA viruses. J Infect Dev Ctries 1: 315–320. [PubMed] [Google Scholar]
- 104. Molnár K, Farkas E (2010) Current results on biological activities of lichen secondary metabolites: a review. Z Naturforsch C 65: 157–173. [DOI] [PubMed] [Google Scholar]
- 105. Shukla V, Joshi GP, Rawat MSM (2010) Lichens as a potential natural source of bioactive compounds: a review. Phytochem Rev 9: 303–314. [Google Scholar]
- 106. Zambare VP, Christopher LP (2012) Biopharmaceutical potential of lichens. Pharm Biol 50: 778–798. [DOI] [PubMed] [Google Scholar]
- 107. Pasciu V, Posadino AM, Cossu A, Sanna B, Tadolini B, et al. (2010) Akt downregulation by flavin oxidase-induced ROS generation mediates dose-dependent endothelial cell damage elicited by natural antioxidants. Toxicol Sci 114: 101–112. [DOI] [PubMed] [Google Scholar]
- 108. Ullah MF, Ahmad A, Khan HY, Zubair H, Sarkar FH, et al. (2013) The prooxidant action of dietary antioxidants leading to cellular DNA breakage and anticancer effects: implications for chemotherapeutic action against cancer. Cell Biochem Biophys 67: 431–438. [DOI] [PubMed] [Google Scholar]
- 109. Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, et al. (2008) Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci USA 105: 11105–11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Weisburg JH, Schuck AG, Silverman MS, Ovits-Levy CG, Solodokin LJ, et al. (2010) Pomegranate extract, a prooxidant with antiproliferative and proapoptotic activities preferentially towards carcinoma cells. Anticancer Agents Med Chem 10: 634–644. [DOI] [PubMed] [Google Scholar]
- 111. Zhang H, Cao D, Cui W, Ji M, Qian X, et al. (2010) Molecular bases of thioredoxin and thioredoxin reductase-mediated prooxidant actions of (-)-epigallocatechin-3-gallate. Free Radic Biol Med 49: 2010–2018. [DOI] [PubMed] [Google Scholar]
- 112. Ashraf MF, Abd Aziz M, Stanslas J, Ismail I, Abdul Kadir M (2013) Assessment of antioxidant and cytotoxicity activities of saponin and crude extracts of Chlorophytum borivilianum . Scientific World Journal 2: 216894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.National Cancer Institute. http://www.cancer.gov Accessed May 29, 2014
- 114. Rao S, Suresh C (2013) In vitro and in vivo effects of the leaf extracts of Cassia tora and Cassia sophera in reducing the cytotoxicity and angiogenesis. British Biotechnology Journal 3: 377–389. [Google Scholar]
- 115. Aslan A, Agar G, Alpsoy L, Kotan E, Ceker S (2012) Protective role of methanol extracts of two lichens on oxidative and genotoxic damage caused by AFB1 in human lymphocytes in vitro. Toxicol Ind Health 28: 505–512. [DOI] [PubMed] [Google Scholar]
- 116. Gulluce M, Agar G, Aslan A, Karadayi M, Bozari S, et al. (2011) Protective effects of methanol extracts from Cladonia rangiformis and Umbilicaria vellea against known mutagens sodium azide and 9 aminoacridine. Toxicol Ind Health 27: 675–682. [DOI] [PubMed] [Google Scholar]
- 117. de Vera JP, Möhlmann D, Butina F, Lorek A, Wernecke R, et al. (2010) Survival potential and photosynthetic activity of lichens under Mars-like conditions: a laboratory study. Astrobiology 10: 215–227. [DOI] [PubMed] [Google Scholar]
- 118. Turkez H, Aydin E, Aslan A (2012) Xanthoria elegans (Link) (lichen) extract counteracts DNA damage and oxidative stress of mitomycin C in human lymphocytes. Cytotechnology 64: 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Meeβen J, Sánchez FJ, Brandt A, Balzer EM, de la Torre R, et al. (2013) Extremotolerance and resistance of lichens: comparative studies on five species used in astrobiological research I. Morphological and anatomical characteristics. Orig Life Evol Biosph 43: 283–303. [DOI] [PubMed] [Google Scholar]
- 120. Meeβen J, Sánchez FJ, Sadowsky A, de la Torre R, Ott S, et al. (2013) Extremotolerance and Resistance of Lichens: Comparative Studies on Five Species Used in Astrobiological Research II. Secondary Lichen Compounds. Orig Life Evol Biosph 43: 501–526. [DOI] [PubMed] [Google Scholar]
- 121. Aubert S, Juge C, Boisson AM, Gout E, Bligny R (2007) Metabolic processes sustaining the reviviscence of lichen Xanthoria elegans (Link) in high mountain environments. Planta 226: 1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bucar F, Schneider I, Ogmundsdóttir H, Ingólfsdóttir K (2004) Anti-proliferative lichen compounds with inhibitory activity on 12(S)-HETE production in human platelets. Phytomedicine 11: 602–606. [DOI] [PubMed] [Google Scholar]
- 123. Nybakken L, Solhaug KA, Bilger W, Gauslaa Y (2004) The lichens Xanthoria elegans and Cetraria islandica maintain a high protection against UV-B radiation in Arctic habitats. Oecologia 140: 211–216. [DOI] [PubMed] [Google Scholar]