Abstract
Men and women respond differently to the subjective effects of cocaine and cocaine-associated cues, which has implications for the development and maintenance of cocaine addiction. Preclinical studies performed in rats, modeling various aspects of cocaine addiction, have largely validated these results, indicating that female rats may be more sensitive to the rewarding properties of cocaine. The molecular mechanisms leading to sex differences in cocaine reward have largely not been determined, although sex hormones are thought to play a role. The mouse is commonly used as a model organism to study the molecular and genetic factors that influence a variety of psychiatric disorders. In particular, the inbred C57BL/6 mouse strain is often used for behavioral studies related to substance abuse. To begin to understand the hormonal, molecular and genetic mechanisms that might affect cocaine reward, we directly compared male and female C57BL/6J mice in cocaine conditioned place preference (CPP), a test that examines the rewarding and cue-associated properties of drugs of abuse. We conditioned mice at three doses of cocaine and examined preference and extinction of preference. We found that the acquisition of cocaine CPP did not differ between male and female mice. However, extinction of cocaine CPP was delayed in male mice compared to females at the lowest dose of cocaine. We conclude that sex differences in cocaine CPP can be observed in C57BL/6J mice at very low doses of cocaine.
Keywords: Conditioned place preference, cocaine, sex differences, C57BL/6, mice, reward
Introduction
A growing body of evidence indicates that there are differences between men and women in the vulnerability to drug abuse. Men report higher rates of recreational drug use and dependence compared to women. However, the percentage of female drug users is on the rise and cocaine abuse poses an increasing risk to the health of women (reviewed in [1]). Women initiate cocaine use earlier than men, are younger when they first enter treatment [2], and abstain from cocaine use for shorter periods of time [3]. Sex differences in cocaine abuse may be attributed to differential behavioral responses to cocaine between males and females. Self-report data have revealed sex differences in the subjective experience of cocaine, including both pleasant (e.g. being “high”) and unpleasant feelings [4–6]. Female drug users are also more sensitive to cocaine-conditioned stimuli and report more intense cocaine cravings than their male counterparts [7,8]. These sex differences in subjective responses to cocaine may be due to organizational differences between the brains of males and females, the activating effects of sex hormones on nervous system function, or a combination of both factors. The mechanisms that cause these differences are currently not well understood, yet are important to guiding treatment strategies for men and women.
One commonly used test for studying the subjective or rewarding effects of drugs of abuse in animals is a classical conditioning experiment known as conditioned place preference (CPP) [9,10]. In this experiment, drug exposure is paired with a specific context and the animals associate the drug-induced state with the environmental stimuli encountered during conditioning. Animals are tested for preference for the previously drug-paired context in the absence of the drug. A few researchers have investigated sex differences in cocaine CPP in rats. Russo et al. [11] found that female Fischer rats develop cocaine CPP after fewer conditioning sessions than males of the same strain. Female rats have also been shown to develop CPP at lower doses of cocaine than male rats, suggesting that females may be more sensitive to cocaine’s rewarding properties [11,12]. However, studies with a different strain of rat determined that the acquisition of cocaine CPP is similar between the sexes but that females exhibit a greater magnitude of reinstatement to cocaine CPP [13].
In order to explore in detail the mechanisms causing sex differences in substance abuse behavior, it is necessary to investigate molecular and genetic mechanisms in conjunction with behavioral models. Yet despite the importance of the mouse as a model organism for genetic research, little work has been done with this species to explore sex differences in behaviors related to substance abuse. To our knowledge, no studies have directly compared male and female mice in cocaine CPP. The C57BL/6J mouse is commonly used for molecular, genetic and behavioral studies and male C57BL/6J mice exhibit a robust CPP to cocaine [14]. Here, we have characterized intact female and male C57BL/6J mice for the acquisition and extinction of cocaine CPP to help guide further genetic studies on sex differences in cocaine abuse.
Methods
Animals
Experimentally naïve, intact 8- to 10-week-old male and female C57BL/6J mice (n = 14–16 per group; Jackson Laboratory, Bar Harbor, ME) were group housed with same-sex cage mates in a temperature- and humidity-controlled environment under a 14 hour light/dark cycle with lights on at 6 am and off at 8 pm. Mice were tested during the light phase. Body weights of females ranged from 16.5–23 grams and males from 20.8–29.5 grams. Cocaine doses were corrected for body weight. A habituation period of at least one week was allowed between the arrival of the mice and the start of the experiment. All mice had access to food and water ad libitum for the duration of the study and were maintained and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The experimental protocol was approved by the University of Illinois at Chicago (UIC) Institutional Animal Care and Use Committee.
Apparatus
Cocaine CPP training and testing were conducted using a modified 48-channel infrared photobeam detector open field apparatus (27.3 cm L x 27.3 cm W x 20.3 cm H) and Activity Monitor software (Med Associates, St. Albans, VT) for automated data collection. Two texturally distinct floor inserts and clear acrylic vertical dividing panels were custom cut by the UIC Scientific Instrument Shop to create a two-chamber choice apparatus. The two floor panels consisted of clear acrylic fluorescent light diffuser panels in the “prismatic” and “grid” textures. Each open field chamber was contained within a compressed wooden cabinet to reduce interference from outside light or sound during training and behavioral trials. All experiments were conducted with the apparatus lights and fans off.
Procedure
A timeline of the CPP protocol is shown in Figure 1. All conditioning sessions and preference tests were performed between 7 a.m. and 11 a.m., with each group tested at the same time every day. On the day before the first conditioning session (habituation, day 1), mice were placed into separate CPP boxes and allowed 30 minutes of unrestricted access between sides. Mice were then assigned to one of the sides for cocaine conditioning using a counterbalanced method so that within each sex and dose, half the mice were conditioned with cocaine on the “prismatic” textured floor and the other half were conditioned on the “grid” textured floor. Over the 8-day conditioning period, each mouse experienced a total of six alternating pairing sessions, three with cocaine and three with saline, beginning with cocaine. On days 2, 6, and 8, each mouse received an intraperitoneal (i.p.) injection of cocaine hydrochloride (Mallinckrodt, Inc., St. Louis, MO, purchased from UIC Pharmacy) at a dose of 2, 5, or 10 mg/kg (10 mL/kg volume in 0.9% sterile saline). The mouse was then immediately placed into the apparatus and confined to the cocaine-paired side (CS+) for 15 minutes. On days 3, 7, and 9, each mouse received an i.p. saline injection before being confined to the saline-paired side (CS-) for 15 minutes. At the end of each session, mice were immediately removed from the CPP chambers and returned to their home cages. On the day of the first preference test (day 10), mice were allowed to freely explore both sides of the CPP chambers for 30 minutes (identical to day 1). After a 2-day rest period, mice were tested for extinction of CPP in 3 subsequent 30-minute trials (days 13, 14, and 20).
Fig 1.
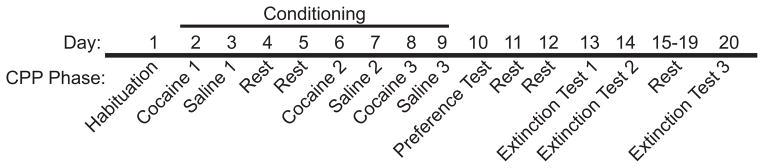
Diagram of cocaine CPP procedure and timeline. Testing day is shown above the long solid black line and CPP phase below. Mice were tested for 30 minutes on day 1 (habituation) and then assigned to one of two compartments with distinct tactile cues for cocaine conditioning in a counterbalanced manner. Three cocaine conditioning sessions (days 2, 6, and 8) and three saline conditioning sessions (days 3, 7, and 9) were conducted for 15 minutes each. Twenty-four hours after the last conditioning session (day 10), mice were tested for preference for 30 minutes as on day 1. Thirty minute extinction tests were performed on days 13, 14, and 20.
Statistical analysis
Data was converted to an initial preference score for each mouse by subtracting the time spent on the CS+ on day 1 from time spent on the CS+ on day 10. Positive scores indicate the development of preference. Preference scores were calculated for the extinction tests by subtracting time spent on CS+ on day 1 from time spent on the CS+ on each extinction day (13, 14, and 20). A 2 by 2 between subjects factorial analysis of variance (ANOVA) was used to analyze effects of sex and dose on initial preference scores. Extinction of preference was analyzed with a mixed factors analysis of covariance (ANCOVA). The grouping variables were sex and dose, the within-subject variable was time, and the covariate was the initial preference score. All follow-up tests were computed using the appropriate error term from the primary mixed factors analysis. Error bars represent standard error of the mean (SEM).
Results
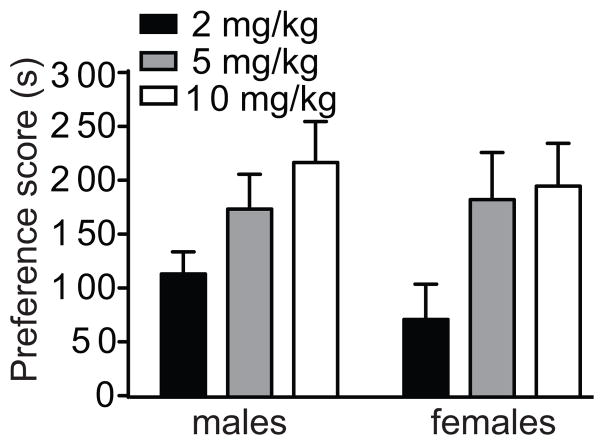
Male and female mice were conditioned for preference using 2, 5, and 10 mg/kg cocaine. Examination of time spent on the CS+ before and after conditioning collapsed across sexes indicated that mice developed significant cocaine CPP at all 3 doses tested (data not shown). The preference scores at each dose and for each sex are shown in Fig. 2. The 2 by 2 between subjects factorial ANOVA of preference scores showed a significant effect of dose with no effects of sex or a dose by sex interaction [dose, F (2, 85)=5.7, p =0.005; sex, F (1, 85)=0.41, p=0.52; dose by sex interaction, F (2, 85)=0.26, p=0.77]. Post-hoc tests indicated a significant difference in preference between 2 and 5 mg/kg cocaine and 2 and 10 mg/kg cocaine, but not between 5 and 10 mg/kg cocaine. Together, these data suggest that male and female C57BL/6J mice initially acquire cocaine CPP equally and that mice develop less preference at 2 mg/kg cocaine.
Fig 2.
Male and female mice acquire cocaine CPP equally. Mice were conditioned with 2, 5, or 10 mg/kg cocaine and tested for preference on day 10. Shown are the preference scores for male and female mice at each dose of cocaine. Positive values indicate the development of preference. There was a significant effect of dose (p=0.005) by ANOVA, but no effect of sex or dose by sex interaction.
We examined the extinction of cocaine CPP by testing for preference on days 13, 14, and 20. The preference scores on days 10 (first preference test), 13, 14, and 20 are shown for each dose in Fig. 3. A mixed factors ANCOVA showed a significant time by sex by dose interaction [F (4, 168)=2.49, p=0.045]. This indicated that the change in preference scores over time depended on the combined influence of sex and dose. The sex by time interaction was significant at 2 mg/kg cocaine [F (2, 168)=4.10, p=0.02], but not at 5 mg/kg [F (2, 168)=4.10, p=0.34] or at 10 mg/kg of cocaine [F (2, 168)=2.01, p=0.14]. Restricting a follow-up analysis to 2 mg/kg, there was a significant sex difference in preference on day 13 [F (1, 168)=6.48, p=0.002] but not on day 14 [F (1, 168)= 1.0, p=0.37] or 20 [F (1, 168)=2.25, p=0.11]. Thus, on day 13 at the 2 mg/kg cocaine dose, males retained preference, whereas females extinguished their preference. Overall, these results indicate that extinction of cocaine CPP occurs equally in both sexes at 5 and 10 mg/kg cocaine and that sex differences in cocaine CPP can be detected with repeated preference/extinction testing at 2 mg/kg cocaine.
Fig 3.
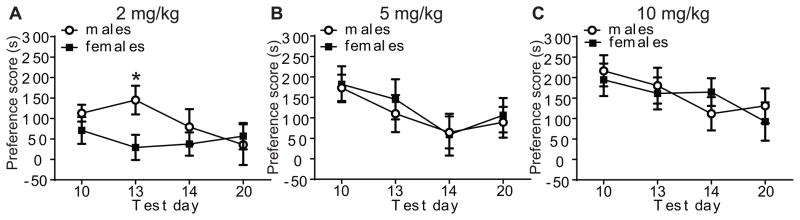
Sex differences in the extinction of cocaine CPP are revealed at 2 mg/kg cocaine. Shown are the preference scores on days 10, 13, 14, and 20 for male and female mice conditioned with 2 mg/kg (A), 5 mg/kg (B), and 10 mg/kg (C) cocaine. ANCOVA indicated a significant time by sex interaction (p=0.02) observed on day 13 after conditioning with 2 mg/kg cocaine, as indicated by the asterisk. No sex differences in extinction were observed at 5 and 10 mg/kg cocaine.
Discussion
In comparing male and female C57BL/6J mice in the acquisition and extinction of cocaine CPP, we found that acquisition does not differ between the sexes, whereas subtle effects of sex on extinction can be detected at low dose (2 mg/kg) cocaine. Acquisition of CPP was tested after conditioning with 3 doses of cocaine. Overall, we did not observe any differences in preference between 5 and 10 mg/kg cocaine, whereas preference at the 2 mg/kg dose was significantly lower than both 5 and 10 mg/kg cocaine. Several groups have investigated dose responses to cocaine CPP in male C57BL/6 mice [14–16]. No differences in preference between 5 and 10 mg/kg cocaine have been observed in these studies, in agreement with the results presented here. However, Cunningham et al. [15] found that C57BL/6J mice develop the same amount of preference with conditioning at 1 mg/kg as at 10 and 30 mg/kg cocaine. This could be due to a greater number of cocaine conditioning sessions in their experiment (4 versus 3), or differences in the training procedure (1 compartment versus 2). It seems likely that differences in the magnitude of cocaine CPP are best seen with low doses of cocaine and fewer conditioning sessions [14]. This may be due to a stronger association of the cocaine-associated cues with repeated pairing sessions and higher doses of cocaine, leading to enhanced memory consolidation during CPP training or retrieval during CPP testing.
Studies examining sex differences in CPP have primarily been performed in rats. Russo et al. found that the acquisition of cocaine CPP occurred at lower doses and with fewer conditioning sessions in females compared to males [11] and Zakharova et al. also observed that the acquisition of cocaine CPP occurred at lower doses in females [12]. However, a study by Bobzean et al. [13] found no sex differences in the acquisition of cocaine CPP over a large range of doses (3 to 25 mg/kg). The differences between these results may be due to a number of factors, including the rat strain, conditioning apparatus, and time of testing. Our data with C57BL/6J mice indicating that there are no sex differences in the acquisition of cocaine CPP are in agreement with Bobzean et al. One caveat to the results presented here is that we did not determine the estrous cycle of mice during the conditioning and testing phases of the experiment, primarily because vaginal lavage can affect cocaine CPP [17]. Estrous cycle length in 2–3 month old C57BL/6 mice can vary widely, with a median length greater than 5 days [18]. The experiment performed here was 20 days long, so mice likely went through 3–4 estrous cycles during this time. Sex differences may have been masked due to fluctuations in the circulating profile of sex hormones in females throughout the conditioning and testing phases. It is apparent that sex hormones affect cocaine reward. In humans, activation of key areas of the brain involved in reward are differentially activated during the menstrual cycle, with more activation occurring during the mid-follicular phase when estrogen levels are rising [19]. Subjective responses to smoked cocaine also vary across the menstrual cycle, with greater positive feelings occurring during the follicular phase [6]. Progesterone attenuates cocaine reward in humans and rats [6,20] and estrogen also plays an important role in cocaine reward. In ovariectomized rats, estradiol itself can induce CPP [21], increase the sensitivity of the brain to reward as measured by intracranial self-stimulation [22], and enhance cocaine CPP [23,24]. The ability of estradiol to enhance cocaine reward may be due to the expression of estrogen receptor alpha (ERα) in the nucleus accumbens, since administration of antisense oligonucleotides targeting ERα into the nucleus accumbens can reduce estradiol-induced CPP [25]. Future studies are aimed at determining whether estradiol specifically plays a role in the acquisition of cocaine CPP by using ovariectomized C57BL/6J mice treated with estradiol.
Differences between male and female mice in the extinction of CPP were observed at the lowest dose of cocaine and only on the second test after conditioning. Notably, we found that cocaine CPP in male mice was retained on the second test day. This could be due to a delay in the extinction of CPP or increased memory consolidation in male mice after the first preference test, although it is difficult to distinguish experimentally between these possibilities. Our results suggest that at a low dose of cocaine, male mice retain cocaine CPP, whereas at this same dose, females maintain little to no preference. Hormonal effects in the brain may account for this difference. Estradiol has been shown to facilitate extinction of cocaine CPP in female rats [24] and may be one factor that can account for the sex difference in extinction of cocaine CPP in mice. No sex differences in extinction of cocaine CPP were observed with 5 and 10 mg/kg cocaine, perhaps because lower doses of cocaine are needed to reveal differences or estrous cycle phase effects are masked due to testing in cycling females. Our extinction data from the 5 and 10 mg/kg doses of cocaine suggest that the rate of extinction depends on the dose of cocaine used during conditioning. At 5 mg/kg cocaine, extinction is clearly evident by the third day of testing, whereas at 10 mg/kg cocaine, partial extinction is only observed on the fourth day of testing. Brabant et al. also saw an effect of dose on the extinction of cocaine CPP in male C57BL/6J mice [14].
Conclusion
C57BL/6J mice are widely used in behavioral studies, and in particular many mutant lines are backcrossed into the C57BL/6 background in order to study the contribution of specific genes to behavioral responses to drugs of abuse. Here we have characterized the response of female C57BL/6J mice to the rewarding properties of cocaine and the extinction of this behavior. We conclude that using low doses of cocaine during conditioning is important for studying the effects of sex hormones on cocaine CPP in C57BL/6 mice. The experiments presented here will help guide experiments examining the molecular and genetic factors that contribute to sex differences in cocaine reward.
Acknowledgments
This research was supported by the National Institute on Drug Abuse (DA033429) to A.W.L. We would like to thank Eric Schmidt at the UIC Scientific Instrument Shop for assistance in the design and fabrication of the CPP chamber inserts and Dr. Amynah Pradhan for helpful discussions and comments on this manuscript. Special thanks to Dr. Leah Rubin for assistance with statistical analysis.
Footnotes
Conflict of Interest: There are no conflicts of interest.
References
- 1.Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- 2.Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch Gen Psychiatry. 1989;46:122–126. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- 3.Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat. 1993;10:63–66. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- 4.Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, et al. Gender differences in response to intranasal cocaine administration to humans. Biol Psychiatry. 1996;39:147–148. doi: 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 5.Lynch WJ, Kalayasiri R, Sughondhabirom A, Pittman B, Coric V, Morgan PT, et al. Subjective responses and cardiovascular effects of self-administered cocaine in cocaine- abusing men and women. Addict Biol. 2008;13:403–410. doi: 10.1111/j.1369-1600.2008.00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans SM, Foltin RW. Does the response to cocaine differ as a function of sex or hormonal status in human and non-human primates? Horm Behav. 2010;58:13–21. doi: 10.1016/j.yhbeh.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elman I, Karlsgodt KH, Gastfriend DR. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. The American journal of drug and alcohol abuse. 2001;27:193–202. doi: 10.1081/ada-100103705. [DOI] [PubMed] [Google Scholar]
- 8.Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- 9.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 10.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 11.Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- 12.Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bobzean SA, Dennis TS, Addison BD, Perrotti LI. Influence of sex on reinstatement of cocaine-conditioned place preference. Brain Res Bull. 2010;83:331–336. doi: 10.1016/j.brainresbull.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Brabant C, Quertemont E, Tirelli E. Influence of the dose and the number of drug-context pairings on the magnitude and the long-lasting retention of cocaine-induced conditioned place preference in C57BL/6J mice. Psychopharmacology (Berl) 2005;180:33–40. doi: 10.1007/s00213-004-2138-6. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham CL, Dickinson SD, Grahame NJ, Okorn DM, McMullin CS. Genetic differences in cocaine-induced conditioned place preference in mice depend on conditioning trial duration. Psychopharmacology (Berl) 1999;146:73–80. doi: 10.1007/s002130051090. [DOI] [PubMed] [Google Scholar]
- 16.Orsini C, Bonito-Oliva A, Conversi D, Cabib S. Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2 inbred strains. Psychopharmacology (Berl) 2005;181:327–336. doi: 10.1007/s00213-005-2259-6. [DOI] [PubMed] [Google Scholar]
- 17.Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–752. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- 18.Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biology of reproduction. 1982;27:327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- 19.Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci U S A. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo SJ, Sun WL, Minerly AC, Weierstall K, Nazarian A, Festa ED, et al. Progesterone attenuates cocaine-induced conditioned place preference in female rats. Brain Res. 2008;1189:229–235. doi: 10.1016/j.brainres.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 21.Frye CA, Rhodes ME. Administration of estrogen to ovariectomized rats promotes conditioned place preference and produces moderate levels of estrogen in the nucleus accumbens. Brain Res. 2006;1067:209–215. doi: 10.1016/j.brainres.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 22.Galankin T, Shekunova E, Zvartau E. Estradiol lowers intracranial self-stimulation thresholds and enhances cocaine facilitation of intracranial self-stimulation in rats. Horm Behav. 2010;58:827–834. doi: 10.1016/j.yhbeh.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Segarra AC, Agosto-Rivera JL, Febo M, Lugo-Escobar N, Menendez-Delmestre R, Puig-Ramos A, et al. Estradiol: a key biological substrate mediating the response to cocaine in female rats. Horm Behav. 2010;58:33–43. doi: 10.1016/j.yhbeh.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twining RC, Tuscher JJ, Doncheck EM, Frick KM, Mueller D. 17beta-estradiol is necessary for extinction of cocaine seeking in female rats. Learning & memory. 2013;20:300–306. doi: 10.1101/lm.030304.113. [DOI] [PubMed] [Google Scholar]
- 25.Walf AA, Rhodes ME, Meade JR, Harney JP, Frye CA. Estradiol-induced conditioned place preference may require actions at estrogen receptors in the nucleus accumbens. Neuropsychopharmacology. 2007;32:522–530. doi: 10.1038/sj.npp.1301124. [DOI] [PubMed] [Google Scholar]