Abstract
Background
The purpose of this study was to examine factors associated with the implementation of case management (CM) interventions in primary health care (PHC) and to develop strategies to enhance its adoption by PHC practices.
Methods
This study was designed as a systematic mixed studies review (including quantitative and qualitative studies) with synthesis based on the diffusion of innovation model. A literature search was performed using MEDLINE, PsycInfo, EMBASE, and the Cochrane Database (1995 to August 2012) to identify quantitative (randomized controlled and nonrandomized) and qualitative studies describing the conditions limiting and facilitating successful CM implementation in PHC. The methodological quality of each included study was assessed using the validated Mixed Methods Appraisal Tool.
Results
Twenty-three studies (eleven quantitative and 12 qualitative) were included. The characteristics of CM that negatively influence implementation are low CM intensity (eg, infrequent follow-up), large caseload (more than 60 patients per full-time case manager), and approach, ie, reactive rather than proactive. Case managers need specific skills to perform their role (eg, good communication skills) and their responsibilities in PHC need to be clearly delineated.
Conclusion
Our systematic review supports a better understanding of factors that can explain inconsistent evidence with regard to the outcomes of dementia CM in PHC. Lastly, strategies are proposed to enhance implementation of dementia CM in PHC.
Keywords: systematic mixed studies review, dementia, case management, primary health care, implementation, diffusion of innovation
Introduction
Dementia is the most common age-related neurodegenerative disorder.1 There are approximately 500,000 people living with dementia in Canada, and over the next 40 years this number will increase by 2.5-fold.2 Dementia is the fourth most common cause of death among patients aged 75 years and over worldwide, the second most burdensome of all brain diseases in Western Europe, and number five among the top ten burdensome diseases in Europe in terms of years of life lost and years living with a disability.3
This vulnerable population is characterized by complex needs that require a comprehensive approach by health care professionals.4 Fragmentation and inefficiency of health services represent major problems for these patients.5,6 As the hub of services, primary health care (PHC) and, in particular, primary care physicians (PCPs), have been internationally recognized as well positioned to serve as these patients’ first contact with the health care system for early diagnosis and management of dementia.7 However, PHC is not yet fully prepared to deal with the diverse needs of these patients.5,6
To address these challenges, case management (CM) has been designed to increase the capacity of PHC to cope with this population, improve the quality of dementia care, and develop cost-effective and efficient ways to coordinate services.8 The Case Management Society of America defines CM as “a collaborative process of assessment, planning, facilitation, care coordination and advocacy for options and services to meet an individual’s and family’s comprehensive health needs through communication and available resources to promote quality cost-effective outcomes”.8 Over the past few decades, several CM demonstration projects have been implemented in PHC to improve clinical outcomes for patients with dementia and to optimize resource utilization, but the research evidence on their outcomes is inconsistent.9–12 It has been shown that barriers to implementation hinder outcomes.13 Callahan et al demonstrated that dementia CM in PHC is unlikely to be successful unless adequate attention is paid to barriers to implementation.14 At this writing, there is no systematic review of the conditions limiting and facilitating successful dementia CM implementation. Thus, our research objectives were to examine the factors associated with the implementation of CM interventions in PHC and develop strategies to enhance its adoption in PHC practices.
Materials and methods
To address our objectives, we conducted a systematic mixed studies review, including studies with diverse designs, including quantitative and qualitative methods.15 Including different forms of evidence produces findings that are more relevant to decision-makers,16 overcomes the issue of a partial portrait based on examining one type of research in isolation,17 and assists the critical analysis of implementation processes from the viewpoint of the targeted participants.15
Data sources
The review was based on a systematic, comprehensive search of four databases (MEDLINE, PsycInfo, EMBASE, and the Cochrane Database of Systematic Reviews) according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.18 Articles published between 1995 (official publication of the Standards of Practice for Case Management)8 and August 31, 2012 were considered for inclusion. The literature search was conducted by a specialized librarian. See Supplementary material for a detailed example of our search in PsycInfo.
Inclusion criteria
CM interventions comprising all the components identified by the Case Management Society of America (case finding and screening, assessment, care planning, implementation and management, monitoring, and review)8 for patients of any age and sex with cognitive impairment or dementia (any type) residing in the community and/or their informal caregivers and receiving CM provided by health care professionals in a range of community settings (eg, patients’ homes, physicians’ offices).19
Quantitative (eg, randomized controlled trials) and qualitative (eg, case studies) studies describing the conditions limiting and facilitating successful CM implementation.
Participants were patients, caregivers, PHC health care professionals (PCPs, case managers, such as nurses and/or social workers, or geriatricians, neurologists, and psychiatrists).
Language of publication was English or French.
Study selection process
Based on the inclusion criteria, two reviewers (VK, IV) independently examined the records (titles and abstracts) obtained from the databases. VK and IV then independently examined full-text copies of the studies corresponding to the selected records. At each step, differences in coding (inclusion/exclusion of a record) were resolved by consensus or referred to a third reviewer (PP). Kappa scores were calculated to estimate interreviewer reliability.20 If more than one publication described the same study, they were treated as one study. All companion articles were also searched.
Data extraction
Information was extracted from each study by two researchers working independently (VK, IV), and comprised author, publication date, region, study design, methodology, methods of data collection and analysis, participant characteristics, sample size, diagnosis, and conditions limiting and facilitating successful CM implementation. Differences arising from the data extraction were resolved by consensus.
Synthesis
In order to describe the conditions limiting and facilitating successful implementation and assemble a broad knowledge base from studies with diverse methodologies and methods, we used a narrative synthesis approach.15 This method “relies primarily on the use of words and text to summarize and explain the findings of the synthesis”.15 First, the narrative data (key sentences eliciting barrier-related and enabler-related research results and researchers’ interpretations of these results) were extracted from all the articles in the sample. These data were analyzed following standard methods for qualitative thematic analysis in order to identify key themes associated with CM implementation.21,22
Second, to better understand the conditions of CM implementation we used a recent modification of the diffusion of innovation model as an organizing framework because it fitted better with our objectives.23,24 This model pertains to addressing the predicament of how to disseminate and sustain interventions in health service delivery and identifying domains in which key factors influence intervention uptake and implementation. It consists of eight interrelated components: the attributes of the innovation (eg, relative advantage, namely clear benefits over existing health care services), the concerns of potential adopters (eg, properties and potential benefits), communication and influence (eg, interpersonal influence), organizational antecedents to innovation (eg, capacity to absorb new knowledge), readiness for innovation (eg, innovation-system fit), the implementation process (eg, human resource issues), linkage (eg, communication within the organization), and the broader context (eg, specific incentives at the national level).
Quality assessment
As recommended by PRISMA,18 the methodological quality of the included studies was assessed independently by two reviewers (VK, PP). We used the Mixed Methods Appraisal Tool, which has been designed and validated for the critical appraisal of studies with diverse designs.25 The tool demonstrated good reliability, with the consistency of the global quality score between reviewers (intraclass correlation) being 0.72 before and 0.94 after discussion.25 The Mixed Methods Appraisal Tool takes into consideration all reported outcomes (primary and secondary). This tool has four criteria for the evaluation of each study design (0 to 4). Thus, to calculate an interrater reliability for several criteria, the weighted kappa calculation is required.26 Studies of lower quality (with a score of 0 or 1) were not excluded from the synthesis, because our primary objective was to gain knowledge on dementia CM and highlight the main limiting and facilitating conditions that will need to be addressed in future research.
Results
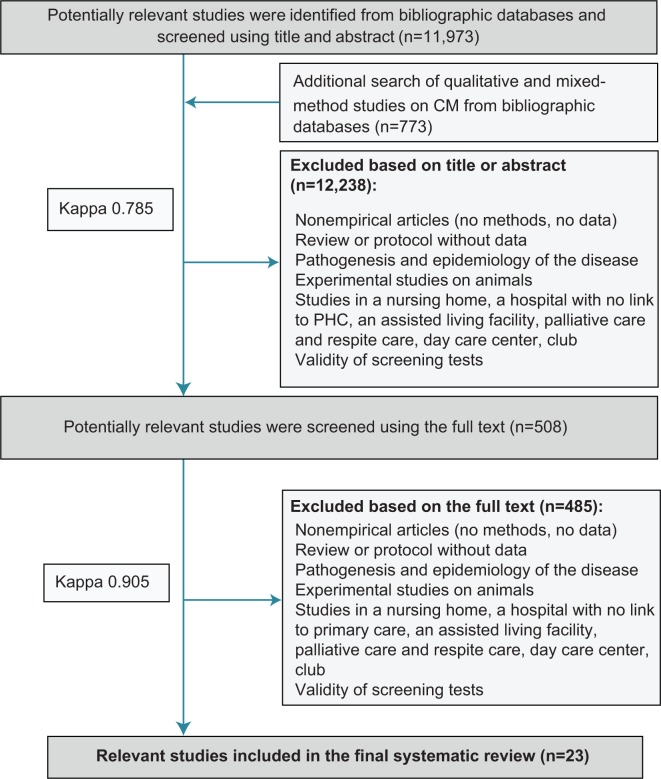
The search yielded 12,746 records (Figure 1). Of these, 12,238 records were not eligible based on the title or abstract (kappa 0.79), and 490 were eliminated based on the full-text publications (kappa 0.91). Twenty-three studies were included in the review (31 publications).
Figure 1.
PRISMA flow chart.
Abbreviations: CM, case management; PHC, primary health care; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Characteristics of the selected studies
Of the included studies, eleven were quantitative studies, comprising nine randomized controlled trials,10–12,27–39 one nonrandomized study,40 and one quantitative descriptive study41 (Table 1), and 12 were qualitative studies, comprising eleven qualitative descriptive studies42–53 and one multiple-case study54 (Table 2). Ten studies were conducted in the USA,10,12,27,36,39,40,43,45,48,50 five in the UK,42,46,47,49,51 and four in the Netherlands.32,41,52,54 The remaining four studies were from Belgium,31 Australia,53 India,34 and People’s Republic of China.35,55 All articles were published in English.
Table 1.
Characteristics of quantitative studies
| Reference | Design of study | Population studied | Sample size | Characteristics of intervention | Health care team composition |
|---|---|---|---|---|---|
| Newcomer et al,11 Miller et al29 (USA) | RCT | Dementia | 8,095 | Assistance with support service; psychological support of caregivers | Case manager (nurse, social worker, mental health worker, gerontology worker) |
| Fortinsky et al12 (USA) | RCT | Dementia | 84 | Education of caregivers; regular follow-up of patients; action plan for caregivers | Case manager (social worker) working with PCP on care plan |
| Schoenmakers et al31 (Belgium) | RCT | Cognitive impairment | 62 | Guidance of the family carer in organizing home care; regular follow-up (home visit, phone calls) | Primary care professional with a bachelor degree working directly with PCP |
| Jansen et al29,30 (the Netherlands) | RCT | Cognitive impairment | 99 | In-home assessment; developed care plan for patient and caregiver with problem identification; liaison to support service; referrals to health professionals if needed | Case manager (nurse specialized in geriatric care) working directly with PCP |
| Dias et al31 (India) | RCT | Dementia | 81 | Education of caregivers about the disease; emotional support for caregivers; improvement of caregivers’ skills; referral to support groups; care plan development based on an agreed protocol, its implementation, and regular follow-up | Case manager (no social work or medical background) working in collaboration with psychiatrist |
| Lam et al32 (People’s Republic of China) | RCT | Dementia | 102 | In-home assessment; home-based program of cognitive stimulation; assistance with support service registration | Case manager (occupational therapist) |
| Mittelman et al33–35 (USA) | RCT | Dementia | 406 | Baseline assessment; individual and family counselling sessions tailored to each caregiver’s situation; care plan development (management of troublesome behavior, communication techniques), its regular reassessment and ad hoc telephone counseling; ongoing emotional support and education | Case manager (social worker) |
| Wright et al36 (USA) | RCT | Dementia | 93 | Problem identification and care plan development; regular medication revision; caregiver support program; assistance with support service | Case manager (nurse) |
| Vickrey et al10 (USA) | RCT | Dementia | 408 | In-home assessment; problem list development with development of a plan, including for the caregiver; regular reassessment; liaison to support services; monitoring and communication via Web-based system | Case manager (social worker) working with PCP on care plan |
| Cherry et al37 (USA) | Nonrandomized study | Dementia | 83 | Comprehensive geriatric assessment; regular follow-ups; assessment of adequacy of family support; development of treatment plan; assistance with support service through referrals; education of caregiver-patient dyads | Case manager (social worker) |
| Verkade et al38 (the Netherlands) | Survey | – | 30 experts in CM | – | – |
Abbreviations: CM, case management; PCP, primary care physicians; RCT, randomized controlled trial.
Table 2.
Characteristics of qualitative studies
| Reference | Design of study | Sample | Characteristics of intervention |
|---|---|---|---|
| Adams et al42 (UK) | Qualitative descriptive/thematic analysis | 14 case managers | CM focused on dementia patients and their caregivers |
| Black et al40,41 (USA) | Qualitative descriptive/thematic analysis | 27 community-based case managers | CM focused on the diseases of older persons, including dementia; focus on advanced care planning skills of case managers as a part of their functions |
| Bogardus et al42 (USA) | Qualitative descriptive/constant comparative method | Ten sets of participants (each set: patient, caregiver, case manager, clinician) | CM focused on dementia patients and their caregivers |
| Gibson et al43 (UK) | Qualitative descriptive/thematic analysis | Ten dyads (patient with mild to moderate dementia and caregiver) receiving service either from a hospital-based memory clinic or a community-based nursing service | Comparison of a community-based and a clinic-based memory service |
| Gladman et al44 (UK) | Qualitative descriptive/thematic analysis with conceptual mapping | Six PCPs, one geriatric psychiatrist, caregivers, patient advocate, team manager, representatives of Alzheimer Association | CM focused on dementia patients and their caregivers |
| Liebel et al45 (USA) | Qualitative descriptive/thematic analysis | 19 patients | Secondary analysis of Medicare primary and consumer-directed care; demonstration designed for patients with disabilities, including cognitive impairment (68%) |
| McCrae et al46 (UK) | Convergent design/thematic analysis | 33 and 27 health care professionals (nurses, occupational therapist, psychiatrists, psychologist, support workers, team leaders) at 6 and 24 months, respectively | Evaluation of “Improving the Quality of Care for Older People in Lambeth” impact from staff perspectives: did it help or hinder them in performing their roles? |
| Netting et al47 (USA) | Qualitative descriptive/thematic analysis | 36 different participants in CM: physicians, case managers, case assistants, practice managers, office staff | CM focused on the diseases of older persons, including dementia |
| Seddon et al48 (UK) | Qualitative descriptive/latent content analysis | 8 care managers and 64 caregivers | CM focused on caregiver’s assessment (ability to care and continue care, coping ability, relationship with a care recipient) |
| Van Eijken et al49 (the Netherlands) | Qualitative descriptive/inductive thematic analysis | 15 PCPs, 6 case managers (nurses), 2 geriatricians, 11 patients and 37 caregivers | CM focused on problems with cognition, mood, behavior, functional decline, mobility, nutrition and urinary incontinence |
| Waugh et al50 (Australia) | Qualitative descriptive/thematic analysis | Five staff workers of the Mercy Community Care agency: 2 managers, 2 case managers, one outreach worker | CM for dementia patients living alone |
| Minkman et al51 (the Netherlands) | Multiple case study/thematic analysis | 9 case managers | CM focused on dementia patients and their caregivers |
Abbreviations: CM, case management; PCP, primary care physicians.
Conditions limiting and facilitating successful CM implementation
The key conditions for implementation are presented in Table 3.
Table 3.
Conditions limiting and facilitating successful CM implementation
| Key factors | Reference
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 12 | 24 | 28 | 29 | 31 | 32 | 33 | 36 | 37 | 38 | 39 | 40 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | |
| Intensity of CM and caseload (n=6) | – | – | – | × | – | – | × | × | – | – | × | – | × | – | – | – | – | – | – | – | – | – | × |
| Inclusion criteria by target population (eg, problem-centered versus age-centered) (n=4) | – | – | × | × | – | – | – | – | – | – | – | – | – | – | – | – | – | – | × | – | – | – | × |
| Attitude of health care professionals, case managers, and patient-caregiver dyad (n=10) | – | – | – | × | × | – | – | – | – | – | × | × | × | – | – | × | – | – | × | × | × | – | × |
| Communication between health care professionals, case managers, patient-caregiver dyad, health care organizations (n=13) | – | – | – | × | – | – | – | – | – | – | × | × | × | × | × | × | × | × | × | × | × | – | × |
| Time constraints (n=3) | – | – | – | – | – | – | – | – | – | – | – | – | × | – | – | × | – | – | – | – | × | – | – |
| Lack of CM integration in the current health care system (n=4) | – | – | – | – | – | – | – | – | – | – | × | – | – | – | – | – | – | – | × | – | × | – | × |
| Turnover of case managers (n=3) | – | × | – | – | – | – | – | – | – | – | × | – | – | – | – | – | – | – | × | – | – | – | – |
| Absence of colocation between case manager and PCP (n=3) | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | × | – | × | – | × |
| Lack of training in geriatrics (n=10) | – | × | – | – | – | × | – | – | – | – | – | – | × | – | × | × | – | – | × | × | × | × | × |
| Duration of CM intervention (n=3) | – | – | – | – | – | × | – | × | × | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Lack of PCP involvement (n=3) | – | × | × | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | × | – | – |
| Engagement period (time to develop trust between the health care professionals) (n=5) | – | – | – | – | – | – | – | – | – | × | – | – | – | – | – | × | – | × | × | – | × | – | – |
| Financial factors (n=1) | × | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
Notes: ×, condition described in the article; –, condition not described.
Abbreviations: CM, case management; PCP, primary care physician.
Conditions limiting and facilitating successful CM implementation and proposed strategies for optimal implementation
Table 4 presents the correspondences between conditions limiting and facilitating successful dementia CM implementation and the components of the diffusion of innovation model.24
Table 4.
Matrix of key limiting and facilitating conditions, components of the diffusion of innovation model, and strategies
| Components of Greenhalgh model | Key limiting and facilitating conditions | Strategies to be used to enhance implementation |
|---|---|---|
| Attributes of CM as an innovation | Intensity of CM and size of caseload | High intensity of CM Optimal caseload (50–60 patients per full-time case manager) |
| Inclusion criteria | Use of patient-oriented approach rather than age/diagnosis-oriented strategy | |
| Concerns of potential adopters | Negative attitudes of health care professionals, case managers, and patient-caregiver dyads toward CM | Information campaign about the value of CM targeting health care professionals involved in dementia health care |
| Regular feedback to tailor the intervention to the needs of health care professionals and the context of the health care facility | ||
| Regular contact with health care professionals to promote the role of case manager | ||
| Communication and influence | Communication between health care professionals, case managers, and patient-caregiver dyads | Clear delineation of the responsibilities of the health care professionals involved in CM (eg, interdisciplinary care protocol) |
| Regular meetings of case managers with interdisciplinary team and PCPs Internet-based care management software system for care planning and coordination | ||
| Web-based access to electronic health records | ||
| Organizational antecedents for innovation | Interorganizational communication | Clear system of communication (eg, referral system) |
| Specific guidelines for referral to support services (eg, AlzheimerAssociation) | ||
| Organizational readiness for innovation | Time constraints | Definition of priorities in the provision of care (simple cases versus more complicated conditions) |
| Sharing of tasks between PCPs and case managers | ||
| Lack of CM integration into current health care system | Integration of CM in the primary health care facility | |
| Implementation process | Turnover of case managers | Appointment of case managers with experience and good communication qualities |
| Training in communication for case managers | ||
| Individual and team training for staff members | ||
| Absence of colocation between case manager and PCP | Location of the case manager in the same facility as PCPs | |
| Lack of training in geriatrics | Educational training (eg, facilitated sessions and decision-support software to improve diagnosis of dementia) | |
| Use of dementia treatment guidelines designed for PHC | ||
| Duration of intervention | At least 12 months of intervention before evaluating the outcomes | |
| Linkage | Lack of PCP involvement or reluctance of PCPs to be involved in care of patients with dementia Engagement period | Information campaign targeting PCPs about the value of CM (champion) |
| Training (eg, facilitated sessions and decision-support software to improve diagnosis of dementia) | ||
| At least 6 months of engagement to develop confidence in the intervention | ||
| Broader context | Financial factors | Capitation-based or mixed remuneration |
Abbreviations: CM, case management; PCP, primary care physician; PHC, health care.
Attributes of CM as an innovation
There are two characteristics of CM interventions that can play important roles in the implementation process, ie, intensity of CM (and more specifically, size of the caseload) and inclusion criteria.
Intensity of CM and caseload
Low intensity (eg, large caseload, infrequent follow-up, low fidelity, partial completion of the designed intervention) was associated with an absence of outcomes for the patient-caregiver dyad.31,35,36,41 A large caseload was considered the factor that changed the proactive nature of dementia care (aimed at preventing a crisis) toward a more reactive approach (dealing with crises).43,44,54 An average caseload of 50–60 patient-caregiver dyads per full-time case manager was perceived as optimal.43,44,54
Inclusion criteria by target population
Case managers considered inclusion of patients with a confirmed diagnosis but without prominent dementia symptoms (early stage of dementia) as a limiting factor toward achieving desired outcomes.27,31,50,54 Recruitment of patients based on diagnosis tends to include participants who will not benefit from CM due to the absence of substantial risk (patients in the early stage of the disease).31 Referral of patients with a problem, regardless of diagnosis and age, was viewed as more beneficial for patients.50
Concerns of potential adopters
In line with the model, the adoption of a CM intervention is a process that is influenced by a negative attitude on the part of health care professionals, case managers, and patient-caregiver dyads toward CM.
Health care professionals expressed doubts over the value of CM within the current health care system.43,44,47–52,54 Health care professionals and members of steering committees expressed great uncertainty and reluctance over the capacity of CM to improve services for elderly persons.43,50,51 Confusion about the case manager’s role accounted for the limitations of the interventions, especially at the beginning of CM implementation.41,43,44,50,51 Despite the fact that health care professionals, especially PCPs (43%), believed that patient care could be improved with the addition of a dementia-trained care coordinator, their reluctance to integrate the CM model persisted.31 Netting et al reported that it took one year before physicians recognized the case manager’s contributions to the care of patients, such as mobilization of resources, focus on psychosocial aspects of the patient’s life, extension of the physician in terms of preventive care, and a reduction in time spent in patient consultations.50
Case managers also contributed to this situation.31,32,42,51 For example, only 63% of case managers clearly explained their role to the patient-caregiver dyads and 25% did not give any detail during the assessment.51 Not surprisingly, negative attitudes on the part of case managers towards CM prevented patients and caregivers from receiving care as it had been initially designed.32 When case managers did not recognize the importance of their role,42 or misinterpreted it, this led them to resist collaborating.31 In such conditions, health care professionals and patients-caregiver dyads expressed doubt about the helpfulness of the CM intervention.30,32
Communication and influence
Individual adoption depends on communication between health care professionals, case managers and patient-caregiver dyads and their interpersonal influence. One of the factors associated with CM implementation was insufficient collaboration between local health care professionals (eg, general practitioner, geriatrician, and case manager, ie, nurse or social worker).41,43,45–48,50,51,54 For example, 52% of case managers indicated that poor communication with health care professionals negatively affected their work.43,44 Case managers had to initiate the first contact, and viewed personal contact as a precondition for effective communication.52 Establishing communication (“learning to interact”) was perceived as time-consuming and required the development of a sensitive strategy tailored to each practice setting’s own culture.50
While trusting relationships between the patient-caregiver dyad and the case manager were considered a prerequisite for achievement of long-term objectives, poor communication between them was also reported.31,41,48 For instance, only 38% of caregivers reported being able to talk about their needs and concerns with the case manager, and only 42% felt that their concerns were understood.51
Organizational antecedents to innovation
According to the model,24 different degrees of innovation adoption by organizations (more or less innovative) may be due to a lack of interorganizational communication. Our results suggest that a close working relationship and permeability of the boundaries between health care services were seen as necessary for support and efficiency of intervention.41,42,49
Organizational readiness for innovation
Our results indicate that an organization may be unprepared to adopt CM due to the time constraints that health care professionals face and poor CM integration into the current health care system.
Time constraints
Pressures on their time prevented case managers from fulfilling their tasks completely (eg, caregiver’s assessment).43,44,47 A similar issue was highlighted for PCPs.52 Case managers believed that more time spent by the PCP on the patient’s problem would facilitate its solution.52
Lack of CM integration in current health care system
While experts stated that CM is impossible unless it is fully integrated into the health care chain,41 case managers were not considered “part of” physicians’ practices.50 Lack of CM integration into the team in the existing health care system impeded reinforcement and assurance of continuity of dementia care.52,54
Implementation process
Critical factors that could affect the adoption of CM were case manager turnover, lack of colocation of case manager and PCP, lack of training in geriatrics, and short duration of intervention.
Turnover of case managers
Turnover of case managers was a source of dissatisfaction with CM.12 Indeed, a sufficient amount of time is needed to establish good relationships between a patient-caregiver dyad and a case manager.12,41,50 This leads to the development of emotional ties that facilitate provision of effective service.
Absence of colocation of case manager and PCP
Absence of case manager colocation in the health care facility (especially in PHC) was seen as preventing full integration of CM into the existing health care practice.50,52,54
Lack of training in geriatrics
The complexity of the work requires that case managers have a range of qualities, such as specific knowledge of dementia and other geriatric conditions, analytical ability (eg, the ability to distinguish the symptoms of dementia from normal aging), a patient-centered as opposed to health care facility-oriented approach, an ability to deliver proactive instead of reactive care, a good awareness of local services, and an understanding of welfare and human rights, suicide risk, and compliance with medications.34,43,44,46,47,51,52,54
Similar issues were reported by PCPs.50,52,54 Absence of the training required to make the diagnosis was a concern for PCPs, especially regarding patients with multiple health problems.50,52,53 PCPs viewed recognizing and evaluating cognitive deficiency as the most challenging task (along with dealing with mood and mobility problems).52 Insufficient knowledge of geriatric diagnostic tools and their use in routine primary care practice were listed as another limiting factor.52 In addition, PCPs showed a lack of initiative or were uncertain about how to counsel (eg, only 30% of them discussed the care plan with a caregiver),12 and were reluctant to prescribe antidementia medications.34
Duration of intervention
Short duration of the intervention seems to be a limiting factor.34,36–39 Dias et al acknowledged that the short follow-up period precluded them from achieving the expected effect.34 Wright et al also indicated that a 12-month period is insufficient to reveal program outcomes: it was solely toward the end of the study (at 12 months) that trends were observed in terms of decreased behavioral symptoms, caregivers recovering from depression, and better health.39
Linkage
CM is more likely to be adopted when the PCPs involved in dementia care and other health care professionals have sufficient time to develop confidence in CM (the “engagement period”).
Lack of involvement of PCP in dementia care
Little or no involvement on the part of PCPs in the care of patients with dementia was perceived as a disadvantage, since PCPs provide support during the early stages of the disease.52 Also, noninvolvement by a patient’s PCP in the intervention has been seen as a limiting factor.27 For instance, an attempt by the Alzheimer Association to collaborate closely with PHC practice resulted in only 30% PCP involvement.12
Engagement period
Confidence in the intervention grew among health care professionals over the first year of implementation.40,47,49,50,52 During the first year, PCPs, case managers, patients, and caregivers developed trusting and working relationships (the “engagement period”). This engagement period is crucial because it leads to improved results in the second year of the intervention.40,49,50
Broader context
In line with the model, CM is more likely to be successful when there are financial incentives and mandates at the national level.24 The adoption and sustainability of CM depends on staff remuneration. Vickrey et al have suggested that fee-for-service remuneration can limit adoption of CM.10 In contrast, capitation under Medicare-managed care is more likely to favor CM becoming the “usual” practice.10
Quality of evidence from the selected studies
The quality of each of the 23 studies was assessed based on Mixed Methods Appraisal Tool criteria.25 Overall, the included studies were of moderate quality (69.6%); only two (8.6%) were of lower quality (score 0 or 1), while five (22%) were of higher quality (all criteria met, Table 5). Two studies were rated as being of lower quality due to unclear descriptions of randomization, allocation concealment, complete outcome data, and rate of dropout,39 the methodology used in data analysis, the relationship of the findings to the context, and the reflexivity of the researchers.53
Table 5.
MMAT quality appraisal for studies with diverse designs
| Reference | Quality appraisal
|
||||
|---|---|---|---|---|---|
| Randomization | Blinding | Outcome data | Dropout rate | Overall | |
| RCTs | |||||
| 10 | 1 | 1 | 1 | 0 | 3 |
| 12 | 0 | 1 | 1 | 0 | 2 |
| 24 | 0 | 0 | 1 | 1 | 2 |
| 28 | 1 | 1 | 0 | 1 | 3 |
| 29 | 1 | 1 | 1 | 1 | 4 |
| 31 | 1 | 0 | 1 | 0 | 2 |
| 32 | 1 | 1 | 1 | 1 | 4 |
| 33 | 1 | 1 | 1 | 1 | 4 |
| 36 | 0 | 0 | 0 | 0 | 0 |
| Reference | Sampling strategy | Representativeness | Appropriate measurements | Response rate | Overall |
| 38 | 1 | 1 | 1 | 1 | 4 |
| Nonrandomized study | |||||
| Reference | Selection bias | Appropriate measurements | Compared groups | Outcome data | Overall |
| 37 | 0 | 0 | 1 | 1 | 2 |
| Quantitative descriptive (survey) | |||||
| 38 | 1 | 1 | 1 | 1 | 4 |
| Reference | Source of data | Methods of analysis | Context | Reflexivity | Overall |
| Qualitative studies | |||||
| 39 | 1 | 1 | 0 | 1 | 3 |
| 40 | 1 | 1 | 0 | 0 | 2 |
| 42 | 1 | 1 | 0 | 0 | 2 |
| 43 | 1 | 1 | 1 | 0 | 3 |
| 44 | 1 | 1 | 1 | 1 | 4 |
| 45 | 1 | 1 | 0 | 0 | 2 |
| 46 | 1 | 0 | 1 | 0 | 2 |
| 47 | 1 | 1 | 1 | 0 | 3 |
| 48 | 1 | 1 | 0 | 0 | 2 |
| 49 | 1 | 1 | 0 | 0 | 2 |
| 50 | 1 | 0 | 0 | 0 | 1 |
| 51 | 1 | 1 | 0 | 0 | 2 |
Notes: 1, criterion met; 0, criterion not met or unable to determine.
Abbreviations: MMAT, Mixed Methods Appraisal Tool; RCTs, randomized controlled trials.
Discussion
This paper reports on the first systematic review to have examined the conditions limiting and facilitating successful implementation of CM interventions designed for patients with dementia and their caregivers in PHC, and proposed strategies for successful adoption of CM based on the diffusion of innovation model.
As CM is intended to provide individualized care to patients through a one-to-one approach,56 the intensity of CM should reflect the needs of patients.57 Caseload is an important component of CM intensity.57 If the caseload is too large, there is the risk of a shift from proactive care (eg, prevention of a health crisis, of avoidable utilization of resources, or of premature deterioration of functional ability) toward a reactive approach (eg, dealing with acute care episodes, crisis management).58 Limiting the average caseload seems to be beneficial for frail elderly patients,57 with a load of 50–60 patients per full-time case manager being suggested57 or 12–15 patients per 8 hours of work.41 However, the load depends on the patients’ demand for care, the amount of activities (CM intensity), and the timing of CM commencement (stage of the disease).41
Another important factor to consider is the target population. There is an ongoing debate about the usefulness (or lack thereof) of CM for all stages of dementia.59 A patient-centered approach as opposed to one based on age/disease inclusion criteria seems to be better, as it allows provision of proactive care to patients at a higher risk for a major problem (eg, a behavioral problem) or at a later stage of dementia.59 Jansen et al found no benefit from CM for patients with modest symptoms of dementia behavioral problems.32 Thus, it seems that patients at the early stage of dementia do not benefit from CM.
Another major factor is to have a clearly defined role for case managers. Clear delineation of responsibilities among CM health care professionals, especially the PCP and the case manager, will help PCPs have more time for patients with more complicated chronic conditions.52 A clear interdisciplinary care protocol may be one solution for delegating certain tasks to case managers (eg, cognitive screening) in a CM intervention.9
Finally, the characteristics of case managers play a role in the success of CM. Stable human resources are necessary for a sustained and successful implementation.24 Fixsen et al considered staff selection as one of the core components of an implementation.60 Effective CM requires individuals with good communication and collaboration skills.61,62
Strengths and limitations of the systematic review
We systematically identified studies relevant to implementation of dementia CM in PHC, appraised the quality of included studies using a validated tool designed for a systematic mixed studies review, and synthesized the findings with a narrative approach. Moreover, we used an organizing framework to propose key implementation strategies. Our search strategy was comprehensive. However, like any review, our findings are limited by the amount of available research evidence.
Conclusion
Our systematic review contributes to a better understanding of the reasons for inconsistent efficacy of dementia CM in PHC. We identified key conditions limiting and facilitating successful CM implementation and key strategies for enhancing the adoption of CM in PHC. In particular, these are optimal caseload (less than 60 patients per full-time case manager), target population (patients with prominent symptoms of dementia), and delineation of the case manager’s role and their characteristics (an interdisciplinary care protocol). Thus, this review can be used to guide the implementation of dementia CM. Further research is needed to test our implementation strategies in a PHC clinical environment.
Supplementary material
Search strategy in PsycInfo
| 1. dementia/or aids dementia complex/or dementia with lewy bodies/or presenile dementia/or semantic dementia/or senile dementia/or vascular dementia/or alzheimer’s disease/or cognitive impairment/or corticobasal degeneration/or creutzfeldt jakob syndrome/or melas/or neurodegenerative diseases/or neurofibrillary tangles/or parkinson’s disease/or picks disease/or pseudodementia/or senile plaques/(65089) |
| 2. exp Cognitive Impairment/(17032) |
| 3. ((cognit* adj1 disorder?) or (cognit* adj1 impairment?)).mp. (26618) |
| 4. pick?? disease.mp. (407) |
| 5. (dementia? or alzheimer*).mp. (54351) |
| 6. lewy body.mp. (865) |
| 7. 1 or 2 or 3 or 4 or 5 or 6 (80731) |
| 8. health care delivery/or case management/or care management/or case coordination/or clinical practice/or “continuum of care”/or evidence based practice/or health care administration/or health care costs/or health care economics/or health care policy/or health care reform/or health care services/or health care utilization/or health service needs/or needs assessment/or outreach programs/or palliative care/or prevention/or private practice/or “quality of care”/or “quality of services”/or treatment/or treatment barriers/or treatment planning/(139434) |
| 9. exp therapeutic processes/or exp therapeutic environment/or treatment termination/(48222) |
| 10. 8 or 9 (183098) |
| 11. (model? or intervention? or program? or process or coordinat*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests and measures] (750405) |
| 12. 10 or 11 (846712) |
| 13. screening/or health screening/or diagnosis/or geriatric assessment/or health promotion/or screening tests/or symptom checklists/(46065) |
| 14. follow*.mp. or exp Followup Studies/(280658) |
| 15. 12 or 13 or 14 (1031166) |
| 16. clinical pathway?.mp. (121) |
| 17. disease management.mp. or exp Disease Management/(3558) |
| 18. exp Teams/or exp Work Teams/(8829) |
| 19. integrated services/or community services/or interdisciplinary treatment approach/or multimodal treatment approach/or social programs/or social services/(21170) |
| 20. 16 or 17 or 18 or 19 (33008) |
| 21. 15 or 20 (1040467) |
| 22. 7 and 21 (36566) |
| 23. limit 22 to (English or French) (35248) |
| 24. exp Primary Health Care/or exp General Practitioners/(13581) |
| 25. (communit* or home*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests and measures] (215688) |
| 26. (primary adj2 care).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests and measures] (19850) |
| 27. exp Family Medicine/or exp Family Physicians/(1552) |
| 28. exp Social Workers/or exp Social Casework/or social work*.mp. (23405) |
| 29. 25 and 28 (5640) |
| 30. nurse?.mp. or exp Nurses/(31729) |
| 31. 25 and 30 (6656) |
| 32. occupational therapist?.mp. or exp Occupational Therapists/(2979) |
| 33. 25 and 32 (712) |
| 34. exp Physical Therapists/or physiotherapist?.mp. (772) |
| 35. pharmacist?.mp. or exp Pharmacists/(1460) |
| 36. 25 and 35 (526) |
| 37. (physician? and (home? or communit* or general or family)).mp. (13177) |
| 38. exp Health Personnel/or health professional?.mp. (84756) |
| 39. 25 and 38 (12752) |
| 40. care navigator?.mp. (2) |
| 41. care navigator?.mp. or Professional Referral/(2205) |
| 42. 24 or 25 or 26 or 29 or 31 or 33 or 36 or 37 or 39 or 41 (241605) |
| 43. ((family adj1 practi*) or (general adj1 practi*)).mp. (10549) |
| 44. 42 or 43 (244710) |
| 45. 7 and 21 and 44 (6440) |
| 46. limit 45 to ((English or French) and yr=“1995-Current”) (5614) |
Acknowledgments
We thank Muriel Gueriton (specialized librarian) for literature search. This study was funded by the Canadian Institute of Health Research (grant reference KRS 119799). The first author (VK) was supported by the Fonds de Recherche du Québec-Santé as a Master’s student.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Alzheimer’s Association Alzheimer’s disease facts and figures. 2013. [Accessed May 15, 2014]. Available from: http://www.alz.org/downloads/facts_figures_2013.pdf.
- 2.Alzheimer Society of Canada Rising tide: the impact of dementia on Canadian society. 2010. [Accessed May 15, 2014]. Available from: http://www.alzheimer.ca/~/media/Files/national/Advocacy/ASC_Rising%20Tide-Executive%20Summary_Eng.pdf.
- 3.Institute of Medicine . Living Well with Chronic Illness: A Call for Public Health Action. Washington, DC, USA: The National Academies Press; 2012. [Google Scholar]
- 4.Knopman D, Donohue J, Gutterman E. Patterns of care in the early stages of Alzheimer’s disease: impediments to timely diagnosis. J Am Geriatr Soc. 2000;48(3):300–304. doi: 10.1111/j.1532-5415.2000.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 5.Pimlott NJ, Persaud M, Drummond N, et al. Family physicians and dementia in Canada: Part 1. Clinical practice guidelines: awareness, attitudes, and opinions. Can Fam Physician. 2009;55(5):506.e1–507.e5. [PMC free article] [PubMed] [Google Scholar]
- 6.Aminzadeh F, Molnar FJ, Dalziel WB, Ayotte D. A review of barriers and enablers to diagnosis and management of persons with dementia in primary care. Can Geriatr J. 2012;15(3):85–94. doi: 10.5770/cgj.15.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Dementia: a public health priority. 2012. [Accessed May 15, 2014]. Available from: http://whqlibdoc.who.int/publications/2012/9789241564458_eng.pdf.
- 8.Case Management Society of America 2012. [Accessed May 15, 2014]. Available from: http://www.cmsa.org.
- 9.Callahan C, Boustani M, Unverzagt F, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148–2157. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- 10.Vickrey B, Mittman B, Connor K, et al. The effect of a disease management intervention on quality and outcomes of dementia care: a randomized, controlled trial. Ann Intern Med. 2006;145(10):713–726. doi: 10.7326/0003-4819-145-10-200611210-00004. [DOI] [PubMed] [Google Scholar]
- 11.Newcomer R, Miller R, Clay T, Fox P. Effects of the Medicare Alzheimer’s disease demonstration on Medicare expenditures. Health Care Financ Rev. 1999;20(4):45–65. [PMC free article] [PubMed] [Google Scholar]
- 12.Fortinsky RH, Kulldorff M, Kleppinger A, Kenyon-Pesce L. Dementia care consultation for family caregivers: collaborative model linking an Alzheimer’s association chapter with primary care physicians. Aging Ment Health. 2009;13(2):162–170. doi: 10.1080/13607860902746160. [DOI] [PubMed] [Google Scholar]
- 13.Grol RP, Bosch MC, Hulscher ME, Eccles MP, Wensing M. Planning and studying improvement in patient care: the use of theoretical perspectives. Milbank Q. 2007;85(1):93–138. doi: 10.1111/j.1468-0009.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callahan C, Boustani M, Weiner M, et al. Implementing dementia care models in primary care settings: The Aging Brain Care Medical Home. Aging Ment Health. 2011;15(1):5–12. doi: 10.1080/13607861003801052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popay J. Moving Beyond Effectiveness in Evidence Synthesis: Methodological Issues in the Synthesis of Diverse Sources of Evidence. London, UK: National Institute for Health and Clinical Excellence; 2006. [Google Scholar]
- 16.Harden A. Mixed-Methods Systematic Reviews: Integrating Quantitative and Qualitative Findings. National Center for the Dissemination of Disability Research (NCDDR), Department of Education’s Office of Special Education and Rehabilitative Services (OSERS); 2010. [Accessed May 15, 2014]. Available from: http://www.ktdrr.org/ktlibrary/articles_pubs/ncddrwork/focus/focus25/Focus25.pdf. [Google Scholar]
- 17.Thomas J, Harden A, Oakley A, et al. Integrating qualitative research with trials in systematic reviews. BMJ. 2004;328(7446):1010–1012. doi: 10.1136/bmj.328.7446.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 19.Canadian Institutes of Health Research Community-based primary health care. 2014. [Accessed May 15, 2014]. Available from: http://www.cihr.ca/e/43626.html.
- 20.Gwet KL. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61(Pt 1):29–48. doi: 10.1348/000711006X126600. [DOI] [PubMed] [Google Scholar]
- 21.Overhage JM, Perkins S, Tierney WM, McDonald CJ. Controlled trial of direct physician order entry: effects on physicians’ time utilization in ambulatory primary care internal medicine practices. J Am Med Inform Assoc. 2001;8(4):361–371. doi: 10.1136/jamia.2001.0080361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casalino L, Gillies RR, Shortell SM, et al. External incentives, information technology, and organized processes to improve health care quality for patients with chronic diseases. JAMA. 2003;289(4):434–441. doi: 10.1001/jama.289.4.434. [DOI] [PubMed] [Google Scholar]
- 23.Greenhalgh T, Stramer K, Bratan T, Byrne E, Mohammad Y, Russell J. Introduction of shared electronic records: multi-site case study using diffusion of innovation theory. BMJ. 2008;337:a1786. doi: 10.1136/bmj.a1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenhalgh T, Robert G, MacFarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. 2004;82(4):581–629. doi: 10.1111/j.0887-378X.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pace R, Pluye P, Bartlett G, et al. Testing the reliability and efficiency of the pilot Mixed Methods Appraisal Tool (MMAT) for systematic mixed studies review. Int J Nurs Stud. 2012;49(1):47–53. doi: 10.1016/j.ijnurstu.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 27.Newcomer R, Spitalny M, Fox P, Yordi C. Effects of the Medicare Alzheimer’s Disease Demonstration on the use of community-based services. Health Serv Res. 1999;34(3):645–667. [PMC free article] [PubMed] [Google Scholar]
- 28.Fox P, Newcomer R, Yordi C, Arnsberger P. Lessons learned from the Medicare Alzheimer Disease Demonstration. Alzheimer Dis Assoc Disord. 2000;14(2):87–93. doi: 10.1097/00002093-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Miller R, Newcomer R, Fox P. Effects of the Medicare Alzheimer’s Disease Demonstration on nursing home entry. Health Serv Res. 1999;34(3):691–714. [PMC free article] [PubMed] [Google Scholar]
- 30.Yordi C, DuNah R, Bostrom A, Fox P, Wilkinson A, Newcomer R. Caregiver supports: outcomes from the Medicare Alzheimer’s disease demonstration. Health Care Financ Rev. 1997;19(2):97–117. [PMC free article] [PubMed] [Google Scholar]
- 31.Schoenmakers B, Buntinx F, Delepeleire J. Supporting family carers of community-dwelling elder with cognitive decline: a randomized controlled trial. Int J Family Med. 2010;2010:184152. doi: 10.1155/2010/184152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansen AP, van Hout HP, Nijpels G, et al. Effectiveness of case management among older adults with early symptoms of dementia and their primary informal caregivers: a randomized clinical trial. Int J Nurs Stud. 2011;48(8):933–943. doi: 10.1016/j.ijnurstu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Jansen AP, van Hout HP, van Marwijk HW, et al. (Cost)-effectiveness of case-management by district nurses among primary informal caregivers of older adults with dementia symptoms and the older adults who receive informal care: design of a randomized controlled trial [ISCRTN83135728] BMC Public Health. 2005;5:133. doi: 10.1186/1471-2458-5-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dias A, Dewey ME, D’Souza J, et al. The effectiveness of a home care program for supporting caregivers of persons with dementia in developing countries: a randomised controlled trial from Goa, India. PLoS One. 2008;3(6):e2333. doi: 10.1371/journal.pone.0002333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lam LC, Lee JS, Chung JC, Lau A, Woo J, Kwok TC. A randomized controlled trial to examine the effectiveness of case management model for community dwelling older persons with mild dementia in Hong Kong. Int J Geriatr Psychiatry. 2010;25(4):395–402. doi: 10.1002/gps.2352. [DOI] [PubMed] [Google Scholar]
- 36.Mittelman MS, Roth DL, Haley WE, Zarit SH. Effects of a caregiver intervention on negative caregiver appraisals of behavior problems in patients with Alzheimer’s disease: results of a randomized trial. J Gerontol B Psychol Sci Soc Sci. 2004;59(1):P27–P34. doi: 10.1093/geronb/59.1.p27. [DOI] [PubMed] [Google Scholar]
- 37.Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592–1599. doi: 10.1212/01.wnl.0000242727.81172.91. [DOI] [PubMed] [Google Scholar]
- 38.Mittelman MS, Roth DL, Coon DW, Haley WE. Sustained benefit of supportive intervention for depressive symptoms in caregivers of patients with Alzheimer’s disease. Am J Psychiatry. 2004;161(5):850–856. doi: 10.1176/appi.ajp.161.5.850. [DOI] [PubMed] [Google Scholar]
- 39.Wright L, Litaker M, Laraia M, DeAndrade S. Continuum of care for Alzheimer’s disease: a nurse education and counseling program. Issues Ment Health Nurs. 2001;22(3):231–252. doi: 10.1080/01612840152053084. [DOI] [PubMed] [Google Scholar]
- 40.Cherry D, Vickrey B, Schwankovsky L, Heck E, Plauchm M, Yep R. Interventions to improve quality of care: the Kaiser Permanente-Alzheimer’s Association Dementia Care Project. Am J Manag Care. 2004;10(8):553–560. [PubMed] [Google Scholar]
- 41.Verkade P, van Meijel B, Brink C, van Os-Medendorp H, Koekkoek B, Francke A. Delphi research exploring essential components and preconditions for case management in people with dementia. BMC Geriatr. 2010;10:54. doi: 10.1186/1471-2318-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams T. A descriptive study of the work of community psychiatric nurses with elderly demented people. J Adv Nurs. 1996;23(6):1177–1184. doi: 10.1111/j.1365-2648.1996.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 43.Black K, Fauske J. Exploring influences on community-based case managers’ advance care planning practices: facilitators or barriers? Home Health Care Serv Q. 2007;26(2):41–58. doi: 10.1300/J027v26n02_03. [DOI] [PubMed] [Google Scholar]
- 44.Black K, Fauske J. Measuring case managers’ advance care planning practice: translating focus group findings to survey development. Care Manag J. 2008;9(4):166–176. doi: 10.1891/1521-0987.9.4.166. [DOI] [PubMed] [Google Scholar]
- 45.Bogardus ST, Jr, Bradley EH, Tinetti ME. A taxonomy for goal setting in the care of persons with dementia. J Gen Intern Med. 1998;13(10):675–680. doi: 10.1046/j.1525-1497.1998.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibson G, Timlin A, Curran S, Wattis J. The impact of location on satisfaction with dementia services amongst people with dementia and their informal carers: a comparative evaluation of a community-based and a clinic-based memory service. Int Psychogeriatr. 2007;19(2):267–277. doi: 10.1017/S1041610206004261. [DOI] [PubMed] [Google Scholar]
- 47.Gladman JR, Jones RG, Radford K, Walker E, Rothera I. Person-centred dementia services are feasible, but can they be sustained? Age Ageing. 2007;36(2):171–176. doi: 10.1093/ageing/afl161. [DOI] [PubMed] [Google Scholar]
- 48.Liebel DV, Powers BA, Friedman B, Watson NM. Barriers and facilitators to optimize function and prevent disability worsening: a content analysis of a nurse home visit intervention. J Adv Nurs. 2012;68(1):80–93. doi: 10.1111/j.1365-2648.2011.05717.x. [DOI] [PubMed] [Google Scholar]
- 49.McCrae N, Banerjee S. Modernizing mental health services for older people: a case study. Int Psychogeriatr. 2011;23(1):10–19. doi: 10.1017/S1041610210001407. [DOI] [PubMed] [Google Scholar]
- 50.Netting F, Williams F. Implementing a case management program designed to enhance primary care physician practice with older persons. J Appl Gerontol. 1998;18(1):25–45. [Google Scholar]
- 51.Seddon D, Robinson CA. Carers of older people with dementia: assessment and the Carers Act. Health Soc Care Community. 2001;9(3):151–158. doi: 10.1046/j.1365-2524.2001.00291.x. [DOI] [PubMed] [Google Scholar]
- 52.van Eijken M, Melis R, Wensing M, Rikkert MO, van Achterberg T. Feasibility of a new community-based geriatric intervention programme: an exploration of experiences of GPs, nurses, geriatricians, patients and caregivers. Disabil Rehabil. 2008;30(9):696–708. doi: 10.1080/09638280701400508. [DOI] [PubMed] [Google Scholar]
- 53.Waugh F. Where does risk feature in community care practice with older people with dementia who live alone? Dementia. 2009;8(2):205–222. [Google Scholar]
- 54.Minkman MM, Ligthart SA, Huijsman R. Integrated dementia care in The Netherlands: a multiple case study of case management programmes. Health Soc Care Community. 2009;17(5):485–494. doi: 10.1111/j.1365-2524.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- 55.Chien W, Lee I. Randomized controlled trial of a dementia care programme for families of home-resided older people with dementia. J Adv Nurs. 2011;67(4):774–787. doi: 10.1111/j.1365-2648.2010.05537.x. [DOI] [PubMed] [Google Scholar]
- 56.Global Action Against Dementia. G8 Dementia Summit Declaration. [Accessed May 15, 2014]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/265869/2901668_G8_DementiaSum-mitDeclaration_acc.pdf.
- 57.Pacala JT, Boult C, Hepburn KW, et al. Case management of older adults in health maintenance organizations. J Am Geriatr Soc. 1995;43(5):538–542. doi: 10.1111/j.1532-5415.1995.tb06102.x. [DOI] [PubMed] [Google Scholar]
- 58.Sheaff R, Boaden R, Sargent P, et al. Impacts of case management for frail elderly people: a qualitative study. J Health Serv Res Policy. 2009;14(2):88–95. doi: 10.1258/jhsrp.2008.007142. [DOI] [PubMed] [Google Scholar]
- 59.MacNeil Vroomen J, Van Mierlo LD, van de Ven PM, et al. Comparing Dutch case management care models for people with dementia and their caregivers: the design of the COMPAS study. BMC Health Serv Res. 2012;12:132. doi: 10.1186/1472-6963-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fixsen DL, Naoom SF, Blase KA, Friedman RM, Wallace F. Implementation Research: A Synthesis of the Literature. Tampa, FL, USA: The National Implementation Research Network; 2005. [Google Scholar]
- 61.Hutt R, Rosen R, McCauley J. Case-Managing Long-Tem Conditions. What Impact Does it Have in the Treatment of Older People? London, UK: King’s Fund; 2004. [Google Scholar]
- 62.Meeks J. A social work case management experience in a managed care setting: the need for effective communication. Home Health Care Manag Pract. 2001;13(6):444–451. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy in PsycInfo
| 1. dementia/or aids dementia complex/or dementia with lewy bodies/or presenile dementia/or semantic dementia/or senile dementia/or vascular dementia/or alzheimer’s disease/or cognitive impairment/or corticobasal degeneration/or creutzfeldt jakob syndrome/or melas/or neurodegenerative diseases/or neurofibrillary tangles/or parkinson’s disease/or picks disease/or pseudodementia/or senile plaques/(65089) |
| 2. exp Cognitive Impairment/(17032) |
| 3. ((cognit* adj1 disorder?) or (cognit* adj1 impairment?)).mp. (26618) |
| 4. pick?? disease.mp. (407) |
| 5. (dementia? or alzheimer*).mp. (54351) |
| 6. lewy body.mp. (865) |
| 7. 1 or 2 or 3 or 4 or 5 or 6 (80731) |
| 8. health care delivery/or case management/or care management/or case coordination/or clinical practice/or “continuum of care”/or evidence based practice/or health care administration/or health care costs/or health care economics/or health care policy/or health care reform/or health care services/or health care utilization/or health service needs/or needs assessment/or outreach programs/or palliative care/or prevention/or private practice/or “quality of care”/or “quality of services”/or treatment/or treatment barriers/or treatment planning/(139434) |
| 9. exp therapeutic processes/or exp therapeutic environment/or treatment termination/(48222) |
| 10. 8 or 9 (183098) |
| 11. (model? or intervention? or program? or process or coordinat*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests and measures] (750405) |
| 12. 10 or 11 (846712) |
| 13. screening/or health screening/or diagnosis/or geriatric assessment/or health promotion/or screening tests/or symptom checklists/(46065) |
| 14. follow*.mp. or exp Followup Studies/(280658) |
| 15. 12 or 13 or 14 (1031166) |
| 16. clinical pathway?.mp. (121) |
| 17. disease management.mp. or exp Disease Management/(3558) |
| 18. exp Teams/or exp Work Teams/(8829) |
| 19. integrated services/or community services/or interdisciplinary treatment approach/or multimodal treatment approach/or social programs/or social services/(21170) |
| 20. 16 or 17 or 18 or 19 (33008) |
| 21. 15 or 20 (1040467) |
| 22. 7 and 21 (36566) |
| 23. limit 22 to (English or French) (35248) |
| 24. exp Primary Health Care/or exp General Practitioners/(13581) |
| 25. (communit* or home*).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests and measures] (215688) |
| 26. (primary adj2 care).mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests and measures] (19850) |
| 27. exp Family Medicine/or exp Family Physicians/(1552) |
| 28. exp Social Workers/or exp Social Casework/or social work*.mp. (23405) |
| 29. 25 and 28 (5640) |
| 30. nurse?.mp. or exp Nurses/(31729) |
| 31. 25 and 30 (6656) |
| 32. occupational therapist?.mp. or exp Occupational Therapists/(2979) |
| 33. 25 and 32 (712) |
| 34. exp Physical Therapists/or physiotherapist?.mp. (772) |
| 35. pharmacist?.mp. or exp Pharmacists/(1460) |
| 36. 25 and 35 (526) |
| 37. (physician? and (home? or communit* or general or family)).mp. (13177) |
| 38. exp Health Personnel/or health professional?.mp. (84756) |
| 39. 25 and 38 (12752) |
| 40. care navigator?.mp. (2) |
| 41. care navigator?.mp. or Professional Referral/(2205) |
| 42. 24 or 25 or 26 or 29 or 31 or 33 or 36 or 37 or 39 or 41 (241605) |
| 43. ((family adj1 practi*) or (general adj1 practi*)).mp. (10549) |
| 44. 42 or 43 (244710) |
| 45. 7 and 21 and 44 (6440) |
| 46. limit 45 to ((English or French) and yr=“1995-Current”) (5614) |