Abstract
Objective
To describe long-term survival in patients with severe acute respiratory distress syndrome (ARDS) and assess differences in patient characteristics and outcomes among those who receive rescue therapies (prone position ventilation, inhaled nitric oxide, or inhaled epoprostenol) versus conventional treatment.
Design and Setting
Cohort study of patients with severe hypoxemia at a University-affiliated Level 1 trauma center.
Patients
Patients diagnosed with severe ARDS within 72 hours of ICU admission between 1/1/2008 and 12/31/2011.
Methods
Data were abstracted from the medical record and included demographic and clinical variables, hospital and ICU length of stay, discharge disposition, and hospital costs. Patient-level data were linked to the Washington State Death Registry. Kaplan-Meier methods and Cox's proportional hazards models were used to estimate survival and hazard ratios.
Main Results
428 patients meeting study inclusion criteria were identified; 62 (14%) were initiated on a rescue therapy. PaO2/FIO2 ratios were comparable at admission between patients treated with a rescue therapy and those treated conventionally, but were substantially lower by 72 hours in those who received rescue therapies (54 ± 17 versus 69 ± 17 mmHg; p<.01). For the entire cohort, estimated survival probability at three years was 55% (95% CI: 51%, 61%). Among 280 hospital survivors (65%), three-year survival was 85% (95% CI: 80%, 89%). The relative hazard of in-hospital mortality was 68% higher among patients who received rescue therapy compared to patients treated conventionally (95% CI: 8%, 162%; p=0.02). For long-term survival, the hazard ratio of death following ICU admission was 1.56 (95% CI: 1.02, 2.37; p=0.04), comparing rescue versus conventional treatment.
Conclusions
Despite high hospital mortality, severe ARDS patients surviving to hospital discharge have relatively good long-term survival. Worsening hypoxemia was associated with initiation of rescue therapy. Patients on rescue therapy had higher in-hospital mortality; however, survivors to hospital discharge had long-term survival that was comparable to other ARDS survivors.
Keywords: critical care, outcomes, acute respiratory distress syndrome, refractory hypoxemia, rescue therapies
Introduction
Each year, more than 175,000 Americans are diagnosed with acute respiratory distress syndrome (ARDS) (1-6). Despite management advances such as the use of lung protective ventilation (7), ARDS continues to be associated with high morbidity and approximately 30-40% mortality (5). Patients who develop severe ARDS are typically under-represented in clinical trials. To our knowledge, there are no long-term outcome studies focused on patients with severe ARDS, including those treated with rescue therapies (6, 8-10).
Patients with severe ARDS may develop life-threatening refractory hypoxemia unresponsive to the use of conventional lung protective ventilation strategies (3). In clinical practice, as an effort to improve oxygenation in these patients, several different “rescue therapies” are often advocated, including inhaled nitric oxide, inhaled epoprostenol and prone position ventilation (3, 11-13). However, randomized controlled trials conducted to date utilized rescue therapies as an adjunctive modality to treat ARDS, rather than a “last resort” intervention for treatment of critical hypoxemia in rapidly deteriorating patients. Additionally, randomized trials typically did not describe long-term survival as an outcome (14-20).
The main objective of this study was to assess the long-term survival in a cohort of patients meeting severe ARDS criteria (5). Our secondary objectives were to describe characteristics and outcomes of patients receiving a rescue therapy compared to those treated conventionally.
Materials and Methods
Ethics Approval and Setting
The University of Washington Institutional Review Board approved this study with a waiver of informed consent. The study setting was Harborview Medical Center (HMC), a 413-bed Level 1 trauma hospital located in Seattle, Washington. HMC is affiliated with the University of Washington and is the only Level 1 trauma center serving Washington, Alaska, Montana and Idaho. There are 88 intensive care unit (ICU) beds distributed among five ICUs (medical/cardiac, trauma/surgical, neurology/neurosurgical, burn and pediatric).
Population Selection and Study Eligibility Criteria
All medical records for patients ages 18 and older, admitted to a HMC ICU between 1/1/2008 and 12/31/2011, who were mechanically ventilated and met criteria for severe ARDS were evaluated for eligibility. Severe ARDS was defined by the presence of a PaO2/FIO2 ratio ≤ 100 mm Hg and the presence of bilateral opacities on chest radiograph not fully explained by effusions, fluid overload, cardiac failure, lung/lobar collapse or nodules (5). To reduce study population heterogeneity, only patients who developed a PaO2/FIO2 ratio ≤ 100 within 72 hours of ICU admission were included.
To verify radiographic criteria, patients meeting inclusion criteria were linked to the HMC Acute Lung Injury Registry maintained by the on-site ARDS Network study coordinators. Among matched patients, we randomly reviewed 25% of the chest x-rays to ensure greater than 95% agreement. Similarly, radiographs and medical records of subjects identified by PaO2/FIO2 criteria but not included in the ALI registry were manually reviewed. Subjects whose medical records suggested congestive heart failure, fluid overload or chronic lung disease as an etiology of the radiographic findings were excluded. Patients placed on inhaled nitric oxide, inhaled epoprostenol or ventilated in the prone position for the treatment of critical hypoxemia were identified within this cohort.
Data collection
Data were electronically and manually abstracted from the HMC electronic medical record. Demographic and clinical admission variables were collected. The simplified acute physiology score II (SAPS II) was calculated for each patient upon ICU admission. For trauma patients, injury severity score (ISS) and abbreviated injury severity (AIS-Head, AIS-Chest) were collected from the HMC Trauma Registry, a database containing comprehensive information for all patients evaluated for traumatic injury at HMC (21).
Clinical and physiologic variables during the ICU stay were collected and included the study qualifying PaO2/FIO2 ratio, the PaO2/FIO2 ratio nadir within the first 24 and 72 hours of ICU admit, days spent with FIO2 > 60%, days of mechanical ventilation, use of vasopressors within the first 24 hours of ICU admission and use of neuromuscular blockade at any time during the ICU admission. We collected ventilator settings recorded in the electronic medical record closest to 0800 each day; we collected tidal volume (mL/kg), PEEP (cm H2O) delivered and mode of ventilation closest to 0800 following the study qualifying PaO2/FIO2 ratio. Daily Sequential Organ Failure Assessment (SOFA) scores were calculated for each ICU day following ICU admission. Mean and max SOFA scores for the entire ICU stay were also calculated. Additional variables associated with exposure to a rescue therapy were collected and are displayed in the electronic supplement.
Costs were estimated from the institutional perspective. For each patient, hospital charges were obtained from hospital billing records. Charges were converted to costs by applying the institutional charge-to-cost ratio (0.668). Dollar values for cost have been adjusted for inflation and are reported in 2012 U.S. dollars.
Data from the WA State Department of Health Center for Health Statistics were obtained for all deaths occurring between 1/1/2008 and 12/31/2012. Data from 2013 were not available at the time. Patient identifying information obtained from the HMC electronic medical record, including name, social security number and date of birth, were linked with the death data in order to ascertain the date, cause and location of death of our study cohort.
Endpoints
The primary endpoint was survival up to 3 years using the initial date of ICU admission as the index time. Secondary endpoints included hospital mortality, ICU and hospital length of stay, discharge disposition and costs.
Statistical Analysis
Baseline demographic characteristics and clinical variables were compared between the patients receiving a rescue therapy versus those managed conventionally using a two-sample Student's t-test with assumption of unequal variances for continuous variables and Fisher's exact test for categorical variables.
For the primary analysis, we assessed overall survival using the date of first ICU admission as the index date. Censored data were assumed to be independent of survival times. Patients who neither died in the hospital nor were located in the WA State Death Registry and had a primary residential address outside WA State were censored at the time of hospital discharge. Because we only linked with the WA State Death Registry, we considered non-WA State residents to be lost to follow-up. Survival curves were estimated using the Kaplan-Meier product limit estimator.
We compared overall survival between patients who received a rescue therapy and those treated conventionally using a Cox proportional hazards model adjusted for age, Caucasian race, admission SAPS II, and primary admission diagnosis of sepsis or pneumonia. Hospital mortality was also compared between groups using Cox regression. We estimated the cause-specific hazard ratio for death before discharge, accounting for the competing risk of hospital discharge. The model was adjusted for the same covariates as above. Cumulative incidence curves were used to illustrate in-patient mortality. We chose this approach because we used a competing risk analysis for this endpoint.
For the long-term survival and hospital mortality outcomes, we also conducted a priori planned secondary analyses using propensity score. For each outcome, we fit the following models: (i) a crude unadjusted model, (ii) a standard adjusted model as described above, and (iii) a propensity score adjusted model. Propensity scores were obtained using logistic regression to model the odds of receiving rescue therapy given the following baseline characteristics: age, gender, BMI, Caucasian race, patient population (medical, trauma, surgical non-trauma), admission diagnosis of sepsis or pneumonia, mechanical ventilation within 24 hours of ICU admit, SAPS II, PaO2/FIO2 ratio at ICU admission, tidal volume delivered, highest glucose and lowest hemoglobin. Injury severity scores were not included as these scores are only pertinent to trauma patients. We calculated the predicted probability of receiving rescue therapy, or the propensity score, for each subject from this model.
The Wilcoxon rank sum test was used to compare ICU length of stay and hospital length of stay between patients treated with rescue therapy versus conventionally. The Fisher's exact test was used to compare discharge disposition. Cost data were analyzed using a two-sample t-test (22).
A two-sided alpha level of .05 was considered statistically significant. Analyses were performed using STATA statistical software, version 12.0 (StataCorp., College Station, TX), and R statistical software, version 2.14.1 (Comprehensive R Archive Network).
Results
Study population
The final cohort included 428 patients with severe ARDS; 62 patients were treated with rescue therapy and 366 were treated conventionally (Figure 1). Demographic and clinical characteristics are displayed in Table 1. The mean age was 51 years (± 17.7, SD). Roughly 85% of patients were admitted to the medical ICU, with sepsis or pneumonia being the most common primary admission diagnosis. The mean study qualifying PaO2/FIO2 ratio for the entire cohort was 76 mm Hg (± 16).
Figure 1.
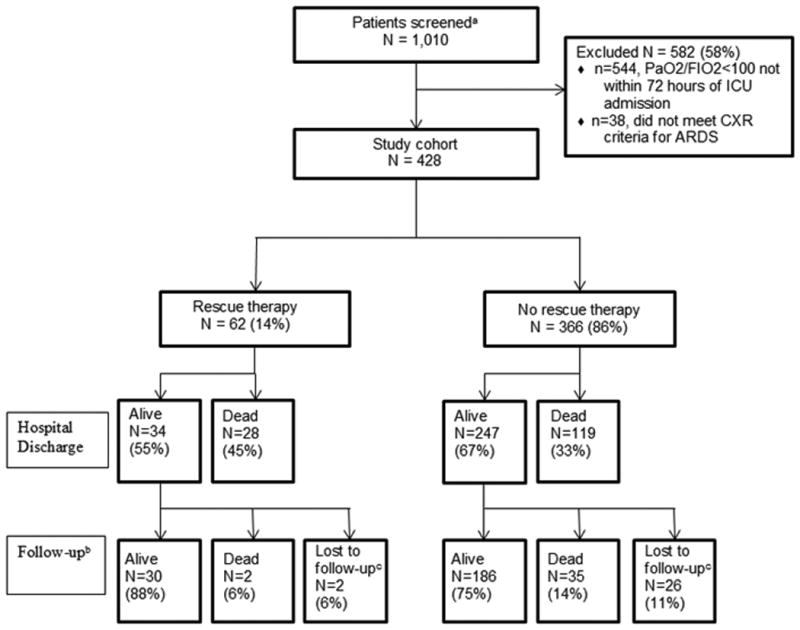
Flow diagram of the study cohort. aIncluded any patient with a PaO2/FIO2 ratio <100 at any time during the first ICU admission; bMedian follow-up duration: 449 days (IQR: 13, 1138); cIncludes subjects that neither appeared in the Washington State Death Registry nor died in the hospital AND had an address of residence outside Washington State.
Table 1. Characteristics of study cohort.
| Characteristics | All patients | Rescue Therapy | No Rescue Therapy | P valuea |
|---|---|---|---|---|
| N (%) | 428 (100) | 62 (14) | 366 (86) | -- |
| Propensity score, mean (SD) | -- | 0.28 (0.18) | 0.12 (0.11) | < 0.01 |
| Age, y, mean (SD) | 51. 0 (17.7) | 41.7 (19.0) | 52.6 (17.0) | < 0.01 |
| Male gender, n (%) | 310 (72) | 42 (68) | 268 (73) | 0.36 |
| Body mass index, mean (SD) | 29.5 (8.7) | 30.1 (10.7) | 29.4 (8.3) | 0.54 |
| Race/Ethnicity, n (%) | 0.01 | |||
| Caucasian | 296 (69) | 36 (58) | 260 (71) | |
| African American | 40 (9) | 5 (8) | 35 (9) | |
| Asian | 46 (11) | 7 (11) | 39 (11) | |
| Hispanic | 15 (4) | 8 (13) | 7 (2) | |
| Native American | 22 (5) | 4 (7) | 18 (5) | |
| Unknown | 9 (2) | 2 (3) | 7 (2) | |
| Patient population, n (%) | 0.57 | |||
| Medical | 363 (85) | 55 (89) | 308 (84) | |
| Trauma | 27 (6) | 4 (6) | 23 (6) | |
| Surgical, non-trauma | 38 (9) | 3 (5) | 35 (10) | |
| Primary admission diagnostic category, n (%) | < 0.01 | |||
| Sepsis or Pneumonia | 141 (33) | 32 (52) | 109 (30) | |
| Trauma | 113 (26) | 10 (16) | 103 (28) | |
| Neurological injury | 42 (10) | 9 (14) | 33 (9) | |
| Other | 132 (31) | 11 (18) | 121 (33) | |
| Mechanically ventilated within 24 hours of ICU admit, n (%) | 163 (38) | 33 (53) | 130 (36) | 0.01 |
| SAPS II, mean (SD) | 60.8 (18) | 60.8 (17.1) | 60.8 (18.3) | 0.99 |
| ISS in trauma patients, mean (SD) (n=169) | 32.1 (15.8) | 35.0 (14.7) | 31.7 (16.0) | 0.36 |
| AIS – Head, mean (SD) (n=102) | 3.44 (1.29) | 3.9 (1.3) | 3.4 (1.3) | 0.15 |
| AIS – Chest, mean (SD) (n=113) | 3.77 (0.76) | 3.9 (0.74) | 3.8 (0.77) | 0.64 |
| PaO2/FIO2 ratio at ICU admission, mean (SD) | 160 (108) | 150 (127) | 162 (104) | 0.47 |
| Study qualifying PaO2/FIO2 ratio, mean (SD)b | 76 (16) | 68 (18) | 78 (16) | < 0.01 |
| ICU length of stay (days), median (IQR) | 15 (8, 25) | 17 (6, 31) | 14 (8, 24) | 0.47 |
| Hospital length of stay (days), median (IQR) | 20 (10, 34) | 21 (8, 36) | 20 (10, 34) | 0.94 |
| Hospital costs, mean (SD)c | 189K (172K) | 218K (193K) | 184K (168K) | 0.20 |
SD: standard deviation; SAPS II: simplified acute physiology score II; ISS: injury severity score; AIS: abbreviated injury severity; ISS and AIS applicable only for trauma patients
Two-sample t-test with assumption of unequal variance or Fisher's exact test, comparing Rescue Therapy versus No Rescue Therapy
Value of first PaO2/FIO2 ratio <100 within 72 hours of admission to ICU
Mean hospital costs, adjusted for inflation and reported in 2012 U.S. Dollars.
Compared to patients treated conventionally, patients treated with a rescue therapy were younger (41.7 years ± 19.0 vs. 52.6 years ± 17.0, p<.01) and more likely to have ARDS secondary to pneumonia or sepsis (p<.01). Baseline severity of illness scores and the admission PaO2/FIO2 ratio were not significantly different between groups (Table 1). However, the study qualifying PaO2/FIO2 ratio - PaO2/FIO2 ratio < 100 mm Hg within 72 hours of ICU admission - was significantly different (68 mm Hg ± 18 versus 78 mm Hg ± 16, rescue compared with conventional therapy respectively, p<.01). The mean tidal volume delivered following the study qualifying PaO2/FIO2 ratio was similar between groups (7.22 mL/kg ± 1.28 versus 6.93 mL/kg ± 1.35, rescue compared with conventional therapy respectively, p=.10) (Table 2).
Table 2. Physiologic variables during ICU stay.
| Rescue Therapy (N = 62) | No Rescue Therapy (N = 366) | P valuea | |
|---|---|---|---|
| Study qualifying PaO2/FIO2 ratio, mean (SD)b | 68 (18) | 78 (16) | <.01 |
| Time from ICU admit to qualifying PaO2/FIO2 ratio (hours), median (IQR) | 8 (4,34) | 14.5 (5,39) | 0.12 |
| Lowest PaO2/FIO2 ratio within 24 hours, mean (SD)c | 93 (83) | 102 (62) | 0.41 |
| Lowest PaO2/FIO2 ratio within 72 hours, mean (SD)d | 54 (17) | 69 (17) | <0.01 |
| Tidal volume, mL/kg, mean (SD)e | 7.22 (1.28) | 6.93 (1.35) | 0.10 |
| PEEP (cm H2O), mean (SD)e | 12.8 (5.4) | 10.3 (4.1) | <.01 |
| Assist control mode of ventilation, n(%) | 60 (97) | 365 (100) | 0.06 |
| Days spent with FIO2>60%, median (IQR) | 8 (4, 17) | 5 (3, 8) | <.01 |
| Total number of ABGs collected, median (IQR)f | 8 (6,12) | 6 (5,9) | 0.01 |
| Number of ABGs with PaO2/FIO2 <100, median (IQR)g | 4 (3,10) | 2 (1,4) | <.01 |
| Days of mechanical ventilation | |||
| median (IQR) | 13 (6,27) | 11 (6,19) | 0.18 |
| mean (SD) | 19.2 (19.3) | 14.9 (14.8) | 0.04 |
| Use of vasopressors, n (%)f | 51 (82) | 240 (66) | 0.01 |
| Use of neuromuscular blockade infusion, n (%)g | 40 (65) | 83 (23) | <0.01 |
| Mean SOFA, mean (SD) | 13.2 (3.0) | 13.9 (2.8) | 0.10 |
| Max SOFA, mean (SD) | 18.0 (3.2) | 18.8 (3.3) | 0.06 |
IQR: Interquartile range; SD: Standard deviation; ABG: Arterial blood gas; SOFA: Sequential Organ Failure Assessment
For continuous variables, two-sample t-test with assumption of unequal variance for mean and Wilcoxon rank sum test statistic for median; Fisher's exact test for categorical variables.
Value of first PaO2/FIO2 ratio <100 within 72 hours of admission to ICU
Lowest PaO2/FIO2 ratio within 24 hours of admission to ICU
Lowest PaO2/FIO2 ratio within 72 hours of admission to ICU
Ventilator settings at 0800 following study qualifying PaO2/FIO2 ratio
Within first 24 hours of ICU admission
At any time during first ICU stay
Among patients treated with a rescue therapy, 36 (58%) were treated with an inhaled therapy only, 13 (21%) with prone position ventilation only, and 13 (21%) with a combination of inhaled therapy and prone position ventilation (Table 3).
Table 3. Characteristics of subjects on rescue therapies.
| Type of rescue therapya | Inhaled therapy onlyb n = 36 | Prone position ventilation only n = 13 | Combination of inhaled therapy and prone position ventilation n = 13 |
|---|---|---|---|
| Age, y, mean ± SD | 42 ± 19 | 47 ± 24 | 36 ± 14 |
| Male gender, n (%) | 26 (72) | 9 (69) | 7 (54) |
| Patient Population, n(%) | |||
| Medical | 34 (94) | 10 (77) | 11 (85) |
| Trauma | 2 (6) | 1 (8) | 1 (8) |
| Surgical, non-trauma | 0 | 2 (15) | 1 (8) |
| Time since ICU admission to initiation of therapyc (hours) | |||
| median (IQR) | 35 (6, 64) | 120 (66,166) | 33 (9,69) |
| mean ± SD | 58 ± 84 | 136 ± 100 | 49 ± 53 |
| PaO2/FIO2 ratio prior to therapy | |||
| median (IQR) | 59 (48, 65) | 177 (104, 268) | 52 (47, 71) |
| mean ± SD | 60 ± 19 | 174 ± 84 | 64 ± 31 |
| Days spent on therapy | |||
| median (IQR) | 3 (2,5) | 1 (1,2) | 6 (4,12) |
| mean ± SD | 4 ± 5 | 2 ± 2 | 9 ± 9 |
| Discharge disposition | |||
| Death, n(%) | 21 (58) | 5 (38) | 2 (15) |
| Hospital costs, mean (SD)d | 188K (202K) | 250K (178K) | 266K (183K) |
SD: standard deviation; IQR: Interquartile range
Categories are mutually exclusive
Inhaled therapy refers to inhaled nitric oxide or inhaled epoprostenol
Time to first therapy
Mean hospital costs, adjusted for inflation and reported in 2012 U.S. Dollars.
Physiologic variables pertinent to degree and onset of hypoxemia are displayed in Table 2. The lowest PaO2/FIO2 ratios within 24 hours of ICU admit were not different, however, the nadir PaO2/FIO2 ratio within 72 hours was lower in the group exposed to a rescue therapy (54 mm Hg ± 17 vs. 69 mm Hg ± 17; p<.01).
Primary endpoint
The Kaplan-Meier plot for overall survival is shown in Figure 2. Median follow-up time from ICU admission was 449 days (IQR: 13, 1138). The estimated survival probability at 3 years was 55% (95% CI: 51%, 61%) for the whole cohort, 51% (95% CI: 40%, 65%) among those initiated on a rescue therapy and 56% (51%, 62%) for patients treated conventionally. In adjusted analyses, overall survival was significantly different between groups (Table 4). Patients treated with a rescue therapy had 56% higher risk of death compared to those treated conventionally (95% CI: 2% – 137%; p = 0.04). Results from unadjusted and propensity score analyses showed similar trends. Most deaths occurred during the hospital admission, with few additional deaths observed post-hospital discharge. Among those surviving to hospital discharge, the estimated 3-year survival probability post-hospital discharge was 85% (95% CI: 80%, 89%) for the entire cohort.
Figure 2.
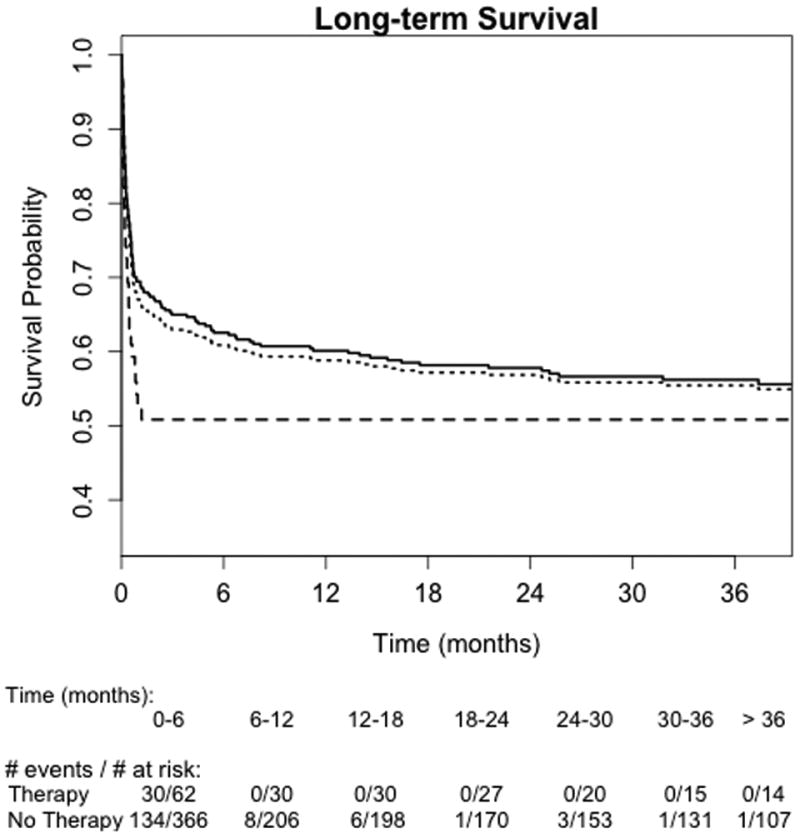
Kaplan-Meier survival curves for overall survival from date of ICU admission. Dotted line, entire cohort; solid line, no rescue therapy; hyphenated line, rescue therapy.
Table 4. Outcomes in patients treated and not treated with a rescue therapy.
| Study Endpoint | All Patients N=428 | Rescue Therapy N=62 | No Rescue Therapy N=366 | Hazard Ratio (95% CI) | P value |
|---|---|---|---|---|---|
| Primary endpoint | |||||
| Overall survival | |||||
| Crude | --- | --- | --- | 1.33 (0.89, 1.98) | 0.16 |
| Standard adjusteda | --- | --- | --- | 1.56 (1.02, 2.37) | 0.04 |
| Propensity score adjusteda,b | --- | --- | --- | 1.48 (0.95, 2.32) | 0.09 |
| Secondary endpoints | |||||
| Hospital mortality | |||||
| Crude | --- | --- | --- | 1.45 (0.96, 2.19) | 0.08 |
| Standard adjusteda | --- | --- | --- | 1.68 (1.08, 2.62) | 0.02 |
| Propensity score adjusteda,b | --- | --- | --- | 1.73 (1.07, 2.79) | 0.03 |
| P-valuec | |||||
| Discharge disposition, n (%) | .05 | ||||
| Death | 148 (35) | 29 (47) | 119 (33) | ||
| Home | 115 (27) | 8 (13) | 107 (29) | ||
| Skilled nursing facility | 94 (22) | 14 (22) | 80 (22) | ||
| Distinct rehab unit | 34 (8) | 6 (10) | 28 (8) | ||
| Other | 37 (9) | 5 (8) | 32 (9) |
Cox proportional hazard model adjusted for age, Caucasian race, admission SAPS II, diagnosis of sepsis or pneumonia.
Cox proportional hazard model adjusted for propensity score which includes Age, Gender, BMI, Caucasian race, Patient population, Admission diagnosis of sepsis or pneumonia, Mechanically ventilated on ICU admit, SAPS II, PaO2/FIO2 ratio at ICU admission, Tidal volume delivered, Highest glucose, Lowest Hemoglobin. ISS, AIS-Head and AIS-Chest not included as only pertinent to trauma patients.
Fisher's exact test
Secondary endpoints
Thirty-five percent of the cohort did not survive to hospital discharge. Among patients treated with a rescue therapy, 47% died in the hospital, while 32% of patients treated conventionally died before hospital discharge. In the rescue therapy group, the 2 post-discharge deaths occurred early (day 1 and day 5). Figure 3 shows cumulative incidence curves for hospital mortality. The risk of in-hospital mortality was 68% higher among patients who received a rescue therapy compared with patients managed conventionally (95% CI: 8% – 162%; p = 0.02, Table 4).
Figure 3.
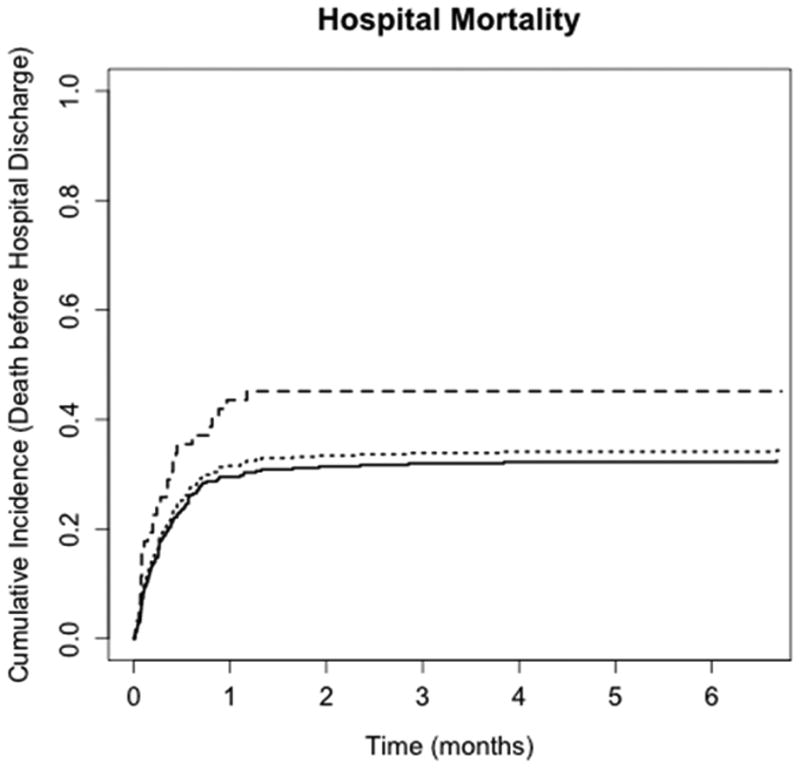
Cumulative incidence curves for in-hospital mortality or death before hospital discharge. Dotted line, entire cohort; solid line, no rescue therapy; hyphenated line, rescue therapy.
The median ICU length of stay was 17 days (IQR: 6, 31) for rescue therapy patients and 14 days (IQR: 3, 16) for patients treated conventionally (p-value = 0.47); median hospital length of stay was 21 days (IQR: 8, 36) and 20 days (IQR: 10, 34) for patients treated with and without rescue therapy (p-value = 0.94). Finally, for patients treated with rescue therapy, mean total hospitalization costs were $218K (SD 193K), compared to $184K (SD 168K) for patients not treated with rescue therapies (p = 0.20).
Discussion
In this study focusing on the long-term survival of 428 patients with severe ARDS, we found that while in-hospital mortality was high, survivors to hospital discharge had good 3-year survival. Patients selected for treatment with a rescue therapy were young, had more progressive hypoxemia and a higher risk of hospital death compared with patients managed conventionally. However, those who survived to hospital discharge also had an equally good chance of living another 3 years. Our data indicate that, unlike the setting of randomized controlled trials, in clinical practice, therapy is not initiated until severe ARDS patients have a declining PaO2/FIO2 ratio, suggesting that physicians are likely to account for the initial response to conventional therapy into their decision to employ rescue treatment (14-16, 18, 19, 23).
There are several possible explanations for the finding that adjusted mortality was higher in the group of patients receiving rescue therapy--either rescue therapies were causing excess death, or there was selection bias present with residual unmeasured confounding. To our knowledge, there are no trials that have found inhaled therapies or prone position ventilation to be associated with a higher risk of death (23, 24). We acknowledge that despite adjusting for potential confounders and secondary propensity adjusted analyses, unmeasured confounding is still possible. However, the most likely explanation for our findings is the presence of selection bias. At our institution there is no specific protocol that triggers the initiation of rescue therapy and this decision is left to the discretion of the attending physician. Therefore, the population selection reflects physician preferences with regard to the type of rescue therapy and the choice of the ARDS population. We used several strategies to minimize selection bias in our study cohort by restricting the inclusion criteria to severe ARDS patients and limiting the eligibility window to the first 72 hours since ARDS onset. In this study, however, we are unable to comment on the independent association between rescue therapies and outcome due to residual confounding and the inability to entirely account for selection bias, two limitations commonly encountered in observational studies.
Context within previous studies
Our study highlights the potential differences between patients selected for rescue therapy treatment in real world clinical practice versus those patients included in randomized, controlled trials. Moreover, prior negative trials of inhaled nitric oxide therapy and prone position ventilation were not limited to severe ARDS patients (14, 15). However, Guerin and colleagues recently reported a mortality benefit to early initiation of prone position ventilation in patients with severe ARDS (defined by PaO2/FIO2 < 150 mm Hg) (20). It remains uncertain if patients in our cohort differed in other ways from those included in randomized trials. Rigorous randomized controlled trials investigating the effectiveness of inhaled rescue therapies in patients with severe ARDS, like the recent study of prone position ventilation are needed (20).
Limitations
We acknowledge the inherent limitations of retrospective observational data. Additionally, due to the single center setting, these data may not generalize to institutions with different practices. We acknowledge that at our institution, we do not routinely use airway pressure release ventilation, while high frequency oscillatory ventilation or extracorporeal membrane oxygenation are not available. Therefore, we cannot comment on these modalities. In this study, we combined three different rescue therapies; therefore, we were unable to determine the association between a given rescue intervention and mortality. Severe ARDS patients, including those placed on a rescue therapy, represent a small proportion of ICU patients, resulting in small sample sizes in this and most prior studies (23, 24). We acknowledge that a larger sample size could have provided more robust data. For example, our sample size did not provide adequate power for the propensity-adjusted model, although the similar effect sizes between our standard and propensity-adjusted models suggests no important difference. Lastly, due to the small sample size, we anticipated that this study would not be powered to detect a difference in costs. Nevertheless, describing clinical practice patterns and long-term outcomes of patients with severe ARDS provides valuable information, and can be used to generate hypotheses for future prospective trials.
Conclusion
Severe ARDS patients have high hospital mortality; however, survivors to hospital discharge have relatively good long-term survival. The subset of severe ARDS patients who have a rapidly declining PaO2/FIO2 ratio and are often identified to be treated with a rescue therapy have even higher hospital mortality. Nonetheless, provided they are discharged alive from the hospital, their long-term survival appears to be comparable to other ARDS survivors. Historically, “rescue” therapies earned this name because they were used as a final effort to improve oxygenation in life-threatening situations (3, 4, 12, 15, 16, 18, 19, 24-26). Future prospective studies should investigate the impact timing of initiation of a rescue therapy in patients who develop early onset severe ARDS may have on survival.
Acknowledgments
Financial Support: This publication was supported by the Department of Anesthesiology & Pain Medicine and the Institute of Translational Health Sciences, University of Washington.
Dr. Khandelwal received grant support from the Department of Anesthesiology & Pain Medicine, University of Washington (Pilot grant from departmental funds). Dr. Hough's institution received grant support from National Institutes of Health. Dr. Bansal consulted for Center for Biomedical Statistics. Dr. Treggiari received support for development of educational presentations from the Cook Medical Group (Advanced airway management). Her institution received grant support from Institute of Translational Health Sciences and NIH/NHLBI.
Dr. Khandelwal: Served as primary author, designed the study protocol, obtained the data, analyzed all the data and wrote the manuscript and its revisions and approved the final version of the manuscript. She attests that no undisclosed authors contributed to the manuscript.
Dr. Hough: Designed the study protocol, reviewed the manuscript and approved the final version of the manuscript.
Dr. Bansal: Analyzed the data, reviewed the manuscript and approved the final version of the manuscript.
Dr. Veenstra: Designed the study protocol, reviewed the manuscript and approved the final version of the manuscript.
Dr. Treggiari: Designed the study protocol, obtained the data, reviewed the manuscript and approved the final version of the manuscript.
Footnotes
Institution where the work was performed: Harborview Medical Center, University of Washington
Copyright Form Disclosures: Dr. Veenstra disclosed that he does not have any potential conflicts of interest.
Disclosures: The authors all attest that they do not have any potential or actual personal or financial involvement with any company or organization with financial interest in the subject matter.
References
- 1.Heyland DK, Groll D, Caeser M. Survivors of acute respiratory distress syndrome: relationship between pulmonary dysfunction and long-term health-related quality of life. Critical care medicine. 2005;33(7):1549–1556. doi: 10.1097/01.ccm.0000168609.98847.50. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. The New England journal of medicine. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Collins SR, Blank RS. Approaches to refractory hypoxemia in acute respiratory distress syndrome: current understanding, evidence, and debate. Respiratory care. 2011;56(10):1573–1582. doi: 10.4187/respcare.01366. [DOI] [PubMed] [Google Scholar]
- 4.Pipeling MR, Fan E. Therapies for refractory hypoxemia in acute respiratory distress syndrome. JAMA : the journal of the American Medical Association. 2010;304(22):2521–2527. doi: 10.1001/jama.2010.1752. [DOI] [PubMed] [Google Scholar]
- 5.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA : the journal of the American Medical Association. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 6.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. The New England journal of medicine. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 7.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. The New England journal of medicine. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 8.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. The New England journal of medicine. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 9.Cheung AM, Tansey CM, Tomlinson G, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2006;174(5):538–544. doi: 10.1164/rccm.200505-693OC. [DOI] [PubMed] [Google Scholar]
- 10.Chiumello D, Taccone P, Berto V, et al. Long-term outcomes in survivors of acute respiratory distress syndrome ventilated in supine or prone position. Intensive care medicine. 2012;38(2):221–229. doi: 10.1007/s00134-011-2445-4. [DOI] [PubMed] [Google Scholar]
- 11.Raoof S, Goulet K, Esan A, et al. Severe hypoxemic respiratory failure: part 2--nonventilatory strategies. Chest. 2010;137(6):1437–1448. doi: 10.1378/chest.09-2416. [DOI] [PubMed] [Google Scholar]
- 12.Siobal MS, Hess DR. Are inhaled vasodilators useful in acute lung injury and acute respiratory distress syndrome? Respiratory care. 2010;55(2):144–157. discussion 157-161. [PubMed] [Google Scholar]
- 13.Esan A, Hess DR, Raoof S, et al. Severe hypoxemic respiratory failure: part 1--ventilatory strategies. Chest. 2010;137(5):1203–1216. doi: 10.1378/chest.09-2415. [DOI] [PubMed] [Google Scholar]
- 14.Angus DC, Clermont G, Linde-Zwirble WT, et al. Healthcare costs and long-term outcomes after acute respiratory distress syndrome: A phase III trial of inhaled nitric oxide. Critical care medicine. 2006;34(12):2883–2890. doi: 10.1097/01.CCM.0000248727.29055.25. [DOI] [PubMed] [Google Scholar]
- 15.Guerin C, Gaillard S, Lemasson S, et al. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2004;292(19):2379–2387. doi: 10.1001/jama.292.19.2379. [DOI] [PubMed] [Google Scholar]
- 16.Gattinoni L, Tognoni G, Pesenti A, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. The New England journal of medicine. 2001;345(8):568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 17.Mancebo J, Fernandez R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2006;173(11):1233–1239. doi: 10.1164/rccm.200503-353OC. [DOI] [PubMed] [Google Scholar]
- 18.Taccone P, Pesenti A, Latini R, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2009;302(18):1977–1984. doi: 10.1001/jama.2009.1614. [DOI] [PubMed] [Google Scholar]
- 19.Adhikari NK, Burns KE, Friedrich JO, et al. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007;334(7597):779. doi: 10.1136/bmj.39139.716794.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerin C, Reignier J, Richard JC, et al. Prone Positioning in Severe Acute Respiratory Distress Syndrome. The New England journal of medicine. 2013 doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y. Cost analysis with censored data. Medical care. 2009;47(7 Suppl 1):S115–119. doi: 10.1097/MLR.0b013e31819bc08a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lumley T, Diehr P, Emerson S, et al. The importance of the normality assumption in large public health data sets. Annual review of public health. 2002;23:151–169. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- 23.Adhikari NK, D P, Lundin S, et al. Inhaled Nitric Oxide Does Not Reduce Mortality in Patients With Acute Respiratory Distress Syndrome Regardless of Severity: Systematic Review and Meta-Analysis. Critical care medicine. doi: 10.1097/CCM.0b013e3182a27909. [DOI] [PubMed] [Google Scholar]
- 24.Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive care medicine. 2010;36(4):585–599. doi: 10.1007/s00134-009-1748-1. [DOI] [PubMed] [Google Scholar]
- 25.Davis JW, Lemaster DM, Moore EC, et al. Prone ventilation in trauma or surgical patients with acute lung injury and adult respiratory distress syndrome: is it beneficial? The Journal of trauma. 2007;62(5):1201–1206. doi: 10.1097/TA.0b013e31804d490b. [DOI] [PubMed] [Google Scholar]
- 26.Kopterides P, Siempos II, Armaganidis A. Prone positioning in hypoxemic respiratory failure: meta-analysis of randomized controlled trials. Journal of critical care. 2009;24(1):89–100. doi: 10.1016/j.jcrc.2007.12.014. [DOI] [PubMed] [Google Scholar]


