Abstract
Objective
To examine pediatric intensivist sedation management, sleep promotion, and delirium screening practices for intubated and mechanically ventilated children.
Design
An international, online survey of questions regarding sedative and analgesic medication choices and availability, sedation protocols, sleep optimization, and delirium recognition and treatment.
Setting
Member societies of the World Federation of Pediatric Intensive and Critical Care Societies were asked to send the survey to their mailing lists; responses were collected from July 2012 to January 2013.
Interventions
Survey
Measurements and Main Results
The survey was completed by 341 respondents, the majority of whom were from North America (70%). Twenty-seven percent of respondents reported having written sedation protocols. Most respondents worked in pediatric intensive care units (PICUs) with sedation scoring systems (70%), although only 42% of those with access to scoring systems reported routine daily use for goal-directed sedation management. The State Behavioral Scale was the most commonly used scoring system in North America (22%), with the COMFORT score more prevalent in all other countries (39%). The most commonly used sedation regimen for intubated children was a combination of opioid and benzodiazepine (72%). Most intensivists chose fentanyl as their first-line opioid (66%) and midazolam as their first-line benzodiazepine (86%), and prefer to administer these medications as continuous infusions. Propofol and dexmedetomidine were the most commonly restricted medications in PICUs internationally. Use of earplugs, eye masks, noise reduction, and lighting optimization for sleep promotion was uncommon. Delirium screening was not practiced in 71% of respondent’s PICUs, and only 2% reported routine screening at least twice a day.
Conclusions
The results highlight the heterogeneity in sedation practices among intensivists who care for critically ill children, as well as a paucity of sleep promotion and delirium screening in PICUs worldwide.
Keywords: sleep, sedation, analgesia, delirium, pediatric, intensive care units, mechanical ventilation, circadian rhythm
INTRODUCTION
Optimal sedation management is an integral component of the comprehensive medical care of a mechanically ventilated child. The heterogeneity in ages and diagnoses in the pediatric intensive care unit (PICU) can create particular challenges in sedation of intubated children, with a myriad of physiologic considerations for each sedative or analgesic medication administered in a given clinical situation. Adequate sedation and analgesia is required for the comfort and safety of the child, as well as to promote patient-ventilator synchrony. The frequent noise and bright lights of the PICU environment and the recurrent interventions by the medical care team add to the stressors that a child experiences when critically ill, and unlike adults, many children cannot cooperate with or understand the need for medical instrumentation and interventions.(1) These factors combined with the development of physiologic tolerance often lead to a cycle of increasing sedative and analgesic medications to maintain a child’s comfort and safety and improve sleep.
Most medications used for sedation and analgesia in the PICU, commonly opioids and benzodiazepines, are known to decrease slow-wave sleep and rapid-eye movement sleep (REM sleep).(2, 3) In addition, benzodiazepines are a strong independent risk factor for the development of delirium.(4, 5) ICU delirium increases morbidity and mortality in critically ill adults, and emerging evidence suggests that delirium may be clinically relevant in critically ill children.(6–9) Normal sleep-wake homeostasis has a critical role in immunity and thermoregulation, as well as prevention of delirium and the development of a catabolic state, which may influence the rate of recovery from critical illness.(2, 10)
There is no universally accepted goal-directed approach to sedation of mechanically ventilated children. The sedative and analgesic medications available for use in the PICU can vary from hospital to hospital, and choice of specific medications may differ in different areas of the world. As care providers change over the course of a child’s PICU admission, variations in sedation goals and approaches may be introduced from both physicians and nurses. In addition to the pharmacologic management of sedation and analgesia, many non-pharmacologic adjunctive approaches have been described in adult and pediatric critical care literature, including sleep promotion and early delirium recognition.(1, 2, 8, 11, 12) To characterize the current state of practice internationally, we designed a detailed survey to describe the experiences and approaches of pediatric intensivists with regard to sedative availability, preferences and strategies, PICU environment, sleep optimization, and delirium recognition and treatment. We hypothesized that there is significant variability in the approaches to sedation of the child requiring long-term mechanical ventilation, and predict that sleep promotion and delirium screening are not routinely practiced in PICUs internationally.
METHODS
On July 5, 2012, the World Federation of Pediatric Intensive and Critical Care Societies (WFPICCS) invited all member societies to send the electronic survey to their membership. The survey was administered by Survey Monkey (www.surveymonkey.com) and was also made available by a direct link from the WFPICCS internet homepage. In September 2012, a reminder was sent in the WFPICCS newsletter. All participants were informed that individual responses would remain anonymous and confidential. The survey closed to further responses on January 15, 2013. Survey questions and topics were developed by content experts in the fields of pediatric critical care medicine and pediatric anesthesiology, and the survey was pilot tested among multiple pediatric intensivists for feedback regarding question clarity and the survey interface. WFPICCS leadership and the Johns Hopkins Institutional Review Board approved the study and final survey for distribution.
The survey was in English and consisted of 40 questions divided into four sections by concept. The first section ascertained particular demographics, including the size and type of hospital, number of years the intensivist had been in practice, and if the institution had a fellowship training program. Respondents were also asked about the physical layout of their PICU. The second section asked about medications available in the PICU, whether any sedative or analgesic agents had restrictions, and their preferences in medication for children who they anticipated to require mechanical ventilation longer than 24 hours. The third section had questions regarding sedation protocols/algorithms used in their PICU and methods used for sedation assessment. The last section gathered information about PICU sleep promotion and delirium screening practices. Questions were closed-ended, multiple-choice design, but many included an “other” option for a free-text response. The questionnaire is included as an appendix to this manuscript.
Data were analyzed with the statistical software package STATA version 11.0 (StataCorp LP, College Station, TX). Characteristics of respondents were summarized with frequencies and proportions for categorical variables and means and standard deviations for continuous variables. Medication preferences and availability were compared across intensivist demographics and other survey responses by using chi-square analysis.
RESULTS
Intensivist and PICU Demographics
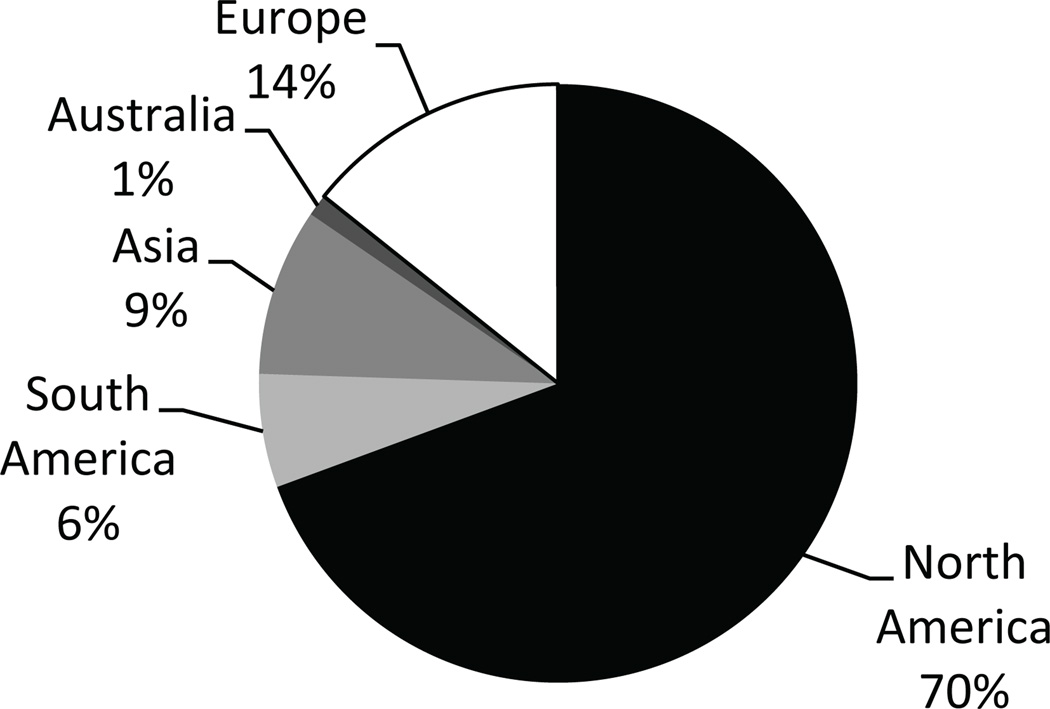
Demographic data for intensivist respondents and the PICU settings are shown in Table 1. In total, 341 respondents participated in the electronic survey through the e-mail link or online invitation. The majority of attending physician respondents (81%) had more than five years of experience caring for critically ill children, and most respondents (56%) reported their primary practice setting as an academic university. Across the sample, the PICUs were reported to accommodate an average of 17.6 (SD, 12.1) critically ill children. As shown in Figure 1, North America was the continent with the largest proportion of respondents (70%), followed by Europe (14%) and Asia (9%).
TABLE 1.
Pediatric Intensivist and Intensive Care Unit Demographics
| Characteristic | No. of Respondents (%) |
|---|---|
| Female sex | 184 (53) |
| Age in years | |
| 21–29 | 15 (4) |
| 30–39 | 99 (29) |
| 40–49 | 113 (33) |
| 50–59 | 103 (30) |
| ≥60 | 16 (5) |
| Care for both children and adults in ICU | 45 (13) |
| Years of practice | |
| 0–5 | 65 (19) |
| 6–10 | 79 (24) |
| 11–15 | 58 (17) |
| 16–20 | 45 (13) |
| >20 | 89 (26) |
| Position | |
| Attending physician | 223 (70) |
| Critical care nurse | 67 (21) |
| Fellow | 20 (6) |
| Nurse practitioner | 8 (3) |
| Fellowship training program in PICU | 186 (54) |
| Number of PICU beds | 17.6 (SD±12.1) |
| Mechanically ventilated children | |
| <20% | 50 (15) |
| 20–40% | 145 (45) |
| 41–70% | 101 (31) |
| >70% | 28 (9) |
| Practice setting | |
| Academic/university | 192 (56) |
| Private/community | 37 (11) |
| Teaching hospital/non-university | 112 (33) |
ICU = intensive care unit; PICU = pediatric intensive care unit.
Figure 1.
Continents represented by survey participants.
Sedation Monitoring of Mechanically Ventilated Children
Written sedation protocols with treatment algorithms were in place in only 27% of the respondents’ PICUs, and of those, 52% of the sample reported that the protocols were physician-driven (Table 2). North American respondents reported a higher percentage of nursing-driven protocols (58%; p=0.02). A physician-driven protocol was defined as one from which the physician directs all medication and dosing changes, in contrast to a nursing-driven protocol where the nurse has the ability to independently titrate medications to the desired level of sedation, within set limits as defined by an initial physician order.
TABLE 2.
Pediatric Intensive Care Unit Sedation Scoring and Protocols
| Survey Item | North America | All Other Countries |
|---|---|---|
| Presence of unit-wide written sedation protocol for all mechanically ventilated patients (n=322 respondents) | ||
| Yes | 46 (20%) | 41 (44%) |
| No | 182 (80%) | 52 (56%) |
| Driver of unit sedation protocol if present (n=84 respondents) | ||
| Nurses | 25 (58%) | 11 (27%) |
| Physicians | 16 (37%) | 28 (68%) |
| Combination | 2 (5%) | 2 (5%) |
| Unit-wide sedation scoring system used for mechanically ventilated patients (n=310 respondents) | ||
| No scoring system | 61 (27%) | 33 (37%) |
| COMFORT | 46 (21%) | 35 (39%) |
| RASS | 23 (10%) | 5 (6%) |
| SBS | 50 (22%) | 2 (2%) |
| Ramsay | 27 (11%) | 13 (14%) |
| Other | 16 (17%) | 2 (2%) |
| Frequency of sedation scoring system use for daily patient care, i.e., goal setting on rounds (n=220 respondents) | ||
| Always or usually | 66 (40%) | 28 (48%) |
| Frequency of bispectral index monitoring for depth of sedation (n=317 respondents) | ||
| Always or usually | 4 (2%) | 7 (8%) |
| Level of satisfaction with the state of sedation practice in primary practice setting (n=291 respondents) | ||
| Satisfied | 128 (60%) | 50 (64%) |
| Neutral | 42 (20%) | 12 (15%) |
| Dissatisfied | 43 (20%) | 16 (21%) |
All parameters refer to children receiving mechanical ventilation in the intensive care unit.
RASS = Richmond Agitation-Sedation Scale; SBS = State Behavioral Scale.
Sedation scoring systems, defined as a tool utilized to assess depth of sedation and set patient-specific goals for sedation, were in place in 70% of the respondents’ PICUs, but only 42% stated that they were used as a routine part of daily rounds and patient-care goals. Eleven percent reported that although a sedation scoring system existed in their PICU, it was never used. Most commonly used sedation scoring scales included COMFORT (37%), State Behavioral Scale (SBS; 24%), and Ramsay (18%). While 72% reported that the bispectral index monitor (BIS) was not available in their unit, 4% reported consistent use of this modality for mechanically ventilated children.
Sedative-Analgesic Medication Preference
In response to a question regarding preferred initial sedative regimen for a child with primary respiratory failure, 72% of survey participants chose a combination of opioid and benzodiazepine, whereas 12% preferred an opioid alone, 8% preferred using an opioid with dexmedetomidine, and only 1% used a combination of benzodiazepine and dexmedetomidine. Less than 1% used dexmedetomidine alone when initiating a sedation regimen. Interestingly, 2% reported the routine use of propofol in the initial sedation regimen, and this was always in combination with an opioid and benzodiazepine. Similarly, 2% used ketamine routinely along with an opioid and benzodiazepine.
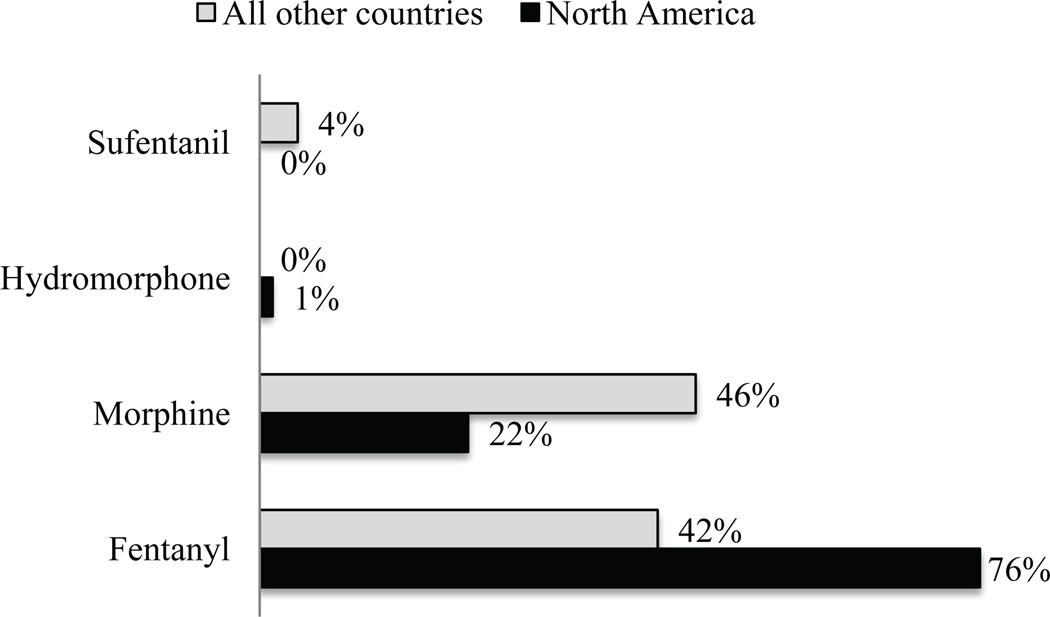
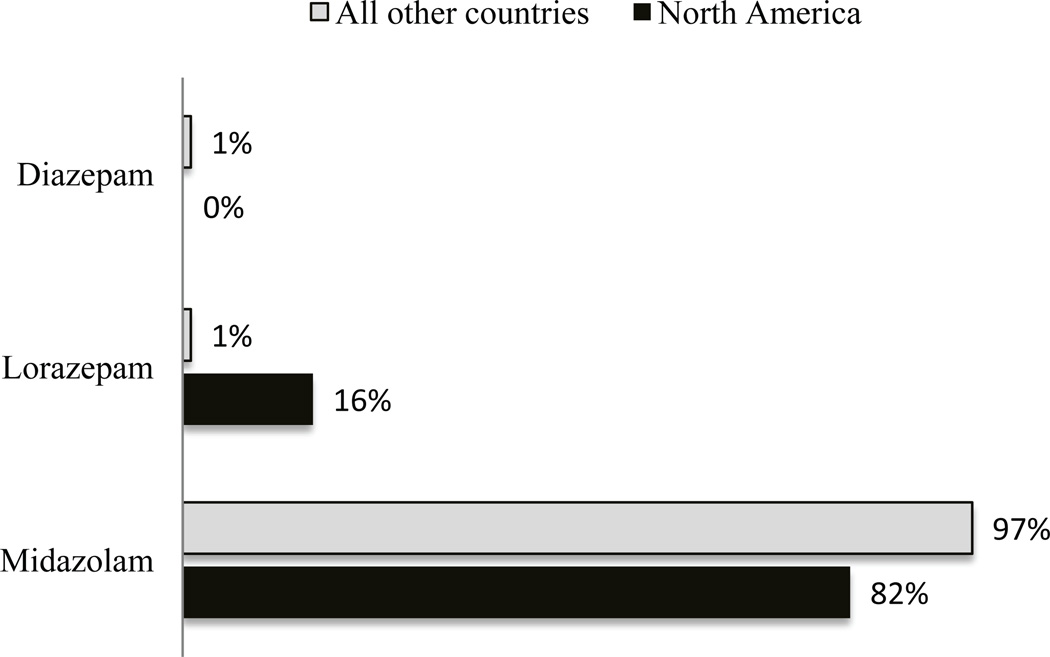
Most respondents (66%) preferred fentanyl as the opioid for analgesia during sedation, whereas 28% preferred morphine, although respondents from countries outside of North America demonstrated a more even preference in comparison—42% for fentanyl and 46% for morphine (Fig. 2; p<0.001). The majority (93%) chooses to administer opioid as a maintenance infusion when it is used as part of a sedation regimen. Midazolam was the benzodiazepine of choice for most respondents (86%; Fig. 3) followed by lorazepam (12%). Similar to opioid administration, most intensivists initiate benzodiazepines as a maintenance infusion (80%), with equal proportions choosing scheduled interval dosing and as-needed dosing (10% each). Propofol and dexmedetomidine were the most commonly restricted drugs for sedation in PICUs. Of 246 respondents who have dexmedetomidine available at their institution, 25% stated they have either duration or indication restrictions.
Figure 2.
Preferred opioid for analgesia in mechanically ventilated children (%); p<0.001 when comparing opioid preference between North America and all other countries.
Figure 3.
Preferred benzodiazepine for sedation in mechanically ventilated children (%); p<0.001 when comparing benzodiazepine preference between North America and all other countries
PICU Layout, Sleep Promotion, and Delirium Screening
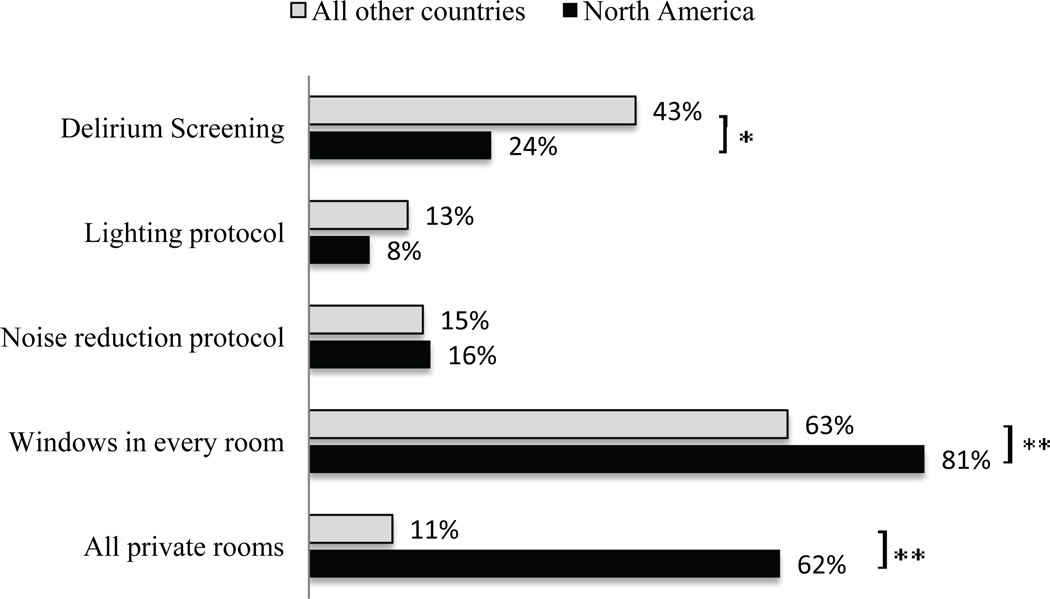
As shown in Figure 4, the majority of North American respondents (62%) reported that their PICU consists of all private rooms, in contrast to 11% for all other countries (p<0.001). Seventy-seven percent of all respondents reported that all patient rooms had windows, and 4% reported no windows in any of the patient rooms. A small proportion reported the presence of unit protocols to optimize noise (16%) and light exposure (9%) for sleep promotion. Of those surveyed, 78% had never observed earplug use in their PICU, and 65% had never observed use of eye masks. Of those who had observed eye mask or earplug use, 5% and less than 1%, respectively, reported consistent use for all mechanically ventilated children.
Figure 4.
Delirium screening, sleep promotion, and pediatric intensive care unit layout (%). p-value for comparison between North America and all other countries *p=0.01 **p <0.001
Seventy-one percent of respondents reported that their unit does not perform routine delirium screening, and only 2% reported that delirium screening is performed on every child at least once per shift. Of the respondents who reported frequent or occasional delirium screening, the only validated delirium screening tool reported was the Pediatric Confusion Assessment Method-ICU (pCAM-ICU, n=6). Multiple respondents listed withdrawal assessment tools such as the WAT-1, SOS (Sophia Observation withdrawal Symptoms scale) and Finnegan as their method of delirium screening. Four respondents reported that their PICU utilizes a unit-specific delirium screening tool.
Free-text responses
Fifty-four respondents provided detailed comments regarding sedation of mechanically ventilated children. One common theme was frustration over inconsistencies in sedation goals. Many intensivists voiced concerns that bedside nurses often want a child to be immobile, without any signs of awareness, leading to oversedation. Several expressed interest in pursuing delirium education due to concerns that agitated, critically ill children may be receiving additional doses of psychoactive medications instead of therapy for what may actually be undiagnosed delirium.
DISCUSSION
Our study is the first international survey to characterize sedation, sleep promotion, and delirium screening practices in the pediatric population. We found that despite the need for, and near universal application of, pharmacologic sedation in the PICU, a minority of programs have formalized methods of sedation assessment, drug choice and delivery, or therapeutic goal direction. Unlike the adult and neonatal ICUs, sleep promotion is exceedingly uncommon in the PICU. Finally, the interplay between sedation, sleep, and ICU delirium is not prioritized in PICU management, even though delirium prevention with nonpharmacologic behavioral interventions including sleep promotion has become a major therapeutic initiative in the care of critically ill adults. Thus, we found significant variation in practice, with many opportunities for future study and therapeutic intervention in a population undergoing active neurocognitive development.
Our finding that less than one-third of respondents utilize a standardized, unit-wide, written sedation protocol for mechanically ventilated children is different from that of a previous survey of PICU fellowship programs in which 66% reported the use of a written sedation policy.(13) This difference is likely due to the survey questions themselves, as we defined the written sedation protocol to be a specific algorithm that is consistently used in the PICU, in contrast to a written sedation policy, which may be more general and used as a guideline. The current study was an international survey that encompassed teaching and non-teaching hospitals, whereas the previous study was limited to only American fellowship program directors with a 59% response rate. Nevertheless, we found that even in PICUs with fellowship programs, the minority (35%) utilized standardized sedation protocols, and sub-group analysis demonstrated no significant difference in the use of sedation protocols (p=0.73) and sedation scoring (p=0.13) between private and university-based respondents.
A growing body of literature has demonstrated the benefit of standardized sedation treatment algorithms in critically ill adults. Sedation protocols in the adult ICU have been shown to decrease days of mechanical ventilation, reduce hospital length of stay, and promote earlier ambulation. Perhaps most importantly, it reduces the incidence and severity of delirium, a negative consequence of critical care.(14–16) A small number of published studies suggest that these benefits extend to pediatrics, and a large multicenter randomized controlled trial is ongoing.(17–19)
Much to our surprise, we found that most intensivists do not utilize formalized methods of sedation assessment to guide drug choice and delivery, although several validated measures exist. The COMFORT score continues to be the most commonly used scoring system overall, although North American respondents report increased usage of the SBS and Richmond Agitation-Sedation Scale (RASS) compared to that in a previous U.S. survey.(13) The SBS was designed and validated in 2006 to define the sedation-agitation continuum in critically ill infants and children to guide goal-directed therapy.(20) RASS, the sedation scale most commonly used in critically ill adults, has not been validated in critically ill children.(21)
Even when respondents stated there was a specific sedation scoring system designated in their PICU, the majority do not consistently use them to set goals for sedation management on a day-to-day basis. This finding is concerning because sedation is not one-size-fits-all. Rather, it requires titration to effect. Thus, the failure to measure the level of sedation and to actually use these measures to guide and titrate therapy may result in oversedation or undersedation, both of which have the potential to produce significant patient morbidity and mortality. Within a complex team framework of nurses, residents, nurse practitioners, fellows, respiratory therapists, and attendings who provide and manage sedation, the use of a streamlined sedation scoring tool in conjunction with a goal-directed treatment algorithm is imperative to facilitate consistent communication and optimal therapy.
A small proportion of care providers utilize automated tools such as the BIS for bedside monitoring of sedation. Initially designed to decrease the incidence of intraoperative awareness during general anesthesia in adults, the BIS monitor uses an algorithmic analysis of the electroencephalogram (EEG) to provide a single, dimensionless number to guide titration of anesthetic agents.(22) Because the algorithm of the BIS monitor is derived from adult EEG data and the EEG of children changes as the brain matures, it remains controversial whether BIS can be generalized to infants and children in the operating room and ICU settings.(23–26)
We found substantial differences in how sedation is provided, particularly in regard to drug preferences and delivery. Although most providers choose a combination of opioid and benzodiazepine as their initial regimen for sedation and analgesia, many opt for opioid alone. Opioids are primarily analgesics that produce sedation and either euphoria or dysphoria as wanted or unwanted side effects. Opioid alone may be chosen by PICU providers because they are less likely to adversely affect hemodynamics and are perceived as short-acting, an erroneous notion given the increased context-sensitive half-life when administered as a continuous infusion.(27, 28) This is especially true for fentanyl, now the most commonly prescribed opioid in North American PICUs, supplanting morphine.(13) Opioid use as a primary sedative agent in the PICU warrants concern due to a lack of anxiolytic and amnestic properties at low doses, leading to dose escalation to achieve the desired level of sedation and increased risk of tolerance and withdrawal. In addition, the child receiving opioid as a primary sedative is exposed to an increased risk of all the adverse effects associated with both long-term and high-dose opioid use, including gastrointestinal dysmotility that may lead to feeding intolerance during a period when optimal nutrition is crucial.
Fentanyl may also be chosen in the PICU due its hepatic clearance, facilitating use in children who have renal insufficiency. Although data are limited, the choice of fentanyl over morphine in the PICU may also be influenced by morphine’s association with histamine release, which may concern care providers when potential hypotension is an undesirable risk.(29, 30) Morphine is the most inexpensive opioid available. Emerging evidence has shown that when used as an infusion it is more favorable than fentanyl with regard to need for dose escalation, incidence of withdrawal, and length of hospital stay.(31, 32) Opioid tolerance and withdrawal pose major challenges for all providers in the PICU, and the predilection for fentanyl may be a trend that needs further evaluation through a randomized controlled trial.
Midazolam is the benzodiazepine most commonly used for sedation in mechanically ventilated children. Benzodiazepines are GABA(A) agonists that are sedative/hypnotic/amnestics with little to no analgesic property. Midazolam has a short plasma half-life, rapid onset, and is amenable to titration as a continuous infusion. Although benzodiazepines provide the benefits of amnesia, anxiolysis, and sedation, one major disadvantage is the increased risk of delirium with prolonged administration.(4, 5, 12, 33) Adult guidelines for long-term sedation recommend lorazepam, based on high-grade evidence that lorazepam infusions require fewer dosage adjustments, require less time to achieve adequate sedation, and provide more predictability for awakening times and time to extubation compared to midazolam.(14, 34, 35) Midazolam pharmacokinetics and pharmacodynamics have been shown to change with age, leading to significant inter-individual variability.(26, 36, 37) To date, direct comparisons of midazolam and lorazepam have not been made in the PICU population, and data are limited on the use of diazepam in the ICU setting.(38) Consistent with other ICU surveys, we found that diazepam is rarely used as a first-line sedative agent in mechanically ventilated children.(13, 38, 39)
Dexmedetomidine, an alpha-2 adrenergic agonist with analgesic, sedative, and anxiolytic properties, has emerged over the last decade as an attractive option for sedation of critically ill children because of its favorable hemodynamic profile and preservation of respiratory function.(40) Compared to all sedatives and analgesics available, dexmedetomidine has been shown to induce an EEG pattern with the most similarities to natural sleep.(41–43) Most respondents did not report restrictions on the use of dexmedetomidine in their units, yet only 10% include it as part of the initial regimen. Although the currently patent-protected dexmedetomidine is more expensive than the commonly used benzodiazepines, studies have demonstrated comparatively less cost because time to extubation and ICU and hospital length of stay are reduced.(44) Dexmedetomidine has been associated with decreased opioid and benzodiazepine administration in critically ill children, as well as decreased inotropic support.(45–47) Despite these favorable qualities, anecdotal evidence suggests that long-term use of dexmedetomidine infusions can result in physiologic tolerance, withdrawal, and adrenal insufficiency.(48–51) Therefore, additional study is warranted.
Our survey demonstrates a clear preference for continuous infusions over scheduled, intermittent dosing of sedatives and analgesics during mechanical ventilation. Continuous infusions are advantageous because they are titratable, maintain steady-state plasma drug levels, decrease line breaks and infection risk, and are less labor-intensive for a busy critical care nurse. Disadvantages include potential for oversedation from drug accumulation and, perhaps more important, an increased risk for tolerance and escalation of sedative and analgesic doses.(52–54) Intermittent, scheduled dosing of sedative and analgesic medications in the appropriate target population may effectively provide many of the purported benefits of sedation interruption, while exposing a child to decreased overall doses of psychoactive medications.(55, 56) These benefits must be considered alongside with the potential increase in nursing workload necessary to care for an awake, critically ill child.(57)
Finally, this study underlines an impending and critical need for sleep promotion and delirium screening in critically ill pediatric patients. Sedatives and analgesics are often increased to improve the subjective assessment of “sleep,” yet these very medications decrease restorative sleep, leading to a vicious cycle of sleep disturbances that manifest as agitation and delirium.(2) A small number of studies that have objectively evaluated sleep in critically ill children have shown significant decreases in slow-wave sleep and REM sleep, which are integral to neuronal development in childhood.(1) Despite the availability of proven, noninvasive, and inexpensive modalities such as earplugs and eye masks to decrease sleep interruptions during nighttime hours, such methods are rarely used in adult and pediatric ICU settings.(11, 58, 59)
A minority of PICUs employ noise-reduction strategies to target WHO-recommended levels of noise (<30 dBA Leq day and nighttime). Several studies have demonstrated that ICU noise levels are frequently greater than 50 dBA throughout a 24-hr period, with several intermittent peaks to >80 dBA.(60, 61) Private rooms provide some protection from general ICU noise and may improve the quality of patient sleep.(62) Light levels are also integral to maintaining circadian rhythmicity during critical illness. Receiving exposure to natural light during the daytime and minimizing nighttime light exposure are necessary for hormonal regulation, specifically melatonin secretion, to optimize the sleep-wake cycle.(63, 64)
Delirium in critically ill children is emerging as an extremely important diagnosis that must be recognized by all pediatric intensivists.(8, 9, 65–67) A combination of critical illness, sedative-analgesic medications, and sleep disruption place the mechanically ventilated child at increased risk for delirium. Consequences can include increased morbidity and mortality from prolonged intubation and weaning of medications. Despite the availability of validated tools for delirium screening and diagnosis, our study draws attention to a PICU culture that does not prioritize this important diagnostic consideration. The low prevalence of PICU delirium screening may be related to multiple factors, including knowledge gaps surrounding the importance of delirium in critically ill children, availability of resources to perform unit-wide screening, and accessibility of therapies for treatment of delirium. Several respondents listed withdrawal screening tools such as the SOS and WAT-1 as their method of delirium screening, highlighting the need for widespread delirium education. Under-recognition of delirium may also be secondary to the reluctance of intensivists to engage the pediatric neuropsychiatrist as yet another consultation team, whose recommendations for nonpharmacologic and pharmacologic therapies may seem challenging for an already burdened patient, family and PICU team.
Most survey results were similar across respondents internationally, although there were some interesting differences. The preference for fentanyl as the first-line opioid is higher in North America compared to all other countries, which may be due to differences in cost considerations or simply PICU culture that has been propagated based on fentanyl’s purported advantages over morphine. North American respondents also reported a higher percentage of nursing-driven sedation protocols, which may reflect an increasing focus on nursing autonomy in patient care as a predictor of greater job satisfaction and improved patient care within a team framework.(68–70)
The current study has some notable limitations. First, the respondents may not be representative of PICU practitioners internationally, as the 341 respondents are a small proportion of all intensivists caring for critically ill children. It is also possible that, as a result of “chain sampling,” several respondents work in the same PICU, which may lead to bias; other PICUs may not have been represented if no staff member received the survey link. Second, a respondent’s practice may differ significantly from others in their center, potentially decreasing generalizability. Additionally, there was a higher proportion of responses from North American intensivists, which may decrease the generalizability of data on the international level. Finally, another limitation stems from questions the survey did not ask. For example, issues such as sedation interruption, rotation, weaning practices, or use of neuromuscular blockade were not included in the survey. Nevertheless, our study delineates the international heterogeneity of practice and differing approaches to sedation, sleep, and delirium in critically ill children.
CONCLUSION
Despite the need for, and near universal application of, pharmacologic sedation in the PICU, only a minority of intensivists utilize formalized methods of sedation assessment, drug choice and delivery, or even therapeutic goal direction. This study highlights the heterogeneity in sedation practices among pediatric intensivists, as well as a paucity of sleep promotion and delirium screening in PICUs worldwide. Thus, numerous opportunities exist for future study and therapeutic intervention in a population undergoing active neurocognitive development.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Tex Kissoon for his support in dissemination of the survey to the WFPICCS membership. We are also grateful to Dr. David Nichols and Dr. Blaine Easley for their integral support of our work to understand the role of sedation, sleep, and delirium as modulators of outcomes in the PICU. This work was supported by the Johns Hopkins CTSA Award Number 5KL2RR025006 from the National Center for Advancing Translational Sciences of the National Institutes of Health. Dr. Punjabi has received research support from the National Institutes of Health (HL075078). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Copyright Form Disclosures:
Dr. Kudchadkar is employed by Johns Hopkins Hospital. Dr. Kudchadkar and her institution received grant support from the Johns Hopkins Institutional KL2 (CTSA) grant. Dr. Yaster consulted for Endo Pharma, Purdue Pharma, and PRA; is employed by JHU; provided expert testimony (legal reviews); and received grant support from NIH and Smiths Medical. Dr. Punjabi received support for article research from NIH (HL075078). His institution received grant support from Resmed and Respironics.
REFERENCES
- 1.Kudchadkar SR, Aljohani OA, Punjabi NM. Sleep of critically ill children in the pediatric intensive care unit: A systematic review. Sleep Med Rev. 2013 doi: 10.1016/j.smrv.2013.02.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kudchadkar S, Sterni L, Yaster M, Easley RB. Sleep in the Intensive Care Unit. Contemporary Critical Care. 2009;7:1–12. [Google Scholar]
- 3.Friese RS. Sleep and recovery from critical illness and injury: A review of theory, current practice, and future directions. Crit Care Med. 2008;36:697–705. doi: 10.1097/CCM.0B013E3181643F29. [DOI] [PubMed] [Google Scholar]
- 4.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Aydogan MS, Korkmaz MF, Ozgül U, et al. Pain, fentanyl consumption, and delirium in adolescents after scoliosis surgery: dexmedetomidine vs midazolam. Paediatr Anaesth. 2013;23(5):446–452. doi: 10.1111/pan.12128. [DOI] [PubMed] [Google Scholar]
- 6.Inouye SK, Rushing JT, Foreman MD, et al. Does delirium contribute to poor hospital outcomes? A three-site epidemiologic study. J Gen Intern Med. 1998;13:234–242. doi: 10.1046/j.1525-1497.1998.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly P, Frosch E. Recognition of delirium on pediatric hospital services. Psychosomatics. 2012;53:446–451. doi: 10.1016/j.psym.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Smith HA, Boyd J, Fuchs DC, et al. Diagnosing delirium in critically ill children: Validity and reliability of the Pediatric Confusion Assessment Method for the Intensive Care Unit. Crit Care Med. 2011;39:150–157. doi: 10.1097/CCM.0b013e3181feb489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schieveld JN, Lousberg R, Berghmans E, et al. Pediatric illness severity measures predict delirium in a pediatric intensive care unit. Crit Care Med. 2008;36:1933–1936. doi: 10.1097/CCM.0b013e31817cee5d. [DOI] [PubMed] [Google Scholar]
- 10.Gabor JY, Cooper AB, Crombach SA, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167:708–715. doi: 10.1164/rccm.2201090. [DOI] [PubMed] [Google Scholar]
- 11.Van Rompaey B, Elseviers MM, Van Drom W, et al. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomized controlled trial in intensive care patients. Crit Care. 2012;16:R73. doi: 10.1186/cc11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandharipande P, Ely EW. Sedative and Analgesic Medications: Risk Factors for Delirium and Sleep Disturbances in the Critically Ill. Crit Care Clin. 2006;22:313–327. doi: 10.1016/j.ccc.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Twite MD, Rashid A, Zuk J, et al. Sedation, analgesia, and neuromuscular blockade in the pediatric intensive care unit: survey of fellowship training programs. Pediatr Crit Care Med. 2004;5:521–532. doi: 10.1097/01.PCC.0000144710.13710.2E. [DOI] [PubMed] [Google Scholar]
- 14.Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Brook AD, Ahrens TS, Schaiff R, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27:2609–2615. doi: 10.1097/00003246-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 17.Grant MJ, Scoppettuolo LA, Wypij D, et al. Prospective evaluation of sedation-related adverse events in pediatric patients ventilated for acute respiratory failure. Crit Care Med. 2012;40:1317–1323. doi: 10.1097/CCM.0b013e31823c8ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deeter KH, King MA, Ridling D, et al. Successful implementation of a pediatric sedation protocol for mechanically ventilated patients. Crit Care Med. 2011;39:683–688. doi: 10.1097/CCM.0b013e318206cebf. [DOI] [PubMed] [Google Scholar]
- 19.Hartman ME, McCrory DC, Schulman SR. Efficacy of sedation regimens to facilitate mechanical ventilation in the pediatric intensive care unit: a systematic review. Pediatr Crit Care Med. 2009;10:246–255. doi: 10.1097/PCC.0b013e31819a3bb9. [DOI] [PubMed] [Google Scholar]
- 20.Curley MA, Harris SK, Fraser KA, et al. State Behavioral Scale: a sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med. 2006;7:107–114. doi: 10.1097/01.PCC.0000200955.40962.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 22.O'Connor MF, Daves SM, Tung A, et al. BIS monitoring to prevent awareness during general anesthesia. Anesthesiology. 2001;94:520–522. doi: 10.1097/00000542-200103000-00025. [DOI] [PubMed] [Google Scholar]
- 23.Kim HS, Oh AY, Kim CS, et al. Correlation of bispectral index with end-tidal sevoflurane concentration and age in infants and children. Br J Anaesth. 2005;95:362–366. doi: 10.1093/bja/aei196. [DOI] [PubMed] [Google Scholar]
- 24.Davidson AJ, McCann ME, Devavaram P, et al. The differences in the bispectral index between infants and children during emergence from anesthesia after circumcision surgery. Anesth Analg. 2001;93:326–330. doi: 10.1213/00000539-200108000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Malviya S, Voepel-Lewis T, Tait AR, et al. Effect of age and sedative agent on the accuracy of bispectral index in detecting depth of sedation in children. Pediatrics. 2007;120:e461–e470. doi: 10.1542/peds.2006-2577. [DOI] [PubMed] [Google Scholar]
- 26.Hammer GB. Sedation and analgesia in the pediatric intensive care unit following laryngotracheal reconstruction. Paediatr Anaesth. 2009;19(Suppl 1):166–179. doi: 10.1111/j.1460-9592.2009.03000.x. [DOI] [PubMed] [Google Scholar]
- 27.Hughes MA, Glass PS, Jacobs JR. Context-sensitive half-time in multicompartment pharmacokinetic models for intravenous anesthetic drugs. Anesthesiology. 1992;76:334–341. doi: 10.1097/00000542-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Santeiro ML, Christie J, Stromquist C, et al. Pharmacokinetics of continuous infusion fentanyl in newborns. J Perinatol. 1997;17:135–139. [PubMed] [Google Scholar]
- 29.Flacke JW, Flacke WE, Bloor BC, et al. Histamine release by four narcotics: a double-blind study in humans. Anesth Analg. 1987;66:723–730. [PubMed] [Google Scholar]
- 30.Grossmann M, Abiose A, Tangphao O, et al. Morphine-induced venodilation in humans. Clin Pharmacol Ther. 1996;60:554–560. doi: 10.1016/S0009-9236(96)90151-4. [DOI] [PubMed] [Google Scholar]
- 31.Franck LS, Vilardi J, Durand D, et al. Opioid withdrawal in neonates after continuous infusions of morphine or fentanyl during extracorporeal membrane oxygenation. Am J Crit Care. 1998;7:364–369. [PubMed] [Google Scholar]
- 32.Anand KJ, Clark AE, Willson DF, et al. Opioid Analgesia in Mechanically Ventilated Children: Results from the Multicenter Measuring Opioid Tolerance Induced by Fentanyl Study. Pediatr Crit Care Med. 2012;14:27–36. doi: 10.1097/PCC.0b013e318253c80e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 34.Pohlman AS, Simpson KP, Hall JB. Continuous intravenous infusions of lorazepam versus midazolam for sedation during mechanical ventilatory support: a prospective, randomized study. Crit Care Med. 1994;22:1241–1247. doi: 10.1097/00003246-199408000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Swart EL, van Schijndel RJ, van Loenen AC, et al. Continuous infusion of lorazepam versus midazolam in patients in the intensive care unit: sedation with lorazepam is easier to manage and is more cost-effective. Crit Care Med. 1999;27:1461–1465. doi: 10.1097/00003246-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 36.de Gast-Bakker DA, van der Werff SD, Sibarani-Ponsen R, et al. Age is of influence on midazolam requirements in a paediatric intensive care unit. Acta Paediatr. 2007;96:414–417. doi: 10.1111/j.1651-2227.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 37.Swart EL, Slort PR, Plotz FB. Growing up with midazolam in the neonatal and pediatric intensive care. Curr Drug Metab. 2012;13:760–766. doi: 10.2174/138920012800840347. [DOI] [PubMed] [Google Scholar]
- 38.Gesin G, Kane-Gill SL, Dasta JF, et al. Diazepam as a component of goal-directed sedation in critically ill trauma patients. J Crit Care. 2011;26:122–126. doi: 10.1016/j.jcrc.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 39.Mehta S, Burry L, Fischer S, et al. Canadian survey of the use of sedatives, analgesics, and neuromuscular blocking agents in critically ill patients. Crit Care Med. 2006;34:374–380. doi: 10.1097/01.ccm.0000196830.61965.f1. [DOI] [PubMed] [Google Scholar]
- 40.Tobias JD. Dexmedetomidine: applications in pediatric critical care and pediatric anesthesiology. Pediatr Crit Care Med. 2007;8:115–131. doi: 10.1097/01.PCC.0000257100.31779.41. [DOI] [PubMed] [Google Scholar]
- 41.Huupponen E, Maksimow A, Lapinlampi P, et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008;52:289–294. doi: 10.1111/j.1399-6576.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 42.Sanders RD, Maze M. Contribution of sedative-hypnotic agents to delirium via modulation of the sleep pathway. Can J Anaesth. 2011;58:149–156. doi: 10.1007/s12630-010-9421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mason KP, O'Mahony E, Zurakowski D, et al. Effects of dexmedetomidine sedation on the EEG in children. Paediatr Anaesth. 2009;19:1175–1183. doi: 10.1111/j.1460-9592.2009.03160.x. [DOI] [PubMed] [Google Scholar]
- 44.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 45.Tobias JD, Berkenbosch JW. Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam. South Med J. 2004;97:451–455. doi: 10.1097/00007611-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Gupta P, Whiteside W, Sabati A, et al. Safety and efficacy of prolonged dexmedetomidine use in critically ill children with heart disease. Pediatr Crit Care Med. 2012;13:660–666. doi: 10.1097/PCC.0b013e318253c7f1. [DOI] [PubMed] [Google Scholar]
- 47.Fagin A, Palmieri T, Greenhalgh D, et al. A comparison of dexmedetomidine and midazolam for sedation in severe pediatric burn injury. J Burn Care Res. 2012;33:759–763. doi: 10.1097/BCR.0b013e318254d48e. [DOI] [PubMed] [Google Scholar]
- 48.Tobias JD. Dexmedetomidine: are tolerance and withdrawal going to be an issue with long-term infusions? Pediatr Crit Care Med. 2010;11:158–160. doi: 10.1097/PCC.0b013e3181ae49af. [DOI] [PubMed] [Google Scholar]
- 49.Weber MD, Thammasitboon S, Rosen DA. Acute discontinuation syndrome from dexmedetomidine after protracted use in a pediatric patient. Paediatr Anaesth. 2008;18:87–88. doi: 10.1111/j.1460-9592.2007.02377.x. [DOI] [PubMed] [Google Scholar]
- 50.Darnell C, Steiner J, Szmuk P, et al. Withdrawal from multiple sedative agent therapy in an infant: is dexmedetomidine the cause or the cure? Pediatr Crit Care Med. 2010;11:e1–e3. doi: 10.1097/PCC.0b013e3181a66131. [DOI] [PubMed] [Google Scholar]
- 51.Tucker EW, Cooke DW, Kudchadkar SR, et al. Dexmedetomidine infusion associated with transient adrenal insufficiency in a pediatric patient: a case report. Case Rep Pediatr. 2013 doi: 10.1155/2013/207907. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hovav E, Weinstock M. Temporal factors influencing the development of acute tolerance to opiates. J Pharmacol Exp Ther. 1987;242:251–256. [PubMed] [Google Scholar]
- 53.Katz R, Kelly HW. Pharmacokinetics of continuous infusions of fentanyl in critically ill children. Crit Care Med. 1993;21:995–1000. doi: 10.1097/00003246-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 54.Tobias JD. Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit Care Med. 2000;28:2122–2132. doi: 10.1097/00003246-200006000-00079. [DOI] [PubMed] [Google Scholar]
- 55.Kress JP, Pohlman AS, O'Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 56.Salgado DR, Favory R, Goulart M, et al. Toward less sedation in the intensive care unit: a prospective observational study. J Crit Care. 2011;26:113–121. doi: 10.1016/j.jcrc.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Mehta S, Burry L, Cook D, et al. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA. 2012;308:1985–1992. doi: 10.1001/jama.2012.13872. [DOI] [PubMed] [Google Scholar]
- 58.Hu RF, Jiang XY, Zeng YM, et al. Effects of earplugs and eye masks on nocturnal sleep, melatonin and cortisol in a simulated intensive care unit environment. Crit Care. 2010;14:R66. doi: 10.1186/cc8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zahr LK, de Traversay J. Premature infant responses to noise reduction by earmuffs: effects on behavioral and physiologic measures. J Perinatol. 1995;15:448–455. [PubMed] [Google Scholar]
- 60.Liu YC, Chen CH, Wang TM, Chi CS. Noise distribution in closed and open spaces in the neonatal intensive care unit. Clinical Neonatology. 2005;12:26–29. [Google Scholar]
- 61.Busch-Vishniac IJ, West JE, Barnhill C, et al. Noise levels in Johns Hopkins Hospital. J Acoust Soc Am. 2005;118:3629–3645. doi: 10.1121/1.2118327. [DOI] [PubMed] [Google Scholar]
- 62.Van Eijk M, Van Den Bossche R, Zwolle S, et al. Quality and quantity of sleep in multiple versus single patient room intensive care units. Crit Care Med. 2010;38:A206. [Google Scholar]
- 63.Shilo L, Dagan Y, Smorjik Y, et al. Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. Am J Med Sci. 1999;317:278–281. doi: 10.1097/00000441-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Perras B, Meier M, Dodt C. Light and darkness fail to regulate melatonin release in critically ill humans. Intensive Care Med. 2007;33:1954–1958. doi: 10.1007/s00134-007-0769-x. [DOI] [PubMed] [Google Scholar]
- 65.Schieveld JN, Leroy PL, van Os J, et al. Pediatric delirium in critical illness: phenomenology, clinical correlates and treatment response in 40 cases in the pediatric intensive care unit. Intensive Care Med. 2007;33:1033–1040. doi: 10.1007/s00134-007-0637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schieveld JN, van der Valk JA, Smeets I, et al. Diagnostic considerations regarding pediatric delirium: a review and a proposal for an algorithm for pediatric intensive care units. Intensive Care Med. 2009;35:1843–1849. doi: 10.1007/s00134-009-1652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Esseveld MM, Leroy PL, Leue C, et al. Catatonia and refractory agitation in an updated flow chart for the evaluation of emotional-behavioral disturbances in severely ill children. Intensive Care Med. 2013;39:528–529. doi: 10.1007/s00134-012-2763-1. [DOI] [PubMed] [Google Scholar]
- 68.Upenieks V. The relationship of nursing practice models and job satisfaction outcomes. J Nurs Adm. 2000;30:330–335. doi: 10.1097/00005110-200006000-00013. [DOI] [PubMed] [Google Scholar]
- 69.Finn CP. Autonomy: an important component for nurses' job satisfaction. Int J Nurs Stud. 2001;38:349–357. doi: 10.1016/s0020-7489(00)00065-1. [DOI] [PubMed] [Google Scholar]
- 70.DuBose JJ, Nomoto S, Higa L, et al. Nursing involvement improves compliance with tight blood glucose control in the trauma ICU: a prospective observational study. Intensive Crit Care Nurs. 2009;25:101–107. doi: 10.1016/j.iccn.2008.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.