Abstract
The aim of this study was to evaluate the efficacy and toxicity of stereotactic body radiation therapy (SBRT) in the treatment of patients with liver metastases. Between August 2006 and July 2011, patients with 1–4 liver metastases were enrolled and treated with SBRT using the CyberKnife® system at Tianjin Medical University Cancer Institute and Hospital. The metastases were from different primary tumors, with a maximum tumor diameter of less than 6 cm. The primary endpoint was local control. Secondary endpoints were overall survival, progression-free survival, distant progression-free survival, and adverse events. Fifty-seven patients with 80 lesions were treated with SBRT. The 1-year and 2-year local control rates were 94.4% and 89.7%, respectively. The difference in local control between patients who received adjuvant treatment before SBRT and those who did not reached statistical significance (P=0.049). The median overall survival for the entire cohort was 37.5 months. According to the primary tumor sites, the median overall survival was not reached. The 2-year overall survival rate was 72.2% in the favorable group (primary tumors originating from the colon, breast, or stomach, as well as sarcomas); however, in the unfavorable group (primary tumors originating from the pancreas, lung, ovary, gallbladder, uterus, hepatocellular carcinoma, as well as olfactory neuroblastoma), the median overall survival and 2-year overall survival rates were 37.5 months and 55.9%, respectively (P=0.0001). Grade 1–2 fatigue, nausea, and vomiting were the most common adverse events, and no grade 3 and higher adverse events were observed. With excellent local control in the absence of severe toxicity, SBRT provides an alternative for patients with 1–4 liver metastases who cannot undergo surgery or other treatments.
Keywords: liver metastasis, stereotactic body radiotherapy, local control
Introduction
The liver is the second most common site for the metastatic spread of cancer, with most liver lesions caused by colorectal cancer, followed by pancreatic and breast cancers.1–3 Surgical resection is currently considered to be the first-line measure for the treatment of liver metastases.4–6 Unfortunately, the feasibility of surgical resection can be limited by a high rate of relapse and the complicated clinical setting, such as tumor size, location, and relationship with major intrahepatic vascular structures, which are frequent causes of interruption or discontinuation of surgical resection.7–10 Even with surgical resection, tumor relapse rates remain very high, seriously affecting the chances of survival of patients with liver metastases.11 Transcatheter arterial chemoembolization and radiofrequency ablation are currently considered to be an alternative or complementary measure for the treatment of metastatic liver cancer; unfortunately, these procedures are associated with their own potentially life-threatening toxicities and complications, which are frequent causes of treatment interruption or discontinuation. Therefore, it seems necessary to have alternative palliative treatment options for patients with liver metastases.
Radiotherapy can be an effective radical treatment strategy for liver metastases. It has been attempted for liver metastases for more than three decades, but its use has been limited by the risk of radiation-induced liver toxicity and the low tolerance of the whole liver to radiotherapy.12,13 However, advances in stereotactic body radiation therapy (SBRT) have enabled high-dose radiotherapy to be directed to the tumor while sparing the noncancerous surrounding liver parenchyma from these high doses. Several recent reports have demonstrated that SBRT is feasible, with promising responses seen for primary hepatocellular carcinoma and liver metastases, but there is a need for additional evidence before its use in clinical practice.14,15
The CyberKnife® (Accuray Incorporated, Sunnyvale, CA, USA), an image-guided robotic radiosurgery system, is a radiation delivery platform capable of detecting and correcting for intrafraction tumor motion as well as adapting to the patient’s breathing pattern and moving the linear accelerator in concert with it.16 Although there is some evidence indicating that SBRT is highly beneficial for treating patients with liver metastases,17–19 it is still unclear whether this evidence is scientifically rigorous enough to recommend routine use of SBRT for the curative treatment of liver metastases. The aim of the present study was to assess the efficacy and safety of SBRT using the CyberKnife® system for the treatment of liver metastases in clinical practice.
Patients and methods
Study design and eligible patients
Consecutive eligible patients with liver metastases were enrolled in this retrospective observational study at the CyberKnife® Center (first-generation division), Tianjin Medical University Cancer Institute and Hospital, between August 2006 and July 2011. All patients were examined by an oncologist before enrollment into the study. The inclusion criteria were defined as follows: patients of any age; liver metastases confirmed cytologically or pathologically, or diagnosed through imaging; Karnofsky performance score ≥70; more than four liver metastases and longest individual tumor diameter less than 6 cm; life expectancy of more than 3 months; unsuitability for surgery due to, eg, old age or poor heart and lung function and received SBRT using CyberKnife® treatment; bilirubin level <3 mg/dL; albumin level >2.5 g/dL; and serum liver enzyme concentration less than twice the upper limit of the normal range. The exclusion criteria included the following: jaundice caused by, eg, obstructive or hemolytic disease; a prolonged prothrombin time induced by hematologic disease; and ascites. Informed consent was obtained from all patients. The study protocol was conducted in accordance with the ethical guidelines of the 1995 Declaration of Helsinki and was approved by the independent ethics committees at Tianjin Medical University Cancer Institute and Hospital.
Patient characteristics
Between August 2006 and July 2011, 57 patients with 80 liver metastases were treated at the CyberKnife® Center, Tianjin Medical University Cancer Institute and Hospital. Forty-two patients were cytologically or pathologically diagnosed and 15 patients were diagnosed on the basis of two imaging studies, including enhanced computed tomography (CT), positron emission tomography/computed tomography (PET/CT), and magnetic resonance imaging. Seventy lesions in 49 patients were assessable for local control; however, eight patients with ten lesions were not assessable because they died within 6 months of treatment. Among the assessable patients, there were nine who had received prior local therapy and 32 who had a history of systemic therapy. In addition, consistent with an earlier study,20 the patients were divided into a favorable prognostic group (primary tumors originating from the colon, breast, or stomach, as well as sarcomas) and an unfavorable prognostic group (primary tumors originating from the pancreas, lung, ovary, gallbladder, uterus, hepatocellular carcinoma, as well as olfactory neuroblastoma). All 57 patients were assessed for progression-free survival, distant progression-free survival, and overall survival. Demographic information and baseline characteristics for all patients are shown in Table 1.
Table 1.
Protocol dose constraints
| Organ/tissue at risk | Dose-limiting |
|---|---|
| Liver | V21 <700 mL |
| Spinal cord | Dmax <22 Gy |
| Kidney | V15 <35%, left kidney Dmax <15 Gy |
| Stomach | V21 <5 mL, Dmax <30 Gy |
| Small intestinal | V16 <5 mL, Dmax <27 Gy |
| Duodenum | V15 <5 mL, Dmax <24 Gy |
Abbreviations: Gy, gray; V, volume; Dmax, maximum dosage.
Treatment schedule
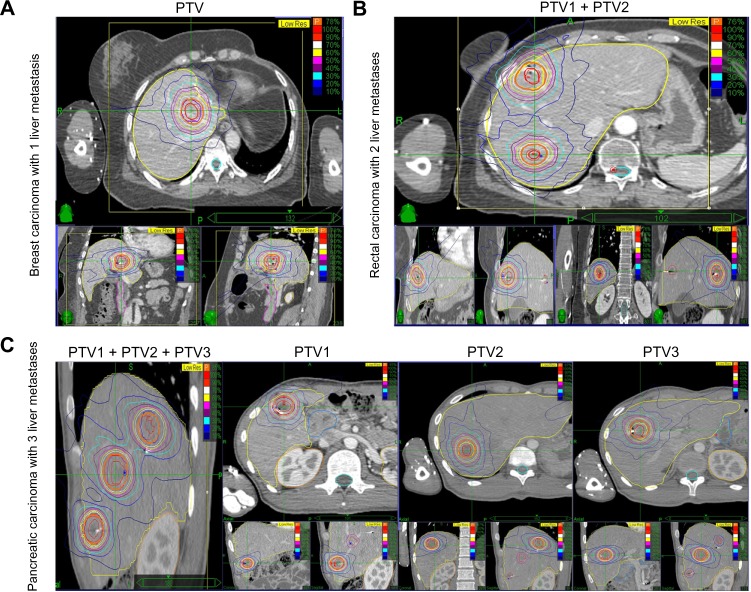
Details of the CyberKnife® treatment technique and its accuracy have been described in our previous publications.21–23 The prescribed dose and fractionation were specified according to lesion location and volume. Briefly, an average of five cylindrical gold fiducial markers (Best Medical International, Springfield, VA, USA) were placed percutaneously either under ultrasound guidance or CT guidance within or around the tumor at a minimum distance of 2 cm. Next, the patients were immobilized using a vacuum mattress before three-dimensional or four-dimensional CT simulation was used in the planning procedure. A set of planning CT images through the liver were obtained after infusion of intravenous radiographic contrast material to highlight the tumor. The images had to have enough margin above and below the tumor according to pretreatment planning CT, PET/CT, and magnetic resonance images. The gross target volume was defined as the liver metastasis and the planning target volume, with an accurate margin according to three-dimensional or four-dimensional CT simulation. The total dose delivered to the tumor and the fractionation schedule were determined by constraints regarding the adjacent normal tissues, as shown in Table 2, and three examples of the dose distribution are shown in Figure 1.
Table 2.
Summary of patient characteristics
| Characteristics | Values (%) |
|---|---|
| Assessable patients (n) | 57 |
| Age (years) | 56 (18–85) |
| Gender (male/female) | 34/23 (60%/40%) |
| Karnofsky performance score ≥70 | 57 (100%) |
| Child-Pugh score | |
| A | 56 (98.25%) |
| B | 1 (1.75%) |
| C | 0 (0%) |
| Primary tumor | |
| Colorectal cancer | 18 (31.58%) |
| Pancreatic cancer | 8 (14.04%) |
| Breast cancer | 7 (12.28%) |
| Lung cancer | 7 (12.28%) |
| Hepatocellular carcinoma | 5 (8.77%) |
| Stomach cancer | 4 (7.02%) |
| Renal cancer | 2 (3.51%) |
| Gallbladder cancer | 2 (3.51%) |
| Ovarian cancer | 1 (1.75%) |
| Esophageal cancer | 1 (1.75%) |
| Sarcoma | 1 (1.75%) |
| Olfactory neuroblastoma | 1 (1.75%) |
| LM location | |
| Right liver | 47 (58.75%) |
| Left liver | 33 (41.25%) |
| Contemporary with other site metastases | |
| Yes | 14 (24.56%) |
| No | 43 (75.44%) |
| Prior local therapy for LM | |
| Surgery | 4 (7.02%) |
| TACE | 2 (3.51%) |
| RFA plus TACE | 2 (3.51%) |
| Surgery plus RFA | 1 (1.75%) |
| None | 48 (84.21%) |
| Prior systemic therapy for LM | |
| Yes | 32 (56.14%) |
| None | 25 (43.86%) |
| Lesions per patient (n) | |
| ≤1 | 39 (68.42%) |
| >1 | 18 (31.58%) |
| Time interval between the first liver metastasis and CyberKnife® treatment (months) | 2.2 (0.3–33.5) |
Notes: Data are presented as the median and ranges; favorable group (primary tumors originating from the colon, breast, or stomach, as well as sarcomas); unfavorable group (primary tumors originating from the pancreas, lung, ovary, gallbladder, uterus, hepatocellular carcinoma, as well as olfactory neuroblastoma).
Abbreviations: LM, liver metastases; RFA, radiofrequency ablation; TACE, trans-catheter arterial chemoembolization.
Figure 1.
Representative planning CT and isodose distributions with SBRT for patients with 1–3 LM.
Notes: Each representative patient had axial, sagittal, and coronal images taken, and red and purple lines indicate GTV and PTV, respectively. (A) A 38-year-old woman treated for a solitary LM from breast carcinoma. SBRT was performed using three fractions of 12 Gy prescribed to the 78% isodose line. (B) A 54-year-old man treated for two LM from rectal carcinoma. SBRT was performed using five fractions of 9 Gy to the 77% isodose line. (C) An 18-year-old man treated for three LM from pancreatic carcinoma. SBRT was performed in three fractions of 12 Gy to the 77% isodose line.
Abbreviations: LM, liver metastasis; CT, computer tomography; GTV, gross tumor volume; PTV, planning target volume; Gy, Gray; SBRT, stereotactic body radiation therapy.
Treatment characteristics
A detailed summary of the treatment planning parameters for all lesions and the assessable lesions is shown in Table 3. The median planning target volume was 27.62 (range 2.50–125.66) cc. The patients received a median of three (range 3–7) fractions with a median dose of 13 (range 6–15) Gray (Gy) per fraction and a total dose of 42 Gy in three fractions (range 39–54 Gy in 3–7 fractions). The median biologically equivalent dose (linear-quadratic model) was 100 (range 67.2–112.5) Gy.24 The dose was prescribed to the median 79% (range 70%–85%) isodose line, which encompassed 95% of the planning target volume.
Table 3.
Summary of CyberKnife® treatment parameters
| All lesions (median)
|
Accessible lesions for local control (median)
|
|||||
|---|---|---|---|---|---|---|
| Left liver | Right liver | Total | Left liver | Right liver | Total | |
| PTV (mL) | 28.09 (8.57–125.66) | 27.15 (2.5–105.01) | 27.62 (2.50–125.66) | 28.21 (8.57–125.66) | 24.69 (2.5–101.28) | 28.25 (2.5–125.66) |
| Prescribed dose (Gy) | 42 (39–54) | 42 (39–50) | 42 (21–54) | 42 (39–54) | 42 (39–50) | 42 (39–54) |
| Number of fractions | 3 (3–6) | 3 (3–7) | 3 (3–7) | 3 (3–6) | 3 (3–7) | 3 (3–7) |
| Dose per fraction (Gy) | 13 (7–15) | 13 (6–15) | 13 (6–15) | 13 (7–15) | 13 (6–15) | 14 (6–15) |
| BED10 (Gy) | 100 (71.4–10.6) | 100 (67.2–112.5) | 100 (67.2–112.5) | 89.7 (71.4–102.6) | 100 (67.2–112.5) | 100 (67.2–112.5) |
| Prescription isodose line, % | 79% (73%–85%) | 79% (75%–84%) | 79% (73%–85%) | 79% (73%–85%) | 79% (75%–84%) | 79% (73%–85%) |
Abbreviations: BED, biologically equivalent dose; Gy, Gray; PTV, planning target volume.
Follow-up and endpoints
The patients were observed at 1 month after completion of treatment, every 3 months for the first year, and every 6 months thereafter until July 2012 (Figure 2). Imaging, including CT, PET/CT, magnetic resonance imaging, adverse events, and compliance were monitored in all patients for the follow-up period using our clinical databases. The primary endpoint was local control rate, and the secondary endpoints were overall survival, progression-free survival, distant progression-free survival, and adverse events. Local control was defined as complete response and partial response, and local tumor response was defined using the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria.25 In addition, local control of liver metastases was assessed at a minimum of 6 months of follow-up after SBRT in order to avoid the uncertainty associated with early transient radiographic changes in the high-dose region. Progression-free survival was defined as the time between the date of SBRT and the date of disease progression or the date of the last follow-up for censored patients. Overall survival was defined as the time between the date of the pathological diagnosis of primaries and the date of death or date of last follow-up for censored patients. Toxicity was determined according to Common Terminology Criteria for Adverse Events version 4.0.26
Figure 2.
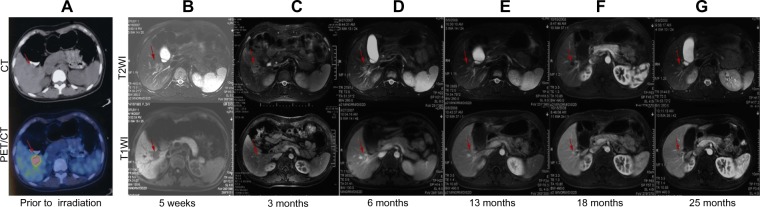
Analysis of initial planning CT and PET/CT as well as follow-up MRI images for a representative patient (a 23-year-old woman treated for a solitary LM from breast carcinoma).
Notes: SBRT was delivered in three fractions of 14 Gy to the 81% isodose line. (A) Initial planning CT and PET/CT images showing a 3.2 cm lesion. (B–G) T1-weighted and T2-weighted MRI images showing a complete response, with decreased attenuation in the surrounding region 6 months after completion of SBRT. The red arrows indicate changes in the liver metastasis after treatment.
Abbreviations: CT, computer tomography; PET/CT, positron emission tomography/computer tomography; MRI, magnetic resonance imaging; LM, liver metastasis; Gy, Gray; PTV, planning target volume; SBRT, stereotactic body radiation therapy.
Statistical analysis
Progression-free survival, distant progression-free survival, and overall survival curves were estimated using a Kaplan-Meier analysis and were compared using the stratified log-rank test, and local control rates were calculated actuarial. Univariate and multivariate analyses were performed using a Cox regression model. A P-value of ≤0.05 was considered to indicate statistical significance. The data were analyzed using Intercooled Stata version 8.2 for Windows software (Stata Corporation, College Station, TX, USA).
Results
Local control, progression-free survival, and distant progression-free survival
The 1-year and 2-year actuarial local control rates for all eligible patients were 94.4% and 89.7%, respectively (Figure 3A). The trend of the 1-year and 2-year tumor-based local control were similar to the patient-based analysis (Figure 3B). Among patients eligible for analysis of local control, those who had undergone systemic treatments before SBRT had a better outcome than those who had not (Figure 3F, P=0.049). Local control was better for patients with liver metastases receiving a biologically equivalent dose >100 Gy than in those receiving a biologically equivalent dose ≤100 Gy, although the difference was not statistically significant (Figure 3G, P=0.11). Furthermore, differences in local control were also statistically insignificant according to primary tumors (Figure 3C, P=0.18), location of liver metastases (Figure 3D, P=0.56), and the time interval between diagnosis of liver metastases and SBRT (Figure 3E, P=0.79). Median progression-free survival and distant progression-free survival for the entire cohort was 12 months and 37 months, respectively (Figure 3H).
Figure 3.
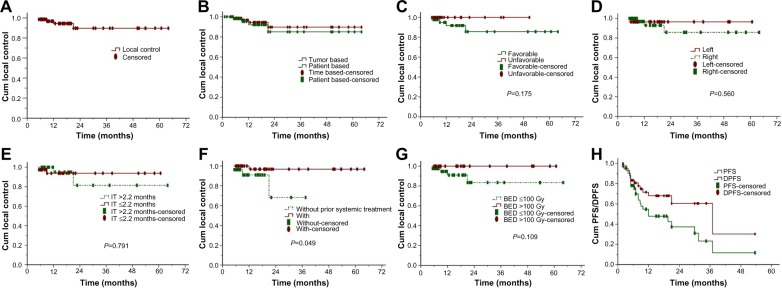
Actuarial local control, PFS, and DPFS of patients with LM.
Notes: (A) Overall local control, (B) local control depending on tumor-based or patient-based analyses, (C) local control depending on primary tumor, (D) local control depending on LM site, (E) local control depending on time between first LM and SBRT, (F) local control depending on prior systematic treatment or lack thereof, (G) local control depending on BED, and (H) PFS and DPFS of patients with LM.
Abbreviations: PFS, progression-free survival; DPFS, distant progression-free survival; LM, liver metastases; BED, biologically equivalent dose; IT, interval time; SBRT, stereotactic body radiation therapy; Gy, Gray; Cum, cumulative.
Overall survival
All patients were followed up until death or July 2012, and 24 patients had died by the last follow-up. Median follow-up duration was 20.5 (range 1–64) for the whole cohort. Median overall survival was 37.5 months, and the 1-year and 2-year overall survival rates were 68.6% and 55.9%, respectively (Figure 4A). In patients with a more favorable prognosis, the median overall survival was not reached and the 1-year and 2-year overall survival rates were 89.6% and 72.2%, respectively. In the rest of the patients, median overall survival was 8.7 months and the 1-year and 2-year overall survival rates were 42.2% and 36.2%, respectively, (Figure 4D, P=0.0001). Patients who had received prior systemic treatment had a slightly longer overall survival compared with those who had not, although this difference was not statistically significant (Figure 4H, P=0.09). In addition, differences in overall survival were not statistically significant for age (Figure 4B, P=0.68), gender (Figure 4C, P=0.62), number of liver metastases (Figure 4E, P=0.39), existence of extrahepatic metastases (Figure 4F, P=0.89), or time between first discovery of liver metastases and SBRT (Figure 4G, P=0.24).
Figure 4.
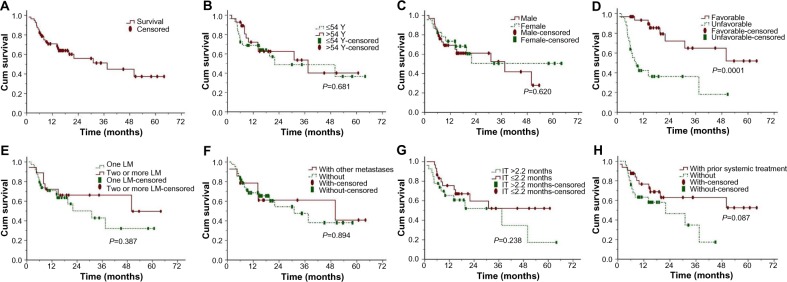
Actuarial overall survival in patients with LM.
Notes: (A) OS in general, (B) OS depending on patient age, (C) OS depending on patient gender, (D) OS depending on primary tumor, (E) OS depending on number of LM, (F) OS depending on extrahepatic metastasis or lack thereof, (G) OS depending on time between first LM and SBRT, and (H) OS depending on prior systematic treatment or no such history.
Abbreviations: OS, overall survival; LM, liver metastasis; IT, interval time; SBRT, stereotactic body radiation therapy; Cum, cumulative; Y, year.
Factors associated with overall survival
Using multivariate analysis, an unfavorable pretreatment prognosis for overall survival was associated with primary tumor site (P=0.0001); however, age, gender, number of liver metastases, metastases at other sites, time interval between diagnosis of liver metastases and SBRT, and prior systemic treatment were not predictive factors according to the multivariate analyses (Table 4). Multivariate analysis was not performed for local control because of the small number of events.
Table 4.
Multivariate analyses for overall survival
| Prognostic factors | β | SE | Wald | P-values | HR | 95% CI |
|---|---|---|---|---|---|---|
| Age | −0.569 | 0.449 | 1.609 | 0.205 | 0.566 | 0.235–1.364 |
| Gender | 0.358 | 0.549 | 0.424 | 0.515 | 1.430 | 0.487–4.194 |
| Primary tumor | 1.940 | 0.551 | 12.389 | 0.0001 | 6.958 | 2.362–20.493 |
| Counts of LM | −0.665 | 0.539 | 1.521 | 0.217 | 0.514 | 0.179–1.480 |
| With other metastases | −0.430 | 0.517 | 0.689 | 0.406 | 0.651 | 0.236–1.794 |
| Interval time† | −0.303 | 0.427 | 0.502 | 0.479 | 0.739 | 0.320–1.706 |
| Prior systemic treatment | 0.442 | 0.548 | 0.650 | 0.420 | 1.556 | 0.531–4.559 |
Note:
Interval time, time between the first liver metastases and CyberKnife® treatment.
Abbreviations: LM, liver metastases; HR, hazards ratio; CI, confidence interval; SE, standard error.
Patterns of failure
None of the eligible patients relapsed within the planning target volume, and a summary of the analysis of failure patterns is provided in Table 5. Twenty-three patients showed no progression after SBRT, but 34 patients (59.6%) experienced intrahepatic and extrahepatic progression within a median of 6 (range 2–53) months after SBRT. Among these 34 patients, 19 (33.3%) experienced extrafield intrahepatic progression, nine (15.8%) experienced extrafield intrahepatic progression only, 23 (40.4%) experienced extrahepatic progression, and 14 (24.6%) experienced extrahepatic progression only.
Table 5.
Treatment response and pattern of failure
| Site of failure | Patients (n=57), n (%) |
|---|---|
| No progression after CyberKnife® SBRT | 23 (40.4%) |
| Progressive disease after CyberKnife® SBRT | 34 (59.6%) |
| Extrafield intrahepatic progression | 19 (33.3%) |
| Extrahepatic progression | 23 (40.4%) |
| Extrafield intrahepatic progression only | 9 (15.8%) |
| Extrahepatic progression only | 14 (24.6%) |
| Progressive disease in patients without previous extra hepatic disease | Patients (n=43), n (%) |
|
| |
| No progression after CyberKnife® SBRT | 20 (46.5%) |
| Progressive disease after CyberKnife® SBRT | 23 (53.5%) |
| Extrafield intrahepatic progression | 17 (39.5%) |
| Extrahepatic progression | 15 (34.9%) |
| Extrafield intrahepatic progression only | 8 (18.6%) |
| Extrahepatic progression only | 6 (14.0%) |
| Progressive disease in patients with previous extrahepatic disease | Patients (n=14), n (%) |
|
| |
| Extrahepatic progression | 10 (71.4%) |
| Intrahepatic progression | 3 (21.4%) |
Abbreviation: SBRT, stereotactic body radiation therapy.
Toxicity
The treatment was well tolerated by all patients. The most common toxicities were grade 1 or 2 fatigue, nausea, and vomiting, and changes in liver function tests, which were corrected by routine treatment. None of the patients developed grade 3 or higher toxicity. In addition, no clinically significant changes were noted on liver function evaluation or physical examination.
Discussion
This study was designed to evaluate the efficacy and safety of SBRT using the CyberKnife® system in the treatment of patients with liver metastases. Our results show that SBRT can achieve local control without severe toxicity in these patients, and provide a rational basis for the clinical use of SBRT in patients with 1–4 liver metastases who cannot undergo surgery or other treatments. However, given the limited sample size and retrospective nature of this study, further studies are needed to elucidate the optimal dose-fractionation schemes for treatment of such patients.
A recent review showed that local control of liver metastases using SBRT is encouraging, with rates ranging from 70% to 100% at 1 year and 60% to 90% at 2 years.27 Our study concurs with the published SBRT data indicating that SBRT achieves good local control. During our study, the 1-year and 2-year actuarial local control rates were 94.4% and 89.7%, respectively. Of note, Stintzing et al demonstrated lower local control rates for colorectal liver metastases than for noncolorectal liver lesions.28
Although the patients in our study who had received systemic treatments prior to SBRT were heterogeneous in terms of drug type, number of rounds of chemotherapy, and time interval between chemotherapy and radiotherapy, the results are consistent with previous studies showing that local control of liver metastases in patients receiving SBRT may depend on prior therapy.29 Therefore, systemic treatment before SBRT does seem to play a role in local control and warrants further investigation. In addition, there was a slight trend towards superiority of receiving a biologically equivalent dose >100 Gy compared with a biologically equivalent dose ≤100 Gy in terms of local control, but the difference was not statistically significant. A multicenter pooled analysis also indicated a relationship between the total dose delivered with SBRT and local control rate; for a three-fraction regimen of SBRT, a prescription dose of at least 48 Gy in three fractions should be considered.19 In addition, differences in local control were not statistically significant according to the primary tumor, location of liver metastases, and the time interval between the diagnosis of liver metastases and SBRT.
The median overall survival after SBRT ranged from 10–34 months, with 2-year overall survival rates ranging from 30% to 83%, with occasional long-term survivors.20,29 Despite the relatively high representation of poor prognostic features in our patients, such as old age and advanced disease, the median overall survival of patients with liver metastases receiving SBRT treatment was 37.5 months and 2-year overall survival rate was 55.9%. These values are comparable with the rates reported for patients with liver metastases from similar primary sites who received surgical treatment. Moreover, our results agree with those reported by Rusthoven et al,20 who demonstrated improved median overall survival after SBRT for liver metastases from favorable primary tumors compared with that for those from unfavorable primary sites. Furthermore, multivariate Cox regression analysis identified that the primary tumor was the only independent prognostic factor that predicted overall survival in patients with liver metastases. In contrast, there is some research that found no significant difference in overall survival between patients with colorectal primary lesions and those with primaries at other sites.30 The reason for this discrepancy is still unclear, but may be related to how well the primary disease has been controlled. In addition, patients with liver metastases who had received prior systemic treatment had longer overall survival than those without such a history, although these differences were not statistically significant. A substantial proportion of patients presented with out-of-field progression at a median time of 6 months after SBRT. Based on a combination of local control and the pattern of failure, it appears that the integration of aggressive local therapy with systemic treatment must play an increasingly important role in the treatment of this disease.
Because of the accuracy of SBRT, normal tissue could be effectively spared and adverse events due to radiation were not encountered. In our study, acute toxicities were for the most part minimal, mainly consisting of grade 1–2 fatigue, nausea, and vomiting, and changes in liver enzyme, bilirubin, and albumin levels, with no grade 3–4 toxicities occurring up to 6 months post-treatment. Therefore, we can conclude that there was no significant correlation between use of SBRT and adverse events. However, the toxicities were retrospectively evaluated, which may have underestimated the frequency of adverse events, and further studies with longer follow-up are necessary to assess the potential immediate benefits of SBRT and identify any toxicity.
This was a retrospective observational study. Given that record-keeping and bias may influence results, prospective studies of liver metastases must be performed to confirm the efficacy and safety of SBRT using the CyberKnife® system. Local control appears to be excellent with SBRT; however, our follow-up was short and the study included a heterogeneous group of patients with a variety of primary tumors, previous treatments, and liver disease status. The optimal dose and fractionation scheme was not determined due to lesion location and volume.
Conclusion
Overall, SBRT is an effective modality with good local control rates and acceptable toxicity for unresectable or medically inoperable liver metastases. Further studies in more favorable patients and with longer follow-up should further elucidate the dose-response relationship, the potential late toxicity profile, and the chances of long-term survival after SBRT. It could also be useful to underline the better prognosis of selected surgical patients compared with those of SBRT in order to help identify the subset of patients most likely to benefit from SBRT.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81201754), the Foundation of National High Technology Research and Development Program (863 Project No. 2012AA022700), the New Teacher Fund for Doctor Station, the Ministry of Education (No. 20121202120014), the Foundation of Tianjin Public Health Bureau (No. 2012KZ067), and the SBRT Foundation of Tianjin Medical University Cancer Institute & Hospital (No. 4-1-3).
Footnotes
Disclosure
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this paper. The authors report no conflicts of interest in this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- 3.Poston GJ. Surgical strategies for colorectal liver metastases. Surg Oncol. 2004;13:125–136. doi: 10.1016/j.suronc.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Yoon SS, Tanabe KK. Surgical treatment and other regional treatments for colorectal cancer liver metastases. Oncologist. 1999;4:197–208. [PubMed] [Google Scholar]
- 5.Robertson DJ, Stukel TA, Gottlieb DJ, Sutherland JM, Fisher ES. Survival after hepatic resection of colorectal cancer metastases: a national experience. Cancer. 2009;115:752–759. doi: 10.1002/cncr.24081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy SK, Barbas AS, Marroquin CE, et al. Resection of non-colorectal non-neuroendocrine liver metastases: a comparative analysis. J Am Coll Surg. 2007;204:372–382. doi: 10.1016/j.jamcollsurg.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Adam R. Chemotherapy and surgery: new perspectives on the treatment of unresectable liver metastases. Ann Oncol. 2003;14(Suppl 2):ii13–ii16. doi: 10.1093/annonc/mdg731. [DOI] [PubMed] [Google Scholar]
- 8.Albert SR, Horvath WL, Sternfeld WC, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol. 2005;23:9243–9249. doi: 10.1200/JCO.2005.07.740. [DOI] [PubMed] [Google Scholar]
- 9.Garden OJ, Rees M, Poston GJ, et al. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55(Suppl 3):iii1–iii8. doi: 10.1136/gut.2006.098053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawlik TM, Schulick RD, Choti MA. Expanding criteria for respectability of colorectal liver metastases. Oncologist. 2008;13:51–64. doi: 10.1634/theoncologist.2007-0142. [DOI] [PubMed] [Google Scholar]
- 11.Khatri VP, Petrelli NJ, Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol. 2005;23:8490–8499. doi: 10.1200/JCO.2004.00.6155. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence TS, Ten Haken RK, Kessler ML, et al. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781–788. doi: 10.1016/0360-3016(92)90651-w. [DOI] [PubMed] [Google Scholar]
- 13.Cheng JC, Wu JK, Huang CM, et al. Radiation induced liver disease after three-dimensional conformal radiotherapy for patients with hepatocellular carcinoma: dosimetric analysis and implication. Int J Radiat Oncol Biol Phys. 2002;54:156–162. doi: 10.1016/s0360-3016(02)02915-2. [DOI] [PubMed] [Google Scholar]
- 14.Katz W, Carey-Sampson M, Muhs AG, Milano MT, Schell MC, Okunieff P. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys. 2007;67:793–798. doi: 10.1016/j.ijrobp.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Mendez Romero A, Wunderink W, Hussain SM, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution phase I–II study. Acta Oncol. 2006;45:831–837. doi: 10.1080/02841860600897934. [DOI] [PubMed] [Google Scholar]
- 16.Adler JR, Jr, Chang SD, Murphy MJ, Doty J, Geis P, Hancock SL. SBRT: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg. 1997;69:124–128. doi: 10.1159/000099863. [DOI] [PubMed] [Google Scholar]
- 17.Goodman KA, Wiegner EA, Maturen KE, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486–493. doi: 10.1016/j.ijrobp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Rule W, Timmerman R, Tong L, et al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol. 2011;18:1081–1087. doi: 10.1245/s10434-010-1405-5. [DOI] [PubMed] [Google Scholar]
- 19.Chang DT, Swaminath A, Kozak M, et al. Stereotactic body radiotherapy for colorectal liver metastases. A pooled analysis. Cancer. 2011;117:4060–4069. doi: 10.1002/cncr.25997. [DOI] [PubMed] [Google Scholar]
- 20.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 21.Yuan Z, Tian L, Wang P, Song Y, Dong Y, Zhuang H. Comparative research on the efficacy of CyberKnife® and surgical excision for stage I hepatocellular carcinoma. Onco Targets Ther. 2013;6:1527–1532. doi: 10.2147/OTT.S51452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian LJ, Zhuang HQ, Yuan ZY. A comparison between CyberKnife® and neurosurgery in solitary brain metastases from non-small cell lung cancer. Clin Neurol Neurosurg. 2013;115:2009–2014. doi: 10.1016/j.clineuro.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Song Y, Zhuang H, et al. Robotic stereotactic irradiation and reirradiation for spinal metastases: safety and efficacy assessment. Chin Med J (Engl) 2014;127:232–238. [PubMed] [Google Scholar]
- 24.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 25.Bogaerts J, Ford R, Sargent D, et al. RECIST Working Party Individual patients data analysis to assess modifications to the RECIST criteria. Eur J Cancer. 2009;45:248–260. doi: 10.1016/j.ejca.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 26.Department of Health and Human Services Common terminology criteria for adverse events (CTCAE), version 4.0. [Accessed March 31, 2014]. Available from: http://www.acrin.org/Portals/0/Administration/Regulatory/CTCAE_4.02_2009-09-15_QuickReference_5x7.pdf.
- 27.Hoyer M, Swaminath A, Bydder S, et al. Radiotherapy for liver metastases: a review of evidence. Int J Radiat Oncol Biol Phys. 2012;82:1047–1057. doi: 10.1016/j.ijrobp.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Vautravers-Dewas C, Dewas S, Bonodeau F, et al. Image-guided robotic stereotactic body radiation therapy for liver metastases: is there a dose response relationship? Int J Radiat Oncol Biol Phys. 2011;81:e39–e47. doi: 10.1016/j.ijrobp.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 29.Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27:1585–1591. doi: 10.1200/JCO.2008.20.0600. [DOI] [PubMed] [Google Scholar]
- 30.Stintzing S, Hoffmann RT, Heinemann V, Kufeld M, Rentsch M, Muacevic A. Radiosurgery of liver tumors: value of robotic radiosurgical devise to treat liver tumors. Ann Surg Oncol. 2010;17:2877–2883. doi: 10.1245/s10434-010-1187-9. [DOI] [PubMed] [Google Scholar]