Summary
Our lab previously described the oncogenic properties of metabotropic glutamate receptor 1 (mGluR1) in melanocytes. mGluR1 transformed immortalized mouse melanocytes in vitro and induced vigorous tumor formation in vivo. Subsequently, we observed the activation of PI3K/AKT in mGluR1-mediated melanocytic tumorigenesis in vivo. In particular, we identified AKT2 being the predominant isoform contributing to the activation of AKT. Suppression of Grm1 or AKT2 using an inducible Tet-R siRNA system resulted in a 60% or 30% reduction respectively in in vivo tumorigenesis. We show that simultaneous down-regulation of Grm1 plus AKT2 results in a reduction of approximately 80% in tumor volumes suggesting that both mGluR1 and AKT2 contribute to the tumorigenic phenotype in vivo. The discrepancy between the mild in vitro transformation characteristics and the aggressive in vivo tumorigenic phenotypes of these stable mGluR1-melanocytic clones led us to investigate the possible involvement of other growth factors. Here, we highlight a potential crosstalk network between mGluR1 and tyrosine kinase, Insulin-like Growth Factor 1 receptor (IGF-1R).
Introduction
Metabotropic glutamate receptor 1 (gene:Grm1;mouse,GRM1;human,protein:mGluR1) is a seven transmembrane receptor that belongs to the superfamily of G-protein coupled receptors (GPCRs). Although previously thought to be functionally relevant only in the central nervous system (CNS), our group has since implicated the role of Grm1 in in vivo mouse models of melanomagenesis (Chen et al., 1996; Pollock et al., 2003; Schiffner et al., 2012). The incidence of melanoma has steadily increased over the past 30 years with an estimation of over 76,000 cases diagnosed in the United States in 2014. Although recent targeted therapy efforts have proven effective, responses are often limited to 6–12 months putting forth the need for new clinically relevant targets or treatment strategies to delay the onset of drug resistance(Flaherty et al., 2010). To date, we have screened over 25 human melanoma cell lines and 170 melanoma biopsies and found approximately 80% of the cell lines and 60% of biopsy samples to express mGluR1 but not in benign nevi or normal melanocytes. Moreover, we have successfully generated several stable mGluR1-expressing mouse melanocytic clones (MASS clones), which exhibited tumorigenic characteristics in vivo (Shin et al., 2008b).
We identified two major signaling pathways that underlie Grm1-mediated melanocytic transformation. The first pathway is MAP Kinase, which is specifically activated in response to L-Quisqualate (Q), a Group I mGluR agonist and suppressed by Bay 36–7620, a specific antagonist of mGluR1. Furthermore, we observed elevated levels of phosphorylated AKT/Protein Kinase B in allografts of MASS clones (Shin et al., 2010). In particular, the AKT2 isoform was differentially activated in excised allografted tumors. Other groups have previously established the role of PI3K/AKT signaling cascade in melanoma development. Stahl and colleagues showed upregulation of AKT3 in melanoma cell lines established from various phases of primary melanoma tumors (Stahl et al., 2004). The targeting of AKT3 by siRNA was sufficient to inhibit human melanoma xenograft tumor progression. However, a recent report demonstrated that loss of PTEN promotes the invasion and migration of melanoma cells via the activation of the AKT2 isoform (Nogueira et al., 2010). Interestingly, we also showed AKT2 and not AKT3 to be the predominant isoform activated in human melanoma biopsy samples (Shin et al., 2010).
While MASS clones exhibit aggressive in vivo phenotype, which included short latency, strong angiogenic activities and invasiveness, the in vitro transformation properties were only modestly altered. As a clue of the discrepancy in aggressiveness between the in vivo and in vitro phenotypes, we observed the hyperactivation of AKT in MASS tumor allografts but not in cultured cells except by stimulation of the receptor with its agonist, L-Quisqualate. As such, we hypothesize that the in vivo microenvironment may contribute in part to promote the tumorigenic phenotype via activation of the PI3K/AKT signaling cascade. It is possible that in vivo, there exist an excessive supply of mGluR’s natural ligand, glutamate, which leads to the constitutive activation of the receptor. Alternatively, we propose the existence of a paracrine supply of growth factors from neighboring stromal cells that feeds into the PI3K/AKT pathway. We screened various exogenous growth factors/ligands including those that activate receptor tyrosine kinases for the ability of one or more of these factors to modulate the activation of ERK and/or AKT. Insulin-like growth factor (IGF-1), a known upstream regulator of AKT, was the only growth factor among those tested that was able to stimulate AKT in cultured MASS clones (Shin et al., 2010). In this report, we set out to determine whether IGF-1 Receptor (IGF-1R) indeed participates in mGluR1-mediated transformation of melan-a cells.
Results
First, we evaluated whether AKT activation is solely contributed by mGluR1 in vivo. To test this, we generated stable inducible siGrm1 siAKT2 MASS clones utilizing a tetracycline-inducible siRNA system. TetO-siAKT2 recombinant DNA was transfected into inducible siGrm1-MASS20 clones from previous studies (Shin et al., 2008b) and several stable clones harboring both siGrm1 and siAKT2 were isolated. Modulation of levels of mGluR1 and AKT2 expression in the presence of doxycyline (analog of tetracycline) was assessed by Western immunoblots (Figure 1A). Previously, suppression of mGluR1 expression in siGrm1-MASS clones led to a 60% reduction in allografted tumors in the presence of the inducer, doxycycline. These in vivo studies suggest that mGluR1 is required in part for the maintenance of tumorigenicity but also points to the involvement of additional factors in promoting melan-a transformation. Suppression of AKT2 isoform in MASS allografts resulted in a decrease in tumor volume in vivo of approximately 30% (Shin et al., 2008a; Shin et al., 2010). Here, we show that simultaneous down-regulation of Grm1 plus AKT2 leads to approximately 80–90% suppression of allografted tumors, these results strongly suggesting that both mGluR1 and AKT2 are involved in the tumorigenic phenotype in vivo (Figure 1B). Furthermore, these findings support our hypothesis that there exists at least one additional factor contributing to AKT2 activation in vivo.
Figure 1. Reduced tumorigenicity in MASS20 cells by siRNA to Grm1 plus AKT2.
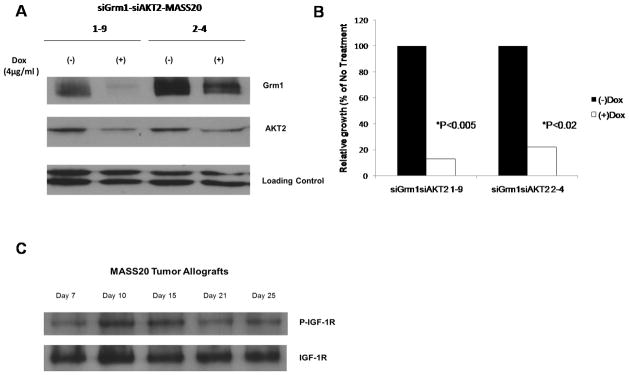
(A) Western immunoblots: siGrm1-siAKT2-MASS20 clones were grown in the presence or absence of the inducer of siRNA, doxycycline, for the indicated time points up to 7 days. Expression of Grm1 and AKT2 was suppressed in the presence of doxycycline (4μg/ml). (B) Simultaneous suppression of Grm1 and AKT2 were assessed by inoculating two different inducible siGrm1-siAKT2-MASS20 clones (106cells/site) into immunodeficient nude mice. The inducer, doxycycline (0.2%w/v) was supplied in the drinking water. Approximately, 80–90% of tumor volumes were suppressed in siGrm1-siAKT2-MASS20 clones. (C) Western immunpblots: Levels of phosphorylated IGF-1R peaked at Day 10 post-initial appearance of MASS20 allografts.
Receptor tyrosine kinases (RTKs) are well-known key constituents of GPCR signaling which facilitates tumor progression in various malignancies by enhancing proliferative and/or cell survival signals (Almendro et al., 2010; Bhola and Grandis, 2008). Previously we identified IGF-1 as being able to activate AKT in cultured MASS20, suggesting the expression and functionality of IGF-1R (or related receptor) in MASS cells (Shin et al., 2010). Now we confirmed the expression of IGF-1R in MASS20 allografts. Interestingly, not only was IGF-1R expressed in MASS20 allografts, the activation of the receptor occurred in a time-dependent manner peaking at Day 10 post initial appearance of tumor (Figure 1C).
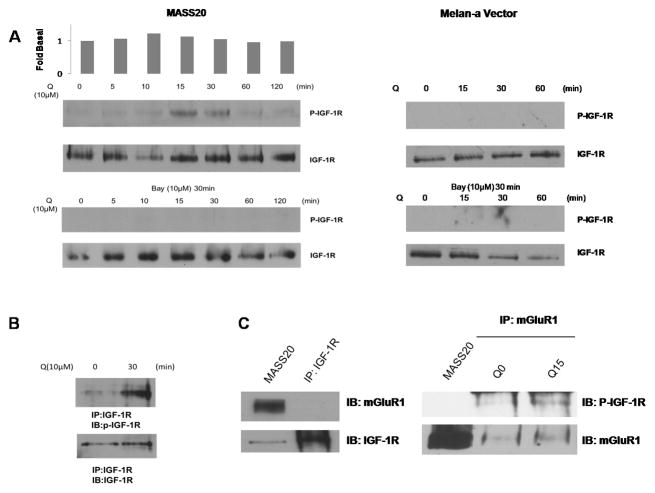
Furthermore, induction of mGluR1 with a Group I mGluR agonist, L-Quisqualate (10μM) in cultured cells caused a rapid transient activation of IGF-1R, which is abolished by pre-incubation with mGluR1-specific antagonist, Bay 36–7620 (10μM) (Figure 2A). Phosphorylation of IGF-1R was not observed in non-tumorigenic melan-a vector cells even when induced by L-Quisqualate. A similar result was achieved with a second stable mGluR1-expressing melanocyte clone, B10BR-SS#14 (Supplementary Figure 1). Because the antibody used to assess IGF-1R phosphorylation cross-reacts with insulin receptor, an immunoprecipitation approach was employed to verify that the 95kD band is indeed IGF-1R. MASS20 cells were induced with L-Quisqualate (10μM) for 30 minutes and extracts were prepared and first immunoprecipitated with IGF-1R antibody followed by immunoblotting using p-IGF-R antibody (Figure 2B). We also found that the phosphorylated form of IGF-1R could be coimmunoprecipitated with mGluR1 only in the presence of L-Quisqualate suggesting that the activation of mGluR1 may allow the recruitment of IGF-1R to the same signaling complex (Figure 2C).
Figure 2. Activation of mGluR1 transactivates IGF-1R.
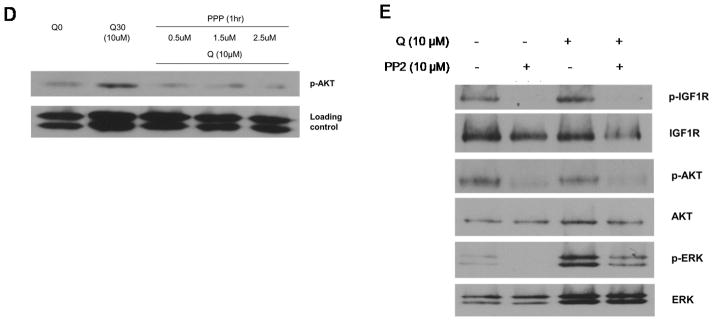
(A) Time-course of L-Quisqualate-(10μM) induced IGF-1R phosphorylation in MASS20 (top panel). Pre-treatment with mGluR1-specific antagonist, Bay 36–7620 (10 μM) abolished this transactivation (middle panel). IGF-1R was not activated by L-Quisqualate in melan-a Vector cells. (B) MASS20 cells treated with or without L-Quisqualate (10μM) for 30min then lysed with Triton-X100 buffer and immunoprecipitated with IGF-1R specific antibody followed by immunoblot with phosphorylated IGF-1R antibody (bottom panel). (C) Western blot analysis of P-IGF-1R coimmunoprecipitated from MASS20 with mGluR1 antibody before and after the activation of mGluR1. (D) MASS20 cells were pre-treated with IGF-1R inhibitor, PPP for 1hr at indicated concentrations before treatment with L-Quisqualate (10μM). (E) MASS20 cells were pretreated with Src inhibitor PP2 (10μM) for 1hr, and then treated with L-Quisqualate (10μM) for 15 min.
Next, we examine whether transactivation of IGF-1R contributes to mGluR1-mediated transformation of mouse melanocytes. First we confirmed our earlier observation that incubation of MASS20 cells with a Group I mGluR agonist, L-Quisqualate (Q) for 30 min led to activation of AKT (Figure 2D). Inclusion of IGF-1R specific inhibitor, PPP, resulted in the absence of AKT phosphorylation above basal levels even in the presence of mGluR1 agonist (Q), suggesting that both mGluR1 and IGF-1R contribute to stimulation of AKT (Figure 2D). Several earlier studies have postulated that Src-family tyrosine kinases serve as intermediates between GPCR and RTKs (Luttrell and Luttrell, 2004). Src is a well-established player in EGFR signaling, directly phosphorylating two key tyrosine residues and allowing mitogenic signals. We assessed the possibility that intracellular tyrosine kinase Src participates as a signaling intermediate for mGluR1-induced IGF-1R tyrosine phosphorylation. Pretreatment of MASS20 with a Src inhibitor, PP2 for 15 minutes (10μM), or the inclusion of a silencing RNA specific for Src followed by stimulation with Q for 15 minutes resulted in the ablation of IGF-1R tyrosine phosphorylation (Figure 2E, Supplementary Figure 2), suggesting that Src is upstream of IGF-1R transactivation. As expected, mGluR1-induced AKT phosphorylation was suppressed with PP2 pre-treatment. Interestingly, MAPK signaling was also disrupted suggesting a divergent pathway following Src kinase activation before reaching IGF-1R (Figure 2E).
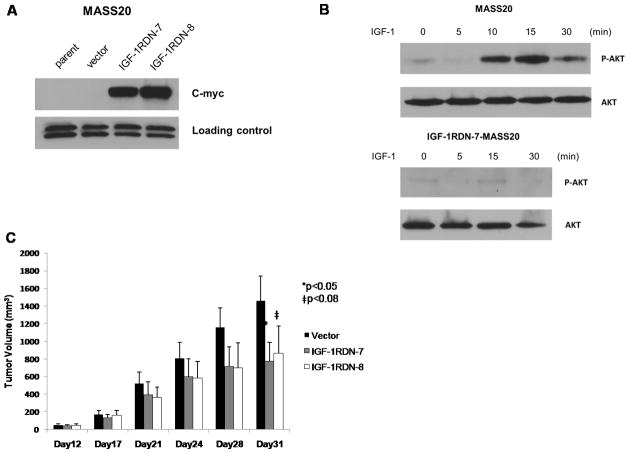
We further confirm the involvement of IGF-1R signaling, by taking advantage of a Myc-tagged dominant-negative IGF-1R construct. MASS20 cells were stably transfected with either empty vector or the truncated IGF-1R mutant. The truncated receptor retains the ligand-binding site but lacks the autophosphorylation domain (Sachdev et al., 2004). Several IGF-1RDN-MASS20 clones were isolated (Figure 3A) and the consequences of the mutated IGF-1R on AKT signaling were assessed. In contrast to the parental MASS20 cells, IGF-1 (5ng/ml) was unable to activate AKT in IGF-1RDN-MASS20 cells (Figure 3B).
Figure 3. Functional IGF-1R is required for mGluR1-mediated tumorigenicity.
(A) Expression of c-Myc-tagged truncated IGF-1R mutant in IGF-RDN-MASS20 clones was visualized by immunoblotting for c-Myc tag. (B) Activation of AKT in IGF-1 treated parental MASS20 cells. Truncated IGF-1R mutant in MASS20 cells inhibits IGF-1(5ng/ml)-induced AKT activation (bottom panel). (C) One vector control and two different IGF-1RDN-MASS20 clones were inoculated into immunodeficient nude mice. Approximately, 35% reduction of tumor volumes in IGF-1RDN-MASS20 clones as compared to Vector-MASS20.
To verify that IGF-1R participates in the tumorigenic phenotype of MASS clones, we subcutaneously injected one vector control-MASS20 and two IGF-1RDN-MASS20 clones into immunodeficient nude mice. Although tumors were detectable in both Vector-MASS20 and IGF-1RDN-MASS20 clones, there was a significant reduction (approximately 40%) in IGF-1RDN-MASS20 tumor volumes by day 21 in comparison to Vector-MASS20 (Figure 3C). The percent inhibition of tumor volume was similar to what we previously observed in MASS20 xenografts following suppression of only AKT2 (6). Taken together, our in vivo data put forth the notion that functional IGF-1/AKT signaling is indeed required for mGluR1-mediated tumorigenesis potentially via the functional transactivation of IGF-1R.
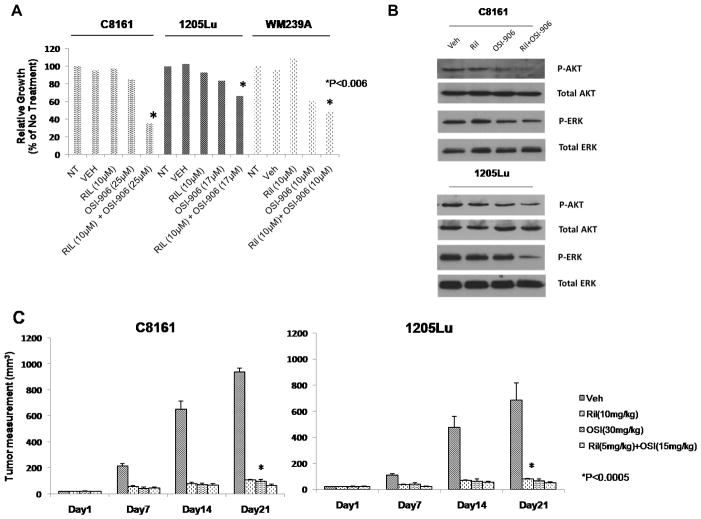
Finally, to evaluate the potential clinical implications in our findings, we explored the efficacy by combining riluzole, an inhibitor of glutamate release and an inhibitor of IGF-1R, Linsitinib (OSI-906) using human melanoma cell lines. B-RAF and N-RAS are among the most common mutations in cutaneous melanoma encompassing 75% and 15% of malignant melanoma cases respectively (Davies et al., 2002; Gorden et al., 2003). The constitutive activation of MAPK pathway in tumors harboring B-RAF and N-RAS mutations are thought to render tumor cells refractory to targeting upstream signaling elements such as mGluR1 or receptor tyrosine kinases. However, we have shown in completed Phase 0 and II trials of single-agent riluzole, an FDA-approved drug for amyotrophic lateral sclerosis, to have anti-tumor activity in patients with advanced melanoma regardless of their BRAF/NRAS status (Yip et al., 2009). We selected a potent inhibitor of IGF-1R that is orally efficacious in pre-clinical models, OSI-906, currently in clinical development for advanced solid tumors (Fassnacht et al., 2011; Mulvihill et al., 2009; Zhao et al., 2012). In in vitro MTT assays all three GRM1-expressing human melanoma cell lines, C8161 (B-RAF wild-type), 1205Lu (B-RAFV600E mutant) and WM239A (B-RAFV600D mutant) displayed enhanced efficacy in suppressing cell growth in the presence of both riluzole and OSI-906 as compared with single agent alone regardless of their B-RAF genotypes (Figure 4A). Previously, we showed a reduction in MAPK signaling in riluzole treated human melanoma cells. Here, we demonstrate enhanced suppression of both phospho-AKT and phospho-ERK with the combination of riluzole and OSI-906 compared to either agent alone (Figure 4B). Given these encouraging in vitro findings, we next assessed the combined efficacy of riluzole and OSI-906 on established xenografted tumors in vivo. Remarkably, the in vivo combinatorial application using only half the optimal dose for single agents showed a synergistic reduction of tumor progression as evident by the decrease in tumor volumes in both B-RAF-wild type and B-RAF-mutant xenografts as compared with vehicle controls (Figure 4C).
Figure 4. Suppression of growth of mGluR1-expressing human melanoma cells with riluzole and OSI-906.
(A) MTT assays of C8161 (B-RAFwild-type), 1205Lu (B-RAFV600E), WM239A (B-RAFV600D) with glutamate release inhibitor, riluzole and IGF-1R inhibitor, OSI-906 or combination of both compounds. (B) Effects of riluzole and OSI-906 on phosphorylated-AKT and phosphorylated-ERK levels. C8161 (Ril; 10μM, OSI; 25μM) and 1205Lu (Ril;10μM, OSI;10μM). (C) Xenografts of C8161 (B-RAFwild-type) and 1205Lu (B-RAFV600E). The groups were no treatment (NT), vehicle (Veh; DMSO), riluzole (10mg/kg), OSI-906 (30mg/kg), or the combination of riluzole (5 mg/kg) and OSI-906 (15mg/kg). All agents were given daily by p.o. gavage. Each treatment group was compared to vehicle group, p<0.0005, t-test, n=10 in each group.
Additionally, we examined tumor biopsies from five patients with advanced metastatic melanoma from both completed Phase 0 and II trials treated with riluzole (Yip et al., 2009). The tumors of all five responders and non-responders were positive for mGluR1 expression. Five sets of paired tumor samples (pre-treatment and post-treatment) were analyzed for phospho-IGF-1R and phospho-AKT levels. Interestingly, reduced phospho-IGF-1R and phospho-AKT levels were detected in responder from both Phase 0 and stable disease in Phase II trials (Patient 9 and Patient 1 respectively) (Figure 5A). In contrast, increased levels of phospho-IGF-1R were detected in tumor biopsies of non-responders, Patient 6 and 12 from Phase 0 and Patient 3 from Phase II (Figure 5A). Correspondingly, Phospho-AKT levels were also increased in non-responders (Patient 12 from Phase 0 and Patient 3 from Phase II). Patient 6 from Phase 0 trial displayed unchanged phospho-AKT levels but elevated phospho-IGF-1R. From these limited results, we speculate that activation of the IGF-1R/PI3K/AKT axis may be associated with resistance to riluzole.
Figure 5. Increased phospho-IGF-1R expression in non-responding patients of Phase 0 and Phase II riluzole trials.
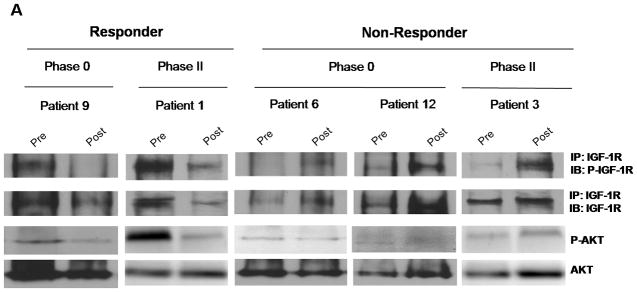
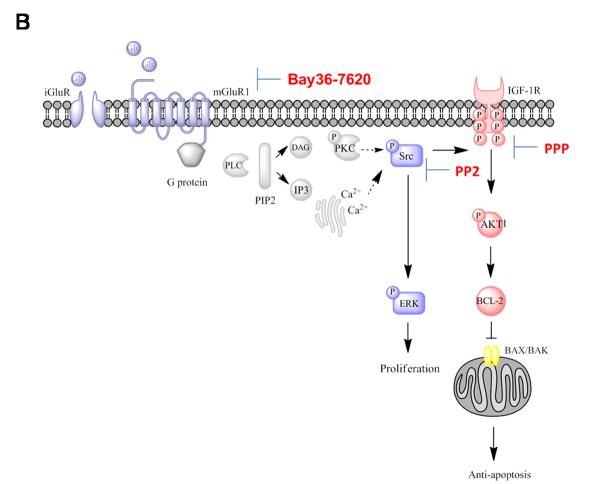
(A) Paired tumor samples from pre-treatment and post-riluzole treatment were analyzed for P-IGF-1R or P-AKT expression by Western immunoblots. A reduction in levels of P-IGF-1R and P-AKT was detected in Phase 0 responder (Patient 9) and Phase II patient with stable disease (Patient 1). In contrast, non-responders in Phase 0 (Patient 6 and 12) and Phase II (Patient 3) riluzole trials showed elevated P-IGF-1R and P-AKT levels or unaltered P-AKT in Patient 6. The blots were subsequently stripped and probed with IGF-1R and AKT antibodies as control. (B) Proposed signaling pathways activated by mGluR1 and mediated by IGF-1R transactivation (Adapted from review article(Teh and Chen, 2012)).
Discussion
Several recent reports by other groups implicate both ionotropic and metabotropic glutamate receptors in oncogenesis; overexpression of mGluR5 and activating mutations in GRIN2A and GRM3 in melanoma (Choi et al., 2011; Prickett et al., 2011). Other noteworthy findings include the identification of over 20 missense mutations in GRM1 in various tumor types, some of which has been characterized to have multiple downstream consequences (Esseltine et al., 2013). Our group was the first to show through insertional mutagenesis resulting in the disruption of intron 3 of Grm1 and the ectopic expression of mGluR1 in melanocytes, which ultimately produced spontaneous metastatic melanomas with 100% penetrance (Pollock et al., 2003).
Conventionally, GPCRS and RTKs were thought to represent distinct signaling units converging only further downstream in the signaling cascade (Natarajan and Berk, 2006). Recently, it is becoming more apparent that they do not operate exclusively but rather cross-communicate with G protein activity occurring either upstream or downstream of RTKs (Delcourt et al., 2007; Natarajan and Berk, 2006). Although crosstalk between GPCRs and RTKs are not novel, this is the first report, which demonstrates the functional transactivation of IGF-1R by mGluR1 in mGluR1 transformed mouse melanocytic cells (Figure 5B). Some other well-characterized examples demonstrating IGF-1R transactivation by GPCRs include angiotensin II type 1(AT1) receptor in smooth muscle cells and GABAB receptor in neuronal cells (Tu et al., 2010; Zahradka et al., 2004). Results from our studies suggest that the mechanism involved is more similar to that exerted by AT1 receptor which is dependent on Src kinase activation (Zahradka et al., 2004). In addition, we also present evidence that activation of the PI3K/AKT pathway occurs via IGF-1R, which in turn contributes to mGluR1-mediated tumorigenesis. This substantiates earlier reports on the cross-coupled signaling between mGluRs and epidermal growth factor receptors (EGFR) with significant implications in glial cell physiology and pathogenesis (Peavy et al., 2001; Sitcheran et al., 2008).
Whether or not melanoma cells produce IGF-1 is controversial. It has been speculated that IGF-1R expressing melanoma cells receive signal from paracrine sources of IGF-1 such as neighboring keratinocytes and fibroblasts because IGF-1R expression correlates with melanoma progression (Kanter-Lewensohn et al., 2000; Satyamoorthy et al., 2001). However, Molhoek and colleagues recently showed the expression of IGF-1 mRNA in melanoma cells (Molhoek et al., 2011) thus, the possibility that melanoma cells produce IGF-1 and create autocrine loops cannot be ruled out. Human relevance of our current studies was put forth in the in vivo tumorigenesis studies with reduced tumor progression by inhibitors to glutamate and IGF-1R signaling. Interestingly, we also observed that biopsies from our completed Phase 0 and II trials, non-responders showed elevated levels of phosphorylated IGF-1R in post-treatment samples in comparison to pre-treatment biopsies (Figure 5A). In contrast, samples from one responder in the Phase 0 trial and one patient with stable disease in the Phase II trial did not show amplified P-IGF-1R in post-treatment samples. Despite the small sample size, these promising results suggest the merit of combining riluzole and OSI-906 to circumvent the challenge of drug resistance. This is in line with our previous observation of patients who did not show clinical or radiologic response in the Phase 0 trial but exhibit high or similar levels of phosphorylated AKT in their post-treatment tumor biopsies (Yip et al., 2009). Similarly, enhanced IGF-1R/PI3K signaling was detected in B-RAF-inhibitor resistant melanomas (Villanueva et al., 2010). Presence of IGF-1 ligand has also been shown to override the suppression of AKT activity by B-RAF inhibitor, PLX4720 (Straussman et al., 2012). Taken together, we propose that IGF-1R activation represents a previously overlooked key pathway involved in the mechanisms by which mGluR1 exerts its transformative properties and warrants further investigation.
Materials and methods
Antibodies and reagents
Anti-phosphorylated AKT (Thr308, Ser473), anti-AKT2 (5B5), anti-phosphorylated ERK1/2 (Thr202/Tyr204), anti-AKT, anti-ERK1/2, anti-Src and anti-IGF-1R antibodies were purchased from Cell Signaling (Davers, MA, USA); IGF-1 (Recombinant Human Insulin Like Growth Factor-1) and anti-phosphorylated IGF-1R antibody were purchased from Invitrogen (Carsbad, CA, USA); anti-mGluR1 was purchased from BD Biosciences (Franklin Lakes, NJ, USA), monoclonal anti-α-tubulin antibody was purchased from Sigma (St. Louis, MO, USA). C-myc tag antibody, L-Quisqualate [(L)-(+)-α-amino-3,5-dioxo-1,2, 4-oxadiazolidine-2-propanoic acid] and Bay 36–7620 [(3aS,6aS)-6anaphtalen-2-ylmethyl-5-methyliden-hexahydro-cyclopental[c]-furan-1-on] were purchased from Tocris (Ellisville, MO, USA). PPP (Picropodophyllin) and PP-2 (4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine) were purchased from Calbiochem (San Diego, CA, USA). Riluzole was obtained from Sigma and OSI-906 (Linsitinib) was generously provided by OSI Pharmaceuticals Inc. and the National Cancer Institute, National Institutes of Health.
Cell culture conditions
Stable Grm1-melanocytic transfectants (MASS and B10SS clones) were produced from introduction of exogenous Grm1 cDNA into immortalized, normal murine melanocytes, melan-a and B10.BR respectively that were provided by Dr. Dorothy Bennett (St. George’s, University of London, UK) and Dr. Ruth Halaban (Yale University, New Haven, CT). UACC903 cells were provided by Dr. Jeffrey Trent (The Translational Genomics Research Center, Phoenix, AZ). 1205Lu cells and WM239A cells were provided by Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA). All cells were maintained in RPMI plus 10% FBS.
A total of 1.25μg siAKT2-Tet° and 1.25μg Zeocin™ plasmid DNA were co-transfected into siGrm1-MASS20-TetR cells(Shin et al., 2008b). Selection of siGrm1-siAKT2-MASS20 stable clones was performed with 400μg/ml of Zeocin™.
Truncated IGF-1R cDNA tagged with the c-myc epitope was kindly provided by Dr. Douglas Yee (Minnesota, University of Minnesota, USA). This construct was transfected into one of the stable-Grm1-melanocytic clones, MASS20. Selection of IGF-1RDN-MASS20 stable clones was performed with 75μg/ml of Zeocin™.
For induction experiments, the conditions were the same as reported (Shin et al., 2008b). Briefly, cells were grown in glutamate/glutamine free RPMI plus 10% dialyzed fetal bovine serum with the supplement of GlutaMax™ (Gibco Life Technologies, Invitrogen, Carsbad, CA, USA) to minimize the effect of glutamate, the natural ligand of Grm1 for 3 days before the cells were plated out in glu/gln free RPMI medium for induction experiments.
Src small interfering RNA (siRNA) transfection in MASS20
A pool of 4 target-specific siRNA designed to target c-Src (sc-29228) and the appropriate control siRNA (sc-37007) was purchased from Santa Cruz Biotechnology. The cells were used 48 hours after transfection.
Western immunoblots
Protein lysates were prepared as described previously (Cohen-Solal et al., 2002). Briefly, media was removed and cells were washed once with ice-cold phosphate buffered saline (PBS). After removal of PBS, extraction buffer was added directly to the plates and cells were collected with a cell scraper. Cell extracts were incubated on ice for 20 min. Cell debris was removed by centrifugation at 14000xg at 4°C for 20 min and supernatant was collected to measure protein concentration and for Western immunoblot analysis.
MTT assays
Each cell line was cultured in 96 well plates at 2x103 per well with the following conditions; no treatment, vehicle (dimethyl sulfoxide, DMSO), riluzole and OSI-906 or a combination of both riluzole and OSI-906. Concentration of each treatment group is stated in the figure legends. Viable cells were measured at Day 4 and Day 7. At designated time points, 0.1 volumes of 5mg/ml Thiazolyl Blue Tetrazolium Bromide (Sigma) in 1x phosphate-buffered saline were added to growth medium, incubated for 4h at 37°C and then solubilized overnight with equal volume of 10% sodium dodecyl sulfate/0.1N HCl. A 96 well plate reader (Infinite 200 Tecan USA, Durham, NC, USA) was used to measure absorbance at 550nm with a reference wavelength of 750nm.
Immunoprecipitation/Coimmunoprecipitation
For immunoprecipitation, protein-A/G-agarose beads were used (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Procedures were performed as per manufacturer’s instructions. Briefly, lysate was incubated with rabbit anti-IGF-1R for 1hr in 4°C. 20μL of Protein-A/G agarose beads were added and incubated overnight at 4°C. After collecting the pellets by centrifugation at 2500 rpm for 5min at 4°C, the pellets were washed four times with Triton-X-100 buffer. Washed pellets were then mixed with 40μl of 1x electrophoresis buffer and boiled for 5min. Samples were centrifuged and loaded for SDS gel electrophoresis.
A similar procedure was carried out for coimmunoprecipitation. Lysates were pre-cleared with protein A/G agarose beads and goat serum before they were incubated with anti-mGluR1 goat polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Subsequently, samples were loaded for SDS gel electrophoresis and probed with a P-IGF-1R antibody.
Allograft assay
The Institutional Animal Care and Use Committee at Rutgers University approved all animal studies. Nude mice were purchased from Taconic (Hudson, NY, USA). To assess the effect of siGrm1 and siAKT2 on Grm1-induced tumorigenesis of MASS clones, 106 cells from each stable siGrm1siAKT-MASS clone were injected subcutaneously into each of the dorsal flanks of 6 week-old male immunodeficient nude mice (Taconic, Hudson, NY, USA). Appearance of the tumor was monitored daily and measured twice a week. When the tumor volumes reach 10mm3, mice were divided randomly into experimental and control groups. Mice in the experimental group were fed with doxycycline (0.2% w/v) containing drinking water and the control group was fed with regular drinking water. Drinking water was changed twice a week. Tumor volumes were measured twice a week with a vernier caliper and calculated by the formula (d2 × D/2), D is the large number and d is the smaller one.
Similarly, to determine the effect of disrupted IGF-1R signaling in MASS clones, 106 cells from one vector control and two stable IGF-1RDN-MASS20 clones were injected subcutaneously into each of the dorsal flanks of 6 week-old male immunodeficient nude mice (Taconic, Hudson, NY, USA). Tumors were monitored and measured as described above.
For the xenograft studies with riluzole and OSI-906, human melanoma cells were injected into both dorsal sites of each mouse at 106 cells per site. Once tumor volumes reached 10 mm3, mice were divided randomly into no treatment and treatment groups. The treatment groups received either vehicle(DMSO), riluzole(10 mg/kg), OSI-906(30 mg/kg) or the combination of riluzole(5 mg/kg) and OSI-906(15 mg/kg) by oral gavage daily. The doses of oral riluzole and OSI-906 were based on published reports (Kim et al., 2012; Lee et al., 2011).
Statistics
Statistical analyses were performed using a paired one-tailed Student’s t-test.
Supplementary Material
Figure S1 Time-course of L-Quisqualate(10μM)-induced IGF-1R phosphorylation in B10SS#14 (top panel). Pre-treatment with mGluR1-specific antagonist, Bay 36–7620 (10μM) abolished this transactivation (middle panel). IGF-1R was not activated by L-Quisqualate in B10.BR parental cells.
Figure S2 (A) Immunoblot with c-Src-specific antibody 48 hours after transfection of small interfering RNA, siCTL (control, scramble RNA) or siSrc showed complete knockdown of Src kinase. (B) Knockdown of Src resulted in the abolishment of IGF-1R activation by mGluR1 agonist, L-Quisqualate.
Significance.
Recent reports implicate metabotropic glutamate receptors in melanomagenesis, activating mutations in GRM3 and overexpression of mGluR5. Our group was the first to show that ectopic expression of mGluR1 can induce spontaneous melanoma development in a transgenic mouse model. Subsequently, PI3K/AKT signaling was identified as one of the downstream targets of mGluR1. Here, we demonstrate a novel pathway for mGluR1-mediated tumorigenesis involving the transactivation of IGF-1R. Disrupted IGF-1R signaling in stable Grm1-melanocytic cells led to the ablation of mGluR1-induced AKT activation and reduced tumor growth. We propose that IGF-1R activation represents a previously overlooked key pathway involved in the mechanisms by which mGluR1 exerts its transformative properties.
Acknowledgments
This work is supported by NCI RO1CA108720, RO1CA124975 and NJCCR 07-1064-CCR-E0. We would like to acknowledge the generosity of Dr. Douglas Yee (Masonic Cancer Center, University of Minnesota) for the IGF-1R constructs. The authors would also like to thank Dr. Brian Wall for advice and technical assistance. The OSI-906 compound was generously provided by OSI Pharmaceuticals Inc. and the National Cancer Institute, National Institutes of Health.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Almendro V, Garcia-Recio S, Gascon P. Tyrosine kinase receptor transactivation associated to G protein-coupled receptors. Curr Drug Targets. 2010;11:1169–80. doi: 10.2174/138945010792006807. [DOI] [PubMed] [Google Scholar]
- Bhola NE, Grandis JR. Crosstalk between G-protein-coupled receptors and epidermal growth factor receptor in cancer. Front Biosci. 2008;13:1857–65. doi: 10.2741/2805. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhu H, Wetzel WJ, Philbert MA. Spontaneous melanocytosis in transgenic mice. J Invest Dermatol. 1996;106:1145–51. doi: 10.1111/1523-1747.ep12340194. [DOI] [PubMed] [Google Scholar]
- Choi KY, Chang K, Pickel JM, Badger JD, 2nd, Roche KW. Expression of the metabotropic glutamate receptor 5 (mGluR5) induces melanoma in transgenic mice. Proc Natl Acad Sci U S A. 2011;108:15219–24. doi: 10.1073/pnas.1107304108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Solal KA, Crespo-Carbone SM, Namkoong J, Mackason KR, Roberts KG, Reuhl KR, Chen S. Progressive appearance of pigmentation in amelanotic melanoma lesions. Pigment Cell Res. 2002;15:282–9. doi: 10.1034/j.1600-0749.2002.02024.x. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Delcourt N, Bockaert J, Marin P. GPCR-jacking: from a new route in RTK signalling to a new concept in GPCR activation. Trends Pharmacol Sci. 2007;28:602–7. doi: 10.1016/j.tips.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Esseltine JL, Willard MD, Wulur IH, Lajiness ME, Barber TD, Ferguson SS. Somatic Mutations in GRM1 in Cancer Alter Metabotropic Glutamate Receptor 1 Intracellular Localization and Signaling. Mol Pharmacol. 2013 doi: 10.1124/mol.112.081695. [DOI] [PubMed] [Google Scholar]
- Fassnacht M, Libe R, Kroiss M, Allolio B. Adrenocortical carcinoma: a clinician’s update. Nat Rev Endocrinol. 2011;7:323–35. doi: 10.1038/nrendo.2010.235. [DOI] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov I, Kim KB, Ribas A, Mcarthur GA, Sosman JA, O’dwyer PJ, Lee RJ, Grippo JF, Nolop K, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden A, Osman I, Gai W, He D, Huang W, Davidson A, Houghton AN, Busam K, Polsky D. Analysis of BRAF and N-RAS mutations in metastatic melanoma tissues. Cancer Res. 2003;63:3955–7. [PubMed] [Google Scholar]
- Kanter-Lewensohn L, Dricu A, Girnita L, Wejde J, Larsson O. Expression of insulin-like growth factor-1 receptor (IGF-1R) and p27Kip1 in melanocytic tumors: a potential regulatory role of IGF-1 pathway in distribution of p27Kip1 between different cyclins. Growth Factors. 2000;17:193–202. doi: 10.3109/08977190009001068. [DOI] [PubMed] [Google Scholar]
- Kim WY, Prudkin L, Feng L, Kim ES, Hennessy B, Lee JS, Lee JJ, Glisson B, Lippman SM, Wistuba Ii, et al. Epidermal growth factor receptor and K-Ras mutations and resistance of lung cancer to insulin-like growth factor 1 receptor tyrosine kinase inhibitors. Cancer. 2012;118:3993–4003. doi: 10.1002/cncr.26656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Wall BA, Wangari-Talbot J, Shin SS, Rosenberg S, Chan JL, Namkoong J, Goydos JS, Chen S. Glutamatergic pathway targeting in melanoma: single-agent and combinatorial therapies. Clin Cancer Res. 2011;17:7080–92. doi: 10.1158/1078-0432.CCR-11-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell DK, Luttrell LM. Not so strange bedfellows: G-protein-coupled receptors and Src family kinases. Oncogene. 2004;23:7969–78. doi: 10.1038/sj.onc.1208162. [DOI] [PubMed] [Google Scholar]
- Molhoek KR, Shada AL, Smolkin M, Chowbina S, Papin J, Brautigan DL, Slingluff CL., Jr Comprehensive analysis of receptor tyrosine kinase activation in human melanomas reveals autocrine signaling through IGF-1R. Melanoma Res. 2011;21:274–84. doi: 10.1097/CMR.0b013e328343a1d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill MJ, Cooke A, Rosenfeld-Franklin M, Buck E, Foreman K, Landfair D, O’connor M, Pirritt C, Sun Y, Yao Y, et al. Discovery of OSI-906: a selective and orally efficacious dual inhibitor of the IGF-1 receptor and insulin receptor. Future Med Chem. 2009;1:1153–71. doi: 10.4155/fmc.09.89. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Berk BC. Crosstalk coregulation mechanisms of G protein-coupled receptors and receptor tyrosine kinases. Methods Mol Biol. 2006;332:51–77. doi: 10.1385/1-59745-048-0:51. [DOI] [PubMed] [Google Scholar]
- Nogueira C, Kim KH, Sung H, Paraiso KH, Dannenberg JH, Bosenberg M, Chin L, Kim M. Cooperative interactions of PTEN deficiency and RAS activation in melanoma metastasis. Oncogene. 2010;29:6222–32. doi: 10.1038/onc.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy RD, Chang MS, Sanders-Bush E, Conn PJ. Metabotropic glutamate receptor 5-induced phosphorylation of extracellular signal-regulated kinase in astrocytes depends on transactivation of the epidermal growth factor receptor. J Neurosci. 2001;21:9619–28. doi: 10.1523/JNEUROSCI.21-24-09619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock PM, Cohen-Solal K, Sood R, Namkoong J, Martino JJ, Koganti A, Zhu H, Robbins C, Makalowska I, Shin SS, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34:108–12. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- Prickett TD, Wei X, Cardenas-Navia I, Teer JK, Lin JC, Walia V, Gartner J, Jiang J, Cherukuri PF, Molinolo A, et al. Exon capture analysis of G protein-coupled receptors identifies activating mutations in GRM3 in melanoma. Nat Genet. 2011 doi: 10.1038/ng.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev D, Hartell JS, Lee AV, Zhang X, Yee D. A dominant negative type I insulin-like growth factor receptor inhibits metastasis of human cancer cells. J Biol Chem. 2004;279:5017–24. doi: 10.1074/jbc.M305403200. [DOI] [PubMed] [Google Scholar]
- Satyamoorthy K, Li G, Vaidya B, Patel D, Herlyn M. Insulin-like growth factor-1 induces survival and growth of biologically early melanoma cells through both the mitogen-activated protein kinase and beta-catenin pathways. Cancer Res. 2001;61:7318–24. [PubMed] [Google Scholar]
- Schiffner S, Chen S, Becker JC, Bosserhoff AK. Highly pigmented Tg(Grm1) mouse melanoma develops non-pigmented melanoma cells in distant metastases. Exp Dermatol. 2012;21:786–8. doi: 10.1111/j.1600-0625.2012.01560.x. [DOI] [PubMed] [Google Scholar]
- Shin SS, Martino JJ, Chen S. Metabotropic glutamate receptors (mGlus) and cellular transformation. Neuropharmacology. 2008a;55:396–402. doi: 10.1016/j.neuropharm.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SS, Namkoong J, Wall BA, Gleason R, Lee HJ, Chen S. Oncogenic activities of metabotropic glutamate receptor 1 (Grm1) in melanocyte transformation. Pigment Cell Melanoma Res. 2008b;21:368–78. doi: 10.1111/j.1755-148X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SS, Wall BA, Goydos JS, Chen S. AKT2 is a downstream target of metabotropic glutamate receptor 1 (Grm1) Pigment Cell Melanoma Res. 2010;23:103–11. doi: 10.1111/j.1755-148X.2009.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitcheran R, Comb WC, Cogswell PC, Baldwin AS. Essential role for epidermal growth factor receptor in glutamate receptor signaling to NF-kappaB. Mol Cell Biol. 2008;28:5061–70. doi: 10.1128/MCB.00578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl JM, Sharma A, Cheung M, Zimmerman M, Cheng JQ, Bosenberg MW, Kester M, Sandirasegarane L, Robertson GP. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–10. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–4. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh JL, Chen S. Glutamatergic signaling in cellular transformation. Pigment Cell Melanoma Res. 2012;25:331–42. doi: 10.1111/j.1755-148X.2012.00983.x. [DOI] [PubMed] [Google Scholar]
- Tu H, Xu C, Zhang W, Liu Q, Rondard P, Pin JP, Liu J. GABAB receptor activation protects neurons from apoptosis via IGF-1 receptor transactivation. J Neurosci. 2010;30:749–59. doi: 10.1523/JNEUROSCI.2343-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, Wubbenhorst B, Xu X, Gimotty PA, Kee D, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–95. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip D, Le MN, Chan JL, Lee JH, Mehnert JA, Yudd A, Kempf J, Shih WJ, Chen S, Goydos JS. A phase 0 trial of riluzole in patients with resectable stage III and IV melanoma. Clin Cancer Res. 2009;15:3896–902. doi: 10.1158/1078-0432.CCR-08-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahradka P, Litchie B, Storie B, Helwer G. Transactivation of the insulin-like growth factor-I receptor by angiotensin II mediates downstream signaling from the angiotensin II type 1 receptor to phosphatidylinositol 3-kinase. Endocrinology. 2004;145:2978–87. doi: 10.1210/en.2004-0029. [DOI] [PubMed] [Google Scholar]
- Zhao H, Desai V, Wang J, Epstein DM, Miglarese M, Buck E. Epithelial-mesenchymal transition predicts sensitivity to the dual IGF-1R/IR inhibitor OSI-906 in hepatocellular carcinoma cell lines. Mol Cancer Ther. 2012;11:503–13. doi: 10.1158/1535-7163.MCT-11-0327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Time-course of L-Quisqualate(10μM)-induced IGF-1R phosphorylation in B10SS#14 (top panel). Pre-treatment with mGluR1-specific antagonist, Bay 36–7620 (10μM) abolished this transactivation (middle panel). IGF-1R was not activated by L-Quisqualate in B10.BR parental cells.
Figure S2 (A) Immunoblot with c-Src-specific antibody 48 hours after transfection of small interfering RNA, siCTL (control, scramble RNA) or siSrc showed complete knockdown of Src kinase. (B) Knockdown of Src resulted in the abolishment of IGF-1R activation by mGluR1 agonist, L-Quisqualate.