Abstract
RAW264.7 cells are one of the major sources of productive inflammatory biomediators, including tumour necrosis factor-α (TNF-α) and interleukin (IL)-6. TNF-α-induced protein 8-like 2 (TIPE2) is an essential negative regulator of Toll-like and T-cell receptors, and the selective expression in the immune system prevents hyper-responsiveness and maintains immune homeostasis. The aim of the present study was to investigate whether atorvastatin upregulates the expression of TIPE2 and further regulates the inflammatory response and oxidation emergency response in RAW264.7 cells. RAW264.7 cells were incubated in Dulbecco’s modified Eagle’s medium containing lipopolysaccharide (LPS) in the presence or absence of atorvastatin. Following incubation, the medium was collected and the levels of TNF-α and IL-6 were measured using an enzyme-linked immunosorbent assay. The cells were harvested, and the mRNA and protein expression levels of TIPE2, macrophage migration inhibitory factor (MIF), IκB and nuclear factor (NF-κB)-κB were analysed using quantitative polymerase chain reaction and western blotting analysis, respectively, the expression of NOS, COX-2 and HO-1 protein were essayed by western blotting analysis, NO and ROS activities were determined. The results revealed that LPS increased the mRNA and protein expression levels of TIPE2, MIF and NF-κB, as well as the production of IL-6 and TNF-α, in a dose and time dependent manner in RAW264.7 cells. Meanwhile, LPS enhanced the expression of NOS and COX-2 protein, blocked HO-1 protein expression, increased NO and PGE2 production and ROS activity in a dose dependent manner in RAW264.7 cells. Atorvastatin significantly increased LPS induced expression of TIPE2, downregulated the expression of NOS, COX-2, MIF and NF-κB and the production of PGE2, NO, IL-6 and TNF-α in a time and dose dependent manner, and increased HO-1 protein expression, reduced ROS production in a dose dependent manner. The observations indicated that atorvastatin upregulated LPS induced expression of TIPE2 and consequently inhibited MIF, NF-κB, NOS and COX-2 expression and the production of NO, PGE2, TNF-α and IL-6, increased HO-1 expression, and inhibited ROS activity in cultured RAW264.7 cells.
Keywords: atorvastatin, RAW264.7 cells, tumour necrosis factor-α-induced protein 8-like 2, macrophage migration inhibitory factor, nuclear factor-κB
Introduction
Sepsis, which results in multiple organ failure, remains a leading cause of mortality and morbidity in intensive care units (1,2). Uncontrolled hyperinflammatory and inappropriate cytokine responses during early sepsis are hypothesised to be the cause of multiple organ dysfunction syndrome (MODS) during sepsis. Thus, controlling inflammation during early sepsis may reduce organ injury and prevent mortality following septic insult. Sepsis is the leading cause of mortality in critically ill patients, and the incidence of sepsis is increasing (3,4). The mortality rate of severe sepsis is very high (up to 70%) and the calculated costs exceed $15 billion per year in the United States (3). The incidence rate of severe sepsis during hospitalisation almost doubled within the last decade and is considerably greater than previously predicted (5). Lipopolysaccharide (LPS), which triggers a systemic inflammatory response (SIR) (1,2), can stimulate RAW264.7 and other inflammatory cells to produce a variety of inflammatory mediators, including tumour necrosis factor-α (TNF-α) and interleukin (IL)-1, 6 and 8. These inflammatory mediators enhance the activity of nuclear factor-κB (NF-κB), resulting in a large number of secondary inflammatory mediators, which aggravates the waterfall effect of SIR, further promoting the development of systemic inflammatory response syndrome (SIRS) into MODS (3).
TNF-α-induced protein 8-like 2 (TIPE2) is necessary for maintaining immune homeostasis (6) and is a member belonging to TNF-α-induced protein-8 (TNFAIP8) family, which is highly expressed in inflammatory tissues, thus, mainly exhibits a negative regulatory effect on the innate immune response (7). Several studies have shown that TIPE2 can inhibit NF-κB expression by virtue of multiple channels, thereby reducing the generation of TNF-α, IL-6 and other proinflammatory mediators and alleviating the sepsis inflammatory response (8).
Statin drugs, including hydroxyl methyl glutaric phthalein coenzyme A (HMG-CoA) reductase inhibitors, are currently the most widely applied and important lipid-regulating drugs. A recent study demonstrated that statins not only exhibit lipid-lowering effects for heart protection, but also inflammatory effects (9). A previous study revealed that statins can inhibit the production of TNF-α, IL-6 and other inflammatory mediators in rats with sepsis (10).
The aim of the present study was to investigate the immune regulatory mechanism of atorvastatin. Since TIPE2 plays a crucial role during the regulatory process of inflammatory immune disorders in severely infected patients, the present study aimed to determine whether atorvastatin inhibited NF-κB expression via the enhancement of TIPE2 expression in LPS-induced RAW264.7 cells, and further decreased the production of proinflammatory factors, including TNF-α and IL-6.
Materials and methods
Materials
A murine RAW264.7 macrophage cell line was obtained from the American Type Culture Collection (Rockville, MD, USA). Dulbecco’s modified Eagle’s medium (DMEM), trypsin, foetal bovine serum (FBS) and LPS were purchased from Sigma-Aldrich (St. Louis, MO, USA). Enhanced chemiluminescence (ECL) reagents and NF-κB and TIPE2 polyclonal antibodies were purchased from BD Pharmingen. (San Jose, CA, USA). Anti-rabbit IgG horseradish peroxidase-conjugated secondary antibodies were obtained from R&D Systems Inc. (Minneapolis, MN, USA). HECAMEG was obtained from Vegatec (Villejuif, France), and TRIzol and electrophoresis reagents were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Atorvastatin was purchased from Pfizer (New York City, NY, USA).
TIPE2 knockdown
TIPE2 siRNA (5′-GAAGTGAAA CTCAGGTCCG-3′) or scrambled control siRNA (5′-AGT GAAACAGTGCAGCTGC-3′) was cloned into the lentiviral vector, pLL3.7, which carries green fluorescent protein (GFP) cDNA. Lentiviruses were produced by cotransfecting 293T cells with pLL3.7 plasmids and packaging vectors (11). To knockdown TIPE2 expression, RAW264.7 were infected with recombinant lentiviruses that carried the TIPE2 siRNA. Cells infected with the same number of recombinant lentiviruses carrying the control siRNA served as wildtype controls. GFP was used to track the virus-infected cells. For the RAW264.7 cell line, infected cells constituted >90% of the total number of cells and the transfected cells were used for the following experiments.
Cell culture
TIPE2 siRNA and control RAW264.7 macrophages were grown in DMEM supplemented with 5% FBS, 100 μg/ml penicillin, 100 μg/ml streptomycin and 50 μg/l amphotericin B. Cells were subcultured in six-well plates and maintained until subconfluence. The medium was then replaced with serum-free culture medium for 24 h prior to the addition of LPS and/or other reagents. The cells were incubated with various concentrations of LPS for 9 h, or 10 ng/ml LPS with various concentrations of atorvastatin for different time periods. For the atorvastatin experiments, the cells were incubated in serum-free medium containing 10 ng/ml LPS in the presence or absence of atorvastatin for 9 h.
RNA isolation and reverse transcription
Total RNA was isolated from cultured RAW264.7 macrophages using the single-step acid guanidinium thiocyanate/phenol/chloroform extraction method. Total RNA (1 μg) was incubated with 200 units Moloney-Murine Leukemia Virus reverse transcriptase in buffer containing 50 mmol/l Tris-HCl (pH 8.3), 75 mmol/l KCl, 3 mmol/l MgCl2, 20 units RNase inhibitor, 1 μmol/l poly(dT) oligomer and 0.5 mmol/l each dNTP in a final volume of 20 μl. The reaction mixture was incubated at 42°C for 1 h and then at 94°C for 5 min to inactivate the enzyme. A total of 80 μl diethyl pyrocarbonate-treated water was added to the reaction mixture prior to storage at −70°C.
Quantitative polymerase chain reaction (qPCR)
RAW264.7 macrophages were homogenised in TRIzol reagent using a Mixer 301. Total RNA was extracted according to the manufacturer’s instructions. RNA samples were electrophoresed in agarose gels and visualised with ethidium bromide for quality control. In total, 3 μg RNA was reverse transcribed with reverse transcriptase for 1 h at 37°C for cDNA synthesis. Quantitative changes in the mRNA expression levels were assessed with a CFX Connect Real-Time PCR Detection System (Bio-Rad, Hercules, USA) using SYBR-Green detection (Aria-tous, Iran). The PCR master mix consisted of 0.5 units Taq polymerase, 2 μl each primer and 3 μl each cDNA sample in a final volume of 20 μl. All the amplifications were repeated three times. The oligonucleotide primer sequences are shown in Table I. β-actin was used as an endogenous control and each sample was normalised on the basis of the β-actin content. The relative quantification of the mRNA expression levels of the target genes was calculated using the 2−ΔΔCt method.
Table I.
Primer sequences of the genes used to validate the microarray analysis by qPCR.
| Gene | Primer | Product (bp) |
|---|---|---|
| TIPE2 | F: 5′-GGGAACATCCAAGGCAAG-3′ R: 5′-AGCTCATCTAGCACCTCACT-3′ |
195 |
| MIF | F: 5′-ATGCCTATGTTCATCGTGAAC-3′ R: 5′-GGCTACGACGAAGGTGGAAC-3′ |
341 |
| NF-κB | F: 5′-GCACGGATGACAGAGGCGTGTATAAGG-3′ R: 5′-GGCGGATGATCTCCTTCTCTCTGTCTG-3′ |
420 |
| IκB | F: 5′-TGCTGAGGCACTTCTGAG-3′ R: 5′-CTGTATCCGGGTGCTTGG-3′ |
421 |
| β-actin | F: 5′-GATTACTGCTCTGGCTCCTGC-3′ R: 5′-GACTCATCGTACTCCTGCTTGC-3′ |
190 |
qPCR, quantitative polymerase chain reaction; TIPE2, tumour necrosis factor-α-induced protein 8-like 2; MIF, macrophage migration inhibitory factor; NF-κB, nuclear factor-κB; F, forward; R, reverse.
Western blot analysis
Protein expression levels of TIPE2, nitric oxide synthase (NOS), haem oxygenase (HO-1) and cyclooxygenase-2 (COX-2) from cultured RAW264.7 cells were quantified using western blot analysis. Briefly, proteins from a 50-μg sample were separated using 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. The membranes were incubated overnight with mouse monoclonal anti-TIPE2 or anti-HO-1 antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or rabbit monoclonal anti-NOS and anti-COX-2 antibodies (Abcam, Cambridge, MA, USA) at 4°C overnight. Following incubation with a secondary antibody, immunoreactive bands were visualised using an ECL detection system. Data were quantified using densitometry following scanning with TINA software (Raytest Isotopenmessgeräete GmbH, Straubenhardt, Germany).
Immunohistochemistry
Immunostaining was performed on RAW264.7 cells following antigen retrieval using Retrievagen A (Zymed Laboratories, Inc., South San Francisco, CA, USA) at 100°C for 20 min, and endogenous peroxidases were quenched with 3% H2O2. RAW264.7 cells were blocked with 2% bovine serum albumin in phosphate-buffered saline, which was followed by staining with primary anti-NF-κB p65 (BD Pharmingen, San Jose, CA, USA) antibodies at room temperature for 1 h. RAW264.7 cells were washed and incubated with secondary antibodies (R&D Systems, Minneapolis, MN, USA), and developed using VECTASTAIN ABC (Vector Laboratories, Inc., Burlingame, CA, USA) and 3,3′-diaminobenzidine (Vector Laboratories, Inc.). Image-Pro Plus analysis software (Media Cybernetics, Inc., Rockville, MD, USA) was used to calculate the NF-κB p65-positive expression in RAW264.7 cells, which was expressed as positive units.
TNF-α, IL-10, IL-6 and prostaglandin E2 (PGE2) assays
Following incubation, the supernatant was collected for the measurement of TNF-α, IL-6 and PGE2. TNF-α, IL-6 and PGE2 levels were assayed in RAW264.7 cells using enzyme-linked immunosorbent assay kits (Assay designs, Inc., Ann Arbor, MI, USA), according to the manufacturer’s instructions.
Determination of nitric oxide (NO) production
Levels of the NO derivative, nitrite, were determined using the test. A nitrite detection kit was used according to the manufacturer’s instructions. The samples were assayed in triplicate and a standard curve using NaNO2 was generated for each experiment for quantification. Briefly, 100-ml samples of medium or standard NaNO2 was mixed with 100 ml Griess reagent in a 96-well plate. After 15 min, the optical density was measured with a microplate reader at 540 nm.
Detection of reactive oxygen species (ROS) production by chemiluminescence
Chemiluminescence of RAW264.7 macrophages was evaluated using the microplate method in Hank’s balanced salt solution (pH 7.4) at room temperature. RAW264.7 cells were incubated with various concentrations of atorvastatin for 9 h in the presence of 10 ng/ml LPS. Next, luminol (Sigma-Aldrich) solution was added to the wells containing 2×105 cells, resulting in a final concentration of 110 μM. After 5 min, phorbol myristate acetate (PMA) solution was added to obtain a concentration of 0.8 μM, in order to stimulate the macrophages. The final volume of each sample was 200 μl. Chemiluminescence was determined for 5 min with luminol alone and following stimulation with PMA for 30 min. The measurement system was equipped with a LB 960 CentroXS3 microplate luminometer (Berthold Technologies GmbH & Co., Wildbad, Germany).
Statistical analysis
Data are expressed as the mean ± standard deviation. Statistical significance was determined using one-way analysis of variance and post-hoc (Bonferroni) test deviations. P<0.05 was considered to indicate a statistically significant difference.
Results
TIPE2 siRNA interference increases NF-κB p65 expression and the secretion of TNF-α and IL-6 in LPS-induced RAW264.7 macrophages
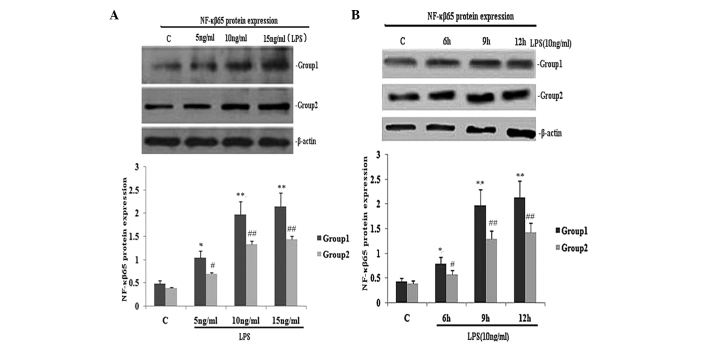
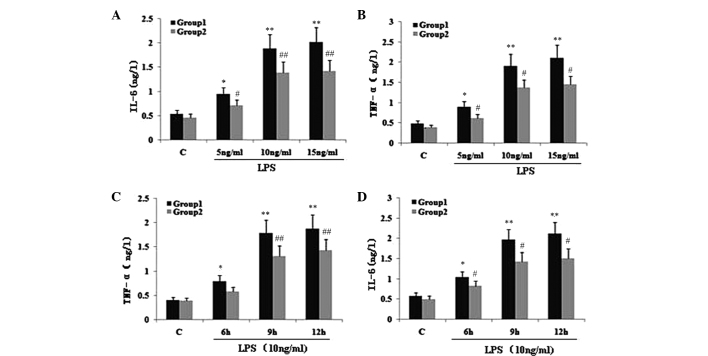
To investigate the effect of TIPE2 on NF-κB p65 expression and the secretion of TNF-α and IL-6, RAW264.7 cells or TIPE2-siRNA RAW264.7 cells were incubated with LPS (10 ng/ml) for various time periods. The expression of NF-κB p65 and the secretion of IL-6 and TNF-α in the two groups increased with LPS at each time point; however, when compared with the RAW264.7 macrophages, the NF-κB p65 expression and secretion of IL-6 and TNF-α were significantly elevated in the TIPE2-siRNA RAW264.7 macrophages at 6, 9 and 12 h (Figs. 1B and 2C and D). Thus, TIPE2 downregulated the expression of NF-κB p65 and decreased the production of IL-6 and TNF-α in a time-dependent manner (Figs. 1B and 2C and D). To determine the concentration-dependent effects of LPS, RAW264.7 cells or TIPE2-siRNA RAW264.7 cells were incubated at various concentrations of LPS for 9 h. LPS increased the expression of NF-κB p65 and the production of IL-6 and TNF-α in a concentration-dependent manner (Figs. 1A and 2A and B). However, when compared with the RAW264.7 macrophages, the NF-κB p65 expression and secretion of IL-6 and TNF-α were significantly elevated in the TIPE2-siRNA RAW264.7 macrophages treated with 5, 10 and 15 ng/ml LPS.
Figure 1.
Effect of TIPE2 on NF-κB p65 protein expression in LPS induced RAW264.7 cells. TIPE2 siRNA and control RAW264.7 cells were incubated with various concentrations of LPS for 9 h, or 10 ng/ml LPS for various time periods. NF-κB p65 protein expression was measured using western blot analysis. Values are expressed as the mean ± standard deviation of three independent experiments. Groups: 1, TIPE2 siRNA RAW264.7 cells; 2, RAW264.7 cells; C, control. (A) Dose and (B) time dependent effects of LPS on NF-κB p65 protein expression in RAW264.7 cells and TIPE2 siRNA RAW264.7 cells. (A) P<0.05, group 1 (5, 10 and 15 mg/ml) vs. group 2 (5, 10 and 15 mg/ml); group 1: *P<0.05, **P<0.01, vs. control; group 2: #P<0.05, ##P<0.01, vs. control. (B) P<0.05, group 1 (6, 9 and 12 h) vs. group 2 (6, 9 and 12 h); group 1: *P<0.05, **P<0.01, vs. control; group 2: #P<0.05, ##P<0.01, vs. control. TIPE2, tumour necrosis factor-α induced protein 8 like 2; NF-κB, nuclear factor κB; LPS, lipopolysaccharide.
Figure 2.
Effect of TIPE2 on proinflammatory factors in LPS induced RAW264.7 cells. TIPE2 siRNA and control RAW264.7 cells were incubated with various concentrations of LPS for 9 h, or 10 ng/ml LPS for various time points. TNF-α and IL-6 levels in the supernatant were assayed using ELISA. Values are expressed as the mean ± standard deviation of three independent experiments. Groups: 1, TIPE2 siRNA RAW264.7 cells; 2, RAW264.7 cells; C, control. (A,B) Dose and (C,D) time dependent effects of LPS on IL-6 and TNF-α levels in RAW264.7 cells and TIPE2 siRNA RAW264.7 cells. (A,B) P<0.05, group 1 (5, 10 and 15 mg/ml) vs. group 2 (5, 10 and 15 mg/ml); group 1: *P<0.05, **P<0.01, vs. control; group 2: #P<0.05, ##P<0.01, vs. control. (C,D) P<0.05, group 1 (6, 9 and 12 h) vs. group 2 (6, 9 and 12 h); group 1: *P<0.05, **P<0.01, vs. control; group 2: #P<0.05, ##P<0.01, vs. control. TIPE2, TNF-α induced protein 8 like 2; LPS, lipopolysaccharide; TNF-α, tumour necrosis factor α; IL, interleukin; ELISA, enzyme linked immunosorbent assay.
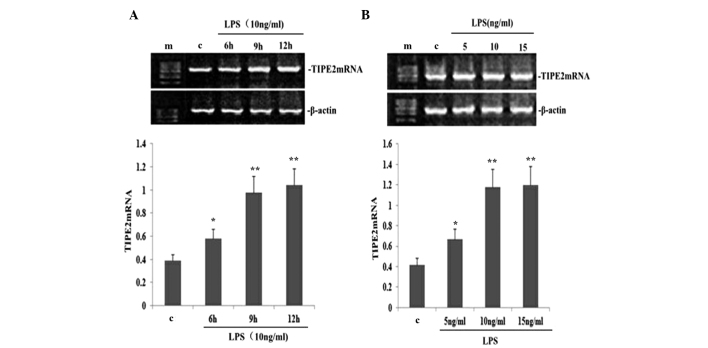
LPS upregulates TIPE2 expression in a dose- and time-dependent manner in RAW264.7 macrophages
To determine the dose-dependent effect on TIPE2 expression in LPS-induced RAW264.7 macrophages, RAW264.7 cells were incubated with various concentrations of LPS for 9 h. LPS increased the expression of TIPE2 mRNA in a concentration-dependent manner (Fig. 3B). Concentrations as low as 5 ng/ml LPS were effective in inducing the mRNA expression of TIPE2. To determine the time-dependent effect, RAW264.7 cells were incubated with 10 ng/ml LPS for various time periods. The expression of TIPE2 was increased by LPS as early as 6 h, which was the earliest time point investigated. Maximum induction was reached after 9 h incubation, which remained stable for at least 12 h, which was the longest time point investigated (Fig. 3A).
Figure 3.
LPS increased TIPE2 mRNA expression in RAW264.7 cells. RAW264.7 cells were incubated with various concentrations of LPS for 9 h, or 10 ng/ml LPS for various time periods. TIPE2 mRNA expression was measured using qPCR. Values are expressed as the mean ± standard deviation of three independent experiments. (A) Time and (B) dose dependent effects of LPS on TIPE2 mRNA expression in RAW264.7 cells. (A) *P<0.05, **P<0.01, vs. control; (B) *P<0.05, **P<0.01, vs. control. TIPE2, tumour necrosis factor α induced protein 8 like 2; LPS, lipopolysaccharide.
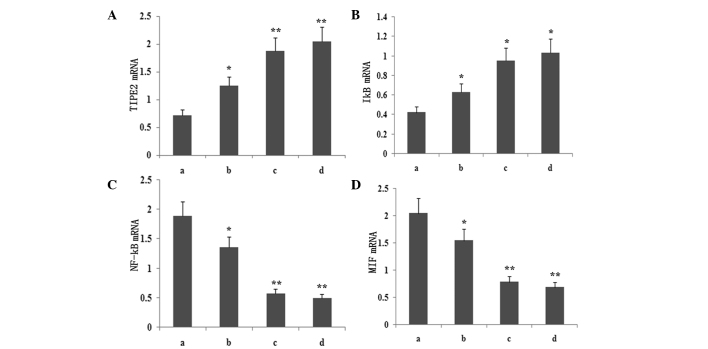
Atorvastatin upregulates LPS-induced TIPE2 and IκB mRNA expression and inhibits LPS-induced NF-κB and MIF mRNA expression in RAW264.7 macrophages
To determine whether atorvastatin affected the LPS-induced gene expression of TIPE2, IκB, MIF and NF-κB, RAW264.7 cells were incubated with various concentrations of atorvastatin for 9 h in the presence of 10 ng/ml LPS. TIPE2, IκB, MIF and NF-κB expression levels were measured using qPCR analysis. PCR analysis indicated that the LPS-induced mRNA expression of TIPE2 and IκB increased significantly with atorvastatin (Figs. 4 and 5). Consistent with this observation, the LPS-induced mRNA expression of NF-κB and MIF was also suppressed by atorvastatin (Figs. 4 and 5). Therefore, atorvastatin upregulated the LPS-induced expression of TIPE2, but decreased NF-κB and MIF expression in a dose-dependent manner.
Figure 4.
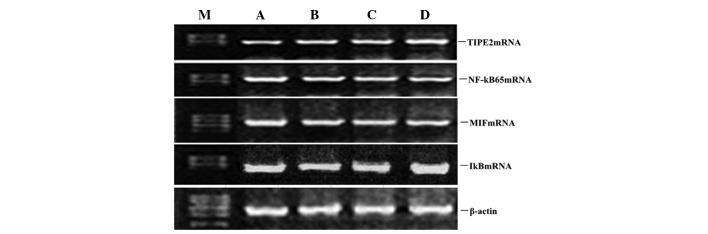
Dose-dependent effect of atorvastatin on LPS-induced TIPE2, MIF, NF-κB and IκB expression in RAW264.7 cells. RAW264.7 cells were incubated with various concentrations of atorvastatin for 9 h in the presence of 10 ng/ml LPS. TIPE2, MIF, NF-κB and IκB mRNA expression levels were measured using qPCR. Representative qPCR analysis shows the mRNA expression levels of TIPE2, MIF, NF-κB and IκB in RAW264.7 cells. M, mark; A, LPS (10 ng/ml); B, LPS (10 ng/ml) + atorvastatin (10 μM); C, LPS (10 ng/ml) + atorvastatin (15 μM); D, LPS (10 ng/ml) + atorvastatin (20 μM); TIPE2, tumour necrosis factor-α-induced protein 8-like 2; LPS, lipopolysaccharide; NF-κB, nuclear factor-κB; MIF, macrophage migration inhibitory factor; qPCR, quantitative polymerase chain reaction.
Figure 5.
Dose-dependent effect of atorvastatin on LPS-induced (A) TIPE2, (B) IκB, (C) NF-κB and (D) MIF expression in RAW264.7 cells. RAW264.7 cells were incubated with various concentrations of atorvastatin for 9 h in the presence of 10 ng/ml LPS. TIPE2, MIF, NF-κB and IκB mRNA expression levels were measured using qPCR. Data are expressed as the mean ± standard error of the mean of three independent experiments.*P<0.05 and **P<0.01, vs. ATV (10, 15 and 20 μM). a, LPS (10 ng/ml); b, LPS (10 ng/ml) + atorvastatin (10 μM); c, LPS (10 ng/ml) + atorvastatin (15 μM); d, LPS (10 ng/ml) + atorvastatin (20 μM); TIPE2, tumour necrosis factor-α-induced protein 8-like 2; LPS, lipopolysaccharide; NF-κB, nuclear factor-κB; MIF, macrophage migration inhibitory factor; qPCR, quantitative polymerase chain reaction.
Atorvastatin increases TIPE2 protein expression, and inhibits COX-2 and NOS protein expression in RAW264.7 macrophages
To observe whether atorvastatin affected LPS-induced TIPE2, COX-2 and NOS protein expression, RAW264.7 cells were incubated with various concentrations of atorvastatin for 9 h in the presence of 10 μg/ml LPS. Protein expression levels of TIPE2, COX-2 and NOS were determined using western blot analysis. The results indicated that the LPS-induced protein expression of COX-2 and NOS was significantly inhibited, and TIPE2 was further increased by atorvastatin in a dose-dependent manner (Figs. 6 and 7).
Figure 6.
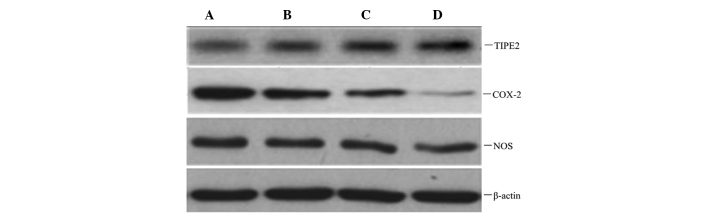
Dose-dependent effect of atorvastatin on LPS-induced TIPE2, COX-2 and NOS expression in RAW264.7 cells. RAW264.7 cells were incubated with various concentrations of atorvastatin for 9 h in the presence of 10 ng/ml LPS. TIPE2, COX-2 and NOS expression levels were measured using western blot analysis. Representative western blot showing the protein expression levels of TIPE2, COX-2 and NOS in RAW264.7 cells. A, LPS (10 ng/ml); B, LPS (10 ng/ml) + atorvastatin (10 μM); C, LPS (10 ng/ml) + atorvastatin (15 μM); D, LPS (10 ng/ml) + atorvastatin (20 μM); TIPE2, tumour necrosis factor-α-induced protein 8-like 2; LPS, lipopolysaccharide; COX-2, cyclooxygenase-2; NOS, nitric oxide synthase.
Figure 7.
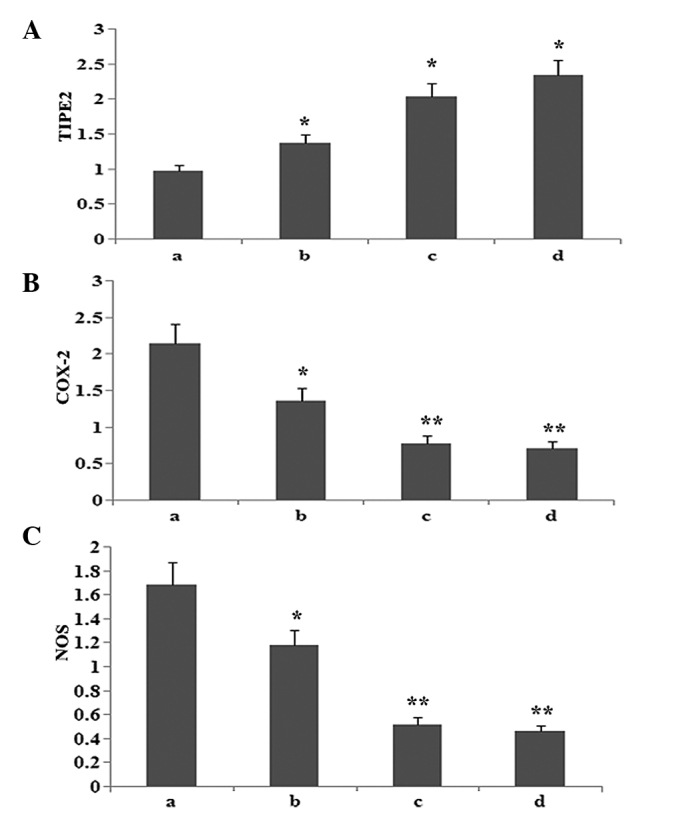
Dose-dependent effect of atorvastatin on LPS-induced (A) TIPE2, (B) COX-2 and (C) NOS expression in RAW264.7 cells. RAW264.7 cells were incubated with various concentrations of atorvastatin for 9 h in the presence of 10 ng/ml LPS. TIPE2, COX-2 and NOS expression levels were measured using western blot analysis. Data are expressed as the mean ± standard error of the mean of three independent experiments.*P<0.05 and **P<0.01, vs. atorvastatin (10, 15 and 20 μM). a, LPS (10 ng/ml); b, LPS (10 ng/ml) + atorvastatin (10 μM); c, LPS (10 ng/ml) + atorvastatin (15 μM); d, LPS (10 ng/ml) + atorvastatin (20 μM); TIPE2, tumour necrosis factor-α-induced protein 8-like 2; LPS, lipopolysaccharide; COX-2, cyclooxygenase-2; NOS, nitric oxide synthase.
Atorvastatin inhibits the production of inflammatory mediators in RAW264.7 macrophages
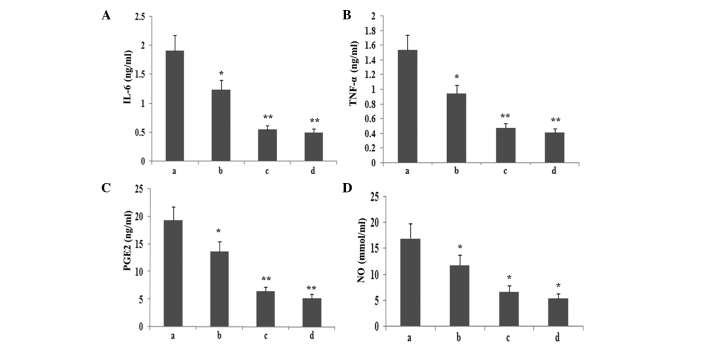
To observe whether atorvastatin affected LPS-induced IL-6, TNF-α, PGE2 and NO expression, RAW264.7 cells were incubated with various concentrations of atorvastatin for 9 h in the presence of 10 ng/ml LPS. The levels of IL-6, TNF-α, PGE2 and NO in the supernatant were assayed. As shown in Fig. 8, atorvastatin reduced IL-6, TNF-α, PGE2 and NO production in a dose-dependent manner.
Figure 8.
Effect of atorvastatin on the LPS-induced production of IL-6, TNF-α, NO and PGE2 in RAW264.7 cells. RAW264.7 cells were incubated with various concentrations of atorvastatin for 9 h in the presence of 10 ng/ml LPS. Following incubation, the supernatant was collected, and IL-6, TNF-α, NO and PGE2 levels were assayed. Data are expressed as the mean ± standard error of the mean of three independent experiments. *P<0.05 and **P<0.01, vs. LPS control. a, LPS (10 ng/ml); b, LPS (10 ng/ml) + atorvastatin (10 μM); c, LPS (10 ng/ml) + atorvastatin (15 μM); d, LPS (10 ng/ml) + atorvastatin (20 μM); TNF-α, tumour necrosis factor-α; IL, interleukin; LPS, lipopolysaccharide; PGE2, prostaglandin E2, NO, nitric oxide.
Atorvastatin increases HO-1 expression and reduces ROS production
To investigate whether atorvastatin affected LPS-induced HO-1 and ROS expression, RAW264.7 cells were incubated with various concentrations of atorvastatin for 9 h in the presence of 10 ng/ml LPS. As shown in Fig. 9, atorvastatin upregulated HO-1 expression, but reduced the production of ROS in a dose-dependent manner.
Figure 9.
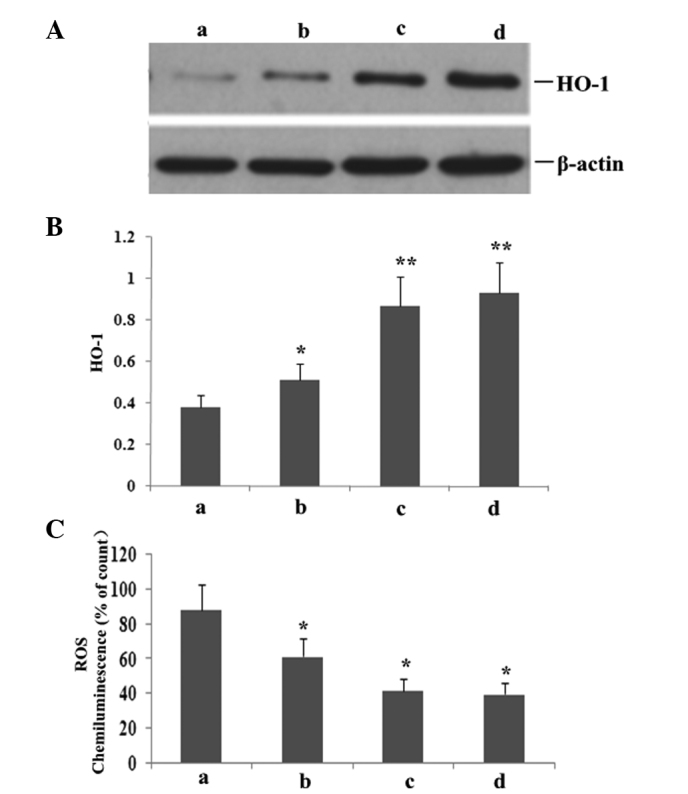
RAW264.7 cells were incubated with various concentrations of atorvastatin for 9 h in the presence of 10 ng/ml LPS. HO-1 and ROS expression levels were measured. (A) Representative western blot shows the protein expression levels of HO-1 in RAW264.7 cells. Statistical summary of the densitometric analysis of (B) HO-1 protein expression and (C) ROS production in RAW264.7 cells. Data are expressed as the mean ± standard error of the mean of three independent experiments.*P<0.05 and **P<0.01, vs. atorvastatin (10, 15 and 20 μM). LPS, lipopolysaccharide; ROS, reactive oxygen species; HO-1, haem oxygenase; a, LPS (10 ng/ml); b, LPS (10 ng/ml) + atorvastatin (10 μM); c, LPS (10 ng/ml) + atorvastatin (15 μM); d, LPS (10 ng/ml) + atorvastatin (20 μM).
Discussion
A largely unopposed early proinflammatory response occurs in severe sepsis, which results in cell death (12). Serum levels of proinflammatory cytokines, including TNF-α, IL-1β and IL-6, peak at early time points during sepsis (13). Monocytes-macrophages are important cells of the innate immune system and are widely present in blood, spleen, liver, lung and other tissues. Upon severe infections, macrophages produce a large number of inflammatory mediators, such as IL-1, TNF-α, NO, IL-8 and IL-6 (14). Excessive inflammatory factors produced by activated macrophages are associated with the pathophysiology of various acute inflammatory diseases, including acute respiratory distress syndrome (ARDS), SIRS and MODS (15–17). The present study revealed that LPS can stimulate the production of various inflammatory factors in a time- and dose-dependent manner, while inhibiting NF-κB expression decreases the generation of TNF-α, IL-6 and other inflammatory factors, which is conducive to inhibiting the further development of sepsis.
NF-κB is considered to play a key role in the production of inflammatory mediators, including proinflammatory cytokines (TNF-α and IL-1), cell adhesion molecules (intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1), immunoreceptors and acute phase protein production (18,19). In resting cells, NF-κB is localised to the cytoplasm as a heterodimer consisting of two polypeptides of 50 (p50) and 65 kDa (p65), which are noncovalently associated with cytoplasmic inhibitory proteins, such as IκB. In response to LPS exposure, viral infection, the expression of specific viral products or other physiological stimuli, IκB undergoes a series of biological events, namely rapid phosphorylation in the N-terminal domain by a large multikinase complex, polyubiquitination and degradation by the 26 S proteasome, which enables translocation of the NF-κB heterodimer to the nucleus (20). Once NF-κB reaches the nucleus, it activates the transcription of several inflammatory enzymes, including COX-2 and inducible NOS (iNOS), by interacting with κB sites in their promoter regions. Furthermore, NO derived from iNOS and PGE2, which is synthesised by COX-2, plays a pivotal role in the pathogenesis of acute and chronic inflammation. Activated NF-κB facilitates the secretion of proinflammatory mediators, such as TNF-α and IL-6. The present study also found that LPS promoted NF-κB p65 activation, inhibited IκB activation, increased proinflammatory mediator production, upregulated COX-2 and NOS expression and enhanced PGE2 and NO expression. However, the increase in TIPE2 expression caused by atorvastatin treatment reduced the production of TNF-α, IL-6, PGE2 and NO via blocking NF-κB p65 activation and upregulating IκB activation.
TIPE2, as a negative regulatory protein, predominantly functions in the regulation of the inflammatory mediator response process (21). Several studies have demonstrated that in TIPE2-deficient rats, weight loss, splenomegaly, leukocytosis and multiple-organ spontaneous inflammatory mediator reactions in multiple organs occur. Following LPS attacks, the excessive response increases, thus, sepsis occurs, which results in premature mortality. According to our previous study, TNF-α and IL-1, -6 and -12 levels are high, and are accompanied with higher levels of IL-10. In addition, the number of T, B and dendritic cells significantly increase (22,23). As indicated by previous studies, TIPE2 may be involved in the regulation of multiple signalling transduction pathways (24,25). TIPE2 can inhibit the activation of c-jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK), and decrease the activation of transcription factor activator protein-1, such that it negatively regulates the JNK and p38 MAPK signalling transduction pathways. In addition, TIPE2 plays a role in the extracellular signal-regulated kinase signalling pathway. It is well established that in a resting state, NF-κB exists as a trimeric complex in cells, connecting the two subunits, p65 and p50, with IκB. Upon TIPE2 deletion or mutation, the inhibitor IκB is degraded from the trimeric complex via protein kinase C phosphorylation, thus, NF-κB can be freely translocated from the cytoplasm to the nucleus. By virtue of the binding site, NF-κB initiates the transcription and translation of a variety of cytokine genes, including TNF-α, IL-1 and IL-6, thereby inducing the activation of inflammatory cells (26). The present study demonstrated that increased NF-κB expression resulted in enhanced expression levels of IL-6, TNF-α and TIPE2 in RAW264.7 cells via a compensatory effect. However, further enhancement of TIPE2 expression significantly lowered NF-κB expression and the production of proinflammatory mediators.
MIF is a proinflammatory mediator that plays a crucial role in sepsis. In human plasma, MIF normally circulates at levels ranging between 2 and 10 ng/ml, but fluctuates in a diurnal rhythm, which reflects neuroendocrine control (27). Plasma MIF concentrations are elevated to extremely high levels in various inflammatory disorders, which is the first indication that MIF may be involved in systemic infection and sepsis. Healy et al reported that macrophages are peripheral sources of MIF that respond to inflammatory stimuli in vivo (28). Subsequently, Donnelly and Rogers (29) demonstrated that increased concentrations of MIF are present in the lungs of individuals with ARDS, and that alveolar macrophages are a source of protein. These studies also demonstrated that MIF enhances, and anti-MIF antibodies attenuate, the secretion of the proinflammatory cytokines, TNF-α and IL-8, from alveolar macrophages. In the present study, LPS was shown to increase MIF expression; however, the increase in MIF expression was inhibited by regulating TIPE2 via atorvastatin treatment.
HO-1 is an inducible enzyme that catalyses the rate-limiting step in the conversion of free haem to carbon monoxide, free iron and biliverdin, which is subsequently catabolised into bilirubin via biliverdin reductase (30). In addition to the primary role in haem degradation, HO-1 has also been recognised to play other important roles in inflammation via HO-1 knockout mice and a human case of genetic HO-1 deficiency (31). According to references, the gaseous molecule CO is responsible for most of the anti-inflammatory actions of HO-1. CO inhibited the production of pro-inflammatory cytokines, such as TNF-α, IL-1β by activating macrophages. Therefore, HO-1 and its enzymatic metabolites are critical regulators of inflammation, with activate macrophages function as critical targets. An imbalance between ROS and the antioxidant defence system results in oxidative stress, which is closely associated with the pathogenesis of sepsis (32). Increased levels of ROS cause direct tissue injury and promote inflammatory responses via the regulation of diverse proinflammatory mediators. In the present study, LPS inhibited HO-1 expression, increased ROS activity and promoted an inflammatory response. However, atorvastatin upregulated HO-1 expression via increasing TIPE2 expression, which decreased ROS activity and prevented an inflammatory response.
Statins are drugs that lower lipid levels typically via the inhibition of HMG-CoA reductase. Research on statins is no longer limited to investigating the lipid lowering effects, with an increasing number of studies investigating the anti-inflammatory effects. Due to the anti-inflammatory effects, statins can inhibit the release of inflammatory factors, thus, statins may exert effects on the inflammatory response against sepsis (33,34). Chello et al (35) used cecal ligation and puncture (CLP) to generate a rat model of sepsis. The study indicated that the average survival rate of mice following statin pretreatment is four times greater compared with controls. Furthermore, Chello et al (36) indicated that simvastatin, in addition to its direct role in the neutrophil proinflammatory effect, can also decrease the levels of C reactive protein (CRP), TNF-α, IL-6 and other proinflammatory factors, thus, being conducive to anti inflammatory effects. Chaudhry et al (37) found that cerivastatin significantly reduced the release of TNF-α and IL-6, improving the survival rate of rats with sepsis. In addition, cerivastatin accelerated bacterial removal in rats 24 h after infection with Salmonella typhi and 48 h with Staphylococcus aureus. The anti inflammatory mechanism of statins is associated with NF-κB. Statins function to reduce the isoprenylation of Rho proteins, preventing Rho proteins from binding to the cell membrane, which decreases the release of NF-κB into the nucleus, thereby decreasing inflammatory cytokine secretion (24) and achieving an anti inflammatory response. Previous studies have found that statins can reduce the binding of bacterial LPS induced C Rel/p65 heterodimers with tissue factor κB sites, thereby regulating inflammatory factors at the gene level, promoting anti inflammatory cytokine (IL-10) synthesis and thereby playing a significant anti inflammatory role (38,39). In a previous study, CLP based sepsis models were generated 18 days after intragastric administration of statins on a 10 mg/kg/day basis. After ~24 h, the serum TNF-α, IL-1 and IL-6 levels were significantly lower compared with the control group and the number of white blood cells and neutrophils had significantly decreased (40), confirming that statins can reduce the level of inflammatory factors in sepsis rats, resulting in anti inflammatory effects (41). Gonzalez et al (42) performed studies on patients with sepsisand demonstrated that at day 14 following oral administration of 80 mg/day simvastatin, the level of CRP gradually decreased and reached a normal level. However, in the control group, the CRP level increased on a daily basis, indicating that statins can reduce the systemic inflammatory response. As indicated by the present study, atorvastatin can promote TIPE2 expression in RAW264.7 cells, inhibit NF-κB, COX-2, MIF and NOS expression, increase IκB and HO-1 expression, significantly reduce the generation of TNF-α and IL-6 and suppress oxidation reactions in a dose- and time-dependent manner.
In conclusion, the production of high levels of proinflammatory mediators is a key factor during the occurrence and development of sepsis. Atorvastatin inhibits NF-κB, MIF, COX-2 and NOS expression, increases HO-1 and IκB expression and significantly reduces the expression of TNF-α and NO proinflammatory factors. Furthermore, prevented PGE2 and IL-6, oxidative stress via an increase in TIPE2 expression, which may contribute to the suppression of the occurrence and development of sepsis upon severe infection.
Acknowledgements
The authors thank Professor Qinqin Huang and Professor Shaoxi Cai for their technical assistance.
Abbreviations
- TIPE2
TNF-α-induced protein 8-like 2
- NF-κB
nuclear factor-κB
- IL
interleukin
- TNF-α
tumour necrosis factor-α
- LPS
lipopolysaccharide
- PBS
phosphate-buffered saline
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme
- MIF
macrophage migration inhibitory factor
- SIR
systemic inflammatory response
- SIRS
systemic inflammatory response syndrome
- MODS
multiple organ dysfunction
- qPCR
quantitative polymerase chain reaction
- NO
nitric oxide
- NOS
nitric oxide synthase
- COX-2
cyclooxygenase-2
- HO-1
haem oxygenase
- PGE2
prostaglandin E2
- ROS
reactive oxygen species
- ARDS
acute respiratory distress syndrome
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- CLP
cecal ligation and puncture
References
- 1.Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The host response to sepsis and developmental impact. Pediatrics. 2010;125:1031–1041. doi: 10.1542/peds.2009-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unuma K, Aki T, Funakoshi T, Yoshida K, Uemura K. Cobalt protoporphyrin accelerates TFEB activation and lysosome reformation during LPS-induced septic insults in the rat heart. PLoS One. 2013;8:e56526. doi: 10.1371/journal.pone.0056526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juskewitch JE, Prasad S, Salas CF, Huskins WC. Reliability of the identification of the systemic inflammatory response syndrome in critically ill infants and children. Pediatr Crit Care Med. 2012;13:e55–e57. doi: 10.1097/PCC.0b013e31822f177a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinel I, Opal SM. Molecular biology of inflammation and sepsis: a primer. Crit Care Med. 2009;37:291–304. doi: 10.1097/CCM.0b013e31819267fb. [DOI] [PubMed] [Google Scholar]
- 6.Woodward MJ, de Boer J, Heidorn S, Hubank M, Kioussis D, Williams O, Brady HJ. TNFAIP8 is an essential gene for the regulation of glucocorticoid-mediated apoptosis of thymocytes. Cell Death Differ. 2010;17:316–323. doi: 10.1038/cdd.2009.125. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Fayngerts S, Wang P, et al. TIPE2 protein serves as a negative regulator of phagocytosis and oxidative burst during infection. Proc Natl Acad Sci USA. 2012;109:15413–15418. doi: 10.1073/pnas.1204525109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freundt EC, Bidere N, Lenardo MJ. A different TIPE of immune homeostasis. Cell. 2008;133:401–402. doi: 10.1016/j.cell.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonopoulos AS, Margaritis M, Lee R, Channon K, Antoniades C. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des. 2012;18:1519–1530. doi: 10.2174/138161212799504803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truwit JD. Statins: a role in infected critically ill patients? Crit Care. 2011;15:145. doi: 10.1186/cc10086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubinson DA, Dillon CP, Kwiatkowski AV, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Gokden N, Mayeux PR. Evidence for the role of reactive nitrogen species in polymicrobial sepsis-induced renal peritubular capillary dysfunction and tubular injury. J Am Soc Nephrol. 2007;18:1807–1815. doi: 10.1681/ASN.2006121402. [DOI] [PubMed] [Google Scholar]
- 13.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 14.Vigil KJ, Adachi JA, Chemaly RF. Viral pneumonias in immunocompromised adult hosts. J Intensive Care Med. 2010;25:307–326. doi: 10.1177/0885066610377969. [DOI] [PubMed] [Google Scholar]
- 15.Hlava N, Niemann CU, Gropper MA, Melcher ML. Postoperative infectious complications of abdominal solid organ transplantation. J Intensive Care Med. 2009;24:3–17. doi: 10.1177/0885066608327127. [DOI] [PubMed] [Google Scholar]
- 16.Zhao J, Yang X, Auh SL, Kim KD, Tang H, Fu YX. Do adaptive immune cells suppress or activate innate immunity? Trends Immunol. 2009;30:8–12. doi: 10.1016/j.it.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KD, Zhao J, Auh S, Yang X, Du P, Tang H, Fu YX. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13:1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López-Rincón G, Pereira-Suárez AL, Del Toro-Arreola S, et al. Lipopolysaccharide induces the expression of an autocrine prolactin loop enhancing inflammatory response in monocytes. J Inflamm (Lond) 2013;10:24. doi: 10.1186/1476-9255-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong IK, Oh da H, Park SJ, et al. Inhibition of NF-κB prevents high glucose-induced proliferation and plasminogen activator inhibitor-1 expression in vascular smooth muscle cells. Exp Mol Med. 2011;43:684–692. doi: 10.3858/emm.2011.43.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin CC, Shih CH, Yang YL, et al. Thrombin induces inducible nitric oxide synthase expression via the MAPK, MSK1, and NF-κB signaling pathways in alveolar macrophages. Eur J Pharmacol. 2011;672:180–187. doi: 10.1016/j.ejphar.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Zhang LJ, Liu X, Gafken PR, Kioussi C, Leid M. A chicken ovalbumin upstream promoter transcription factor I (COUP-TFI) complex represses expression of the gene encoding tumor necrosis factor alpha-induced protein 8 (TNFAIP8) J Biol Chem. 2009;284:6156–6168. doi: 10.1074/jbc.M807713200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H, Gong S, Carmody RJ, et al. TIPE2, a novel negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 2008;133:415–426. doi: 10.1016/j.cell.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laliberté B, Wilson AM, Nafisi H, et al. TNFAIP8: a new effector for Galpha(i) coupling to reduce cell death and induce cell transformation. J Cell Physiol. 2010;225:865–874. doi: 10.1002/jcp.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antosz H, Choroszyńska D. Negative regulation of Toll-like receptor signalling. Postepy Hig Med Dosw (Online) 2013;67:339–350. doi: 10.5604/17322693.1046538. (In Polish) [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Wang J, Fan C, et al. Crystal structure of TIPE2 provides insights into immune homeostasis. Nat Struct Mol Biol. 2009;16:89–90. doi: 10.1038/nsmb.1522. [DOI] [PubMed] [Google Scholar]
- 26.Li D, Song L, Fan Y, et al. Down-regulation of TIPE2 mRNA expression in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin Immunol. 2009;133:422–427. doi: 10.1016/j.clim.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Zeng X, Chen S, et al. Characterization, epitope identification and mechanisms of the anti-septic capacity of monoclonal antibodies against macrophage migration inhibitory factor. Int Immunopharmacol. 2011;11:1333–1340. doi: 10.1016/j.intimp.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Healy ZR, Liu H, Holtzclaw WD, Talalay P. Inactivation of tautomerase activity of macrophage migration inhibitory factor by sulforaphane: a potential biomarker for anti-inflammatory intervention. Cancer Epidemiol Biomarkers Prev. 2011;20:1516–1523. doi: 10.1158/1055-9965.EPI-11-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donnelly LE, Rogers DF. Novel targets and drugs in inflammatory lung disease. Curr Opin Pharmacol. 2008;8:219–221. doi: 10.1016/j.coph.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Tu YP, Chuang SJ, Chen SC, et al. Simvastatin induces the expression of hemeoxygenase-1 against ischemia-reperfusion injury on the testes in rats. Toxicol Lett. 2011;207:242–250. doi: 10.1016/j.toxlet.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Park JS, Jung JS, Jeong YH, et al. Antioxidant mechanism of isoflavone metabolites in hydrogen peroxide-stimulated rat primary astrocytes: critical role of heme oxygenase-1 and NQO1 expression. J Neurochem. 2011;119:909–919. doi: 10.1111/j.1471-4159.2011.07395.x. [DOI] [PubMed] [Google Scholar]
- 32.Nassar NN, Li G, Strat AL, Abdel-Rahman AA. Enhanced hemeoxygenase activity in the rostral ventrolateral medulla mediates exaggerated hemin-evoked hypotension in the spontaneously hypertensive rat. J Pharmacol Exp Ther. 2011;339:267–274. doi: 10.1124/jpet.111.183368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kagami S, Kanari H, Suto A, et al. HMG-CoA reductase inhibitor simvastatin inhibits proinflammatory cytokine production from murine mast cells. Int Arch Allergy Immunol. 2008;146(Suppl 1):61–66. doi: 10.1159/000126063. [DOI] [PubMed] [Google Scholar]
- 34.Trezzi M, Blackstone EH, Sun ZY, et al. Statin therapy is associated with fewer infections after cardiac operations. Ann Thorac Surg. 2013;95:892–900. doi: 10.1016/j.athoracsur.2012.11.071. [DOI] [PubMed] [Google Scholar]
- 35.Chello M, Anselmi A, Spadaccio C, et al. Simvastatin increases neutrophil apoptosis and reduces inflammatory reaction after coronary surgery. Ann Thorac Surg. 2007;83:1374–1380. doi: 10.1016/j.athoracsur.2006.10.065. [DOI] [PubMed] [Google Scholar]
- 36.Chello M, Mastroroberto P, Patti G, D’Ambrosio A, Morichetti MC, Di Sciascio G, Covino E. Simvastatin attenuates leucocyte-endothelial interactions after coronary revascularisation with cardiopulmonary bypass. Heart. 2003;89:538–543. doi: 10.1136/heart.89.5.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhry MZ, Wang JH, Blankson S, Redmond HP. Statin (cerivastatin) protects mice against sepsis related death via reduced proinflammatory cytokines and enhanced bacterial clearance. Surg Infect (Larchmt) 2008;9:183–194. doi: 10.1089/sur.2006.077. [DOI] [PubMed] [Google Scholar]
- 38.Fraunberger P, Gröne E, Gröne HJ, Walli AK. Simvastatin reduces endotoxin induced nuclear factor kappaB activation and mortality in guinea pigs despite lowering circulating low density lipoprotein cholesterol. Shock. 2009;32:159–163. doi: 10.1097/SHK.0b013e318193c514. [DOI] [PubMed] [Google Scholar]
- 39.Lin H, Xiao Y, Chen G, et al. HMG CoA reductase inhibitor simvastatin suppresses Toll like receptor 2 ligand induced activation of nuclear factor kappa B by preventing RhoA activation in monocytes from rheumatoid arthritis patients. Rheumatol Int. 2011;31:1451–1458. doi: 10.1007/s00296-010-1510-6. [DOI] [PubMed] [Google Scholar]
- 40.Meijvis SC, van de Garde EM, Rijkers GT, Bos WJ. Treatment with anti inflammatory drugs in community acquired pneumonia. J Intern Med. 2012;272:25–35. doi: 10.1111/j.1365-2796.2012.02554.x. [DOI] [PubMed] [Google Scholar]
- 41.Fogerty MD, Efron D, Morandi A, Guy JS, Abumrad NN, Barbul A. Effect of preinjury statin use on mortality and septic shock in elderly burn patients. J Trauma. 2010;69:99–103. doi: 10.1097/TA.0b013e3181df61b1. [DOI] [PubMed] [Google Scholar]
- 42.González CM, Luna AH, Morales MP, Sanchez JA, Guzman CO, Granillo GF. Statin anti-inflammatory therapy in septic patients. Critical Care. 2008;12(Suppl5):P26–P35. [Google Scholar]