Abstract
The present study investigated the association of thyroid dysfunction (TD) with the distribution of chronic hepatitis C virus (HCV) infection in untreated patients. A total of 1,012 cases of HCV-infected patients were collected from different regions, of which 209 patients demonstrated a type of TD (chronic thyroiditis complicated with hyperthyroidism, chronic thyroiditis complicated with hypothyroidism, subclinical hyperthyroidism, subclinical hypothyroidism, hyperthyroidism, hypothyroidism or chronic thyroiditis). The results showed the existence of geographical differences in the types of TD present with HCV infection. The female patients had a higher incidence of autoimmune-related TD than the male patients. High levels of HCV RNA expression were most common in all HCV-infected patients, regardless of the presence of TD. High and medium expression levels of HCV RNA were more prevalent in the patients with autoimmune-related TD. Relative analysis of the HCV RNA levels showed that the pathogenesis of TD was not correlated with the HCV RNA expression levels; however, it may have been associated with autoimmunity. The HCV-infected patients with TD were most commonly middle-aged, whereas young adults were the largest group of patients with HCV and normal thyroid function. Among all HCV genotypes, type 1b was the most common HCV genotype and type 2 was the second most common. Types 3 and 6 were scarce in this study population. No associations were identified between HCV genotypes and thyroid disease. The data of liver function showed that HCV-infected patients with TD had a higher liver dysfunction rate compared with that of the patients with normal thyroid function. Therefore, liver dysfunction may be associated with thyroid disease. This study supports the potential of individualized treatment for HCV-infected patients.
Keywords: thyroid dysfunction, liver dysfunction, hepatitis type C, Chinese population
Introduction
Hepatitis C (HCV) is the principal cause of chronic liver disease, cirrhosis and hepatocellular carcinoma (HCC). It was estimated in 2001 that the number of new cases of HCV infection worldwide is >3.5 million per year (1). In China, a country with a high incidence of HCV infection, there are ~40 million individuals infected with HCV, with a prevalence of ~3.2% (2). HCV infection has been verified to be associated with numerous autoimmune disorders, including thyroid dysfunction (TD), autoantibody formation and autoimmune idiopathic thrombocytopenic purpura (3,4). TD is one of the complex diseases common in patients with chronic HCV infection and is triggered by interactions between genetic, epigenetic and microenvironmental factors (5). Therefore, the association between the epidemiological factors of HCV infection and possible properties of TD requires investigation in patients with HCV infection. Although the high incidence of TD in patients with HCV receiving interferon-α therapy has been well verified in a previous study (6), the correlation between TD and HCV-infected patients without interferon treatment remains under debate and is explored in the present study.
TD may be divided into chronic thyroiditis complicated with hyperthyroidism, chronic thyroiditis complicated with hypothyroidism, subclinical hyperthyroidism, subclinical hypothyroidism, hyperthyroidism, hypothyroidism and chronic thyroiditis for clinical diagnosis. An increased incidence of clinical and subclinical autoimmune thyroiditis has been observed in patients with chronic HCV infection compared with that in healthy controls (7). Hypothyroidism is more common in patients with chronic HCV infection than in normal controls or patients with chronic hepatitis B infection (8). However, the reports on the correlation between chronic HCV infection and TD are limited, and are mainly focused on HCV patients during interferon treatment (9) or overall TD incidence (10). To the best of our knowledge, o study has investigated on the correlation between chronic HCV infection and each type of TD. TD has been reported to have an occurrence of 2–13% in patients with HCV infection, and is more frequent in females (11,12). The prevalence of TD in HCV-infected patients has been frequently reported; however, the correlation of multiple factors associated with TD, including gender, age, geographical location, HCV genotype and RNA expression levels, with HCV infection remains unknown, particularly in the Chinese population. The present study aimed to investigate the correlation between various types of TD and the epidemiological factors of HCV-infected patients, including geographical distribution, HCV RNA expression levels, HCV genotype, gender, age and liver function in different regions of China.
Patients and methods
Patient population
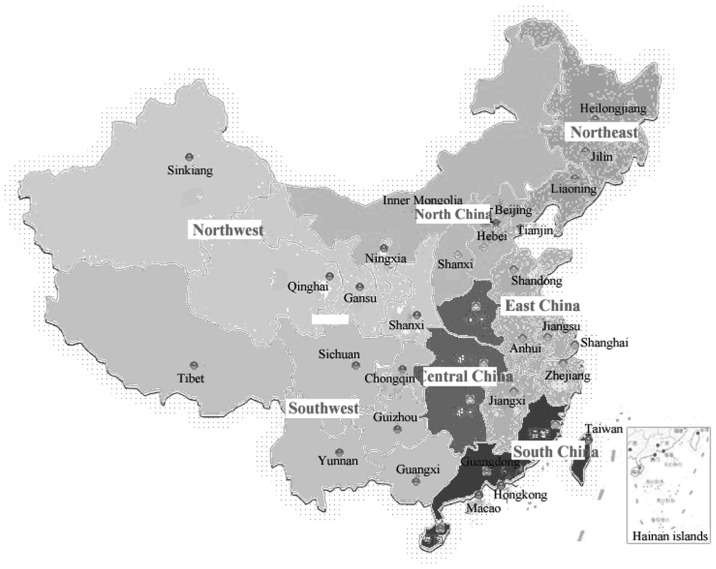
The registration ID for the present study is the ClinicalTrials.gov identifier NCT01293279. A total of 1,012 patients infected with HCV, including 209 patients with a type of TD, were randomly recruited for this study. The patients were treated at 28 hospitals in different regions of China (Table I) and the age, gender and HCV RNA expression were considered. All the patients were free of cirrhosis and HCC, and had not been treated with interferon. The study was approved by the ethics committees of all hospitals at which the patients were recruited. Written informed consent was obtained from each patient. The hospitals were divided territorially into North, Northeast, Southwest, South, Central, Northwest and East China (Fig. 1). Among the 1,012 patients with HCV infection, there were 92 cases in North China, 91 in Southwest China, 104 in South China, 224 in Central China, 162 in Northwest China, 252 in East China and 89 in Northeast China.
Table I.
Regional information of the Chinese patients with HCV infection enrolled in this study.
| Region | Hospital | No. of patients |
|---|---|---|
| North | Peking University People’s Hospital | 59 |
| Beijing Friendship Hospital | 33 | |
| Northeast | Shengjing Hospital of China Medical Hospital | 34 |
| The First Hospital of Jilin University | 34 | |
| The Second Affiliated Hospital of Harbin Medical University | 21 | |
| Southwest | West China Hospital, Sichuan | 22 |
| The First Affiliated Hospital of Kunming Medical College | 40 | |
| Southwest Hospital | 29 | |
| South | The First Affiliated Hospital of Guangxi Medical University | 40 |
| Nanfang Hospital | 14 | |
| The Third Affiliated Hospital of Sun Yat-sen University | 50 | |
| Central | Henan Provincial People’s Hospital | 95 |
| The First Affiliated Hospital of Zhengzhou University | 28 | |
| People’s Hospital of Hubei Wuhan University | 38 | |
| Affiliated Tongji Hospital of Tongj Medical College of Huazhong University of Science and Technology | 32 | |
| The Second Xiangya Hospital of Central South University | 31 | |
| Northwest | Tangdu Hospital | 40 |
| The First Affiliated Hospital of Shanxi Medical University | 37 | |
| The First Affiliated Hospital of Lanzhou University | 58 | |
| Ningxia People’s Hospital | 27 | |
| East | The First Affiliated Hospital of Nanchang University | 37 |
| The First Affiliated Hospital of Anhui Medical University | 19 | |
| The Second Hospital of Shangdong University | 41 | |
| The First Affiliated Hospital of Medical College Zhejiang University | 33 | |
| The First Affiliated Hospital of Fujian Medical University | 11 | |
| Shanghai Ruijin Hospital | 52 | |
| The First Affiliated People’s Hospital of Shanghai Jiaotong University | 2 | |
| Jiangsu Province Hospital | 57 |
HCV, hepatitis C virus.
Figure 1.
Regional distribution of the patients with HCV recruited in this study. Twenty-eight hospitals were divided territorially into North, Northeast, Southwest, South, Central, Northwest and East China. There were 92 HCV-infected patients in North China, 91 in Southwest China, 104 in South China, 224 in Central China, 162 in Northwest China, 252 in East China and 89 in Northeast China. HCV, hepatitis C virus.
HCV genotypes and RNA expression levels
The specific primers and typing probes for the HCV genotyping were designed according to the 5′ non-coding region (nt299-1) of HCV gene sequences published in GenBank. The HCV RNA levels in the plasma samples of the patients with HCV genotypes 1b, 2, 3, 6 were detected by an Abbott RealTime HCV assay (Abbott Laboratories, Des Plaines, IL, USA), and total RNA was isolated from the plasma of the HCV-infected patients using an RNeasy Mini kit and a RNase-Free DNase set (Qiagen, Hilden, Germany). The PCR products were dot-blot hybridized to determine the HCV genotypes [Versant HCV Genotype 2.0 assay (LiPA); Siemens Healthcare Diagnostics, Tarrytown, NY, USA].
Thyroid function
Peripheral blood samples were collected from fasting patients and were stored at −70°C until use. In each patient, the plasma levels of thyroid-stimulating hormone (TSH), thyroxine (T4), triiodothyronine (T3), free thyroxine (FT4), free triiodothyronine (FT3), and anti-thyroid peroxidase (anti-TPO) antibodies (Ab) were measured by the immune-chemiluminescense-assay method (ADVIA Centaur® CP immunoassay System; Siemens AG, Erlangen, Germany). Anti-thyroglobulin Ab (anti-Tg Ab) was determined by the Immulite® 1,000 Systems method (Siemens AG). Thyroid function was defined according to the criteria taken from Surks et al (13). Therefore, the subjects were divided into chronic thyroiditis complicated with hyperthyroidism, chronic thyroiditis complicated with hypothyroidism, subclinical hyperthyroidism, subclinical hypothyroidism, hyperthyroidism, hypothyroidism and chronic thyroiditis with normal thyroid function (Table II). Hyperthyroidism was defined with TSH levels <0.1 mIU/l and elevated FT4 level. Subclinical hyperthyroidism was defined as TSH levels between 0.1 to 0.4 mIU/l and normal FT4 levels. Hypothyroidism was defined as a TSH level ≥10 mIU/l independently of the FT4 value. Subclinical hypothyroidism was defined as a TSH level of 4.5 to 10 mIU/l, with an FT4 level between 10 to 25 pmol/l. Positive anti-TPO antibodies (>35 IU/ml) and anti-Tg antibodies (>40 IU/ml) were indications for complicated chronic thyroiditis.
Table II.
Criteria of the different types of TD.
| Types of TD | Tg | Tb | TSH | T3 | T4 | FT3 | FT4 |
|---|---|---|---|---|---|---|---|
| Chronic thyroiditis complicated with hypothyroidism | + | + | ↑ | ↓/not | ↓ | ↓/not | ↓ |
| Subclinical hypothyroidism | − | − | ↑ | NOR | NOR | NOR | NOR |
| Chronic thyroiditis complicated with hyperthyroidism | + | + | ↓ | ↑ | ↑ | ↑ | ↑ |
| Subclinical hyperthyroidism | − | − | ↓ | NOR | NOR | NOR | NOR |
| Chronic thyroiditis with normal thyroid function | + | + | NOR | NOR | NOR | NOR | NOR |
| Hypothyroidism | − | − | ↑ | ↓ | ↓ | ↓/not | ↓/not |
| Hyperthyroidism | − | − | ↓ | ↑ | ↑ | ↑ | ↑ |
TD, thyroid dysfunction; Tg, anti-thyroglobulin; Tb, anti-thyroid peroxidase; TSH, thyroid-stimulating hormone; T3, triiodothyronine; T4, thyroxine; F, free; +, positive; -, negative; ↑, elevated; ↓, reduced; NOR, normal. Hyperthyroidism was defined with TSH levels <0.1 mIU/L and elevated FT4 level. Subclinical hyperthyroidism was defined as TSH levels between 0.1 to 0.4 mIU/l and normal FT4 levels. Hypothyroidism was defined as a TSH level ≥10 mIU/l independently of the FT4 value. Subclinical hypothyroidism was defined as a TSH level of 4.5 to 10 mIU/l, with an FT4 level between 10 to 25 pmol/l. Positive anti-TPO antibodies (>35 IU/ml) and anti-Tg antibodies (>40 IU/ml) were indications for complicated chronic thyroiditis.
Liver function
The liver function was tested in each HCV patient. The concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and albumin (ALB) were determined by automated clolrimetric method on Beckman SYNCHRON LX20 system (Beckman-Coulter, Fullerton, CA, USA). Reference ranges were: ALT 5–40 U/l, AST 8–40 U/l, ALB 35~55 g/l.
Statistical analysis
Student’s t-test, χ2 test and Fisher’s exact test were performed to compare the differences among the groups of patients. Two-tailed P<0.05 was considered to indicate a statistically significant difference. The statistical analyses were performed with the statistical package SPSS, version 13.0 (SPSS, Inc., Chicago, IL, USA).
Results
In this study, all 1,012 HCV-infected patients treated at 28 hospitals in 7 regions of China were randomly recruited (Fig. 1; Table I). The gender ratio of males to females was 552/460. The HCV-infected patients were divided into four groups according to age: The elderly (≥60 years old), middle-aged (45–59 years old), young adults (18–44 years old), and teenagers (<18 years old). The HCV genotypes 1b, 2, 3 and 6 were detected in the recruited patients. Furthermore, the HCV RNA expression levels were detected and divided into high (RNA≥1×107 copies/ml), medium (1×105≤RNA<1×107 copies/ml) and low (RNA<1×105 copies/ml).
Incidence of TD in patients with HCV infection in different regions
The distribution of various types of TD in HCV-infected patients was investigated. The study showed that North (28.3%) and Northwest (26.5%) China accounted for the highest proportions of TD in patients with HCV infection, and the lowest proportions were observed in the patients in Southwest (15.7%), South (14.4%) and Central (17.4%) China. Of the various types of TD, hyperthyroidism had the lowest incidence in the TD patients with HCV infection. Subclinical hypothyroidism had the highest incidence of all the types of TD; the highest proportions of patients with subclinical hypothyroidism were in North (13.0%) and North West (14.2%) China and the lowest proportion was in South China (1.9%). Chronic thyroiditis with normal thyroid function was observed at the highest proportions in North East (10.1%) and North (8.7%) China (Table III).
Table III.
Geographical distribution of each type of TD in patients with HCV infection from different regions of China (%).
| Types of TD | North | Northeast | Southwest | South | Central | Northwest | East |
|---|---|---|---|---|---|---|---|
| Chronic thyroiditis complicated with hyperthyroidism | 0 | 0 | 0 | 3.8 | 0.9 | 1.2 | 1.2 |
| Hyperthyroidism | 1.1 | 0 | 0 | 0 | 0 | 0 | 0.4 |
| Subclinical hyperthyroidism | 0 | 0 | 0 | 1.0 | 0 | 0.6 | 0.8 |
| Chronic thyroiditis complicated with hypothyroidism | 1.1 | 4.5 | 3.4 | 1.0 | 2.8 | 2.5 | 3.6 |
| Hypothyroidism | 4.3 | 0 | 1.1 | 0 | 1.8 | 3.1 | 1.6 |
| Subclinical hypothyroidism | 13.0 | 7.9 | 6.7 | 1.9 | 8.0 | 14.2 | 7.1 |
| Chronic thyroiditis with normal thyroid function | 8.7 | 10.1 | 4.5 | 6.7 | 4.0 | 4.9 | 6.0 |
| Total | 28.2 | 22.5 | 15.7 | 14.4 | 17.5 | 26.5 | 20.7 |
TD, thyroid dysfunction; HCV, hepatitis C virus.
Distribution of the HCV genotypes and RNA expression levels in TD patients with HCV infection
Numerous studies have verified the association between autoimmune disorders and HCV infection (14,15). Therefore, the HCV genotypes and RNA expression levels in patients with autoimmune-related TD and other types of thyroid disease were detected in the present study. The data showed that the patients with medium expression levels of HCV RNA were the predominant population of those with chronic thyroiditis complicated with hypothyroidism (54%, 15/28). Patients with high and low HCV RNA expression levels were the main population of those with hypothyroidism, with percentages of 56 (10/18) and 38% (7/18), respectively. The proportion of patients with HCV and high and medium HCV RNA expression levels was higher in those with autoimmune-related TD than in the patients with normal thyroid function (Table IV). HCV genotyping shows that among all genotypes, 1b was the most common genotype in HCV-infected patients with TD and without TD, and the percentages were 61% and 58%, respectively (Table V).
Table IV.
HCV RNA expression levels in HCV-infected patients with different types of TD.
| RNA expression levels (%) | |||
|---|---|---|---|
|
|
|||
| Types of TD | High | Medium | Low |
| Chronic thyroiditis complicated with hyperthyroidism | 55 (6/11) | 45 (5/11) | 0 |
| Hyperthyroidism | 100 (2/2) | 0 | 0 |
| Subclinical hyperthyroidism | 50 (2/4) | 50 (2/4) | 0 |
| Chronic thyroiditis complicated with hypothyroidism | 32 (9/28)a | 54 (15/28) | 14 (4/28) |
| Hypothyroidism | 56 (10/18) | 6 (1/18)a | 38 (7/18)a |
| Subclinical hypothyroidism | 53 (46/86) | 38 (33/86) | 9 (7/86) |
| Chronic thyroiditis with normal thyroid function | 48 (29/60) | 42 (25/60) | 10 (6/60) |
| Normal thyroid function | 53 (430/803) | 41 (326/803) | 6 (49/803) |
Significant differences from the normal thyroid function with HCV infection group.
TD, thyroid dysfunction; HCV, hepatitis C virus.
Table V.
Distribution of HCV genotypes in patients with TD and HCV infection.
| HCV genotype (%) | ||||
|---|---|---|---|---|
|
|
||||
| Types of TD | 1b | 2 | 3 | 6 |
| Chronic thyroiditis complicated with hyperthyroidism | 64 (7/11) | 18 (2/11) | 9 (1/11) | 9 (1/11) |
| Hyperthyroidism | 100 (2/2) | 0 | 0 | 0 |
| Subclinical hyperthyroidism | 50 (2/4) | 0 | 25 (1/4) | 25 (1/4) |
| Chronic thyroiditis complicated with hypothyroidism | 61 (17/28) | 32 (9/28) | 3.5 (1/28) | 3.5 (1/28) |
| Hypothyroidism | 50 (9/18) | 33 (6/18) | 17 (3/18) | 0 |
| Subclinical hypothyroidism | 60 (52/86) | 35 (30/86) | 2.3 (2/86) | 2.3 (2/86) |
| Chronic thyroiditis with normal thyroid function | 67 (40/60) | 23 (14/60) | 6.7 (4/60) | 3.3 (2/60) |
| Normal thyroid function | 58 (473/803) | 24 (190/803) | 10.3 (83/803) | 7.7 (57/803) |
HCV, hepatitis C virus; TD, thyroid dysfunction.
Distribution of TD in patients with HCV infection of different genders and ages
The data showed that in all recruits, the numbers of the elderly, middle-aged, young adults and teenagers accounted for 14.8 (150/1,012), 37.7 (382/1,012) and 47.3 (480/1,012) (the two teenagers were excluded from the TD analysis), respectively. In all HCV-infected patients with TD, the middle-aged group (43.5%) accounted for the largest number, which increased to 72.2% in the HCV-infected patients with hypothyroidism. However, young adults were the main group (52.2%) of HCV-infected patients without TD. Gender differences were identified in the numbers of HCV-infected patients with TD. The percentage of female patients was significantly higher than that of the males in the total number of patients with TD. However, the percentage of males was higher than that of females in the number of HCV-infected patients without TD (Table VI).
Table VI.
Distribution (%) of the types of TD in patients with HCV infection in different gender and age groups.
| Types of TD | Male | Female | Elderly | Middle-aged | Young adult |
|---|---|---|---|---|---|
| Chronic thyroiditis complicated with hyperthyroidism | 45 (5/11) | 55 (6/11) | 27.3 (3/11) | 36.4 (4/11) | 36.4 (4/11) |
| Hyperthyroidism | 0 | 100 (2/2) | 50 (1/2) | 0 | 50 (1/2) |
| Subclinical hyperthyroidism | 75 (3/4) | 25 (1/4) | 25 (1/4) | 25 (1/4) | 50 (2/4) |
| Chronic thyroiditis complicated with hypothyroidism | 29 (8/28)a | 71 (20/28) | 28.6 (8/28)a | 50.0 (14/28)a | 24.1 (6/28)a |
| Hypothyroidism | 28 (5/18)a | 72 (13/18) | 5.6 (1/18) | 72.2 (13/18)a | 22.2 (4/18)a |
| Subclinical hypothyroidism | 48 (41/86) | 52 (45/86) | 27.9 (24/86)a | 39.5 (34/86) | 31.4 (27/86)a |
| Chronic thyroiditis with normal thyroid function | 40 (24/60)a | 60 (36/60) | 25 (15/60)a | 43.3 (26/60) | 31.7 (19/60)a |
| Normal thyroid function | 58 (466/803) | 42 (337/803) | 12.1 (97/803) | 35.1 (290/803) | 51.8 (416/803) |
Significant differences from the normal thyroid function group.
TD, thyroid dysfunction; HCV, hepatitis C virus.
Association of liver function with TD in HCV-infected patients
The prevalence of liver disorders in patients with TD and HCV infection was also investigated in the present study. The data showed that the incidence of a liver disorder in the patients with chronic thyroiditis complicated with hypothyroidism (46.5%) was lower than that in patients with other types of TD. The patients with hypothyroidism had the highest percentage of liver disorder (69%) among all HCV-infected patients. The results for hyperthyroidism are of no statistical significance due to the small sample size. Although lower ALB levels were common in HCV-infected patients with normal thyroid function, patients with low levels of ALB were scarce in those with any type of TD (Table VII).
Table VII.
Liver function with TD in HCV infected patients.
| Liver function | |||
|---|---|---|---|
|
|
|||
| Types of TD | ALT↑ | AST↑ | ALB↓ |
| Chronic thyroiditis complicated with hyperthyroidism | 7/11 | 5/11 | 0 |
| Hyperthyroidism | 0 | 1/2 | 1/2 |
| Subclinical hyperthyroidism | 1/4 | 3/4 | 1/4 |
| Chronic thyroiditis complicated with hypothyroidism | 14/28 | 12/28 | 3/28 |
| Hypothyroidism | 13/18 | 12/18 | 1/18 |
| Subclinical hypothyroidism | 48/86 | 50/86 | 8/86 |
| Chronic thyroiditis with normal thyroid function | 35/60 | 36/60 | 7/60 |
| Normal thyroid function | 517/803 | 448/803 | 64/803 |
TD, thyroid dysfunction; HCV, hepatitis C virus; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; ↑, elevated; ↓, reduced.
Discussion
HCV infection is a major cause of chronic liver disease worldwide. Varied data on the prevalence of HCV infection are frequently reported in studies (16,17). These discrepancies reflect not only the distinct epidemiological characteristics of patients with HCV, but also the differences in the methodologies used. Taking into account the impact of external and anthropic factors, all samples were analyzed in the same center in the present study.
A previous study has demonstrated that the distribution of the HCV genotypes demonstrates geographical differences, and this impact should be considered in an epidemiological study (18). The HCV genotypes are considered a major determinant of the response to treatment in HCV infection (19,20). Therefore, the status of HCV infection and the HCV genotype are important in the etiological diagnosis, clinical treatment and vaccine development of HCV infection in specific regions. However, no significant difference in the distribution of HCV genotypes was identified between the TD and normal thyroid function groups in the present study. This indicates that the geographical differences in the TD incidence in patients with HCV infection is not likely to be caused by the HCV genotype.
Certain studies have shown that hypothyroidism and thyroid autoimmunity disorder are more common in patients with HCV, even in the absence of interferon treatment (5,21). However, the present study shows that subclinical hypothyroidism is the most prevalent type of TD in untreated HCV patients, while patients with hyperthyroidism account for the lowest proportion of those with HCV infection. Furthermore, the HCV RNA expression levels in patients with different types of TD were detected. The data confirm the existence of an association between TD and the HCV RNA expression levels. Patients with medium expression levels of HCV RNA are the predominant population of those with chronic thyroiditis complicated with hypothyroidism (53.7%, 15/28), but are scarce in those with hypothyroidism. Low levels of HCV RNA are common in patients with hypothyroidism. This result indicates that the pathogenesis of TD is not likely to be associated with the HCV RNA expression levels but may be associated with autoimmunity. In HCV-infected patients, autoimmunity is an important pathogenesis of TD development through the actions of thyroid autoantibodies and certain chemokines (22,23) The detailed mechanisms require investigation in the future.
Previous studies have shown that gender is one of the most common risk factors that predict the development of TD during interferon therapy (7,24,25). TD is more common in females than in males in different regions all over the world, which has been verified in the majority of conditions caused by HCV infection and other diseases (26,27). In the present study, it was also identified that the number of females was significantly larger than that of the males in the cohort of patients with TD and HCV infection. However, in China HCV infection is more prevalent in males than in females (28), leading to markedly more male patients than female patients. Hsieh et al reported that the female gender is a predisposing factor for TD in patients with HCV infection receiving interferon treatment (29), which is supported by the findings of the present study in Chinese patients. The incidence of TD in patients with HCV of different age groups was also investigated. The results show that patients with TD are mainly distributed in the middle-aged group. Furthermore, the percentage of patients with TD among middle-aged patients with HCV infection is highest in those with hypothyroidism (72.2%). Cappola et al reported the percentage of each type of TD in 3,233 normal elderly adults. The study demonstrated that subclinical hypothyroidism was the most prevalent type and accounted for 15.3% of all recruited cases (30), which is in accordance with the results of the present study.
The incidence of liver disorders in TD patients was investigated and it was identified that the percentage of patients with liver disorder was highest in those with hypothyroidism (69%) than in other types of TD (P<0.05). A correlation between hypothyroidism and liver disorders may exist in HCV-infected patients. However, as patients with chronic thyroiditis complicated with hypothyroidism show a lower percentage of liver disorders than those with hypothyroidism, the higher incidence of liver disorder in patients with hypothyroidism may be unrelated to autoimmune disorder.
In conclusion, this study investigated the correlation between the TD subtypes and the geographical distribution, HCV RNA expression levels, HCV genotype, gender, age and liver function in HCV-infected patients of China. It was demonstrated that the highest incidence of TD in HCV-infected patients was in those from North and Northwest China. The HCV-infected patients with TD exhibited a higher frequency of high and medium RNA levels compared with that in the patients with normal thyroid function. Middle-aged patients account for the largest number of all HCV-infected patients with TD, while young adults are the main group of HCV-infected patients without TD. Hypothyroidism in patients with HCV is associated with a higher incidence of liver disorders. This study indicates differences in age, gender and geographical distribution among various types of TD in HCV-infected patients, which may aid the success of individualized treatment for HCV-infected patients.
Acknowledgements
This study was supported by grants from the National Science and Technology Major Project for Infectious Diseases Control during the 11th Five-Year Plan period (2008ZX10002-012 and 2008ZX10002-013) and 12th Five-Year Plan period (2012ZX10002003), the Natural Science Foundation of Gansu Province (0803RJZA057), the Fundamental Research Funds for the Central Universities (lzujbky-2009-150) and from Bristol-Myers Squibb. Operational support and statistical analyses were provided by ReSearch Pharmaceutical Services, Inc. (Beijing, China).
References
- 1.Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol. 2013;28(Suppl 1):7–10. doi: 10.1111/jgh.12220. [DOI] [PubMed] [Google Scholar]
- 2.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Hampel H, Yeh C, Rabeneck L. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439–1445. doi: 10.1053/jhep.2002.37191. [DOI] [PubMed] [Google Scholar]
- 4.Andrade LJ, Atta AM, D’Almeida Junior A, Paraná R. Thyroid dysfunction in hepatitis C individuals treated with interferon-alpha and ribavirin - a review. Braz J Infect Dis. 2008;12:144–148. doi: 10.1590/s1413-86702008000200009. [DOI] [PubMed] [Google Scholar]
- 5.Antonelli A, Ferri C, Fallahi P, Ferrari SM, Ghinoi A, Rotondi M, Ferrannini E. Thyroid disorders in chronic hepatitis C virus infection. Thyroid. 2006;16:563–572. doi: 10.1089/thy.2006.16.563. [DOI] [PubMed] [Google Scholar]
- 6.Tomer Y, Blackard JT, Akeno N. Interferon alpha treatment and thyroid dysfunction. Endocrinol Metab Clin North Am. 2007;36:1051–1166. x–xi. doi: 10.1016/j.ecl.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vezali E, Elefsiniotis I, Mihas C, Konstantinou E, Saroglou G. Thyroid dysfunction in patients with chronic hepatitis C: virus- or therapy-related? J Gastroenterol Hepatol. 2009;24:1024–1029. doi: 10.1111/j.1440-1746.2009.05812.x. [DOI] [PubMed] [Google Scholar]
- 8.Antonelli A, Ferri C, Pampana A, Fallahi P, Nesti C, Pasquini M, Marchi S, Ferrannini E. Thyroid disorders in chronic hepatitis C. Am J Med. 2004;117:10–13. doi: 10.1016/j.amjmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Nair Kesavachandran C, Haamann F, Nienhaus A. Frequency of thyroid dysfunctions during interferon alpha treatment of single and combination therapy in hepatitis C virus-infected patients: a systematic review based analysis. PLoS One. 2013;8:e55364. doi: 10.1371/journal.pone.0055364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danilovic DL, Mendes-Correa MC, Chammas MC, Zambrini H, Barros RK, Marui S. Thyroid disturbance related to chronic hepatitis C infection: role of CXCL10. Endocr J. 2013;60:583–590. doi: 10.1507/endocrj.ej12-0321. [DOI] [PubMed] [Google Scholar]
- 11.Tran A, Quaranta JF, Benzaken S, Thiers V, Chau HT, Hastier P, Regnier D, Dreyfus G, Pradier C, Sadoul JL, et al. High prevalence of thyroid autoantibodies in a prospective series of patients with chronic hepatitis C before interferon therapy. Hepatology. 1993;18:253–257. [PubMed] [Google Scholar]
- 12.Rodríguez-Torres M, Ríos-Bedoya CF, Ortiz-Lasanta G, Marxuach-Cuétara AM, Jiménez-Rivera J. Thyroid dysfunction (TD) among chronic hepatitis C patients with mild and severe hepatic fibrosis. Ann Hepatol. 2008;7:72–77. [PubMed] [Google Scholar]
- 13.Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, Franklyn JA, Hershman JM, Burman KD, Denke MA, Gorman C, Cooper RS, Weissman NJ. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–238. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 14.Khairy M, El-Raziky M, El-Akel W, Abdelbary MS, Khatab H, El-Kholy B, Esmat G, Mabrouk M. Serum autoantibodies positivity prevalence in patients with chronic HCV and impact on pegylated interferon and ribavirin treatment response. Liver Int. 2013;33:1504–1509. doi: 10.1111/liv.12227. [DOI] [PubMed] [Google Scholar]
- 15.Yang DH, Ho LJ, Lai JH. Useful biomarkers for assessment of hepatitis C virus infection-associated autoimmune disorders. World J Gastroenterol. 2014;20:2962–2970. doi: 10.3748/wjg.v20.i11.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oliveira-Filho AB, Sawada L, Pinto LC, Locks D, Bahia SL, Castro JA, Hermes RB, Brasil-Costa I, Amaral CE, Lemos JA. Epidemiological aspects of HCV infection in non-injecting drug users in the Brazilian state of Pará, eastern Amazon. Virol J. 2014;11:38. doi: 10.1186/1743-422X-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh P, Kaur R, Kaur A. Frequency distribution of Hepatitis C virus in different geographical regions of Punjab: Retrospective study from a tertiary care centre in North India. J Nat Sci Biol Med. 2014;5:56–58. doi: 10.4103/0976-9668.127288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viazov S, Zibert A, Ramakrishnan K, Widell A, Cavicchini A, Schreier E, Roggendorf M. Typing of hepatitis C virus isolates by DNA enzyme immunoassay. J Virol Methods. 1994;48:81–91. doi: 10.1016/0166-0934(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 19.Sibbing B, Nattermann J. Hepatitis C virus infection and genetic susceptibility to therapy. J Gastrointestin Liver Dis. 2011;20:397–406. [PubMed] [Google Scholar]
- 20.Zeuzem S, Berg T, Moeller B, Hinrichsen H, Mauss S, Wedemeyer H, Sarrazin C, Hueppe D, Zehnter E, Manns MP. Expert opinion on the treatment of patients with chronic hepatitis C. J Viral Hepat. 2009;16:75–90. doi: 10.1111/j.1365-2893.2008.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ploix C, Verber S, Chevallier - Queyron P, Ritter J, Bousset G, Monier JC, Fabien N. Hepatitis C virus infection is frequently associated with high titers of anti-thyroid antibodies. Int J Immunopathol Pharmacol. 1999;12:121–126. [PubMed] [Google Scholar]
- 22.Yang R, Shan Z, Li Y, Fan C, Li C, Teng W. Prevalence of thyroid autoantibodies in hepatitis C and hepatitis B infection in China. Intern Med. 2011;50:811–815. doi: 10.2169/internalmedicine.50.4870. [DOI] [PubMed] [Google Scholar]
- 23.Danilovic DL, Mendes-Correa MC, Chammas MC, Zambrini H, Barros RK, Marui S. Thyroid disturbance related to chronic hepatitis C infection: role of CXCL10. Endocr J. 2013;60:583–590. doi: 10.1507/endocrj.ej12-0321. [DOI] [PubMed] [Google Scholar]
- 24.Dalgard O, Bjøro K, Hellum K, Myrvang B, Bjøro T, Haug E, Bell H. Thyroid dysfunction during treatment of chronic hepatitis C with interferon alpha: no association with either interferon dosage or efficacy of therapy. J Intern Med. 2002;251:400–406. doi: 10.1046/j.1365-2796.2002.00974.x. [DOI] [PubMed] [Google Scholar]
- 25.Friedrich-Rust M, Theobald J, Zeuzem S, Bojunga J. Thyroid function and changes in ultrasound morphology during antiviral therapy with pegylated interferon and ribavirin in patients with chronic hepatitis C. J Viral Hepat. 2009;16:168–177. doi: 10.1111/j.1365-2893.2008.01059.x. [DOI] [PubMed] [Google Scholar]
- 26.Andrade LJ, Atta AM, D’Almeida Junior A, Paraná R. Thyroid dysfunction in hepatitis C individuals treated with interferon-alpha and ribavirin - a review. Braz J Infect Dis. 2008;12:144–148. doi: 10.1590/s1413-86702008000200009. [DOI] [PubMed] [Google Scholar]
- 27.Chirico V, Antonio L, Vincenzo S, Luca N, Valeria F, Basilia P, Luciana R, Carmelo S, Teresa A. Thyroid dysfunction in thalassaemic patients: ferritin as a prognostic marker and combined iron chelators as an ideal therapy. Eur J Endocrinol. 2014;170:X3. doi: 10.1530/EJE-13-0627. [DOI] [PubMed] [Google Scholar]
- 28.Tao YL, Tang YF, Qiu JP, Cai XF, Shen XT, Wang YX, Zhao XT. Prevalence of hepatitis C infection among intravenous drug users in Shanghai. World J Gastroenterol. 2013;19:5320–5325. doi: 10.3748/wjg.v19.i32.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh MC, Yu ML, Chuang WL, Shin SJ, Dai CY, Chen SC, Lin ZY, Hsieh MY, Liu JF, Wang LY, Chang WY. Virologic factors related to interferon-alpha-induced thyroid dysfunction in patients with chronic hepatitis C. Eur J Endocrinol. 2000;142:431–437. doi: 10.1530/eje.0.1420431. [DOI] [PubMed] [Google Scholar]
- 30.Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]