Abstract
In eukaryotic cells, the cytoplasm and the nucleus are separated by a double-membraned nuclear envelope (NE). Thus, transport of molecules between the nucleus and the cytoplasm occurs via gateways termed the nuclear pore complexes (NPCs), which are the largest intracellular channels in nature. While small molecules can passively translocate through the NPC, large molecules are actively imported into the nucleus by interacting with receptors that bind nuclear pore complex proteins (Nups). Regulatory factors then function in assembly and disassembly of transport complexes. Signaling pathways, cell cycle, pathogens, and other physiopathological conditions regulate various constituents of the nuclear transport machinery. Here, we will discuss several findings related to modulation of nuclear transport during physiological and pathological conditions, including tumorigenesis, viral infection, and congenital syndrome. We will also explore chemical biological approaches that are being used as probes to reveal new mechanisms that regulate nucleocytoplasmic trafficking and that are serving as starting points for drug development.
Nuclear Transport in Health
Transport of molecules of less than 50 kD can passively occur through the NPC. However large molecules, including proteins, require receptors for trafficking through the NPC. Proteins usually contain specific motifs termed Nuclear Localization Sequences (NLSs) and Nuclear Export Sequences (NESs) that are recognized by transport receptors termed karyopherins, importins (α and β transportin, snurportin, etc.), or exportins (Crm1/XPO/exportin 1, etc.). The receptor-cargo complexes interact with nuclear pore complex proteins (nucleoporins or Nups) and are translocated through the NPC. Once import complexes reach the nucleoplasmic side of the NPC, the GTPase Ran binds the transport receptor and the cargo is released to exert its function in the nucleus. In contrast, RanGTP enhances the interaction of transport receptors with cargos destined for nuclear export. The export complex is then translocated through the NPC and dissociated at the cytoplasmic side by the actions of the GTPase-activating protein RanGAP and other factors [1].
Regarding transport of RNA, a subset of mRNAs, miRNAs, and tRNAs can also bind export receptors that utilize RanGTP in a similar manner as transport of proteins [2]. On the other hand, bulk mRNA nuclear export is mediated by transport receptors that do not belong to the karyopherin family of proteins and do not require Ran. Bulk mRNA export is driven by the heterodimer NXF1(TAP) - NXT1(p15) (Mex67 and Mtr2 respectively in yeast) that is recruited to the mRNA by the TREX complex [3]. Once the mRNP reaches the cytoplasmic side, the ATP-dependent RNA helicase Dbp5 promotes the release of the mRNP into the cytoplasm. This step is regulated by the mRNA export factor Gle1 and inositol hexakisphosphate(IP6) [3]. NXF1(TAP) - NXT1(p15) heterodimer has structure similarity to the transport factor NTF2 [4], which imports RanGDP into the nucleus [1]. This NTF2-like domain of the NXF1-NXT1 heterodimer, together with another domain at the C-terminus of NXF1, interact with FG repeats on nucleoporins to mediate nuclear export of mRNAs [4].
Nucleocytoplasmic Trafficking in Cell Proliferation and Tumorigenesis
An elegant mode for regulation of nuclear transport is achieved by post-translation modifications [5]. An example of such regulation can be found in the NF-κB signaling pathway, a major regulator of immunity and cell proliferation, which is involved in tumorigenesis and response to viral infection [6]. Briefly, in basal conditions, NF-κB binds to its inhibitory protein IκB. Since IκB masks the NF-κB NLS, this heterodimer is mostly cytoplasmic. As a response to stress or extracellular cues sensed by plasma membrane receptors, IκB is phosphorylated and targeted for degradation. The exposed NF-κB NLS will then interact with karyopherins leading to rapid import of NF-κB into the nucleus where it will regulate transcription of various genes. This allows a rapid response to stress conditions and emphasizes the importance of regulated nucleocytoplasmic trafficking in health and disease. Other regulated nuclear import and export mechanisms are used by various key signaling pathways such as the p53 pathway [7], interferon (IFN) response pathway [8], and hormone activated pathways [9]. Since there are ~20 karyopherins in humans that can differentially recognize cargos, nuclear transport regulation may serve as an efficient and specific way to control different pathways upon activation by diverse stimuli. Thus, regulated transport is important to signaling and cellular response to environment and stress. In turn, disruptions of transport can lead to disease.
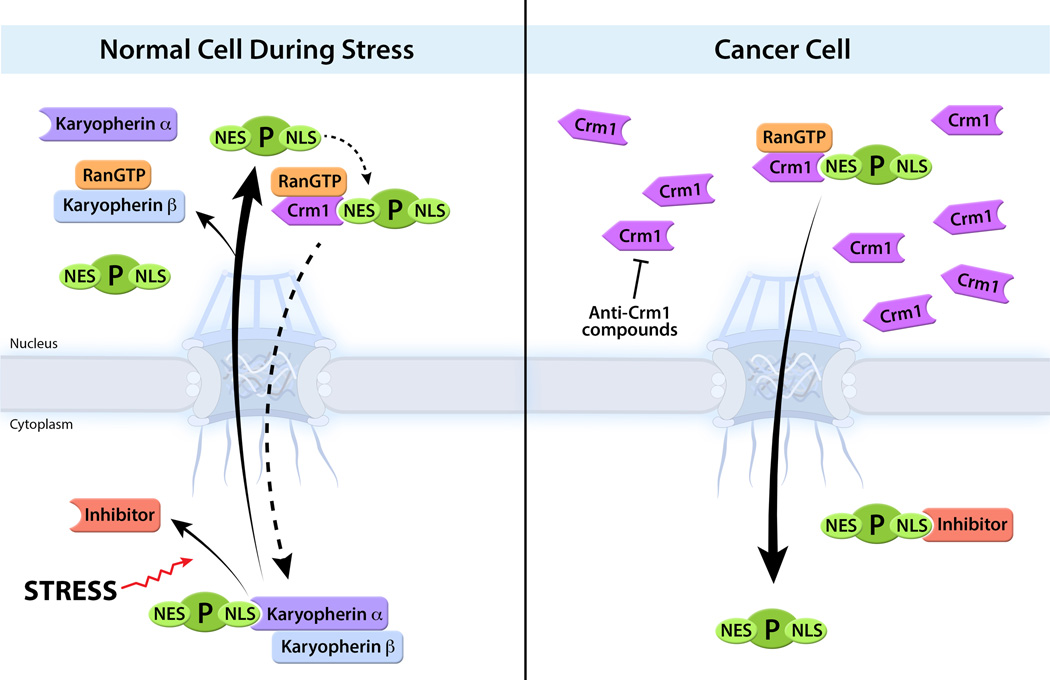
Since key oncogenes and tumor suppressors function in the nucleus and have NLSs and NESs, unbalanced nucleocytoplasmic shuttling of these factors are correlated with tumorigenesis. Examples include p53, FoxO, topo-IIα and the NF-κB inhibitor IκB, which interact with karyopherins/importins as they enter the nucleus and bind Crm1 (exportin-1 or XPO1) when they exit the nucleus. It has been shown that Crm1 is highly overexpressed in many different types of malignancies including gliomas, osteosarcomas, and leukemias [10–13]. Various findings have led to the model that overexpression of Crm1 enhances nuclear export of tumor suppressors and therefore prevents their accumulation and function in the nucleus. This outcome was specifically demonstrated in certain cases of acute myeloid leukemia (AML) where a mutation was found in the tumor suppressor nucleophosmin (NPM1) [14]. This mutation creates a novel NES that enhances Crm1 binding. Abnormal Crm1-mediated nuclear export of NPM1 removes it from the nucleus and prevents its suppression function on cell proliferation [14–16]. Given the putative pivotal role of Crm1 in a broad spectrum of malignancies, there is an ongoing effort to specifically inhibit this export factor. In fact, Crm1 inhibitors such as leptomycin B (LMB) and derivatives were shown to preferentially induce apoptosis of malignant cells when compared to normal cells, at specific concentrations [17]. However, these inhibitors were not effective in vivo because of off-target effects and high cytotoxicity [18]. Recently, new highly specific Crm1 inhibitors were developed and are termed small molecule drug-like selective inhibitors of nuclear export (SINEs) [19]. Treatment of cells with these Crm1 inhibitors lead to nuclear accumulation of p53, FoxO and IκB, among other factors, and induce preferential killing of various malignant cells over normal cells in vitro and in vivo [19–22]. While the mode of action of these inhibitors may not be restricted to this set of molecules, SINEs are now being tested in clinical trials for cancer therapy, illustrating the importance of nuclear transport mechanisms for the development of new therapeutic strategies (Figure 1).
Figure 1. Abnormal Nuclear Export of Proteins in cancer cells.
Upon genotoxic stress, various proteins (P) including tumor suppressors, such as p53, accumulate in the nucleus to regulate intranuclear processes. The translocation of these proteins into the nucleus involves recognition of the protein’s nuclear localization sequence (NLS) by a karyopherin or importin, which in some cases bind a second karyopherin. In the nucleus, the karyopherin(s) is dissociated from the cargo through the action of RanGTP. Certain proteins involved in cell proliferation have their NLS masked by inhibitors, which are dissociated upon various stimuli. This effect allows recognition of the NLS by karyopherins and protein import into the nucleus. Some of these proteins also have a nuclear export sequence (NES), which interacts with the export receptor Crm1 (XPO1). This interaction is enhanced by RanGTP, which is followed by subsequent translocation of the export complex to the cytoplasm. In certain types of cancer, Crm1 is overexpressed and promotes nuclear export of proteins, including tumor suppressors, inducing cell proliferation. Anti-Crm1 compounds are being tested for cancer therapeutics.
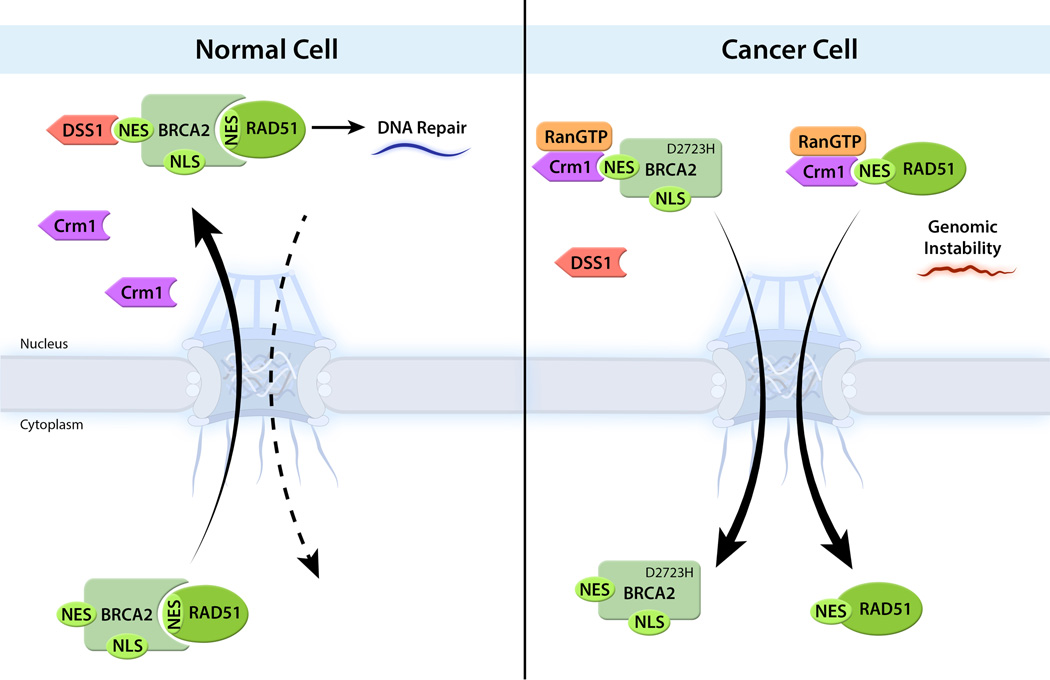
Recently, impaired regulation of nucleocytoplasmic trafficking was found in a BRCA2 mutant that predisposes individuals to various cancers including breast, ovarian and pancreatic cancers [23]. BRCA2 is a tumor suppressor that guides RAD51 to ssDNA foci where they function in DNA repair through homologous recombination. Thus, individuals with BRCA2 mutations are prone to cancer owing to genomic instability. One of the most common mutation in breast cancer is BRCA2D2723H. The region where this mutation occurs was shown to interact with the 26S proteasome complex subunit DSS1. DSS1 masks BRCA2 NES and the mutated BRCA2D2723H exposes the NES to Crm1, which exports it to the cytoplasm. In addition, BRCA2 interaction with RAD51 is also affected due to cytoplasmic redistribution of BRCA2D2723H. This effect exposes the NES of RAD51, driving its localization to the cytoplasm. Thus, these consecutive abnormal exposures of NESs perturb the nucleocytoplasmic equilibrium of important DNA repair factors and cause severe effects on genome stability (Figure 2).
Figure 2. Disruption of BRCA2-RAD51 nucleocytoplasmic trafficking in cancer cells.
During genotoxic stress in normal cells, RAD51 is recruited by BRCA2 to sites of DNA repair. The interaction of BRCA2 with RAD51 masks the RAD51 NES, preventing its export to the cytoplasm. DSS1, which binds BRCA2, masks BRCA2 NES and inhibit Crm1 mediated export. The masking of the two NESs inhibits cytoplasmic redistribution of the BRCA2-RAD51 complex. An abundant mutation in breast cancers (BRCA2D2723H) was found to prevent DSS1 association with BRAC2. As an outcome, BRCA2-NES is unmasked and is exported by Crm1-RanGTP. The remaining nuclear RAD51 are also redistributed to the cytoplasm, preventing its ability to function during DNA damage.
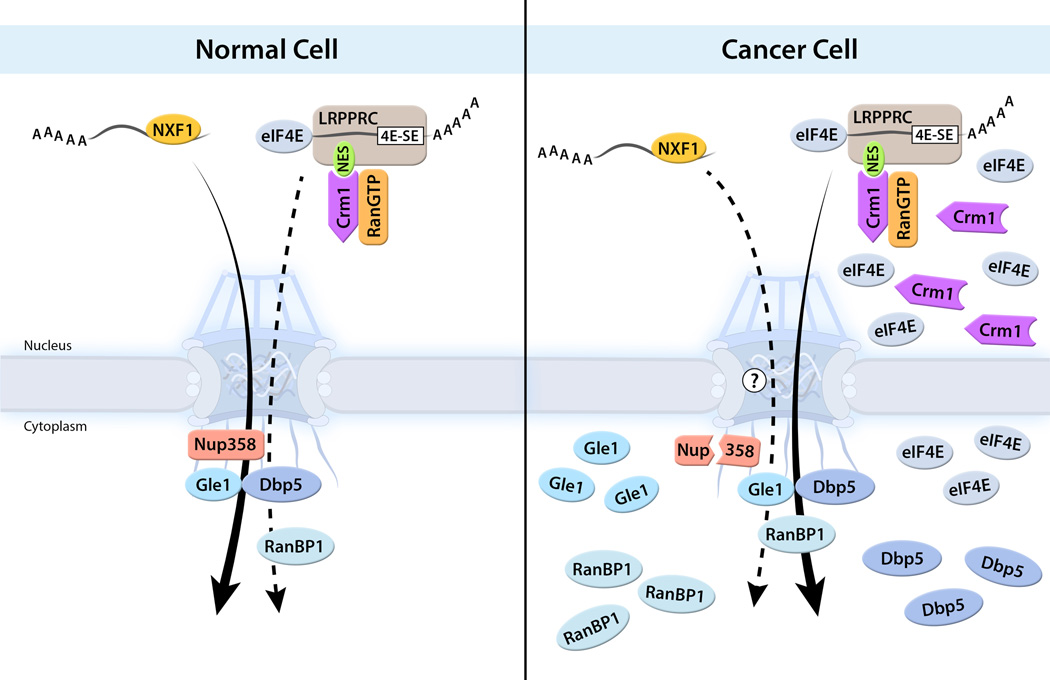
In addition to nuclear export of proteins, Crm1 was shown to export specific classes of mRNAs, which require translation initiation factor 4E (eIF4E). This class of mRNAs contain a 50-nucleotide structural element in their 3’ UTR termed the eIF4E sensitivity element (4E-SE) [24,25]. Among 4E-SE containing mRNAs are many known regulators of cell proliferation including c-Myc, Hdm2, NBS1, ODC, and Cyclin D1. Nuclear export of these mRNAs is dependent on eIF4E and enhanced by eIF4E overexpression (Figure 3). eIF4E is elevated in many cancers including acute myeloid leukemia (AML) [26]. Recently, eIF4E overexpression was linked to changes at the cytoplasmic side of the NPC [27] (Figure 3). Overexpression of eIF4E reduced Nup358 levels [27], which is a major constituent of the cytoplasmic filaments of the NPC. This condition favors eIF4E-dependent mRNA export. Additionally, eIF4E overexpression led to increased levels of RanBP1, Gle1, and Dbp5 [27], which are key soluble factors that participate in cargo release from the cytoplasmic filaments of the NPC. In addition to its role in mRNA export, eIF4E is a well-known translation factor that interacts with the 7-methly guanosine (m(7)G) cap on mRNAs. Knockdown of eIF4E with siRNA or treatment of cells with ribavirin, a nucleoside inhibitor that mimics m(7)G cap, disrupted eIF4E-mediated NPC modifications and eIF4E-dependent mRNA export [27]. Importantly, ribavirin is in phase II clinical trial for AML [26]. Taking together, these findings link specific mRNA export and NPC reprogramming with translation and transformation induced by eIF4E. It will also be interesting to assess the impact of changes in Gle1, Dbp5, and Nup358 upon eIF4E overexpression on NXF1-mediated mRNA export as these are important factors for this pathway.
Figure 3. eIF4E-mediated mRNA export and its link to tumorigenesis.
eIF4E mediates export of a subset of mRNAs, which contain the 4E-SE RNA element that is recognized by LRPPRC bound to eIF4E. LRPPRC contains an NES that interacts with Crm1-RanGTP, which translocates the mRNP to the cytoplasm. Among the 4E-SE mRNAs, there are important proliferation factors. In many cancer cells, eIF4E and Crm1 levels are elevated resulting in abnormal increase in nuclear export of various mRNAs, including the ones that regulate cell proliferation, which promotes their translation in the cytoplasm. eIF4E up-regulation leads to Nup358 degradation and increased levels of Dbp5, Gle1 and RanBP1. The crosstalk between the bulk mRNA export machinery and the eIF4E mRNA export pathway will be interesting to investigate.
Down-regulation of specific nucleoporins can also alter nuclear export of specific classes of mRNAs. Mice expressing low levels of the nucleoporin Nup96 present defects in nuclear export of subsets of mRNAs involved in immunity and cell cycle regulation [28,29]. More recently, another member of the mRNA export machinery was shown to function in processing and export of a specific class of mRNAs. THOC5, a member of the THO complex and mRNA export (TREX) complex, is localized in the nucleus and is exported to the cytoplasm during M-CSF-induced bone marrow-derived macrophage differentiation [30]. THOC5 mediates processing and export of subsets of mRNAs including well-known regulators of myeloid differentiation [30]. THOC5 thus functions in the maintenance of hematopoiesis and is involved in leukemogenesis [30].
Nucleoporin action in stress and oncogenesis outside the NPC
Aside from nucleocytoplasmic transport, it has been shown that some nucleoporins have additional functions inside the nucleus, some of which are directly related to development, stress, and tumorigenesis. One example is Nup98 that shuttles between the NPC and the nucleoplasm and regulates transcription of subsets of genes involved in development and cell cycle [31,32]. In addition, chromosomal translocations that lead to fusion proteins between Nup98 and transcription factors are known to be associated with leukemogenesis [33]. One example is Nup98 fusion with plant homeodomain (PHD) fingers that recognizes H3K4me3/2 marks. This fusion protein supports tumorigenesis by impairing the removal of H3K4me3 that activate transcription of Hox(s), Gata3, Meis1, Eya1 and Pbx1. This effect abolishes differentiation and causes oncogenesis [34]. The question then is what happens to Nup96? The Nup98 and Nup96 proteins are encoded by the same mRNA, which generates a Nup98-Nup96 precursor protein that yields the mature Nup98 and Nup96 proteins [35]. Thus, the chromosomal translocation involving Nup98 would likely disrupt Nup96 expression. Low Nup96 levels regulate nuclear export of subsets of mRNAs involved in immunity and cell cycle regulation [28,29]; therefore, abnormal Nup96 levels may contribute to the disease phenotypes observed in the Nup98 fusions with transcription factors.
Surprisingly, wild-type Nup98 was linked to oncogenesis in an unexpected manner. In a focused siRNA screen targeting nuclear transport factors, Nup98 was shown to be required for up-regulation of p21 mRNA upon p53 induction by genotoxic stress [36,37]. Nup98 specifically bound the 3’UTR of p21 mRNA preventing its degradation by the exosome. Similarly to p21, Nup98 also targeted a subset of mRNAs upon activation of p53, including 14–3-3σ, further demonstrating the significance of Nup98 in the p53 response. A reduction in both wild-type Nup98 and p21 levels was found in hepatocellular carcinomas, which correlates with a potential role for Nup98 in preventing tumorigenesis.
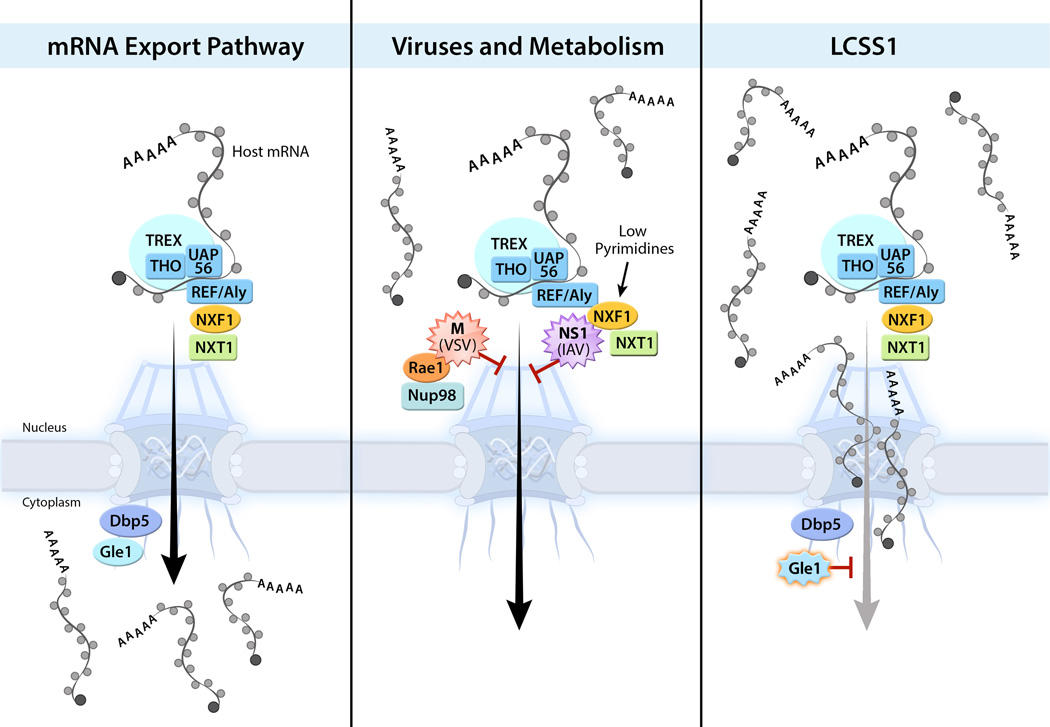
Nup98 also interacts with the mRNA export factor Rae1, which is targeted by the vesicular stomatitis virus (VSV) matrix (M) protein during infection [38,39] (Figure 4). This complex can function in interphase and mitosis to inhibit mRNA export [38–42] or cause death in metaphase [43], respectively. Since tumor cells have high mitotic index, death in mitosis may contribute to VSV oncolytic function. In another scenario, influenza virus also causes reduction of Nup98 levels to promote virus replication [44] (see also below). In this case, the targeting of Nup98 by the virus prevents proper host mRNA export, including mRNAs that encode antiviral factors [44]. These findings point to roles of Nup98 in response to various stress conditions where it can promote export of mRNAs that encode antiviral factors and stabilize mRNAs upon p53 activation. In both circumstances, a genome-wide search for Nup98 interacting mRNAs would be important for systematic understanding of its functions.
Figure 4. mRNA nuclear export in viral infection, metabolism, and congenital syndrome.
Bulk mRNA export is mediated by the TREX complex, which consists of THO, UAP56, and Aly/Ref. The association of Aly with mRNA recruits the mRNA export receptor heterodimer NXF1-NXT1, which mediates export of mRNAs by interacting with Nups at the NPC. Influenza virus NS1 protein or VSV M protein inhibit mRNA export. Low levels of pyrimidine induced by a DHODH inhibitor up-regulates NXF1 and release mRNA export block mediated by these viral proteins. Mutation in the mRNA export factor Gle1 disrupts its function in mRNA export and causes the lethal congenital contracture syndrome-1 (LCCS1).
mRNA export in viral infection and metabolism
Many viruses, including cytoplasmic replicating or nuclear replicating viruses, have been shown to target the nuclear transport machinery (for a complete review please see references “45,46”. Regulation of the nuclear transport machinery can facilitate major proviral outcomes: reduce competition with host factors for gene expression and prevent host antiviral responses. In some cases it was demonstrated that the up-regulation of the nuclear transport machinery promotes antiviral response. One example is VSV, which is an RNA virus that replicates in the cytoplasm and has the M protein that inhibits mRNA nuclear export, as mentioned above [38–42]. This effect prevents expression of host mRNAs that encode antiviral factors and makes the translation machinery available for expression of viral mRNAs. The mechanism of action of M protein is discussed elsewhere [45,46]. As a counterattack, the nuclear transport machinery can be up-regulated by antiviral cytokines, such as interferons, to antagonize the mRNA export block and promote antiviral response [47,48].
An important human pathogen that disrupts host mRNA nuclear export is influenza A virus. This is achieved by the action of the virus non-structural protein 1 (NS1), which targets the mRNA processing [49,50] and export machineries [42,44]. Regarding mRNA export, NS1 binds and forms an inhibitory complex with NXF1, NXT1 (p15), Rae1 and E1B-AP5, which restricts cellular mRNA export. Furthermore, NS1 down-regulates Nup98 levels, which further contributes to the mRNA export inhibition [44]. Once again, this effect prevents expression of antiviral factors and promotes viral replication. Given the importance of NS1-mediated host mRNA export block to favor viral replication, NS1 is seen as an attractive target for development of novel antiviral therapeutics and for probing novel cellular mechanisms. In the last few years, high throughput screens were performed to identify small molecules that could antagonize NS1-mediated inhibition of host gene expression [51,52]. In one screen, an NS1 antagonist was shown to rescue interferon expression by NS1 thereby restoring antiviral response [51]. As mentioned above, interferon can up-regulate mRNA export, which reverts viral-mediated export block [47,48]. This effect would lead to expression of mRNAs encoding antiviral factors, which would contribute to the restoration of antiviral response. In another screen, an antagonist of NS1 inhibited replication of VSV and influenza virus by inducing the expression of REDD1, an inhibitor of the mTORC1 pathway that is required for influenza virus replication [52,53]. The relationship between this mechanism and the effect of NS1 on nucleocytoplasmic trafficking is not yet known. However, another compound identified in the same screen revealed a new link between the pyrimidine biosynthesis pathway and mRNA nuclear export [42]. This compound, a quinoline carboxylic acid, directly inhibited the host enzyme dihydroorotate dehydrogenase (DHODH) [42,54] which is essential for de novo pyrimidine biosynthesis but not for pyrimidine synthesis via the salvage pathway. This specific and partial inhibition allows the use of DHODH inhibitors at concentrations that effectively prevent virus replication without causing cytotoxicity. The inhibition of DHODH led to increase in NXF1 levels, which reverted the mRNA export block mediated by both NS1 and VSV M proteins. mRNAs encoding antiviral factors were then released from the nuclear block by the up-regulation of NXF1, leading to inhibition of virus replication (Figure 4). The mechanism by which pyrimidine levels elevate expression of NXF1 is not known and hopefully this will be uncovered in future studies.
mRNA export in congenital contracture syndrome
Another interesting disease related to defect in mRNA export is human lethal congenital contracture syndrome-1 (LCCS1). It is caused by a proline-phenylalanine-glutamine peptide insertion in the coiled-coil domain of Gle1 [55], a key mRNA export factor. As mentioned above, Gle1 functions in mRNA export as an important regulator of the RNA-dependent ATPase activity of Dbp5, which mediates the key step for mRNA release at the cytoplasmic side of the NPC [3]. Disruption of Dbp5 function leads to nuclear accumulation of mRNAs and anchored mRNPs at the nuclear periphery, as shown by live cell imaging approaches [56]. Recently, the abnormal behavior of Gle1 mutant in LCCS1 was attributed to disruption of Gle1 oligomerization that impairs its function in mRNA export but not its role in translation [57]. These studies again demonstrate a crucial function for mRNA nuclear export in human development and disease (Figure 4).
In sum, these findings together point to the nuclear transport machinery as a key driver of various disease states when it is abnormally regulated. These results also reveal key pressure points within this machinery that can be targeted by compounds, which can both uncover novel molecular mechanisms as well as serve as starting points for drug development. Future studies on the connections between the nuclear transport machinery and different cellular conditions such as the cell cycle, signaling, and pathogens will likely reveal new facets of pathophysiology that can be useful to devise new therapeutic strategies.
Acknowledgements
We thank Angela Diehl for outstanding figure design. This work was supported by NIH R01AI079110, R01AI089539 and CPRIT RP121003-RP120718-P2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
• papers of special interest
- 1.Wente SR, Rout MP. The nuclear pore complex and nuclear transport. Cold Spring Harb. Perspect. Biol. 2010;2:a000562. doi: 10.1101/cshperspect.a000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Köhler A, Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nat. Rev. Mol. Cell Biol. 2007;8:761–773. doi: 10.1038/nrm2255. [DOI] [PubMed] [Google Scholar]
- 3.Natalizio BJ, Wente SR. Postage for the messenger: designating routes for nuclear mRNA export. Trends Cell Biol. 2013;23:365–373. doi: 10.1016/j.tcb.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fribourg S, Braun IC, Izaurralde E, Conti E. Structural basis for the recognition of a nucleoporin FG repeat by the NTF2-like domain of the TAP/p15 mRNA nuclear export factor. Mol. Cell. 2001;8:645–656. doi: 10.1016/s1097-2765(01)00348-3. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler EC, Ghosh S. Regulating inducible transcription through controlled localization. Sci. STKE. 2005;2005:re6. doi: 10.1126/stke.2842005re6. [DOI] [PubMed] [Google Scholar]
- 6.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 7.Chène P. Inhibiting the p53-MDM2 interaction: an important target for cancer therapy. Nat. Rev. Cancer. 2003;3:102–109. doi: 10.1038/nrc991. [DOI] [PubMed] [Google Scholar]
- 8.Reich NC, Liu L. Tracking STAT nuclear traffic. Nat. Rev. Immunol. 2006;6:602–612. doi: 10.1038/nri1885. [DOI] [PubMed] [Google Scholar]
- 9.Vandevyver S, Dejager L, Libert C. On the trail of the glucocorticoid receptor: into the nucleus and back. Traffic. 2012;13:364–374. doi: 10.1111/j.1600-0854.2011.01288.x. [DOI] [PubMed] [Google Scholar]
- 10.Shen A, Wang Y, Zhao Y, Zou L, Sun L, Cheng C. Expression of CRM1 in human gliomas and its significance in p27 expression and clinical prognosis. Neurosurgery. 2009;65:153–159. doi: 10.1227/01.NEU.0000348550.47441.4B. discussion 159–160. [DOI] [PubMed] [Google Scholar]
- 11.Turner JG, Dawson J, Sullivan DM. Nuclear export of proteins and drug resistance in cancer. Biochem. Pharmacol. 2012;83:1021–1032. doi: 10.1016/j.bcp.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner JG, Sullivan DM. CRM1-mediated nuclear export of proteins and drug resistance in cancer. Curr. Med. Chem. 2008;15:2648–2655. doi: 10.2174/092986708786242859. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y, Dong Y, Lin F, Zhao H, Shen Z, Chen P, Sun Y-J, Tang L-N, Zheng S-E. The expression of CRM1 is associated with prognosis in human osteosarcoma. Oncol. Rep. 2009;21:229–235. [PubMed] [Google Scholar]
- 14.Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, La Starza R, Diverio D, Colombo E, Santucci A, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N. Engl. J. Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 15.Colombo E, Bonetti P, Lazzerini Denchi E, Martinelli P, Zamponi R, Marine J-C, Helin K, Falini B, Pelicci PG. Nucleophosmin is required for DNA integrity and p19Arf protein stability. Mol. Cell. Biol. 2005;25:8874–8886. doi: 10.1128/MCB.25.20.8874-8886.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falini B, Gionfriddo I, Cecchetti F, Ballanti S, Pettirossi V, Martelli MP. Acute myeloid leukemia with mutated nucleophosmin (NPM1): any hope for a targeted therapy? Blood Rev. 2011;25:247–254. doi: 10.1016/j.blre.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Mutka SC, Yang WQ, Dong SD, Ward SL, Craig DA, Timmermans PBMWM, Murli S. Identification of nuclear export inhibitors with potent anticancer activity in vivo. Cancer Res. 2009;69:510–517. doi: 10.1158/0008-5472.CAN-08-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao C, Lu C, Chen L, Koty PP, Cobos E, Gao W. p53-Dependent anticancer effects of leptomycin B on lung adenocarcinoma. Cancer Chemother. Pharmacol. 2011;67:1369–1380. doi: 10.1007/s00280-010-1434-6. [DOI] [PubMed] [Google Scholar]
- 19. Lapalombella R, Sun Q, Williams K, Tangeman L, Jha S, Zhong Y, Goettl V, Mahoney E, Berglund C, Gupta S, et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood. 2012;120:4621–4634. doi: 10.1182/blood-2012-05-429506. In this study, abnormal nucleocytoplasmic distribution of tumor suppressors and reversal of this effect by a new class of Crm1 inhibitors was demonstrated in chronic lymphocytic leukemia.
- 20.Turner JG, Dawson J, Emmons MF, Cubitt CL, Kauffman M, Shacham S, Hazlehurst La, Sullivan DM. CRM1 Inhibition Sensitizes Drug Resistant Human Myeloma Cells to Topoisomerase II and Proteasome Inhibitors both In Vitro and Ex Vivo. J. Cancer. 2013;4:614–625. doi: 10.7150/jca.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azmi AS, Al-Katib A, Aboukameel A, McCauley D, Kauffman M, Shacham S, Mohammad RM. Selective inhibitors of nuclear export for the treatment of non-Hodgkin’s lymphomas. Haematologica. 2013;98:1098–1106. doi: 10.3324/haematol.2012.074781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt J, Braggio E, Kortuem KM, Egan JB, Zhu YX, Xin CS, Tiedemann RE, Palmer SE, Garbitt VM, McCauley D, et al. Genome-wide studies in multiple myeloma identify XPO1/CRM1 as a critical target validated using the selective nuclear export inhibitor KPT-276. Leukemia. 2013 doi: 10.1038/leu.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jeyasekharan AD, Liu Y, Hattori H, Pisupati V, Jonsdottir AB, Rajendra E, Lee M, Sundaramoorthy E, Schlachter S, Kaminski CF, et al. A cancer-associated BRCA2 mutation reveals masked nuclear export signals controlling localization. Nat. Struct. Mol. Biol. 2013;20:1191–1198. doi: 10.1038/nsmb.2666. In this study, it was found that the molecular mechanism by which BRCA2D2723H enhances tumorigenesis is specifically linked to the aberrant exposure of both its NES and the NES of its binding partner RAD51. This prevents nuclear accumulation of the BRCA2-RAD52 complex and promotes genomic instability.
- 24.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KLB. eIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3’UTR. J. Cell Biol. 2005;169:245–256. doi: 10.1083/jcb.200501019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KLB. eIF4E is a central node of an RNA regulon that governs cellular proliferation. J. Cell Biol. 2006;175:415–426. doi: 10.1083/jcb.200607020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assouline S, Culjkovic B, Cocolakis E, Rousseau C, Beslu N, Amri A, Caplan S, Leber B, Roy D-C, Miller WH, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood. 2009;114:257–260. doi: 10.1182/blood-2009-02-205153. [DOI] [PubMed] [Google Scholar]
- 27. Culjkovic-Kraljacic B, Baguet A, Volpon L, Amri A, Borden KLB. The oncogene eIF4E reprograms the nuclear pore complex to promote mRNA export and oncogenic transformation. Cell Rep. 2012;2:207–215. doi: 10.1016/j.celrep.2012.07.007. This study shows that overexpresstion of eIF4E enhances eIF4E-dependent mRNA export and modulates the levels of nucleoporin and transport factors at the cytoplasmic side of the nuclear pore complex.
- 28.Chakraborty P, Wang Y, Wei J-H, van Deursen J, Yu H, Malureanu L, Dasso M, Forbes DJ, Levy DE, Seemann J, et al. Nucleoporin levels regulate cell cycle progression and phase-specific gene expression. Dev. Cell. 2008;15:657–667. doi: 10.1016/j.devcel.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faria AMC, Levay A, Wang Y, Kamphorst AO, Rosa MLP, Nussenzveig DR, Balkan W, Chook YM, Levy DE, Fontoura BM a. The nucleoporin Nup96 is required for proper expression of interferon-regulated proteins and functions. Immunity. 2006;24:295–304. doi: 10.1016/j.immuni.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Tran DD, Saran S, Dittrich-Breiholz O, Williamson aJ, Klebba-Färber S, Koch a, Kracht M, Whetton aD, Tamura T. Transcriptional regulation of immediate-early gene response by THOC5, a member of mRNA export complex, contributes to the MCSF-induced macrophage differentiation. Cell Death Dis. 2013;4:879. doi: 10.1038/cddis.2013.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capelson M, Liang Y, Schulte R, Mair W, Wagner U, Hetzer MW. Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell. 2010;140:372–383. doi: 10.1016/j.cell.2009.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalverda B, Pickersgill H, Shloma VV, Fornerod M. Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell. 2010;140:360–371. doi: 10.1016/j.cell.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Köhler A, Hurt E. Gene regulation by nucleoporins and links to cancer. Mol. Cell. 2010;38:6–15. doi: 10.1016/j.molcel.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 34.Wang GG, Song J, Wang Z, Dormann HL, Casadio F, Li H, Luo J-L, Patel DJ, Allis CD. Haematopoietic malignancies caused by dysregulation of a chromatin-binding PHD finger. Nature. 2009;459:847–851. doi: 10.1038/nature08036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fontoura BM, Blobel G, Matunis MJ. A conserved biogenesis pathway for nucleoporins: proteolytic processing of a 186-kilodalton precursor generates Nup98 and the novel nucleoporin, Nup96. J. Cell Biol. 1999;144:1097–1112. doi: 10.1083/jcb.144.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yarbrough ML, White Ma, Fontoura BMa. Shaping the p53 response with nucleoporins. Mol. Cell. 2012;48:665–666. doi: 10.1016/j.molcel.2012.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singer S, Zhao R, Barsotti AM, Ouwehand A, Fazollahi M, Coutavas E, Breuhahn K, Neumann O, Longerich T, Pusterla T, et al. Nuclear Pore Component Nup98 Is a Potential Tumor Suppressor and Regulates Posttranscriptional Expression of Select p53 Target Genes. Mol. Cell. 2012;48:799–810. doi: 10.1016/j.molcel.2012.09.020. This study reveals a potential tumor supressor function for wild-type Nup98 by protecting specific p53-inducible mRNAs from degradation
- 38.Von Kobbe C, van Deursen JM, Rodrigues JP, Sitterlin D, Bachi A, Wu X, Wilm M, Carmo-Fonseca M, Izaurralde E. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol. Cell. 2000;6:1243–1252. doi: 10.1016/s1097-2765(00)00120-9. [DOI] [PubMed] [Google Scholar]
- 39.Faria Pa, Chakraborty P, Levay A, Barber GN, Ezelle HJ, Enninga J, Arana C, van Deursen J, Fontoura BM. VSV disrupts the Rae1/mrnp41 mRNA nuclear export pathway. Mol. Cell. 2005;17:93–102. doi: 10.1016/j.molcel.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 40.Her LS, Lund E, Dahlberg JE. Inhibition of Ran guanosine triphosphatase-dependent nuclear transport by the matrix protein of vesicular stomatitis virus. Science. 1997;276:1845–1848. doi: 10.1126/science.276.5320.1845. [DOI] [PubMed] [Google Scholar]
- 41.Enninga J, Levay A, Fontoura BMA. Sec13 shuttles between the nucleus and the cytoplasm and stably interacts with Nup96 at the nuclear pore complex. Mol. Cell. Biol. 2003;23:7271–7284. doi: 10.1128/MCB.23.20.7271-7284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang L, Das P, Schmolke M, Manicassamy B, Wang Y, Deng X, Cai L, Tu BP, Forst CV, Roth MG, et al. Inhibition of pyrimidine synthesis reverses viral virulence factormediated block of mRNA nuclear export. J. Cell Biol. 2012;196:315–326. doi: 10.1083/jcb.201107058. This paper uncovers a link between pyrimidine metabolism and regulation of mRNA nuclear export by viral proteins. It shows that DHODH inhibitors release mRNA export block induced by viral proteins.
- 43.Chakraborty P, Seemann J, Mishra RK, Wei J-H, Weil L, Nussenzveig DR, Heiber J, Barber GN, Dasso M, Fontoura BM a. Vesicular stomatitis virus inhibits mitotic progression and triggers cell death. EMBO Rep. 2009;10:1154–1160. doi: 10.1038/embor.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satterly N, Tsai P-L, van Deursen J, Nussenzveig DR, Wang Y, Faria Pa, Levay A, Levy DE, Fontoura BMa. Influenza virus targets the mRNA export machinery and the nuclear pore complex. Proc. Natl. Acad. Sci. U. S. A. 2007;104:1853–1858. doi: 10.1073/pnas.0610977104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yarbrough ML, Mata M a, Sakthivel R, Fontoura BMA. Viral Subversion of Nucleocytoplasmic Trafficking. Traffic. 2013 doi: 10.1111/tra.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuss SK, Mata Ma, Zhang L, Fontoura BMa. Nuclear imprisonment: viral strategies to arrest host mRNA nuclear export. Viruses. 2013;5:1824–1849. doi: 10.3390/v5071824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castelló A, Izquierdo JM, Welnowska E, Carrasco L. RNA nuclear export is blocked by poliovirus 2A protease and is concomitant with nucleoporin cleavage. J. Cell Sci. 2009;122:3799–3809. doi: 10.1242/jcs.055988. [DOI] [PubMed] [Google Scholar]
- 48.Enninga J, Levy DE, Blobel G, Fontoura BMa. Role of nucleoporin induction in releasing an mRNA nuclear export block. Science. 2002;295:1523–1525. doi: 10.1126/science.1067861. [DOI] [PubMed] [Google Scholar]
- 49.Nemeroff ME, Barabino SM, Li Y, Keller W, Krug RM. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3’end formation of cellular pre-mRNAs. Mol. Cell. 1998;1:991–1000. doi: 10.1016/s1097-2765(00)80099-4. [DOI] [PubMed] [Google Scholar]
- 50.Chen Z, Li Y, Krug RM. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3’-end processing machinery. EMBO J. 1999;18:2273–2283. doi: 10.1093/emboj/18.8.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basu D, Walkiewicz MP, Frieman M, Baric RS, Auble DT, Engel DA. Novel influenza virus NS1 antagonists block replication and restore innate immune function. J. Virol. 2009;83:1881–1891. doi: 10.1128/JVI.01805-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mata MA, Satterly N, Versteeg GA, Frantz D, Wei S, Williams N, Schmolke M, Peña-Llopis S, Brugarolas J, Forst CV, et al. Chemical inhibition of RNA viruses reveals REDD1 as a host defense factor. Nat. Chem. Biol. 2011;7:712–719. doi: 10.1038/nchembio.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.König R, Stertz S, Zhou Y, Inoue A, Hoffmann H-H, Bhattacharyya S, Alamares JG, Tscherne DM, Ortigoza MB, Liang Y, et al. Human host factors required for influenza virus replication. Nature. 2010;463:813–817. doi: 10.1038/nature08699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das P, Deng X, Zhang L, Roth MG, Fontoura BMA, Phillips MA, De Brabander JK. SAR Based Optimization of a 4-Quinoline Carboxylic Acid Analog with Potent Anti-Viral Activity. [Internet] ACS Med. Chem. Lett. 2013;4:517–521. doi: 10.1021/ml300464h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nousiainen HO, Kestilä M, Pakkasjärvi N, Honkala H, Kuure S, Tallila J, Vuopala K, Ignatius J, Herva R, Peltonen L. Mutations in mRNA export mediator GLE1 result in a fetal motoneuron disease. [Internet] Nat. Genet. 2008;40:155–157. doi: 10.1038/ng.2007.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hodge Ca, Tran EJ, Noble KN, Alcazar-Roman AR, Ben-Yishay R, Scarcelli JJ, Folkmann AW, Shav-Tal Y, Wente SR, Cole CN. The Dbp5 cycle at the nuclear pore complex during mRNA export I: dbp5 mutants with defects in RNA binding and ATP hydrolysis define key steps for Nup159 and Gle1. Genes Dev. 2011;25:1052–1064. doi: 10.1101/gad.2041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Folkmann AW, Collier SE, Zhan X, Ohi MD, Wente SR. Gle1 Functions during mRNA Export in an Oligomeric Complex that Is Altered in Human Disease. Cell. 2013;155:582–593. doi: 10.1016/j.cell.2013.09.023. This paper shows that the mutation in Gle1 that causes human lethal congenital contracture syndrome-1 (LCCS1) disrupts its ability to oligomerize. This effect impairs Gle1 function in mRNA export but not in translation.