Abstract
The active metabolite of vitamin D, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), suppresses the proliferation while promoting the differentiation of keratinocytes through the vitamin D receptor (VDR). β-catenin, on the other hand, promotes proliferation and blocks epidermal differentiation, although it stimulates hair follicle differentiation. In intestinal epithelia VDR binds β-catenin and blocks its proliferative effects. In this study we investigated the role of 1,25(OH)2D3 /VDR on β-catenin regulated gene transcription during keratinocyte proliferation and differentiation. 1,25(OH)2D3 suppressed promoter reporter activity driven by synthetic and natural TCF/β-catenin response elements. Over-expression of VDR further suppressed these TCF/β-catenin promoter activities. 1,25(OH)2D3 also suppressed the mRNA expression of the β-catenin regulated gene Gli1 through VDR. These data was consistent with our previous observations that VDR silencing resulted in keratinocyte hyperproliferation with increased expression of Gli1 in vitro, whereas VDR null skin showed hyperproliferation in vivo. In contrast, 1,25(OH)2D3 induced expression of another β-catenin regulated gene, PADI1, important for both epidermal and hair follicle differentiation. Deletion of VDR resulted in defects in hair differentiation in vivo, with decreased expression of β-catenin regulated hair differentiation genes such as PADI1, hair keratin KRT31 and calcium binding protein S100a3. These genes possess vitamin D response elements (VDRE) adjacent to TCF/β-catenin response elements and are regulated by both VDR and β-catenin signaling. Therefore, we propose that VDR and β-catenin interact reciprocally to promote VDR stimulation of genes involved with differentiation that contain both VDR and β-catenin response elements while inhibiting β-catenin stimulation of genes involved with proliferation. Thus the major finding of this study is that while 1,25(OH)2D3/VDR inhibits the actions of β-catenin to promote keratinocyte proliferation, 1,25(OH)2D3/VDR promotes the ability of β-catenin to stimulate hair follicle differentiation.
Keywords: VDR, β-catenin, keratinocyte, proliferation, differentiation
1. Introduction
Keratinocyte proliferation and differentiation are regulated by several factors including vitamin D and Wnt/β-catenin signaling. The active metabolite of vitamin D, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), suppresses the proliferation while promoting the differentiation of keratinocytes through the vitamin D receptor (VDR). 1,25(OH)2D3 binds to the vitamin D receptor (VDR) and activates transcription of target genes through its binding to genomic sites, termed VDR response elements (VDREs) [1]. β-catenin, on the other hand, promotes proliferation and blocks epidermal differentiation, although it stimulates hair follicle differentiation during anagen induction. Activation of Wnt/β-catenin signaling stimulates translocation of β-catenin to the nucleus where it activates transcription of target genes through its binding to genomic sites termed TCF/LEF (TCF) response elements together with TCF/LEF proteins [2]. Since the molecular interaction between VDR and β-catenin was revealed, mutual regulation between VDR and β-catenin signaling has been proposed [3]. In colon cancer cells, VDR has been shown to bind to β-catenin, and reduce its transcriptional activity in a ligand dependent fashion [4]. It has also been reported that 1,25(OH)2D3 stimulates β-catenin transcriptional activity through VDR, and putative VDREs were identified in a number of β-catenin target genes in the proximal or distal side of TCF response elements suggesting interaction of VDR and β-catenin at the transcriptional level [5]. A good example of this is the ability of VDR to function as an effecter for β-catenin regulated hair differentiation [5]. Here, we further investigated the role of VDR on β-catenin signaling and discovered a reciprocal role of VDR on β-catenin signaling in keratinocytes.
The role of 1,25(OH)2D3/VDR on β-catenin transcriptional activity was examined by using a β-catenin promoter reporter (TOP glow) in primary keratinocytes. The mRNA levels of β-catenin target genes such as Gli1 that possess TCF/LEF response elements and promote proliferation were measured. The mRNA levels of differentiation genes including peptidyl arginine deiminase 1 (PADI1), PADI3, calcium binding protein S100a3 and Dlx2 that possess putative VDRE adjacent or distal to TCF response elements in their promoters were also evaluated [5]. The role of VDR was investigated by manipulating VDR levels. VDR was either over-expressed or blocked in keratinocyte cultures. The in vivo role of VDR had previously been examined using VDR null mice that have defects in hair differentiation resulting in alopecia as well as hyper-proliferation of the epidermal and hair follicle keratinocytes [6]. Similarly the in vitro role of VDR had previously been examined by VDR silencing that caused hyper-proliferation of keratinocytes and differentiation defects [7]. In this study the expression levels of β-catenin regulated differentiation genes in VDR null mice were evaluated and compared to control mice in the context of our previously reported results. Our data demonstrated a reciprocal effect of 1,25(OH)2D3/VDR on β-catenin transcriptional activity depending on whether the genes encoded proteins involved with keratinocyte proliferation or with differentiation [5].
2. Material and methods
2.1 Keratinocyte culture
Human keratinocytes were isolated from neonatal-human foreskin and grown in keratinocyte growth medium 154CF (KGM, Cascade) as described [7]. Second passage keratinocytes were cultured with keratinocyte growth medium containing 0.03mM calcium to maintain them in a proliferative state. They were treated with EtOH or 10−8M 1,25(OH)2D3.
2.2 VDR blockage by Cre-lox strategy in mouse keratinocyte
Expression of VDR in mouse keratinocytes is deleted by Cre-lox strategies. Mouse keratinocytes were isolated from floxed VDR mice and cultured according to the manufacture’s protocol (Cellntech). They were cultured at low density in PCT epidermal keratinocyte culture medium (CnT07) containing 0.07mM calcium until colonies with self-renewal capability formed. Cells were then maintained in extended culture until they were naturally immortalized to show significant proliferation potential. Cells were frozen, then propagated for each experiment to obtain consistent properties. VDR was removed by infecting them with 2–5 pfu/cell of adenovirus containing Cre recombinase. Cells were also infected by the same titer of control DNR adenovirus. The titer of Cre adenovirus was determined to obtain more than 90% removal of VDR expression by QPCR analysis.
2.3 QPCR analysis
The levels of mRNA expression were evaluated by real time PCR (QPCR) as described [7]. Total RNA was isolated from cultured keratinocytes, or from back skin of VDR null and littermate control mice using RNeasy RNA purification kit (Qiagen, Valencia, CA), and cDNA was prepared using a reverse transcription kit (Applied Biosystems, Foster City, CA). The mRNA expression was measured using SYBR green master mix (Applied Biosystems) with QPCR instruments 7300 (Applied Biosystems). Relative expression of these genes compared with mitochondrial ribosomal protein L19 (keratinocyte) or GAPDH (mouse skin) was calculated. Primers for QPCR analysis were designed using Primer Express (Applied Biosystem)[8][7].
2.4 Promoter reporter assay
Human keratinocytes were transfected with luciferease constructs linked to a natural TCF promoter derived from Cyclin D1 (Cyclin D1 TK promote) or synthetic TCF promoter (TOPglow), or its mutated control (FOPglow). The cells were cotransfected with CMV hVDR vector (+) to overexpress VDR, or with control pcDNA vector (−). Proliferating keratinocytes maintained with 0.07mM calcium (6 wells) were transfected using 6.4 ul Enhancer, 8–20 μl Effectene, 1 ug cDNA of promoter reporter, 100 ng cotransfectant, and 32 ng control vector of Renilla luciferase-thymidine kinase promotor (pRL-TK) (Promega Corp., Madison, WI) according to the manufacturer’s protocol (QIAGEN, Valencia, CA). Cells were then treated with 1,25(OH)2D3 or EtOH overnight. The firefly and renilla luciferase activities were measured by dual luciferase kit (Promega), and ratios of firefly to renilla luciferase activity were calculated (relative luciferease).
3. Results and Discussion
3.1 1,25(OH)2D3and VDR suppressed transcriptional activity of β-catenin/TCF
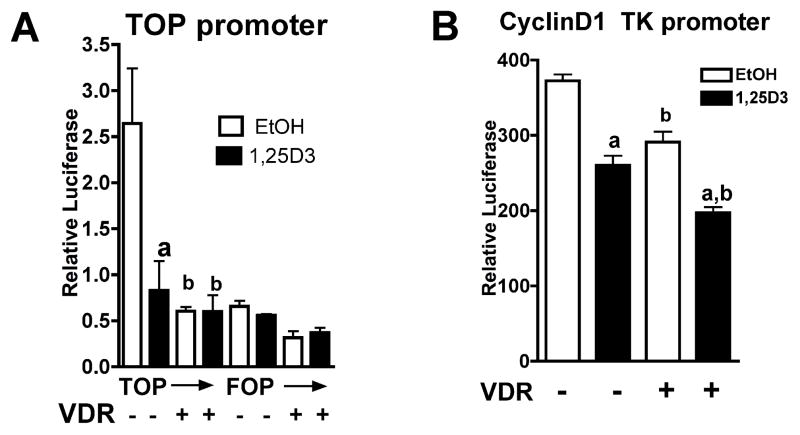
We first investigated the role of VDR on the transcriptional activity of β-catenin signaling. Keratinocytes were transfected with a luciferease construct driven by tandem repeats of synthetic TCF response elements (TOPglow), and its point mutant (FOPglow). Other keratinocytes were transfected with a reporter construct linked to tandem repeats of Cyclin D1 derived TCF response elements (TK-CyclinD1). The cells were cotransfected with CMV hVDR vector (+) to overexpress VDR, or control pcDNA vector (−). The cells were then treated with 1,25(OH)2D3 or EtOH, and the luciferase activity was measured. 1,25(OH)2D3 decreased transcriptional activity of TOP glow but not FOP glow (Fig. 1A). 1,25(OH)2D3 also decreased TK-Cyclin D1 activity (Fig. 1B). When VDR is over-expressed, transcriptional activity of both TOP glow and TK-Cyclin D1 were further suppressed. These results together with previous reports [3, 4] indicated that 1,25(OH)2D3 and VDR regulate β-catenin signaling through TCF mediated transcriptional regulation in keratinocytes (Fig 1 A and B). These results are consistent with our previous observation that 1,25(OH)2D3 and VDR suppressed mRNA expression of β-catenin regulated Cyclin D1 and Gli1 in human keratinocytes [7].
Fig. 1. 1,25(OH)2D3and VDR suppressed transcriptional activity of TCF promoter reporters.
Human keratinocytes were transfected with constructs where firefly luciferase is linked to tandem repeats of synthetic TCF/LEF elements (TOP glow) or its mutant FOP glow (A), or natural TCF/LEF elements derived from the Cyclin D1 promoter (B). Cells were cotransfected with control promoter pRL-TK linked to renilla luciferase. VDR was over-expressed by cotransfection with CMV hVDR vector (VDR +) or cells were treated with control pcDNA vector as control (−). The cells were then treated with 1,25(OH)2D3 or EtOH. The dual luciferase activities were measured, and relative luciferase activity was shown by calculating ratios of firefly to renilla luciferase activity. The error bars enclose mean +/− SD of triplicate cultures. Statistical significance compared with EtOH controls is shown by a, and with no VDR group is shown by b ( p<0.05)(A, B). Statistical significance compared to no VDR group with and without 1,25(OH)2D3 is shown by ab (B) (p<0.05).
3.2 1,25(OH)2D3 represses mRNA expression of the β-catenin target gene of Gli1 but induces the β-catenin target gene PADI1 in vitro
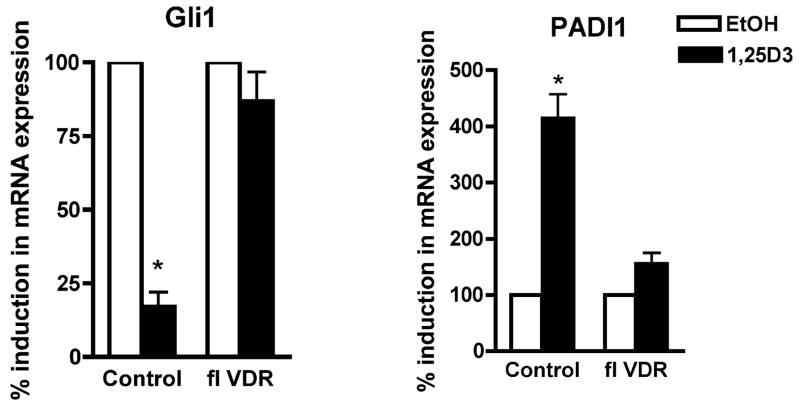
Next, we evaluated how 1,25(OH)2D3 and VDR influence β-catenin regulated genes to control epidermal proliferation and differentiation. We developed a mouse keratinocyte system, where expression of VDR is manipulated by Cre-lox strategy. Mouse keratinocytes were first isolated from floxed VDR mice and cultured until self-renewing colonies arise. Cells were maintained in long term cultures until they are naturally immortalized. Floxed VDR cells were infected by adenovirus carrying cre recombinase (Cre) to removed the VDR gene. The same cells were infected by the same titer of control adenovirus (control). Gene expression of Cre infected cells was compared to that of control cells by using QPCR. VDR expression was reduced by Cre to less than 10 % of control. Cells were then treated with 1,25(OH)2D3. The expression of Gli1 was repressed by 1,25(OH)2D3 in the control cells but not in the cre treated cells indicating that 1,25(OH)2D3 repressed Gli1 through VDR. In contrast to suppression of the proliferation associated gene Gli1, 1,25(OH)2D3 induced the mRNA expression of the β-catenin target gene PADI1 in the same keratinocytes. When VDR was deleted, 1,25(OH)2D3 action was aborted again indicating its action through VDR (Fig. 2). These results indicate that 1,25(OH)2D3 and VDR repress Gli1, a β-catenin regulated gene associated with proliferation but induce PADI1, a β-catenin regulated gene associated with epidermal and hair follicle differentiation.
Fig. 2. 1,25(OH)2D3 repressed mRNA expression of Gli1 but induced PADI1 through VDR.
Mouse keratinocytes were isolated from floxed VDR mice. Cells were either infected by DNR adenovirus (control) or Cre adenovirus to block VDR expression (fl VDR). They were then treated with either EtOH (open bars) or 1,25(OH)2D3 (closed bars). Total RNA was isolated from using RNeasy RNA purification kit (Qiagen, Valencia, CA). The mRNA levels of Gli1 and PADI1 were measured by QPCR. Relative expression of these genes compared with mitochondrial ribosomal protein L19 was calculated. The mRNA levels were normalized to control gene L19, and relative expression to EtOH control group was expressed as a percentage (%). The fold changes compared with EtOH vehicle were calculated. The data are shown as mean ± SD of three different preparations of keratinocytes. Statistical significance (* p<0.05) compared with EtOH controls is shown with asterisks.
3.3 VDR ablation reduces β-catenin target genes involved in hair differentiation in vivo
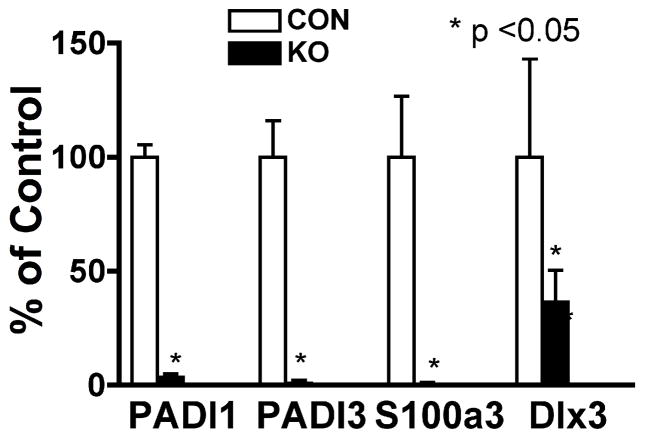
Next, we examined the role of VDR on β-catenin signaling in vivo in VDR null mice. These mice develop defects in hair follicle differentiation causing complete alopecia as described previously [6]. The back skin was excised from VDR null mice (KO) and littermate controls (CON) at 11 wk, when signaling components of the Wnt/b-catenin pathway, Lef1 and Wnt3a, start to change [9]. At this time histological abnormalities caused by VDR ablation including utricle and cyst formation were obvious but mice were still in the early stages of hair loss. The mRNA levels of β-catenin regulated genes of PADI1, PADI3, S100A3, and Dlx3 essential for hair follicle differentiation were evaluated. Their expression was markedly decreased in VDR null mice compared to littermate control mice, suggesting that VDR ablation may reduce β-catenin regulated differentiation associated target genes in vivo (Fig. 3). although these changes also reflect the absence of normal hair follicles. Thus it is not clear whether the changes in expression of these genes is causal or secondary to the loss of hair follicle cycling.
Fig. 3. mRNA expression of β-catenin regulated genes to control hair follicle differentiation were reduced in VDR KO mice.
Total RNA was isolated from the whole skin of littermate controls (open bars) and VDR null mice (closed bars) at 11 weeks of age. The mRNA levels of PADI1, PADI3, S100A3, and Dlx3 were measured by QPCR. Relative expression of these genes compared to GAPDH was calculated. Relative expression to control mice was expressed as a percentage (%). Data are represented as mean and standard deviation (SD) of three mice in each group. All data were analyzed by t-test, and statistical significance compared to control group is shown with asterisks (*P <0.05).
3.4 Reciprocal role of 1,25(OH)2D3/VDR on β-catenin/TCF transcriptional regulation
In this study, we showed that 1,25(OH)2D3/VDR suppressed the transcriptional activity of β-catenin, and repressed mRNA expression of Gli1. These results indicate that 1,25(OH)2D3/VDR exerts biological action through β-catenin by inhibiting its transcriptional regulation of proliferation associated genes. In contrast, 1,25(OH)2D3/VDR stimulates β-catenin signaling to induce keratinocyte differentiation through stimulation of the transcription of hair differentiation genes. Taken together with our former observations that VDR deletion results in hyperproliferation and differentiation defects [6] [7], we propose a model suggesting a reciprocal role for VDR in regulating β-catenin target genes (Fig. 4). 1,25(OH)2D3/VDR may compete with TCF/LEF proteins for β-catenin to repress transcription of genes involved in proliferation. In contrast, 1,25(OH)2D3 through VDR may stimulate the transcription of genes to induce differentiation by cooperatively binding to adjacent VDRE sites to TCF/LEF sites (Fig. 4).
Fig. 4. A reciprocal role of 1,25(OH)2D3/VDR in β-catenin/TCF target genes.
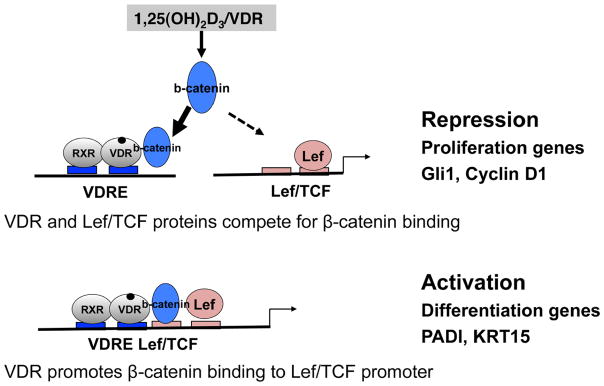
A model to show 1,25(OH)2D3/VDR repression of β-catenin/TCF stimulation of genes involved with proliferation as VDR and TCF/LEF proteins compete for β-catenin binding (upper panel). In contrast, VDR activates β-catenin to promote keratinocyte differentiation by promoting β-catenin binding to the TCF/LEF response elements in genes with adjacent VDREs that mediate differentiation (lower panel).
Highlights.
1,25(OH)2D3/VDR inhibits β-catenin mediated transcription to promote keratinocyte proliferation.
1,25(OH)2D3/VDR promotes β-catenin transcription to stimulate hair differentiation.
VDR and β-catenin interact reciprocally to regulate keratinocytes.
Acknowledgments
We thank Ms Sally Pennypacker for assistance with the keratinocyte cultures, and Chak K Fong for technical assistance. We are also grateful to Dr Z. Xie, S. Yamada, and Dr Amano for their support and discussion. This work was supported by the National Institutes of Health grant R01 AR050023 (DDB), DOD grant CA110338 (DDB), and a VA Merit Review (DDB).
Abbreviations
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
- VDR
vitamin D receptor
- VDRE
vitamin D response element
- Gli 1
glioma-associated oncogene homolog 1
- PADI 1
peptidyl arginine deiminase 1
- TCF
T-cell factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13(3):325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 2.Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22(17):2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, Zinser G, Valrance M, Aranda A, Moras D, Norman A, Welsh J, Byers SW. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Molecular cell. 2006;21(6):799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 4.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Munoz A. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. The Journal of cell biology. 2001;154(2):369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM. The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS ONE. 2008;3(1):e1483. doi: 10.1371/journal.pone.0001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikle DD, Elalieh H, Chang S, Xie Z, Sundberg JP. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207(2):340–353. doi: 10.1002/jcp.20578. [DOI] [PubMed] [Google Scholar]
- 7.Oda Y, Chalkley RJ, Burlingame AL, Bikle DD. The transcriptional coactivator DRIP/mediator complex is involved in vitamin D receptor function and regulates keratinocyte proliferation and differentiation. The Journal of investigative dermatology. 2010;130(10):2377–2388. doi: 10.1038/jid.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oda Y, Hu L, Bul V, Elalieh H, Reddy JK, Bikle DD. Coactivator MED1 ablation in keratinocytes results in hair-cycling defects and epidermal alterations. The Journal of investigative dermatology. 2012;132(4):1075–1083. doi: 10.1038/jid.2011.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teichert A, Elalieh H, Bikle D. Disruption of the hedgehog signaling pathway contributes to the hair follicle cycling deficiency in Vdr knockout mice. J Cell Physiol. 2010;225(2):482–489. doi: 10.1002/jcp.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]