Abstract
Background
Sedentary behavior is associated with adverse health effects. To prevent sedentary behavior and limit health risks, insights into associated determinants are essential. Sedentary behavior should be viewed as a distinct health behavior, therefore its determinants should be independently identified.
Purpose
This study examines the prospective associations between a wide-range of midlife determinants and objectively measured sedentary time in old age.
Methods
Data from 565 participants (aged 73–92 years) of the AGESII-Reykjavik Study were used. Participants wore an accelerometer (ActiGraph GT3X) on the right hip for 7 consecutive days. On average 31 years earlier (during midlife) demographic, socioeconomic, lifestyle and biomedical factors were collected. Linear regression models were used to examine prospective associations between midlife determinants and sedentary time (<100 counts per minute) in old age.
Results
After adjustment for sex, age, follow-up time, minutes of moderate to vigorous physical activity, BMI, health status, mobility limitation and joint pain in old age, the midlife determinants not being married, primary education, living in a duplex or living in an apartment (vs. villa), being obese and having a heart disease were associated with, respectively, on average 15.3, 12.4, 13.5, 13.3, 21.8, 38.9 sedentary minutes more per day in old age.
Conclusions
This study shows that demographic, socioeconomic and biomedical determinants in midlife were associated with considerably more sedentary time per day in old age. These results can indicate the possibility of predicting sedentariness in old age, which could be used to identify target groups for prevention programs reducing sedentary time in older adults.
Keywords: Accelerometry, Sedentary Lifestyle, Older Adults, Longitudinal Studies, Socioeconomic Factors, Biomedical Factors
INTRODUCTION
During the last years there has been growing interest in sedentary behavior as a risk factor for adverse health effects, independent of physical activity. Sedentary behavior, such as sitting, lying down, watching TV and using the computer, has been associated with mortality(8, 20, 21, 23, 29) and metabolic and cardiovascular risk factors(2, 9, 13, 18, 19, 32).
Sedentary time increases with age and older adults spend up to 80% of their waking time being sedentary(1, 6, 11, 24). To prevent this lifestyle and limit its health risks, it is important to identify determinants which contribute to a (highly) sedentary lifestyle among older adults.
Determinants of physical activity and in particular moderate to vigorous physical activity (MVPA), such as health status and self-efficacy, have been studied extensively(3). However, sedentary behavior should be viewed as a distinct health behavior which differs from a lack of MVPA, and therefore its determinants should be independently identified. To date, only a few studies have prospectively examined the determinants of self-reported (7, 25) or objectively measured sedentary time(10, 14). The number of determinants investigated in these studies were limited and the study populations were middle aged.
To develop prevention programs for sedentary behavior, it is of importance to study a broad spectrum of determinants over the lifetime that affects sedentary time. Therefore, this study examines over a period of three decades the prospective associations between a wide-range of midlife determinants (demographic, socioeconomic, lifestyle, biomedical) and objectively measured sedentary time in old age, in a subsample of the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study.
METHODS
Study population
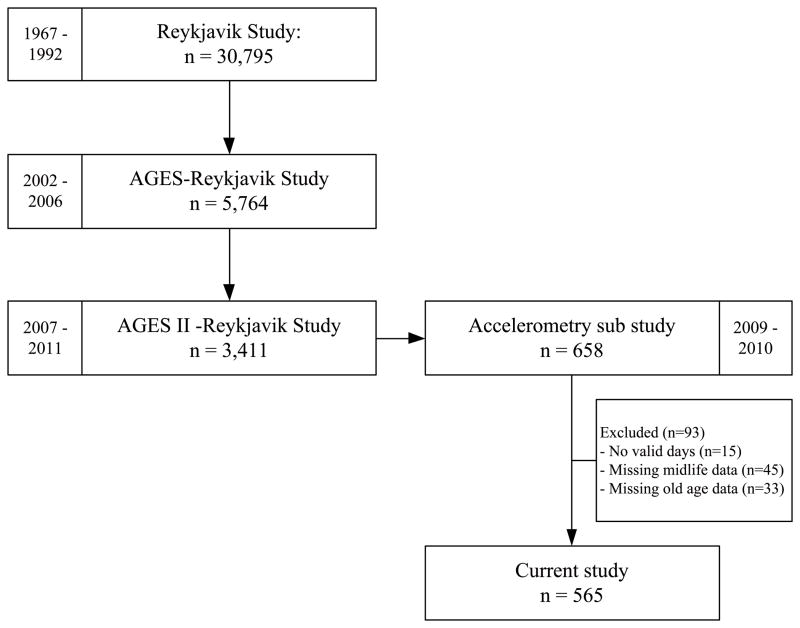
For this study a subsample of the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study cohort was used. The AGES-Reykjavik Study originates from the Reykjavik Study, which was established in 1967 and comprised a random sample of 30,795 participants born in 1907–1935 and residing in Reykjavik, Iceland(17). Measurements for the Reykjavik Study were conducted in the period between 1967 and 1992. From 2002 until 2006 the measurements for the AGES-Reykjavik Study took place in 5,764 participants of the original cohort(17). Measurements for the follow-up AGESII-Reykjavik Study took place in 2007–2011 among 3,411 participants. From April 2009 to June 2010, an accelerometry sub study was performed in which 658 participants who did not have severe cognitive dysfunction (MMSE≤20) were asked to wear an accelerometer for seven consecutive days(1). After excluding participants who did not record at least one valid day (at least 10h of monitoring) (n=15) or missing data of the measurements during the Reykjavik Study, AGES-Reykjavik Study or AGESII-Reykjavik Study (n=78), a total of 565 participants (aged 73 to 92 years) were included in the current analyses (Figure 1). All participants gave written informed consent.
Figure 1.
Flow of participants
The AGES Reykjavik Study was approved by the institutional review boards of the National Institute on Aging, the National Bioethics Committee (VSN: 00-063) and the Data Protection Authority.
Measurements
Sedentary time in old age
Sedentary time was assessed using an accelerometer (ActiGraph GT3X, Ft. Walton Beach, FL, USA), which was attached to a belt and was worn on the right hip. Participants were instructed to wear the accelerometer for seven consecutive days and only remove the monitor before going to bed and during showering, bathing, swimming and other water activities. The triaxial ActiGraph recorded movement on the vertical, anteroposterior and mediolateral axes. Raw accelerometry data were processed and averaged by minute using customized software written in MATLAB R2006a (The MathWorks, Inc.; Natick MA, USA) to obtain relevant outcome variables. Non-wear time was defined as any interval ≥60 consecutive minutes of zero counts in all three axes, in which a period up to two minutes of nonzero counts under 100 in the vertical axis was allowed(1). A valid day was defined as at least 10h of monitoring. The outcome measure used in this analysis was the percentage of wear time minutes per day spent sedentary (0–99 counts per minute (cpm) in the vertical axis).
Midlife determinants
A variety of midlife determinants collected as part of the Reykjavik Study were used for the analysis, including demographic characteristics, socioeconomic factors, lifestyle factors, and biomedical factors. Data were collected by questionnaires (detailed medical history, health-related behavior), physical examinations, and laboratory tests.
Demographic characteristics of sex, age and marital status were derived from a questionnaire. Marital status was categorized as married/not married, with the categories single, widow(er), divorced and separated collapsed into not married, because of the small numbers in these categories. Due to the large number of individuals without this information (n=178), this group was included as separate category.
Socioeconomic factors included level of education, housing type, and occupation, which were derived from a questionnaire. Level of education was categorized into college/university, secondary and primary. Housing type was categorized into villa, duplex, and apartment; and occupation was categorized into professional work, light work, manual labor, and homemaker.
Lifestyle factors included smoking status, physical activity, active commuting, and occupation activity, and were derived from a questionnaire. These factors were categorized into never smoker, previous smoker and current smoker; active/inactive; active commuting/not active commuting; and for occupational activity ‘on the move’, standing, and sitting respectively.
Biomedical factors included body mass index (BMI), weight status, hypertension, cholesterol levels, triglyceride levels, heart disease, type 2 diabetes, arthritis, lung disease and lung function (FVC, FEV1, FEV1/FVC ratio). Height and weight were measured during the physical examinations and BMI was calculated as kg/m2. Weight status was categorized as BMI <25, BMI 25–30 and BMI ≥30. Hypertension was defined as self-reported hypertension and/or a systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg(5). Cholesterol and triglyceride levels in blood were obtained by laboratory tests. The presence of heart disease, diabetes, arthritis and lung disease were derived from a questionnaire about whether participants had ever sought a doctor or were treated in a hospital for a disease. Lung function was obtained by spirometry. The spirometric reference values were calculated using the NHANES III/Hankinson equations(16) and the FEV1/FVC ratio was calculated using the observed values.
Covariates
Covariates were measured by means of questionnaires and accelerometry in old age as part of the AGES II-Reykjavik Study and included sex, age, follow-up time, daily minutes of MVPA (MVPA≥2020 cpm), BMI (kg/m2), health status, mobility limitation, and joint pain. Follow-up time was calculated as the time between midlife measurements and AGES II measurements. Health status was defined as the number of the following conditions: cancer (measured as part of AGES I-Reykjavik Study), arthritis, osteoporosis, Parkinson’s disease, heart disease, asthma, chronic obstructive pulmonary disease (COPD), and depression. Mobility limitation was defined as any difficulty walking 500m or climbing ten steps without resting. Joint pain was defined as pain, aching or stiffness in one or more of the following joints: back (upper, middle, under), hips, knees, ankles, and toes.
Statistical analysis
Descriptive characteristics were summarized as mean and SD or percentages. Chi-square tests and analysis of variance (ANOVA) were used to examine differences between men and women in the categorical and continuous midlife determinants. Gender differences in the accelerometry variables were examined using ANOVA. Linear regression analysis was used to study whether the midlife determinants predicted the amount of sedentary time in old age. For each determinant the analyses were run separately, with the determinant as the exposure variable and the percentage of minutes per day spent sedentary as the outcome. All models were adjusted for sex, age and follow-up time (Model 1). Model 2 was additionally adjusted for MVPA (min/day) to understand whether the midlife determinants were related to sedentary time in old age independent of MVPA. Model 3 was additionally adjusted for BMI, health status, mobility limitation and joint pain in old age to exclude the effects of these conditions on sedentary time. Interactions of all midlife determinants with gender were not statistically significant (p>0.10) and therefore the main analyses were conducted for men and women together. All analyses were conducted with IBM SPSS Statistics 20.0. Significance level was set at <0.05.
RESULTS
Descriptive characteristics of the study sample are presented in Table 1. In midlife, women were significantly older than men and were less often married. Women also had a lower educational level, lived more often in an apartment, were less often employed and reported more being ‘on the move’ during work than men. Women smoked less than men, had a lower BMI, were less overweight, had less hypertension and lower triglyceride levels (all p<0.05). Self-reported physical activity and active commuting did not significantly differ between men and women.
Table 1.
Descriptive characteristics of the study sample in midlife
| Determinants | total | men | women | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Demographic | Sex (n) | 565 | 222 | (39.3%) | 343 | (60.7%) | ||
| Age (years) | 48.8 | (6.2) | 46.9 | (5.2) | 50.0 | (6.4) | <0.001 | |
| Marital status (%) | <0.001 | |||||||
| married | 60.7 | 81.1 | 47.5 | |||||
| not married | 7.8 | 5.9 | 9.0 | |||||
| missing | 31.5 | 13.0 | 43.5 | |||||
| Socio-economic | Level of education (%) | <0.001 | ||||||
| college/university | 20.5 | 31.1 | 13.7 | |||||
| secondary | 50.1 | 50.0 | 50.1 | |||||
| primary | 29.4 | 18.9 | 36.2 | |||||
| Housing type (%) | 0.023 | |||||||
| villa | 33.6 | 36.9 | 31.5 | |||||
| duplex | 21.1 | 24.8 | 18.7 | |||||
| apartment | 45.3 | 38.3 | 49.8 | |||||
| Occupation group (%) | <0.001 | |||||||
| professional work | 17.9 | 36.0 | 6.1 | |||||
| light work | 24.4 | 27.9 | 22.2 | |||||
| manual labor | 14.5 | 33.3 | 2.3 | |||||
| housemaker | 43.2 | 2.8 | 69.4 | |||||
| Lifestyle | Smoking status (%) | <0.001 | ||||||
| never smoker | 44.3 | 32.4 | 51.9 | |||||
| previous smoker | 19.6 | 21.2 | 18.7 | |||||
| current smoker | 36.1 | 46.4 | 29.4 | |||||
| Physical activity (%active) | 28.0 | 27.9 | 28.0 | 0.988 | ||||
| Active commuting (%active) | 11.3 | 8.1 | 13.4 | 0.052 | ||||
| Occupation activity (%) | <0.001 | |||||||
| on the move | 65.1 | 40.1 | 81.3 | |||||
| standing | 7.4 | 16.2 | 1.7 | |||||
| sitting | 27.5 | 43.7 | 16.9 | |||||
| Biomedical | BMI (kg/m2) | 25.0 | (3.5) | 25.4 | (3.3) | 24.7 | (3.6) | 0.009 |
| Weight status (%) | 0.001 | |||||||
| BMI < 25 | 55.9 | 47.3 | 61.5 | |||||
| 25 ≤ BMI < 30 | 35.4 | 45.0 | 29.2 | |||||
| BMI ≥ 30 | 8.7 | 7.7 | 9.3 | |||||
| Hypertension (%) | 30.3 | 44.6 | 21.0 | <0.001 | ||||
| Cholesterol (% > 5,0 mmol/L) | 88.1 | 89.2 | 87.5 | 0.535 | ||||
| Triglyceride (% > 1,7mmol/L) | 10.6 | 18.5 | 5.5 | <0.001 | ||||
| Heart disease (%) | 1.1 | 0.5 | 1.5 | 0.254 | ||||
| Diabetes type 2 (%) | 0.5 | 0.5 | 0.6 | 0.832 | ||||
| Arthritis (%) | 0.5 | 0.5 | 0.6 | 0.832 | ||||
| Lung disease (%) | 1.4 | 0.9 | 1.7 | 0.405 | ||||
| Lung function (%) | ||||||||
| FVC (observed < 80% predicted) | 20.0 | 18.0 | 21.3 | 0.343 | ||||
| FEV1 (observed < 80% predicted) | 20.9 | 18.5 | 22.4 | 0.256 | ||||
| FEV1/FVC (< 0,7) | 10.4 | 9.5 | 11.1 | 0.539 | ||||
Values are mean (SD), unless stated otherwise.
Table 2 shows the physical activity characteristics assessed by accelerometry of the participants in old age (mean age is 80 years (SD 4.7)). Almost 50% of the participants (49.9%) provided seven valid days of data (>10 hours of monitoring), with 28.5% providing six days, 9.6% 5 days, 4.1% 4 days and 3.9% 3 days. Participants spent most of their wear time (75%) being sedentary, over 10 hours a day in both sexes. Men were significantly more sedentary than women, but recorded also more minutes in MVPA.
Table 2.
Physical activity characteristics of the study sample in old age
| Age (years) | total | men | women | p-value | |||
|---|---|---|---|---|---|---|---|
| 80.0 | (4.7) | 79.7 | (4.3) | 80.2 | (5.0) | ||
| Number of valid days (>10h weartime) | 6.0 | (1.6) | 6.0 | (1.4) | 6.0 | (1.4) | 0.828 |
| Weartime on valid day (hours) | 13.7 | (1.5) | 13.9 | (1.6) | 13.6 | (1.4) | 0.034 |
| Daily counts per minute | 128.5 | (69.8) | 132.5 | (77.0) | 126.0 | (64.8) | 0.283 |
| Daily sedentary time (hours/day) | 10.3 | (1.5) | 10.6 | (1.5) | 10.1 | (1.4) | <0.001 |
| % sedentary time | 75.3 | 77.0 | 74.3 | <0.001 | |||
| Daily MVPA (min/day) | 6.7 | (10.0) | 9.5 | (12.8) | 4.9 | (7.2) | <0.001 |
| % MVPA | 0.8 | 1.1 | 0.6 | <0.001 | |||
Values are mean (SD), unless stated otherwise.
In Table 3, the results of the linear regression analyses with the midlife determinants of sedentary time are presented. After adjusting for sex, age, and follow-up time (Model 1), level of education, living in an apartment, current smoker status, BMI, obesity (BMI≥30), heart disease and lung function (FVC and FEV1) were significantly associated with a higher amount of sedentary time per day in old age. After additionally adjusting for minutes of MVPA (Model 2), primary education, living in a duplex or apartment, BMI, obesity, heart disease and FVC remained statistically significant associated with more sedentary time. These variables except BMI and FVC remained significantly associated with a higher percentage of sedentary time after adjusting for BMI, health status, mobility limitation and joint pain in old age (Model 3). Not being married was also significantly associated with more sedentary time in this model.
Table 3.
Associations between midlife determinants and the amount of time spent being sedentary (% sedentary minutes of wear time) in old age
| Determinants | Model | Model | Model | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||
| B | p-value | B | p-value | B | p-value | ||
| Demographic | Marital status | ||||||
| married | ref | ||||||
| not married | 2.07 | 0.125 | 2.39 | 0.062 | 2.48 | 0.046 | |
| missing | 0.86 | 0.457 | 1.28 | 0.242 | 1.08 | 0.306 | |
| Socio-economic | Level of education | ||||||
| college/university | ref | ref | ref | ||||
| secondary | 1.84 | 0.048 | 1.51 | 0.087 | 0.90 | 0.295 | |
| primary | 2.43 | 0.019 | 2.51 | 0.011 | 2.00 | 0.038 | |
| Housing type | |||||||
| villa | ref | ||||||
| duplex | 1.56 | 0.109 | 2.69 | 0.004 | 2.19 | 0.016 | |
| apartment | 2.46 | 0.002 | 2.67 | <0.001 | 2.16 | 0.004 | |
| Occupation group | |||||||
| professional | ref | ref | ref | ||||
| light work | 0.75 | 0.513 | 1.16 | 0.288 | 0.99 | 0.349 | |
| manual labor | 0.43 | 0.728 | 1.19 | 0.314 | 0.87 | 0.450 | |
| housewife | 0.61 | 0.632 | 1.01 | 0.407 | 0.66 | 0.574 | |
| Lifestyle | Smoking status | ||||||
| never smoker | ref | ref | ref | ||||
| previous smoker | 0.46 | 0.626 | 0.12 | 0.892 | −0.28 | 0.756 | |
| current smoker | 1.95 | 0.017 | 1.05 | 0.178 | 0.39 | 0.613 | |
| Physical activity | −0.68 | 0.384 | −0.39 | 0.604 | −0.20 | 0.784 | |
| Active commuting | −0.23 | 0.837 | 0.27 | 0.800 | 0.25 | 0.804 | |
| Occupation activity | |||||||
| on the move | ref | ref | ref | ||||
| standing | 0.50 | 0.727 | 0.06 | 0.965 | 0.03 | 0.980 | |
| sitting | 1.21 | 0.159 | 0.69 | 0.395 | 0.93 | 0.236 | |
| Biomedical | BMI (kg/m2) | 0.33 | 0.001 | 0.22 | 0.021 | 0.21 | 0.078 |
| Weight status | |||||||
| BMI < 25 | ref | ref | ref | ||||
| 25 ≤ BMI < 30 | 1.14 | 0.132 | 0.41 | 0.573 | 0.04 | 0.955 | |
| BMI ≥ 30 | 4.94 | <0.001 | 3.87 | 0.002 | 3.53 | 0.009 | |
| Hypertension | −0.20 | 0.802 | 0.11 | 0.883 | 0.14 | 0.846 | |
| Cholesterol | 0.39 | 0.723 | 0.75 | 0.472 | 0.92 | 0.358 | |
| Triglyceride | 1.23 | 0.292 | 0.81 | 0.463 | 0.77 | 0.474 | |
| Heart disease | 8.22 | 0.016 | 7.55 | 0.020 | 6.30 | 0.045 | |
| Diabetes type 2 | −3.77 | 0.434 | −4.27 | 0.350 | −3.17 | 0.476 | |
| Arthritis | −3.77 | 0.434 | −4.27 | 0.350 | −3.17 | 0.476 | |
| Lung disease | 3.12 | 0.293 | 4.56 | 0.105 | 4.58 | 0.094 | |
| Lung function | |||||||
| FVC (% of predicted value) | −0.07 | 0.003 | −0.05 | 0.039 | −0.04 | 0.114 | |
| FEV1 (% of predicted value) | −0.05 | 0.018 | −0.04 | 0.087 | −0.02 | 0.244 | |
| FEV1/FVC | 2.04 | 0.631 | 1.19 | 0.767 | 1.56 | 0.689 | |
Regression results are presented as unstandardized coefficients (B). Model 1: adjusted for sex, age and follow-up time; Model 2 is additionally adjusted for MVPA (min/day); Model 3 is additionally adjusted for BMI, health status, mobility limitation and joint pain in old age.
The percentages of sedentary time (B) shown in table 3 correspond with an average of 15.3 (95% CI 0.3–30.4) sedentary minutes more per day in old age for participants who were not married at midlife compared those who were married; 12.4 (0.7–24.0) sedentary minutes more for participants with primary education compared to college/university educated; 13.5 (2.6–24.5) and 13.3 (4.4–22.2) sedentary minutes more for participants living in a duplex or apartment resp. compared to those living in a villa; 21.8 (5.4–38.2) sedentary minutes more for obese participants compared to those with BMI≤25; and 38.9 (0.9–76.9) sedentary minutes more for participants with a heart disease.
In additional analyses, weight status and heart disease were further adjusted for marital status, level of education and housing type at midlife; this did not alter the results (data not shown).
DISCUSSION
To our knowledge, this is the first study that examined the prospective associations between midlife determinants from four different domains (demographic, socioeconomic, lifestyle and biomedical) and objectively measured sedentary time in old age over a period of three decades. The midlife determinants marital status, level of education, housing type, weight status and heart disease were statistically significantly associated with a higher amount of sedentary time per day in old age, even after adjusting for sex, age, follow-up time, MVPA, BMI, health status, mobility limitation and joint pain.
The demographic factor not being married and the socioeconomic factors, primary education, living in a duplex or living in an apartment, were associated with, respectively, on average 15.3, 12.4, 13.5 and 13.3 minutes more sedentary time in old age. Smoking at midlife was the only lifestyle factor that was associated with sedentary time, but exclusively in the unadjusted model. Spending most working hours sitting was not significantly associated with more sedentary time in old age. Finally, two biomedical factors, being obese and having a heart disease, were associated with on average 21.8 and 38.9 minutes more sedentary time per day in old age.
To date only a few studies have prospectively examined the association of determinants of objectively measured sedentary time. A study among middle aged participants (mean 54 years) investigated associations between two demographic factors (age, sex), two socioeconomic factors (level of education, employment grade), two lifestyle factors (smoking status, self-reported physical activity), two biomedical factors (BMI, general perceived health) and sedentary time, and found that age and education were associated with sedentary time after 13 years of follow-up(14). Ekelund et al.(10) examined the associations between sedentary time and biomedical factors (body weight, BMI, fat mass, waist circumference) in middle aged participants (mean 49 years). After 5.6 years of follow-up, body weight, BMI, fat mass and waist circumference predicted an increase in the amount of sedentary time.
BMI was also described as a determinant of a sedentary lifestyle in a study that measured BMI and a sedentary lifestyle at the ages of 41, 44, 46 and 54, although sedentariness was not measured objectively in that study(25). Obesity was prospectively associated with self-reported time spent watching TV per week in a cohort of middle-aged active employees.(30) Ding et al.(7) examined individual, social and environmental correlates of change in self-reported TV viewing time which was used as a measure for sedentary time. They described associations between education, self-reported physical activity, living in a low-walkable neighborhood and TV viewing time after 4 years follow-up in a population aged 20–65 years.
Recently, several studies have been conducted in which determinants of self-reported physical inactivity were examined(4, 12, 22, 28). However, sedentary behavior should be assumed as a distinct health behavior with its own nature and physiology, which differs from a lack of physical activity and should therefore be studied independently(15, 26, 31). More studies examining the determinants of sedentary behavior are warranted in order to better advise the development of public health guidelines and prevention programs, as emerging evidence shows the adverse health consequences of sedentary behavior(27).
A major strength of the present study was the objective measurement of sedentary time with accelerometry in a large sample of older adults of which 92% provided at least 4 valid days. The amount of sedentary time (75%) presented in this study is comparable with the amounts described in other studies with older adults, which also used the same cut point to define sedentary time(6, 11, 24). Using a hip-worn accelerometer to define sedentary time may, however, not accurately separate sitting time from standing time. Another strength of this study was adjustment for important confounders such as health status, mobility limitation and joint pain in the final analyses, which exclude the possibility that these factors account for or contribute to a higher amount of sedentary time, although this could have resulted in over adjustment, as the confounders may be part of the pathway between the midlife determinants and sedentary time or could be a result of sedentary time. Furthermore, the longitudinal design of the study with a follow-up time of approximately 31 years is an important strength.
Some limitations of the study should also be considered. First, the midlife data were collected between 1967 and 1992, although this limitation was in part addressed by adjusting all analyses for follow-up time. Further, the determinants could easily have changed during that time period. The measurements in midlife were predominantly collected by means of questionnaires, which could possibly have resulted in misclassification of the lifestyle or biomedical factors.
Second, the associations between the biomedical factors and the amount of sedentary time are possibly bidirectional as, for example, obesity leads to a more sedentary lifestyle, but more sedentary time could also increase BMI. In this study sedentary time was measured exclusively in old age, therefore it might be possible that obesity or heart disease resulted from a more sedentary lifestyle. However, an extra analysis in which these factors were additionally adjusted for physical activity in midlife (a proxy for sedentary time in midlife) did not change the results (data not shown). Furthermore, studies have shown that BMI at baseline predicted sedentary time at follow-up whereas the reverse association was not established(10, 25). These findings support the hypothesis that the direction of the association is mainly from the biomedical factors to sedentary time.
Third, although this study adjusted for a broad spectrum of confounders, it is possible that some unmeasured confounders, such as psychosocial factors (anxiety, isolation), account for some of the presented associations, which could be explored in further research. Also, this study was conducted in a relatively healthy subsample of the AGESII-Reykjavik Study and therefore the results might not be representative for the general older population. Finally, as sedentary time was collected over one week, the data may not truly reflect habitual behavior.
To conclude, this study shows that midlife determinants across several domains: demographic and socioeconomic factors—not being married, lower educational level, poorer housing—and biomedical factors including being obese and having a heart disease, were associated with considerably more sedentary time per day in old age. These results can indicate the possibility of predicting sedentariness in old age, years before this behavior manifests. This information could be used to identify target groups for prevention programs aimed at reducing sedentary time and decreasing the risk of sedentary-related adverse health effects.
Acknowledgments
The researchers are indebted to the participants for their willingness to participate in the study.
This study has been funded by NIH contract N01-AG-12100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). A Koster has received funding from the European Union Seventh Framework Programme (FP7-PEOPLE-2011-CIG) under grant agreement PCIG09-GA-2011-293621.
Footnotes
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
The results of the present study do not constitute endorsement by American College of Sports Medicine.
References
- 1.Arnardottir NY, Koster A, Van Domelen DR, et al. Objective measurements of daily physical activity patterns and sedentary behaviour in older adults: Age, Gene/Environment Susceptibility-Reykjavik Study. Age ageing. 2012;0:1–7. doi: 10.1093/ageing/afs160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bankoski A, Harris TB, McClain JJ, et al. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes care. 2011;34(2):497–503. doi: 10.2337/dc10-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJF, Martin BW. Correlates of physical activity: why are some people physically active and others not? Lancet. 2012;380(9838):258–71. doi: 10.1016/S0140-6736(12)60735-1. [DOI] [PubMed] [Google Scholar]
- 4.Borodulin K, Mäkinen TE, Leino-Arjas P, et al. Leisure time physical activity in a 22-year follow-up among Finnish adults. Int J Behav Nutr Phys Act. 2012;9(1):121–6. doi: 10.1186/1479-5868-9-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 6.Davis MG, Fox KR, Hillsdon M, Sharp DJ, Coulson JC, Thompson JL. Objectively measured physical activity in a diverse sample of older urban UK adults. Med Sci Sports Exer. 2011;43(4):647–54. doi: 10.1249/MSS.0b013e3181f36196. [DOI] [PubMed] [Google Scholar]
- 7.Ding D, Sugiyama T, Winkler E, Cerin E, Wijndaele K, Owen N. Correlates of change in adults’ television viewing time: a four-year follow-up study. Med Sci Sports Exerc. 2012;44(7):1287–92. doi: 10.1249/MSS.0b013e31824ba87e. [DOI] [PubMed] [Google Scholar]
- 8.Dunstan DW, Barr ELM, Healy GN, et al. Television viewing time and mortality: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Circulation. 2010;121(3):384–91. doi: 10.1161/CIRCULATIONAHA.109.894824. [DOI] [PubMed] [Google Scholar]
- 9.Edwardson CL, Gorely T, Davies MJ, et al. Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PloS one. 2012;7(4):e34916. doi: 10.1371/journal.pone.0034916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekelund U, Brage S, Besson H, Sharp S, Wareham NJ. Time spent being sedentary and weight gain in healthy adults: reverse or bidirectional causality? Am J Clin Nutr. 2008;88:612–7. doi: 10.1093/ajcn/88.3.612. [DOI] [PubMed] [Google Scholar]
- 11.Evenson KR, Buchner DM, Morland KB. Objective measurement of physical activity and sedentary behavior among US adults aged 60 years or older. Prev Chronic Dis. 2012;9(2):110109. [PMC free article] [PubMed] [Google Scholar]
- 12.Golubic R, Ekelund U, Wijndaele K, et al. Rate of weight gain predicts change in physical activity levels: a longitudinal analysis of the EPIC-Norfolk cohort. Int J Obes. 2012:1–6. doi: 10.1038/ijo.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grøntved A, Hu FB. Television Viewing and Risk of Type 2 Diabetes, Cardiovascular Disease, and All-CauseMortality. JAMA. 2011;305(23):2448–55. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamer M, Kivimaki M, Steptoe A. Longitudinal patterns in physical activity and sedentary behaviour from mid-life to early old age: a substudy of the Whitehall II cohort. J Epidemiol Community Health. 2012;66(12):1110–5. doi: 10.1136/jech-2011-200505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton MT, Healy GN, Dunstan DW, Zderic TW, Owen N. Too little exercise and too much sitting: Inactivity physiology and the need for new recommendations on sedentary behavior. Cur Cardiovasc Risk Rep. 2008;2(4):292–8. doi: 10.1007/s12170-008-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 17.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165(9):1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Healy G, Wijndaele K, Dunstan D, et al. Objectively measured sedentary time, physical activity, and metabolic risk. Diabetes care. 2008;31(2):369–71. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 19.Healy GN, Matthews CE, Dunstan DW, Winkler EaH, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur Heart J. 2011;32(5):590–7. doi: 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc. 2009;41(5):998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- 21.Koster A, Caserotti P, Patel KV, et al. Association of Sedentary Time with Mortality Independent of Moderate to Vigorous Physical Activity. PLoS One. 2012;7(6):e37696. doi: 10.1371/journal.pone.0037696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakerveld J, Dunstan D, Bot S, et al. Abdominal obesity, TV-viewing time and prospective declines in physical activity. Prev Med. 2011;53(4–5):299–302. doi: 10.1016/j.ypmed.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Matthews C, George S, Moore S, et al. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am J Clin Nutr. 2012;95:437–45. doi: 10.3945/ajcn.111.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews CE, Chen KY, Freedson PS, et al. Amount of Time Spent in Sedentary Behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167(7):875–81. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortensen LH, Siegler IC, Barefoot JC, Grønbaek M, Sørensen TIA. Prospective associations between sedentary lifestyle and BMI in midlife. Obesity. 2006;14(8):1462–71. doi: 10.1038/oby.2006.166. [DOI] [PubMed] [Google Scholar]
- 26.Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105–13. doi: 10.1097/JES.0b013e3181e373a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owen N. Sedentary behavior: Understanding and influencing adults’ prolonged sitting time. Prev Med. 2012;55(6):535–9. doi: 10.1016/j.ypmed.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Petersen L, Schnohr P, Sørensen TIA. Longitudinal study of the long-term relation between physical activity and obesity in adults. Int J Obes Rela Metab Disord. 2004;28(1):105–12. doi: 10.1038/sj.ijo.0802548. [DOI] [PubMed] [Google Scholar]
- 29.Van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting time and all-cause mortality risk in 222 497 Australian adults. Arch Intern Med. 2012;172(6):494–500. doi: 10.1001/archinternmed.2011.2174. [DOI] [PubMed] [Google Scholar]
- 30.Pulsford RM, Stamatakis E, Britton AR, Brunner EJ, Hillsdon MM. Sitting behavior and obesity: evidence from the Whitehall II study. Am J Prev Med. 2013;44(2):132–8. doi: 10.1016/j.amepre.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tremblay MS, Colley RC, Saunders TJ, Healy GN, Owen N. Physiological and health implications of a sedentary lifestyle. Appl Physiol Nutr Metab. 2010;35(6):725–40. doi: 10.1139/H10-079. [DOI] [PubMed] [Google Scholar]
- 32.Wilmot EG, Edwardson CL, Achana Fa, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55(11):2895–905. doi: 10.1007/s00125-012-2677-z. [DOI] [PubMed] [Google Scholar]