Abstract
Objectives
Psychosocial factors have previously been linked with survival and mortality in cancer populations. Little evidence is available about the relationship between these factors and outcomes in gynaecologic cancer populations, particularly endometrial cancer, the fourth most common cancer among women. This study examined the relationship between several psychosocial factors prior to surgical resection and risk of all-cause mortality in women with endometrial cancer.
Design
The study utilized a non-experimental, longitudinal design.
Methods
Participants were 87 women (Mage = 60.69 years, SDage = 9.12 years) who were diagnosed with T1N0–T3N2 endometrial cancer and subsequently underwent surgery. Participants provided psychosocial data immediately prior to surgery. Survival statuses 4–5 years post-diagnoses were abstracted via medical record review. Cox regression was employed for the survival analysis.
Results
Of the 87 women in this sample, 21 women died during the 4- to 5-year follow-up. Adjusting for age, presence of regional disease and medical comorbidity severity (known biomedical prognostic factors), greater use of an active coping style prior to surgery was significantly associated with a lower probability of all-cause mortality, hazard ratio (HR) = 0.78, p = .04. Life stress, depressive symptoms, use of self-distraction coping, receipt of emotional support and endometrial cancer quality of life prior to surgery were not significantly associated with all-cause mortality 4–5 years following diagnosis.
Conclusions
Greater use of active coping prior to surgery for suspected endometrial cancer is associated with lower probability of all-cause mortality 4–5 years post-surgery. Future research should attempt to replicate these relationships in a larger and more representative sample and examine potential behavioural and neuroendocrine/immune mediators of this relationship.
Although the biologic factors that influence disease progression and mortality in cancer have been well-studied, comparatively less is known about the psychosocial and biobehavioural factors that influence clinical outcomes in cancer populations. In recent years, models have been developed that outline the potential mechanisms by which psychosocial factors may be associated with clinical outcomes in cancer. These testable models are derived from empirical research and posit that psychosocial factors, such as stress and coping, are associated with tumorigenesis through the effects of stress hormones on immunity (Antoni et al., 2006). Studies that support this model have found that higher levels of perceived stress and chronic stress have been associated with greater likelihood of developing pre-cancers (Pereira et al., 2003), greater stromal and tumour expression of proteinase factors supporting angiogenesis and invasion in the tumour microenvironment (Lutgendorf et al., 2008), shorter disease-free interval following cancer treatment (Palesh et al., 2007) and increased risk of cancer recurrence (Ramirez et al., 1989). Alternately, high levels of social well-being (Lutgendorf et al., 2002) and social support (Lutgendorf et al., 2008) have been associated with lower levels of vascular endothelial growth factor and proteinase factors known to stimulate tumour growth and progression. With regard to other psychosocial factors and mortality, studies have shown that greater depressive symptoms (Onitilo, Nietert, & Egede, 2006; Pinquart & Duberstein, 2010) and depressive or avoidant coping styles (Faller & Bülzebruck, 2002; Tian, Chen, & Hang, 2009) are associated with increased mortality, while active coping styles (Faller & Bülzebruck, 2002), emotional support (Ell, Nishimoto, Mediansky, Mantell, & Hamovitch, 1992; Waxler-Morrison, Hislop, Mears, & Kan, 1991; Weihs et al., 2005) and better global quality of life are associated with a decreased risk of mortality and longer survival across several cancer populations. In gynaecologic cancer populations, specifically ovarian cancer, better quality of life (Carey et al., 2008) and higher social attachment, a type of emotional support (Lutgendorf et al., 2012), are associated with longer survival times. However, other findings do not support the above relationships between psychosocial factors and mortality across cancer populations, most notably in areas of stressful life events (Barraclough et al., 1992; Graham, Ramirez, Love, Richards,& Burgess, 2002) and quality of life (Montazeri, 2009). Nevertheless, research suggests that implementing psychotherapeutic interventions to reduce distress is associated with reduced risk of developing pre-cancer (Antoni et al., 2008), as well as longer survival times and longer time to disease recurrence in breast cancer (Andersen et al., 2008; Spiegel, Kraemer, Bloom, & Gottheil, 1989). Although these studies provide support for the relationship between psychosocial factors and mortality in cancer, the majority of the research has been conducted in breast, lung and head/neck cancer populations. Among women, comparatively little research has examined these relationships in high prevalence female cancers other than breast cancer, such as endometrial cancer. In addition to being the most common gynaecologic cancer, endometrial cancer is the fourth most common type of cancer affecting women and the eighth leading cause of cancer-related death among women in the United States (American Cancer Society, 2011; Howlader et al., 2011), thus meriting further study.
The present study examined the relationship between psychosocial factors and 5-year mortality in women diagnosed with endometrial cancer. Specifically, this study examined the relationship between pre-surgical psychosocial factors and 5-year all-cause mortality. Consistent with the literature, greater perceived negative impact of stressful events, depressive symptoms and use of a self-distraction coping strategy were hypothesized to be associated with a greater probability of all-cause mortality, whereas use of an active coping strategy, emotional support and greater quality of life were hypothesized to be associated with a lower probability of all-cause mortality.
Patients and methods
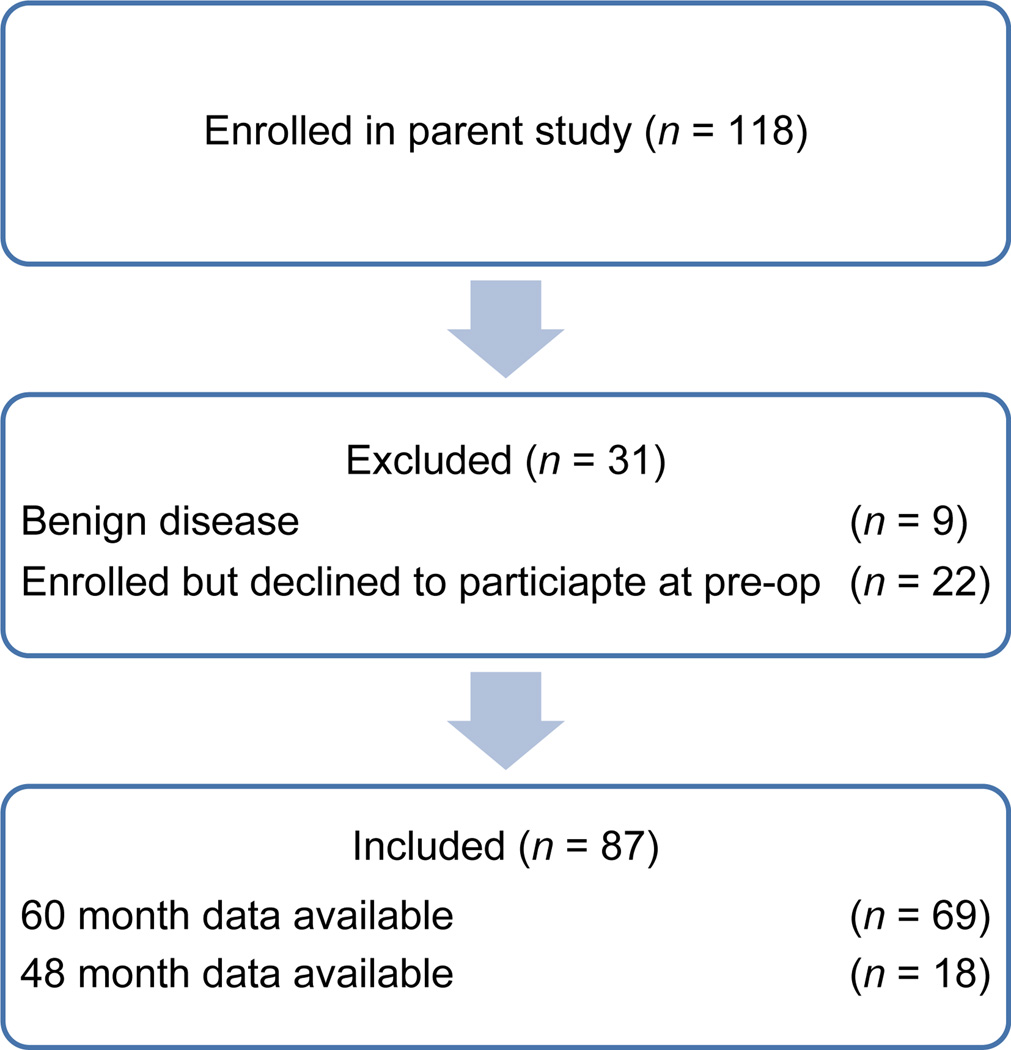
Participants for the present study were 87 women enrolled in a larger, parent study (N = 118) examining psychoneuroimmunologic relations in women with suspected endometrial cancer during the perioperative period between 2004 and 2009 (American Cancer Society Chris DiMarco Institutional Research Grant to the University of Florida/Shands Cancer Center, PI: W. Stratford May; National Cancer Institute, R03 CA 117480, PI: Deidre B. Pereira). For the parent study, inclusion criteria were the following: (1) women with suspected primary endometrial cancer who were 18 or older, (2) undergoing a TAH-BSO or surgical resection, and (3) fluent in spoken English. Exclusion criteria were the following: (1) a diagnosis of recurrent endometrial cancer, (2) metastasis from another site, (3) undergoing preoperative chemotherapy or radiotherapy, and (4) a current psychotic disorder or suicidal ideation. Briefly, participants in the parent study completed psychosocial interviews and questionnaires, as well as blood and saliva sampling for quantitation of cytokines and saliva, during the perisurgical period. Parent study participants were eligible for the present study if they (1) were ultimately diagnosed with endometrial cancer, (2) contributed at least partial psychosocial data, and (3) were confirmed to be alive or deceased within 48–60 months following diagnoses. Thirty-one of the 118 women enrolled in the parent study were excluded for the present study due to either a diagnosis of benign endometrial disease or for declining to complete study procedures and consequent lack of at least partial psychosocial data. The 87 participants for the present study were followed via medical chart and tumour registry review for 48–60 months following surgical diagnosis and staging for assessment of survival status. All study procedures were conducted in accordance with the rules and regulations of the local IRB.
Psychosocial assessment
Negative impact of stressful life events
Negative impact of stressful life events was assessed using a modified version (Leserman et al., 2005; Pereira et al., 2010) of the Life Experiences Survey (LES; Sarason, Johnson, & Siegel, 1978). The original LES is composed of 60 items that refer to life changes relevant to the experiences of the general population over the prior year. This modified version is a 50-item questionnaire that assesses the incidence and impact of events considered to be moderate or severe life stressors among individuals with a chronic/life-limiting illness over the previous 6 months. The following domains are included: Changes in or problems with close relationships, death or illness among family and friends, work or financial problems, illness, accidents, injury and other life changes. The life events are rated for their perceived negative impact on a scale ranging from 0 (not stressful) to 4 (extremely stressful). The minimum and maximum of possible scores ranges from 0 to 200 on the perceived negative impact scale. The scale had high reliability, Cronbach’s α = .81.
Depression
Depression was assessed using the Structured Interview Guide for the Hamilton Anxiety/Depression Scales (SIGH-AD; Williams, 1988), which assesses symptoms of depression and anxiety over the prior week. The original SIGH-AD is composed of 31 items with a 17-item depression subscale and a 14-item anxiety subscale. The present study used an abbreviated version that consisted of a 15-item depression subscale and a 7-item anxiety subscale that excluded items that may be associated with endometrial cancer symptomatology, such as genital symptoms (how has your interest in sex been?) and loss of weight. Only the depression subscale was used in the present study. The minimum possible depression score was 0, while the maximum depression score was 48. The depression subscale had high reliability, Cronbach’s α = .81, as well as high concurrent validity with the Beck Depression Inventory-II (Beck, Steer, & Brown, 1996) collected on a subset of participants in this sample, r(38) = .77, p < .001.
Coping
Coping strategies utilized in response to a suspected/confirmed cancer diagnosis were assessed using the Brief COPE (Carver, 1997). This 28-item questionnaire contains between 11 and 14 subscales, including self-distraction, active coping, substance use, use of emotional support, acceptance, humour, planning, venting, religion, denial, behavioural disengagement and positive reframing. Response choices are on a scale ranging from 1 (I haven’t been doing this at all) to 4 (I have been doing this a lot). The minimum and maximum possible subscale scores range from 2 to 8 for the 2-item subscales and from 3 to 12 for the 3-item subscales. The Brief COPE had high reliability in the present study, Cronbach’s α = .81.
Exploratory factor analysis of all 28 items was utilized to determine the factor structure of the Brief COPE in the present sample. The final analysis resulted in a 10-factor solution, with each factor composed of one to four items. The following items loaded onto factor labelled Active Coping (α = .57): ‘I’ve been taking action to try to make the situation better’ (.69), ‘I’ve been concentrating my efforts on doing something about the situation I’m in’ (.60) and ‘I’ve been trying to come up with a strategy, or plan, about what to do’ (.62). The first two items comprise the Acting Coping subscale, while the last item contributes to the Planning subscale, as described by Carver (1997). Because the planning item loaded more highly onto the Acting Coping subscale in the present study, and Carver (1997) noted that active coping and planning items often load onto one factor, the planning item was retained onto the Active Coping subscale in the present study. The following items loaded onto a factor labelled Self-Distraction (α = .41): ‘I’ve been turning to work or other activities to take my mind off things’ (.51) and ‘I’ve been doing something to think about it less – like going to movies, watching TV, reading, daydreaming, sleeping, or shopping doing other activities to avoid thinking about it’ (.67). These two items comprised the Self-Distraction subscale in Carver (1997), as well. Both the Acting Coping and Self-Distraction subscale scores were used as predictors in the present study.
Emotional support
Emotional support was assessed using the Sources of Social Support Scale (SSSS; Carver, 2004), a measure of relational health. The SSSS is a 50-item questionnaire that assesses the frequency of emotional, tangible and negative support received from spouse/partner, friends, adult women family members, other family members and healthcare providers. Emotional support is assessed by a 20-item subscale, which had high reliability, Cronbach’s α = .89. Response choices are on a scale ranging from 1 (not at all) to 5 (a lot). The minimum and maximum of possible scores ranges from 0 to 100 for the emotional support subscale.
Endometrial cancer specific quality of life
Quality of life was assessed using the Functional Assessment of Cancer Therapy for Endometrial Cancer (FACT-En). The FACT was developed specifically for use in cancer populations for patients receiving treatment and has well-established reliability and validity (Cella et al., 1993). In the present study, reliability was good, Cronbach’s α = .69. The FACT-En is a 43-item questionnaire that assesses different domains of well-being, including physical, social, emotional and functional well-being, over the past week. Additionally, there is a subscale of items assessing physical concerns specific to endometrial cancer. Participants were asked to rate the extent to which each statement applied to them. Response choices are on a scale ranging from 0 (not at all) to4(very much). The minimum and maximum of possible scores ranges from 0 to 172 for global quality of life.
Demographic and biobehavioural variables
Participant demographic characteristics were assessed using a modified version of the MacArthur Sociodemographic Questionnaire (Adler, Epel, Castellazzo, & Ickovics, 2000). Demographic variables used in subsequent analyses include items on race, ethnicity, subjective socioeconomic status and objective socioeconomic status; the latter of which was determined by creating a composite variable including level of education, occupation and household income (Adler et al., 2000). Biobehavioural information collected from relevant medical records included tumour stage (T1–T2 vs. T3), tumour histology (endometrioid type vs. all others) and body mass index (BMI). The presence and severity of comorbid health conditions was assessed using the Charlson Comorbidity Index (Charlson, Pompei, Ales, & McKenzie, 1987). This measure provides a weighted index that assesses the number and severity of comorbid conditions. Health conditions are assigned a weight of 1, 2, 3, or 6 depending on the seriousness and risk of mortality. The weights directly correspond to the relative risk of mortality associated with the particular comorbid condition, rounded to the nearest digit. For each participant, these weights are added to obtain a cumulative score of health comorbidity.
Longitudinal survival assessment
Once participants were 48–60 months post-surgery, survival data (alive or deceased; if alive, date of last contact with the oncology data centre; if deceased, date of death) were obtained from the hospital’s oncology data centre. For the few participants without follow-up data through the data centre, the Social Security Death Index was accessed to determine participant status.
Statistical analyses
An a priori power analysis was based on values of effect size and event rates from the literature (Howlader et al., 2011; Onitilo et al., 2006; Pinquart & Duberstein, 2010; Satin, Linden, & Phillips, 2009) to determine the number of participants needed to achieve adequate power for the survival analysis. This power analysis determined that for B = .15, p (event rate) = .32, α = .05, a total of 78 participants would be needed to obtain power at .80. Given that 87 participants from the parent study met inclusion criteria for the present study, it was determined that the study was adequately powered and statistical procedures were commenced.
Chi-square and t-tests were then conducted to determine whether the included and excluded participants significantly differed on demographic and biobehavioural variables. After the psychosocial data were examined for incomplete data, comparative tests were again conducted to determine whether participants with complete versus incomplete data differed on the aforementioned variables. These analyses yielded no significant differences (Little’s MCAR test: χ2 = 46.09, df = 40, p = .24); thus, the data were determined to be missing completely at random. Potential control variables were determined using Cox regression and included age at diagnoses, tumour histology, tumour stage, BMI and Charlson Comorbidity Index scores. Multiple imputation was then used to predict plausible values for all missing data (Sterne et al., 2009). The imputation model utilized the observed psychosocial variables and relevant control variables as predictors with patient status as the outcome. The imputation was performed ten times, and no significant differences were found between the imputed and complete data; however, two psychosocial variables, depression and negative impact of stress events were found to be non-normally distributed and were consequently normalized using the Blom transformation (Blom, 1958). Cox regression was then performed on all ten data sets, and the results averaged to determine whether psychosocial factors were significantly associated with the probability of mortality 5 years post-diagnosis. The relationship between mortality and each psychosocial variable was tested separately, yielding a total of six Cox regression equations adjusted for relevant biobehavioural control variables.
Results
A total of 118 women met the eligibility requirements for participation in the parent study between 2004 and 2008 and were subsequently enrolled. Thirty-one of these 118women were excluded from the present study: 9 had a diagnosis of benign gynaecologic disease; 22 were enrolled but later declined to participate further in study procedures and therefore had no psychosocial data. The remaining 87 women were ultimately diagnosed with endometrial cancer, contributed at least some psychosocial data and were confirmed to have died or to be alive at 48–60 months post-diagnosis (Figure 1). Results of comparison tests revealed that the 22 women who declined participation lived significantly closer to the study’s medical centre than the current sample. No other significant differences were found between groups on demographic or biobehavioural variables. The 87 included participants ranged in age from 36 to 84 (M = 60.7 years, SD = 9.1 years). The majority of participants identified as Caucasian (89.7%) and were diagnosed with T1 or T2 endometrial cancer (86.2%; Table 1). Nine participants received adjuvant treatment following surgery: five (three endometrioid; two non-endometrioid) received radiation therapy, three (two endometrioid; one non-endometrioid) received chemotherapy and one (nonendometrioid) received radiation therapy and chemotherapy. Of the 87 participants, 66 (75.9%) survived 48–60 months post-diagnosis, while 21 (24.1%) were deceased within 5 years of diagnosis. Descriptive statistics for all psychosocial predictors are presented in Table 2.
Figure 1.
CONSORT diagram showing participants included in the current study.
Table 1.
Participant characteristics
| Characteristic | N | % | M | SD |
|---|---|---|---|---|
| Age, years | 87 | 60.69 | 9.12 | |
| Perceived SES | 81 | 5.56 | 2.01 | |
| Composite SES | 73 | 0.06 | 0.83 | |
| Charlson Comorbidity Index score | 86 | 2.63 | 0.90 | |
| Body mass index | 87 | 36.25 | 11.10 | |
| Race | ||||
| Caucasian | 78 | 89.7 | ||
| Other | 9 | 10.3 | ||
| Cancer stage | ||||
| T1N0–T2N0 | 75 | 86.2 | ||
| T3N0, N1, or N2 | 12 | 13.8 | ||
| Histology | ||||
| Endometrioid | 75 | 86.2 | ||
| Non-endometrioid | 12 | 13.8 |
Note. SES = socioeconomic status.
Table 2.
Descriptive statistics of psychosocial variables
| Construct | N | % | M | SD | Median | Range | α |
|---|---|---|---|---|---|---|---|
| Negative impact of stressful life events | 81 | 93.1 | 3.01 | 0.96 | 3.04 | 1.4–5.5 | .82 |
| Depression symptoms | 85 | 97.7 | 3.00 | 0.97 | 3.00 | 0.65–5.5 | .81 |
| Self-distraction | 72 | 82.8 | 4.98 | 1.62 | 5.00 | 2–8 | .41 |
| Active coping | 71 | 81.6 | 8.65 | 2.21 | 9.00 | 3–12 | .57 |
| Emotional support | 75 | 86.2 | 65.67 | 19.64 | 68.79 | 0–100 | .89 |
| Endometrial cancer specific quality of life | 72 | 82.8 | 130.80 | 23.64 | 133.07 | 46.5–170.5 | .69 |
Associations among control variables and mortality
Tumour stage, specifically comparing T3 disease to T1 and T2, age at diagnosis, and Charlson Comorbidity Index scores were significantly associated with patient status, or the probability of mortality within 5 years post-diagnosis. Specifically, presence of regional disease was significantly associated with a greater probability of mortality, HR = 4.17 (95% CI 1.68, 10.36), p < .01, as was older age at diagnosis, HR = 1.05 (95% CI 1.00, 1.10), p = .04. Greater medical comorbidity was marginally associated with a greater probability of mortality, HR = 1.43 (95% CI 0.98, 2.10), p = .07. Thus, presence of regional disease, age, and medical comorbidity were adjusted for in the Cox regression analyses. Tumour histology and BMI were not significantly associated with patient status and, as such, were not used as covariates (Table 3).
Table 3.
Unadjusted Cox proportional HRs for overall mortality in patients with endometrial cancer
| Variable name | R | 95% CI | p |
|---|---|---|---|
| Age | 1.05 | 1.00–1.10 | .04 |
| Histology | 1.67 | 0.56–4.96 | .36 |
| Presence of regional disease | 4.17 | 1.68–10.36 | <.01 |
| Charlson Comorbidity Index score | 1.43 | 0.98–2.10 | .07 |
| Body mass index (BMI) | 0.98 | 0.94–1.02 | .25 |
Note. HR= hazard ratio; CI = confidence interval.
Associations among psychosocial factors and mortality
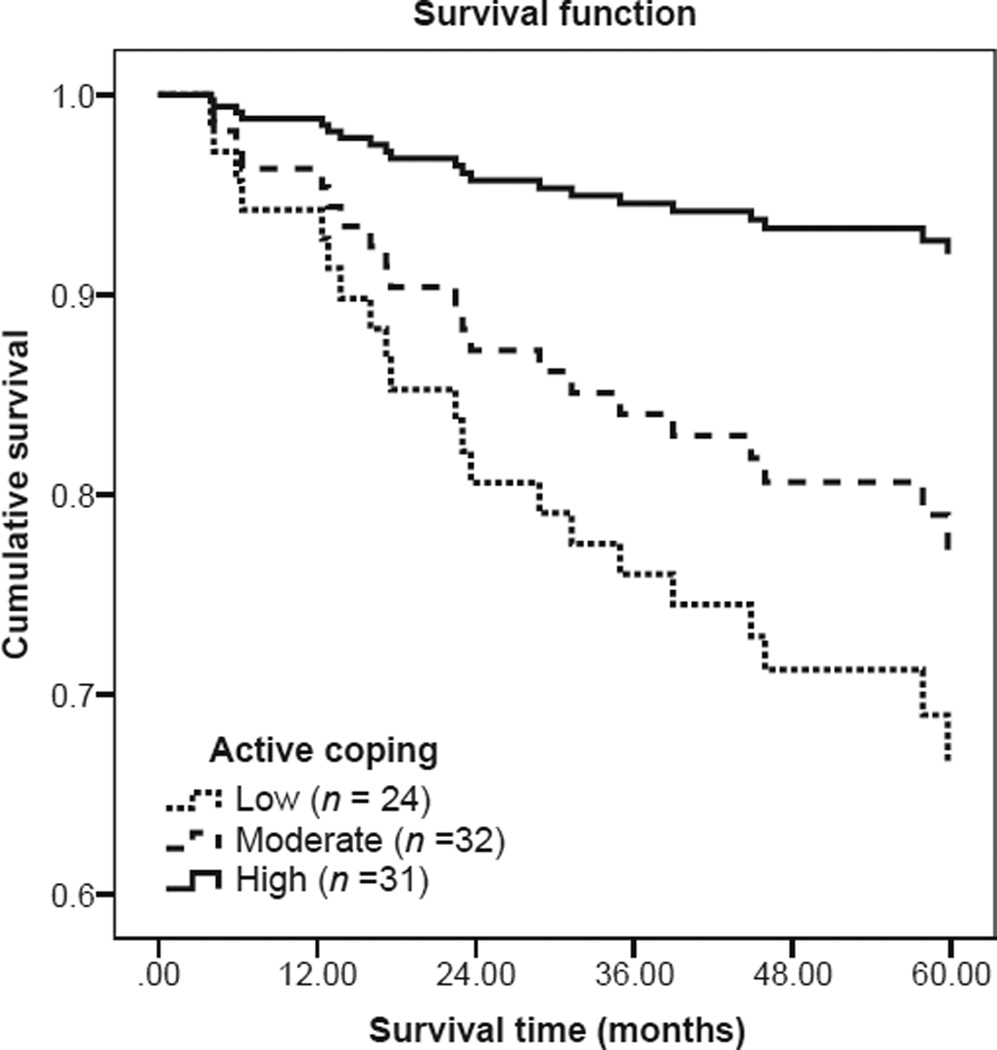
Across all six models (Table 4), Cox regression analyses revealed that older age at diagnosis and presence of regional disease were significantly or nearly significantly associated with a higher probability of all-cause mortality; however, medical comorbidity was no longer associated with mortality. Among the six psychosocial factors examined (Table 4), active coping was found to be significantly associated with a lower probability of all-cause mortality within 5 years of diagnosis, HR = 0.78 (95% CI 0.61, 0.99), p = .04.1 The direction of this relationship was such that more frequent use of active coping prior to surgery was associated with a greater probability of survival across 5 years post-diagnosis. Figure 2 demonstrates the Cox regression survival curve for active coping. For illustrative purposes, a categorical active coping variable was created from the continuous variable using terciles based on participant scores on the active coping scale (range 3–12). This resulted in three distinct participant groups: High use of active coping (score of 3–7; N = 31), moderate use of active coping (score of 8–9; N = 32), and low use of active coping (score of 10–12; N = 24). As shown in Figure 2, individuals with high use of active coping had a lower probability of mortality compared to those with moderate or low use of active coping. In contrast with hypotheses, after adjusting for age, presence of regional disease and medical comorbidity, there was no significant association between 5-year all-cause mortality and any of the following predictors: (1) negative impact of stressful life events, (2) depression, (3) use of self-distraction coping, (4) emotional support, or (5) global quality of life (Table 4).
Table 4.
Cox proportional hazards model for overall mortality in patients with endometrial cancer adjusted for age, presence of regional disease, and medical comorbidity severity
| Model number | Psychosocial predictor | HR | 95% CI | p |
|---|---|---|---|---|
| 1 | Negative impact of stressful life events | 0.72 | 0.43–1.20 | .21 |
| 2 | Depression symptoms | 0.86 | 0.54–1.37 | .52 |
| 3 | Self-distraction | 0.96 | 0.72–1.27 | .76 |
| 4 | Active copinga | 0.78 | 0.61–0.99 | .04 |
| 5 | Emotional support | 0.98 | 0.95–1.00 | .08 |
| 6 | Endometrial cancer specific quality of life | 0.99 | 0.97–1.01 | .18 |
Note. HR= hazard ratio; CI = confidence interval.
Covariates: Age: HR = 1.04 (95% CI 0.99, 1.10), p = .10; presence of regional disease: HR = 6.80 (95% CI 2.39, 19.33), p < .001; medical comorbidity: HR = 1.38 (95% CI 0.88, 2.15), p = .16.
Figure 2.
Survival time for participants with high levels of active coping versus moderate and low levels of active coping. Cox regression adjusted for covariates indicates that participants who endorsed higher levels of active coping had a lower probability of mortality than participants who endorsed moderate or low levels of active coping.
Discussion
The current study suggests that, contrary to the model of biobehavioural influences on tumour growth and cancer outcomes (Antoni et al., 2006), negative impact of stressful live events and depression are not significantly associated with all-cause mortality in women diagnosed with endometrial cancer. However, more frequent use of active coping is significantly associated with a lower probability of all-cause mortality within 5 years of diagnosis. This is consistent with previous research that indicates the use of engaged coping strategies, such as active coping, is associated with longer survival in cancer populations (Faller & Bülzebruck, 2002).
The correlational relationship between active coping and all-cause mortality may imply that women who are more actively engaged in the treatment process could demonstrate better stress management skills and adherence to treatment, which may be associated with quicker and less complicated recovery and possibly a lower probability of disease recurrence or progression. Additionally, active coping could potentially moderate the relationship between stress and neuroendocrine regulation, thereby affecting immune system functioning and biologic responses to cancer (Antoni et al., 2006). The clinical implications of this finding suggest that it may be important to assess coping strategies in women undergoing cancer surgery and design/implement effective coping interventions for those that may only infrequently use active coping strategies.
The remaining psychosocial factors were not significantly associated with all-cause mortality. However, it is notable that emotional support was approaching significance, indicating that greater emotional support at the time of diagnosis might be associated with a lower probability of all-cause mortality, which is consistent with previous research (Ell et al., 1992; Waxler-Morrison et al., 1991; Weihs et al., 2005) and the recent findings by Lutgendorf et al. (2012) in ovarian cancer. It is possible that while these factors were not related to all-cause mortality, they could be significant predictors of cancer-specific mortality. This could not be assessed in the current study given the limitations of the databases utilized. Additionally, similar to findings regarding quality of life and mortality (Carey et al., 2008), psychosocial factors assessed after surgery over the intervening years of treatment and recovery may be more important with regard to predicting survival and mortality in gynaecologic cancers given the natural fluctuation in psychosocial functioning over time.
In this study, the biobehavioural covariates, specifically presence of regional disease, age at diagnosis and frequency of health comorbidities, were consistently significant predictors of all-cause mortality. These findings are consistent with the inherent prognostic nature of the biobehavioural factors and suggest that the current study had adequate external validity, such that factors that are expected to be related to mortality in medical settings were related to all-cause mortality in the study. Of note, these findings were obtained via multiple imputation, which in addition to preserving sample size and increasing power, creates a more realistic data set that is generalizable to the population. Generalizability in the current study is also supported by the survival rates in the sample population. Looking at all-cause mortality, the approximate 5-year survival rate for participants in the study was 76%, a number that compares favourably to the published national statistics (American Cancer Society, 2011; Howlader et al., 2011), suggesting that the survival rate found in the current study is consistent with the survival rate found in local/regional endometrial cancer. Thus, the current findings can be generalized to women who have undergone surgery for a diagnosis of T1–T3 endometrial cancer. Finally, it is also notable that the effect size of the relationship between active coping and survival in the current study, HR = 0.78, is stronger than the effect size found by Lutgendorf et al. (2012) for the relationship between social attachment and survival.
The current study has several limitations. First of all, these findings are only generalizable to women diagnosed at surgery with non-metastatic endometrial cancer. It is unclear whether these psychosocial–mortality relationships would also emerge in women with Stage IV disease, who have lower 5-year survival rates overall compared to women with early-stage disease. However, it should be noted that only a small proportion of women with endometrial cancer are diagnosed at surgery with Stage IV disease (7.5%; Ries et al., 2007), so the present study is generalizable to the vast majority of women diagnosed with endometrial cancer. However, the results of this study cannot be generalized to women with recurrent endometrial cancer.
A second limitation concerns the fact that Cox regression analyses were not adjusted for the use or dose of any adjuvant treatments. However, adjuvant therapy was administered for only a small percentage of participants. Furthermore, the majority of these participants had advanced stage and/or non-endometrioid cancer, and presence of regional disease and histologic type were considered as covariates.
A third limitation is that it is unclear whether the results of the current study can be generalized to other gynaecologic cancers, such as ovarian cancer; or other common endocrine-responsive cancers, such as breast cancer. Although the overall 5-year survival rate for endometrial cancer exceeds that of ovarian cancer, the 5-year survival rates for both cancers are virtually equivalent by stage of disease (American Cancer Society, 2011). Additionally, the relationship between active coping and survival in the present study is comparable to findings by Lutgendorf et al. (2012) regarding social support and survival in ovarian cancer. Nevertheless, future research should examine these relationships within the general gynaecologic cancer population to determine whether such generalizability is possible.
Given that the majority of the participants in the current study’s sample were non-Hispanic Caucasian, these findings are also not generalizable to other racial groups or Latinas. Previous research suggests that racial or ethnic minority groups endorse different types of coping strategies in response to a cancer diagnosis (Culver, Arena, Antoni, & Carver, 2002); thus, the current finding that active coping is associated with longer survival in a mostly Caucasian sample might not extend to African-American or Hispanic populations. Future research should seek to replicate and expand upon these findings within more inclusive and representative samples.
Another important consideration of this study is that it was only a subset of the parent study population. Of the parent study, 22 participants who were recruited subsequently declined to participate in study procedures, the majority citing stress and being overwhelmed with the diagnosis and impending surgery as reasons for being unable to fully participate. Given these reasons, it is possible that this subset of participants were in significantly more distress than the sample included in the current study and their exclusion could explain why several of the psychosocial factors were not found to be significantly associated with all-cause mortality.
Although a significant relationship was found between active coping and mortality, there are likely other factors that mediate the relationship between coping and mortality in the cancer population. Coping itself has been found to moderate the relationship between perceptions of stress and mood (Antoni et al., 2006). Therefore, it is possible that there is an interaction between perceived negative impact of stressful life events and active coping, instead of a main effect of active coping on mortality. Unfortunately, this could not be assessed in the current study due to the limitations in the databases utilized. Future research is needed on these relationships in gynaecologic cancers. This study also highlights the importance of further research into psychosocial and biologic factors related to active coping that might be predictive of longer survival and decreased mortality. Research has suggested that psychosocial factors are not only associated with survival and mortality outcomes, but also time to disease progression and/or disease recurrence (Graham et al., 2002; Ramirez et al., 1989). Expanding on the research between psychosocial factors and clinical outcomes may lead to the development of interventions that could impact disease factors and health-related quality of life in women diagnosed with endometrial and other gynaecologic cancers.
Statement of contribution.
What is already known on this subject?
Psychosocial factors have previously been linked with clinical outcomes in a variety of cancer populations. With regards to gynecologic cancer, the majority of the research has been conducted in ovarian cancer and examines the protective role of social support in mortality outcomes.
What does this study add?
Demonstrates association between active coping during perioperative period and 5 year survival.
Demonstrates psychosocial–survival relationship exists independent of biobehavioral factors.
Acknowledgements
This research was supported by an American Cancer Society (IRG-01-188-01) and the National Cancer Institute (R03 CA 117480).
Footnotes
Presented at the 33rd Annual Meeting of the Society of Behavioral Medicine, New Orleans, LA, 11–14 April 2012.
This result was obtained using an empirically derived, 3-item active coping subscale. Analyses were also conducted using the 2-item active coping subscale published by Carver (1997). Similar results emerged. Using this 2-item subscale, active coping was marginally associated with mortality, HR = 0.71, p = .058 (95% CI 0.50, 1.01).
Conflicts of interests
The authors indicated no potential conflicts of interest.
References
- Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy white women. Health Psychology. 2000;19:586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer facts & figures. Atlanta, GA: American Cancer Society; 2011. [Google Scholar]
- Andersen BL, Yang H, Farrar WB, Golden-Kreutz DM, Emery CF, Thornton LM, Carson WE. Psychologic intervention improves survival for breast cancer patients: A randomized clinical trial. Cancer. 2008;113:3450–3458. doi: 10.1002/cncr.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Snood AK. The influence of bio-behavioural factors on tumour biology: Pathways and mechanisms. Nature Reviews Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Pereira DB, Marion I, Ennis N, Peake M, Rose R, O’Sullivan M. Stress management effects on perceived stress and cervical intraepithelial neoplasia in low-income HIV infected women. Journal of Psychosomatic Research. 2008;65:389–401. doi: 10.1016/j.jpsychores.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough J, Pinder P, Cruddas M, Osmond C, Taylor I, Perry M. Life events and breast cancer prognosis. British Medical Journal. 1992;304:1078–1081. doi: 10.1136/bmj.304.6834.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Blom G. Statistical elements and transformed beta variables. New York, NY: Wiley; 1958. [Google Scholar]
- Carey MS, Bacon M, Tu D, Butler L, Bezjak A, Stuart GC. The prognostic effects of performance status and quality of life scores on progression-free survival and overall survival in advanced ovarian cancer. Gynecologic Oncology. 2008;108:100–105. doi: 10.1016/j.ygyno.2007.08.088. [DOI] [PubMed] [Google Scholar]
- Carver CS. You want to measure coping but your protocol’s too long: Consider the Brief COPE. International Journal of Behavioral Medicine. 1997;4:92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- Carver CS. The sources of social support survey. 2004. Unpublished Instrument. [Google Scholar]
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Harris J. The Functional Assessment of Cancer Therapy Scale: Development and validation of the general measure. Journal of Clinical Oncology. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. Retrieved from http://jco.ascopubs.org. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, McKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Culver JL, Arena PL, Antoni MH, Carver CS. Coping and distress among women under treatment for early stage breast cancer: Comparing African-Americans, Hispanics and Non-Hispanic Whites. Psycho-Oncology. 2002;11:495–504. doi: 10.1002/pon.615. [DOI] [PubMed] [Google Scholar]
- Ell K, Nishimoto R, Mediansky L, Mantell J, Hamovitch M. Social relations, social support and survival among patients with cancer. Journal of Psychosomatic Research. 1992;36:531–541. doi: 10.1016/0022-3999(92)90038-4. [DOI] [PubMed] [Google Scholar]
- Faller H, Bülzebruck H. Coping and survival in lung cancer: A 10-year follow-up. American Journal of Psychiatry. 2002;159:2105–2107. doi: 10.1176/appi.ajp.159.12.2105. [DOI] [PubMed] [Google Scholar]
- Graham J, Ramirez A, Love S, Richards M, Burgess C. Stressful life experiences and risk of relapse of breast cancer: Observational cohort study. British Medical Journal. 2002;324:1420–1423. doi: 10.1136/bmj.324.7351.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2008. Bethesda, MD: National Cancer Institute; 2011. Retrieved from http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- Leserman J, Whetten K, Lowe K, Stangl D, Swartx MS, Thielman NM. How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the south. Psychosomatic Medicine. 2005;67:500–507. doi: 10.1097/01.psy.0000160459.78182.d9. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, De Geest K, Bender D, Ahmed A, Goodheart MJ, Dahmoush L, Sood AH. Social influences on clinical outcomes of patients with ovarian cancer. Journal of Clinical Oncology. 2012;30:2885–2890. doi: 10.1200/JCO.2011.39.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Johnsen ER, Cooper B, Anderson B, Sorosky JI, Buller RE, Sood AK. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95:808–813. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Lamkin DM, Jennings NB, Arevalo JMG, Penedo F, DeGeest K, Sood AK. Biobehavioral influences on matrix metalloprotinase expression in ovarian carcinoma. Clinical Cancer Research. 2008;14:6839–6846. doi: 10.1158/1078-0432.CCR-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: An overview of the literature from 1982 to 2008. Health and Quality of Life Outcomes. 2009;7(23):102. doi: 10.1186/1477-7525-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onitilo AA, Nietert PJ, Egede LE. Effect of depression on all-cause mortality in adults with cancer and differential effects by cancer site. General Hospital Psychiatry. 2006;28:396–402. doi: 10.1016/j.genhosppsych.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Palesh O, Butler LD, Koopman C, Giese-Davis J, Carlson R, Spiegel D. Stress history and breast cancer recurrence. Journal of Psychosomatic Research. 2007;63:233–239. doi: 10.1016/j.jpsychores.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira DB, Antoni MH, Danielson A, Simon T, Efantis-Potter J, Carver C, O’Sullivan M. Life stress and cervical squamous intraepithelial lesions in women with human papillomavirus and human immunodeficiency virus. Psychosomatic Medicine. 2003;65:427–434. doi: 10.1097/01.psy.0000041620.37866.89. [DOI] [PubMed] [Google Scholar]
- Pereira DB, Sannes T, Dodd SM, Jensen SE, Morgan LS, Chan EKL. Life stress, negative mood states, and antibodies to heat shock protein 70 in endometrial cancer. Brain, Behavior, and Immunity. 2010;24:21–214. doi: 10.1016/j.bbi.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M, Duberstein PR. Depression and cancer mortality: A meta-analysis. Psychological Medicine. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez AJ, Craig TKJ, Watson JP, Fentiman IS, North WRS, Rubens RD. Stress and relapse of breast cancer. British Medical Journal. 1989;298:291–293. doi: 10.1136/bmj.298.6669.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J, editors. SEER survival monograph: Cancer survival among adults: U.S. SEER program, 1988–2001, patient and tumor characteristics (NIH Pub. No. 076215) Bethesda, MD: National Cancer Institute; 2007. Retrieved from http://seer.cancer.gov/publications/survival. [Google Scholar]
- Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life change: Development of the Life Experiences Survey. Journal of Consulting and Clinical Psychology. 1978;46:932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- Spiegel D, Kraemer HC, Bloom JR, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. The Lancet. 1989;334:888–891. doi: 10.1016/s0140-6736(89)91551-1. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, White IR, Carlon JB, Spratt M, Royston P, Kenward MG, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. British Medical Journal. 2009;339:157–160. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Chen Z, Hang L. The effects of psychological status of the patients with digestive system cancers on prognosis of the disease. Cancer Nursing. 2009;32:230–235. doi: 10.1097/NCC.0b013e31819b59c0. [DOI] [PubMed] [Google Scholar]
- Waxler-Morrison N, Hislop TG, Mears B, Kan L. Effects of social relationships of survival for women with breast cancer: A prospective study. Social Science & Medicine. 1991;33:177–183. doi: 10.1016/0277-9536(91)90178-f. [DOI] [PubMed] [Google Scholar]
- Weihs KL, Simmens SJ, Mizrahi J, Enright TM, Hunt MR, Siegal RS. Dependable social relationships predict overall survival in stages II and III breast carcinoma patients. Journal of Psychosomatic Research. 2005;59:299–306. doi: 10.1016/j.jpsychores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Archives of General Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]