Abstract
OBJECTIVES
This study examined differences in gestational weight gain for women in CenteringPregnancy (CP) group prenatal care versus individually delivered prenatal care.
METHODS
We conducted a retrospective chart review and used propensity scores to form a matched sample of 393 women (76% African-American, 13% Latina, 11% White; average age 22 years) receiving prenatal care at a community health center in the South. Women were matched on a wide range of demographic and medical background characteristics
RESULTS
Compared to the matched group of women receiving standard individual prenatal care, CP participants were less likely to have excessive gestational weight gain, regardless of their pre-pregnancy weight (b = −.99, 95% CI [−1.92, −.06], RRR = .37). CP reduced the risk of excessive weight gain during pregnancy to 54% of what it would have been in the standard model of prenatal care (NNT = 5). The beneficial effect of CP was largest for women who were overweight or obese prior to their pregnancy. Effects did not vary by gestational age at delivery. Post-hoc analyses provided no evidence of adverse effects on newborn birth weight outcomes.
CONCLUSIONS
Group prenatal care had statistically and clinically significant beneficial effects on reducing excessive gestational weight gain relative to traditional individual prenatal care.
Keywords: birth weight, pregnancy, weight gain, women, obesity
In 2009, the Institute of Medicine (IOM) issued new recommendations for healthy weight gain during pregnancy based on pre-pregnancy body mass index (BMI), recommending that underweight women (BMI<18.5) gain 28–40 pounds during pregnancy, healthy weight women (BMI=18.5–24.9) gain 25–35 pounds, overweight women (BMI=25.0–29.9) gain 15–25 pounds, and obese women (BMI>30) gain 11–20 pounds (1). Maternal weight gain below these guidelines is associated with low neonatal birth weight, and weight gain above these guidelines is associated with numerous detrimental outcomes for mother and child, including gestational diabetes mellitus, infant macrosomia, persistent maternal obesity postpartum, and pediatric obesity (2,3,4,5,6). Yet an estimated 45% of U.S. women gain weight during pregnancy in excess of these guidelines (7).
Although much is known about the cascade of adverse effects related to excessive weight gain during pregnancy, existing interventions to prevent it are so limited in number and effectiveness that both the IOM (1) and the Agency for Healthcare Research and Quality (AHRQ) (8) have identified this topic as a major research gap. Although interventions have been tested to facilitate healthy weight gain during pregnancy (with varying levels of cultural tailoring, individualization, and participant burden), to date, prenatal care delivery models have not been sufficiently evaluated for their efficacy.
Standard prenatal care typically involves approximately 13 one-on-one visits of 15–20 minutes duration with a primary care provider. This care model generally focuses on conducting health assessments and lab tests, early detection of medical problems, and health education. Providers typically counsel on appropriate prenatal nutrition, exercise, and weight gain targets. An alternative prenatal care model, prenatal care in a group setting, emphasizes health education, group support, and self-assessment. The CenteringPregnancy (CP) model is one standardized group-delivered prenatal care model (9), which typically involves groups of 8–12 women of similar gestational age who meet for 10 group sessions of 90–120 minutes. At the beginning of each CP session, women participate in personal prenatal assessments by weighing themselves, measuring blood pressure, determining gestational age, and editing their charts. During this assessment period, women have time to individually discuss with providers their progress, charts, or other issues (e.g., scheduling individual prenatal sessions for issues requiring additional privacy). Each CP session also includes two formal group discussion/education periods and a midsession break with refreshments. The group education periods focus on a variety of wellness topics, are led by a practitioner, and often supplemented with handouts, worksheets, and videos. Women in CP typically keep weekly food journals and learn to set physical activity and nutrition goals. The CP curriculum also has sessions dedicated to prenatal nutrition and exercise, and the group format is intended to provide social support and facilitate collective problem-solving around barriers to behavior change. Although women who receive traditional prenatal care typically receive information about prenatal nutrition and exercise, CP programs give women opportunities to discuss their health, problem-solve, socialize, and develop support networks with other pregnant women as part of their medical care.
Only one randomized controlled trial has compared gestational weight gain outcomes for CP versus traditional individual prenatal care participants (10). No significant differences in weight gain were found between groups. However, the study did not examine differences in weight gain by pre-pregnancy BMI (e.g., healthy, overweight, obese), which is problematic given that pre-pregnancy BMI is the single best predictor of weight gain during pregnancy (11,12,13), and that clinical recommendations for appropriate weight gain vary by BMI category. A second (non-randomized) study found that CP clients gained significantly more weight during pregnancy than traditional care patients (14). Another non-randomized study found no significant differences between groups in the likelihood of gaining excess weight, but women in CP were less likely than women in traditional care to gain weight below the recommended amount (15). Both studies used non-equivalent comparison groups, and neither examined differences in weight gain by pre-pregnancy BMI categories.
Given the limited and inconsistent findings to date (16), the purpose of this study was to compare gestational weight gain for similar women enrolled in group (CP) versus traditional prenatal care. Secondary objectives were to explore whether the association between prenatal care model and gestational weight gain varied according to women’s pre-pregnancy BMI and gestational age at birth. To explore possible adverse effects, we also conducted post-hoc analyses comparing newborn birth weights based on mothers’ type of prenatal care. We are unaware of any controlled studies that have examined these differential effects of prenatal care model on gestational weight gain by pre-pregnancy BMI or gestational age. The present study therefore contributes to the literature by examining whether group prenatal care has a beneficial effect on gestational weight gain, and whether those effects are more or less pronounced in certain groups of women.
METHODS
Sample
The sample included all obstetric patients who received prenatal care (CP or traditional) at an independent faith-based community health center in a Southern metropolitan area between 2008 and 2011. Patients were offered the option to participate in CP at their initial prenatal care appointment. Women were deemed ineligible for CP by the health clinic staff if they did not speak English (however, multilingual women were eligible) or were considered at high risk due to the following conditions: prior cesarean birth, prior low birth weight infant, diabetes, lupus, heart disease, clotting problems, seizures, kidney disorder, cervical incompetence, or mental health issues. The health center was an approved CP site, had a dedicated group space for CP, and had two providers responsible for delivering CP (one certified nurse-midwife and one physician) at the time of data collection (17).
We extracted de-identified data from records for all women who received prenatal care and delivered between mid-2008 and late-2011 (17). Gestational weight gain data were extracted from medical charts for 569 prenatal care recipients with singleton births, 242 who received CP care and 327 who did not. Data were collected and managed using REDCap, a secure, web-based application designed to support data capture for research studies (18). All research activities were conducted in accord with prevailing ethical principles. The retrospective chart review used to collect medical record data on background and weight gain outcomes was part of a contracted evaluation and was therefore not human subjects research. The current study is a secondary analysis of the de-identified dataset that was compiled during that contract evaluation, and thus the Vanderbilt University Institutional Review Board (IRB) Committee exempted this secondary analysis from IRB review because it met criteria set forth in federal regulations at 45 CFR 46.101(b)(4).
Measures
Healthcare providers measured weight and height at the first and last prenatal care visits. We calculated BMI from weight (pounds) and height (inches) measurements as
Total gestational weight gain was calculated as the difference in weight measurements between the first and last prenatal care visits, as documented in the medical charts.
Gestational weight gain was measured with a three-category variable (1 = low weight gain, 2 = healthy weight gain, 3 = high weight gain) indicating whether gestational weight gain was under, within, or over the range recommended by the IOM (1) and based on pre-pregnancy BMI and total gestational weight gain.
Prenatal care format was measured with a binary variable (1 = CP group prenatal care; 0 = TC traditional individual care). Participants who attended at least one CP prenatal care session were classified as CP participants. Participants who only attended traditional individual prenatal care sessions, and attended no CP sessions, were classified as TC participants. This approach is conservative and consistent with the intention-to-treat principle. Many CP participants attended individually delivered prenatal care sessions in addition to group sessions (e.g., to discuss issues requiring more privacy or other issues that could not be fully addressed during the CP personal assessment periods). The fact that many CP participants also attended individual prenatal care sessions does not reflect a contamination or cross-over problem. Rather, this reflects the fact that CP (as a prenatal care package) primarily involves group sessions and also includes supplementary individual sessions on an as-needed basis.
Background demographic and medical history variables were extracted from medical charts for use as covariates in the propensity score and final outcome models (statistical methods described below). The following background variables were used in the propensity score estimation models: maternal age, race, Spanish language speaker (i.e., multilingual Spanish/English), education level, marital status, government insurance, current employment, gravidity, height, gestational age and weight at entry into care, pre-pregnancy BMI, systolic blood pressure, histories of non-gestational diabetes, depression, drug use, abnormal Pap smear, pulmonary problems, alcohol use, gynecological surgery, hypertension, kidney problems, operations, blood transfusions, and trauma.
Newborn health outcomes were also collected from retrospective chart reviews. Preterm delivery was measured with a binary variable (1 = gestational age < 37 weeks; 0 = gestational age ≥ 37 weeks). Newborn birth weight was measured as total birth weight (grams) and scored as a binary variable for low birth weight (1 = birth weight < 2,500 grams; 0 = birth weight ≥ 2,500 grams).
Data Analysis
Because the study was retrospective, we could not assign women randomly to prenatal care conditions. Therefore, we used propensity scores to create a statistically matched group of women who received CP versus TC (19,20). Propensity score methods attempt to reduce the impact of selection bias and confounding on estimated causal treatment effects in non-randomized observational studies (19). Random assignment permits causal inferences because it ensures that treatment status is independent of baseline characteristics, whether observed or unobserved. Propensity score methods attempt to permit causal inferences by matching or balancing groups on baseline characteristics, but can only do so on observed, i.e., measured, characteristics.
Propensity scores were estimated as the predicted probability of women participating in CP (versus TC), based on a logistic regression model that controlled for background demographic and medical history variables. Patients were excluded from the final matched sample if their estimated propensity score fell outside the common support region where the distributions of the propensity scores for the two groups overlapped (nCP = 84; nTC = 92). The restriction to the common support region was used to ensure conservative estimates of CP effects, such that CP participants were only compared with TC participants with similar background characteristics. By retaining all CP participants who could be matched with TC participants (or vice versa), we maximized our statistical power to detect effects by maintaining the largest overall sample size.
The quality of the matching on individual variables incorporated in the propensity scores was assessed by examining pre- and post-matching means, standardized mean differences, and variance ratios (21,22,23,24); results indicated acceptable covariate balance was achieved (see (17) for standardized mean differences and variance ratios for all variables). The final matched sample included 393 prenatal care recipients (nCP = 158; nTC = 235) balanced on background covariates; this balancing permitted a fair comparison of gestational weight gain across groups.
To address the primary goals of the study, we estimated the main effects of prenatal care on gestational weight gain outcomes for the matched sample using weighted multinomial logistic regression models. All analyses used inverse propensity score weighting, with sampling weights equal to 1/propensity score for CP participants and 1/(1−propensity score) for TC participants. The purpose of using propensity score techniques was to reduce any bias associated with observed baseline differences between the CP and TC groups. To safeguard against any remaining imbalance between groups on key background characteristics, and for face validity purposes, all outcome analyses additionally adjusted for maternal age, race, gravidity, and total number of prenatal care visits attended.
To address the secondary objectives of the study (i.e., to explore variability in prenatal care format effects), we used multiplicative interaction terms to examine whether the effect of CP on gestational weight gain varied according to (1) pre-pregnancy BMI (healthy, overweight, or obese) and (2) gestational age at delivery (preterm or term). Finally, post-hoc analyses were used to explore possible adverse effects of prenatal care format on newborn birth weight. We used propensity score weighted ordinary least squares and logistic regression models to examine the effects for total birth weight and low birth weight outcomes.
To facilitate interpretation of results, we used results from the multinomial logistic regression models to estimate predicted probabilities of excessive gestational weight gain for CP and TC participants, split by pre-pregnancy BMI category (25). These predicted probabilities were then translated into three different effect size metrics, again to aid interpretation: absolute risk differences, risk ratios, and the number needed to treat (26). Absolute risk differences were calculated as the difference in the risk of excessive gestational weight gain for the two prenatal care groups. Risk ratios were calculated as the ratio of risks of excessive gestational weight gain for the two groups. The number needed to treat was calculated as the inverse of the absolute risk difference.
RESULTS
Table 1 presents results from the logistic regression model that generated the propensity scores used to match the two groups of participants. After matching, participants were an average age of 22 years; 76% were African American, 13% were Latina, 11% were White; 88% were on public insurance. No women were underweight prior to pregnancy, 43% were a healthy weight prior to pregnancy, 30% were overweight, and 27% were obese. Women in CP prenatal care gained an average of 27.66 pounds during pregnancy, and women in TC gained an average of 24.96 pounds. Overall, women gained an average of 26 pounds during pregnancy; 29% gained less than the recommended amount of weight during pregnancy, 36% gained weight within recommended guidelines, and 35% gained weight in excess of the IOM recommendations. Table 2 presents background demographic and medical history characteristics for the two groups before and after the propensity score matching.
Table 1.
Logit coefficients and odds ratios from propensity score estimation model predicting participation in CP group prenatal care (n = 569)
| Covariate | b | se | 95% CI | OR |
|---|---|---|---|---|
| Height at entry | .14 | .15 | (−.16, .43) | 1.15 |
| Weight at entry | −.02 | .03 | (−.08, .03) | .98 |
| Pre-pregnancy BMI | .16 | .17 | (−.17, .50) | 1.17 |
| Maternal age | −.03 | .03 | (−.08, .03) | .97 |
| Black, non-Hispanic | .13 | .33 | (−.51, .78) | 1.14 |
| Hispanic | −.15 | .99 | (−2.08, 1.79) | .86 |
| Primary language: Spanish | .17 | .98 | (−1.75, 2.1) | 1.19 |
| Single | −.19 | .28 | (−.74, .36) | .83 |
| Education | .13 | .09 | (−.04, .30) | 1.14 |
| Employed | .27 | .24 | (−.19, .74) | 1.31 |
| Public insurance | .14 | .30 | (−.45, .74) | 1.15 |
| Gravidity | −.21 | .08** | (−.36, −.07) | .81 |
| Gestational age at entry | −.06 | .02** | (−.09, −.02) | .94 |
| Systolic blood pressure | .00 | .01 | (−.02, .01) | 1.00 |
| Abnormal pap history | −.23 | .26 | (−.73, .28) | .79 |
| Alcohol use history | .92 | .38* | (.17, 1.67) | 2.51 |
| Blood transfusion history | .79 | .64 | (−.47, 2.05) | 2.20 |
| Depression/psychiatric illness history | −.22 | .36 | (−.94, .49) | .80 |
| Diabetes, non-gestational history | .08 | .77 | (−1.43, 1.58) | 1.08 |
| Drug use risk history | .16 | .26 | (−.35, .66) | 1.17 |
| Pulmonary problems | .36 | .33 | (−.29, 1.01) | 1.43 |
| Gynecological surgery history | .44 | .60 | (−.73, 1.62) | 1.55 |
| Hypertension history | −.17 | .45 | (−1.04, .70) | .84 |
| Kidney disease/UTIs history | .91 | .44* | (.05, 1.77) | 2.48 |
| Operations/hospitalizations history | .03 | .25 | (−.46, .51) | 1.03 |
| Trauma/violence history | −.68 | .59 | (−1.83, .46) | .51 |
| No medical history risk factors | .12 | .24 | (−.35, .58) | 1.13 |
| Constant | −8.25 | 9.61 | (−27.09, 10.59) |
p < .05.
p < .01.
b = logit coefficient, se = standard error of logit coefficient, OR = odds ratio (exp[b]), BMI = body mass index, UTI = urinary tract infection. Results are from a logistic regression model predicting type of prenatal care received (1= CP, 0 = traditional care).
Table 2.
Characteristics of unmatched and matched participants, by type of prenatal care
| Unmatched sample | Matched sample | ||||
|---|---|---|---|---|---|
|
| |||||
| CP n = 242 | TC n = 327 | CP n = 158 | TC n = 235 | All n = 393 | |
| Gestational weight gain (GWG) | |||||
| Average GWG, pounds (Mean; SD) | 25.50 (13.99) | 21.32 (14.50) | 27.66 (13.34) | 24.96 (13.47) | 26.05 (13.47) |
| Low GWG (%) | 30.17 | 44.04 | 23.42 | 32.76 | 29.01 |
| Healthy GWG (%) | 33.47 | 29.36 | 34.81 | 36.17 | 35.62 |
| Excessive GWG (%) | 36.36 | 26.61 | 41.77 | 31.06 | 35.37 |
| Maternal weight background | |||||
| Weight at entry (Mean; SD) | 157.92 (37.87) | 157.52 (38.96) | 157.97 (34.72) | 153.91 (37.33) | 155.54 (36.31) |
| Pre-pregnancy BMI (Mean; SD) | 27.43 (6.16) | 27.38 (6.31) | 27.39 (5.82) | 26.76 (6.01) | 27.01 (5.93) |
| Healthy (%) | 39.67 | 42.51 | 37.97 | 46.81 | 43.26 |
| Overweight (%) | 60.33 | 57.49 | 32.28 | 28.09 | 29.77 |
| Obese (%) | 28.93 | 29.66 | 29.75 | 25.11 | 26.97 |
| Demographic characteristics | |||||
| Age (Mean; SD) | 22.16 (5.29) | 22.83 (5.64) | 22.07 (5.20) | 22.59 (5.82) | 22.38 (5.58) |
| Black, non-Hispanic (%) | 78.10 | 75.84 | 81.01 | 72.77 | 76.08 |
| Hispanic (%) | 11.98 | 14.07 | 10.13 | 15.74 | 13.49 |
| Primary language: Spanish (%) | 11.57 | 13.45 | 10.13 | 14.47 | 12.72 |
| Single (%) | 82.23 | 81.65 | 83.54 | 81.28 | 82.19 |
| Education (Mean; SD) | 3.17 (1.47) | 2.87 (1.24) | 3.18 (1.43) | 2.79 (1.13) | 2.95 (1.27) |
| Employed (%) | 26.78 | 21.77 | 28.18 | 21.16 | 23.99 |
| Public insurance (%) | 87.19 | 85.02 | 89.24 | 86.81 | 87.79 |
| Pregnancy and birth characteristics | |||||
| Gestational diabetes (%) | 2.48 | 4.59 | 1.90 | 4.68 | 3.56 |
| Gravidity (Median; SD) | 1 (1.56) | 2 (1.77) | 1 (1.47) | 2 (1.59) | 2 (1.56) |
| Gestational age at entry (Mean; SD) | 13.66 (4.52) | 15.17 (5.50) | 13.61 (4.41) | 14.93 (5.20) | 14.40 (4.93) |
| Preterm, this pregnancy (%) | 8.26 | 16.82 | 4.43 | 8.94 | 7.12 |
| Total number of prenatal care visits (Mean; SD) | 17.03 (5.83) | 8.38 (4.13) | 19.63 (3.95) | 9.82 (3.12) | 13.76 (5.94) |
| Medical history characteristics | |||||
| Abnormal pap (%) | 16.53 | 18.65 | 17.72 | 15.74 | 16.54 |
| Alcohol use (%) | 11.16 | 4.59 | 8.23 | 4.26 | 5.85 |
| Blood transfusion (%) | 2.48 | 1.83 | 2.53 | .85 | 1.53 |
| Depression/psychiatric illness (%) | 7.02 | 8.56 | 7.59 | 8.51 | 8.14 |
| Diabetes, non-gestational (%) | 1.24 | 1.53 | 1.27 | 1.70 | 1.53 |
| Drug use risk (%) | 17.77 | 15.30 | 18.99 | 15.32 | 16.79 |
| Gynecological surgery (%) | 3.31 | 2.14 | 2.53 | 1.28 | 1.78 |
| Hypertension (%) | 4.54 | 6.42 | 4.43 | 5.53 | 5.09 |
| Kidney disease/UTIs (%) | 6.61 | 3.36 | 4.43 | 3.40 | 3.82 |
| Operations/hospitalizations (%) | 22.31 | 21.10 | 19.62 | 17.87 | 18.58 |
| Pulmonary problems (%) | 10.33 | 7.03 | 10.76 | 6.81 | 8.40 |
| Trauma/violence (%) | 2.07 | 3.98 | 3.16 | 5.11 | 4.33 |
CP = Centering Pregnancy prenatal care; TC = traditionally delivered prenatal care; SD = standard deviation; n = sample size; BMI = body mass index; UTI = urinary tract infection.
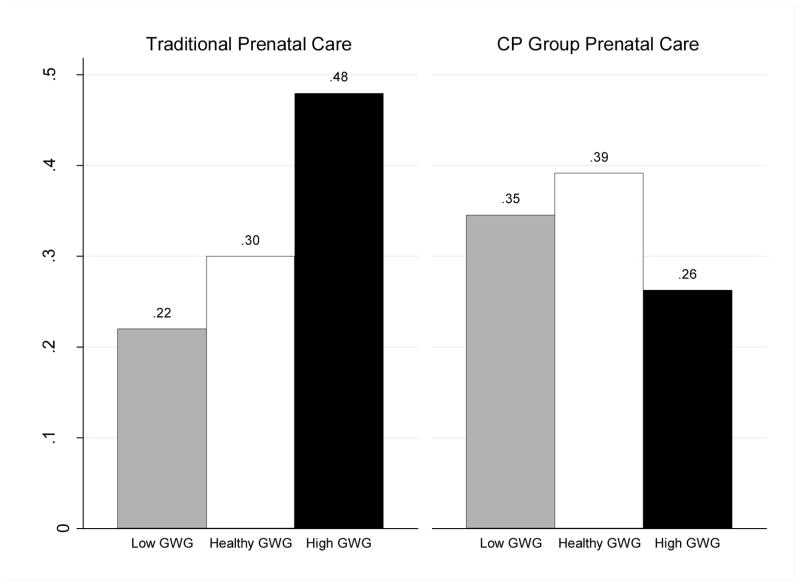
Table 3 presents coefficients and relative risk ratios from multinomial logistic regression models predicting low, healthy, or excessive gestational weight gain. Model I estimated the main effect of CP versus TC on type of gestational weight gain. Models II – III addressed the exploratory aims of the study, and included multiplicative interaction terms between CP prenatal care receipt and pre-pregnancy BMI category and gestational age at birth, respectively. Women in CP were significantly less likely than participants in TC to have excessive gestational weight gain, relative to healthy gestational weight gain (b = −.99, p = .04, 95% CI [−1.92, −.06], RRR = .37). Results also indicated no significant differences between CP and TC groups in the relative risk of low gestational weight gain versus healthy weight gain (b = .25, p = .60, 95% CI [−.67, 1.17], RRR = 1.28). Figure 1 depicts the results from Model I as predicted probabilities for low, healthy, and high gestational weight gain. Participants in TC were less likely to have low or healthy gestational weight gain compared to CP participants.
Table 3.
Coefficients and relative risk ratios from multinomial logistic regression models predicting type of gestational weight gain (GWG)
| Low GWG vs. Healthy GWG | High GWG vs. Healthy GWG | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| b | se | 95% CI | RRR | b | se | 95% CI | RRR | |
| Model I: Main effects | ||||||||
| CP prenatal care (vs. TC) | .25 | .47 | (−.67, 1.17) | 1.28 | −.99 | .47* | (−1.92, −.06) | .37 |
| Model II: Pre-pregnancy BMI Interaction | ||||||||
| CP prenatal care | .07 | .58 | (−1.06, 1.21) | 1.08 | − 1.07 | .57† | (−2.19, .04) | .34 |
| Overweight | −.75 | .41† | (−1.55, .05) | .47 | 1.21 | .42** | (.39, 2.02) | 3.34 |
| Obese | −.02 | .43 | (−.87, .82) | .98 | 1.44 | .47** | (.51, 2.37) | 4.22 |
| CP X overweight | 1.16 | .69† | (−.19, 2.51) | 3.20 | .20 | .63 | (−1.05, 1.44) | 1.22 |
| CP X obese | −.28 | .71 | (−1.68, 1.12) | .76 | −.96 | .66 | (−1.40, 1.21) | .91 |
| Model III: Gestational age interaction | ||||||||
| CP prenatal care | .26 | .48 | (−.68, 1.20) | 1.30 | − 1.04 | .49* | (−2.00, −.08) | .35 |
| Preterm | −.56 | .53 | (−1.61, .48) | .57 | − 1.47 | .72* | (−2.87, −.06) | .23 |
| CP X preterm | .20 | 1.14 | (−2.04, 2.45) | 1.23 | 1.52 | 1.12 | (−.67, 3.72) | 4.58 |
GWG = gestational weight gain, b = logit coefficient, se = standard error of logit coefficient, RRR = relative risk ratio (exp[b]). Coefficients are from multinomial logistic regression models predicting type of gestational weight gain (low, healthy, high per 2009 IOM clinical guidelines) with healthy weight gain as the outcome reference category. All models additionally adjusted for maternal age, race, gravidity, and number of prenatal care visits attended. All models weighted using weights of 1/propensity score for CP participants and 1/(1−propensity score) for traditional care participants.
p < .10.
p < .05.
p < .01.
Figure 1.
Predicted probabilities of type of gestational weight gain (GWG), by format of prenatal care.
Note. GWG – gestational weight gain. Predicted probabilities estimated from multinomial logistic regression models shown in Model I, Table 3.
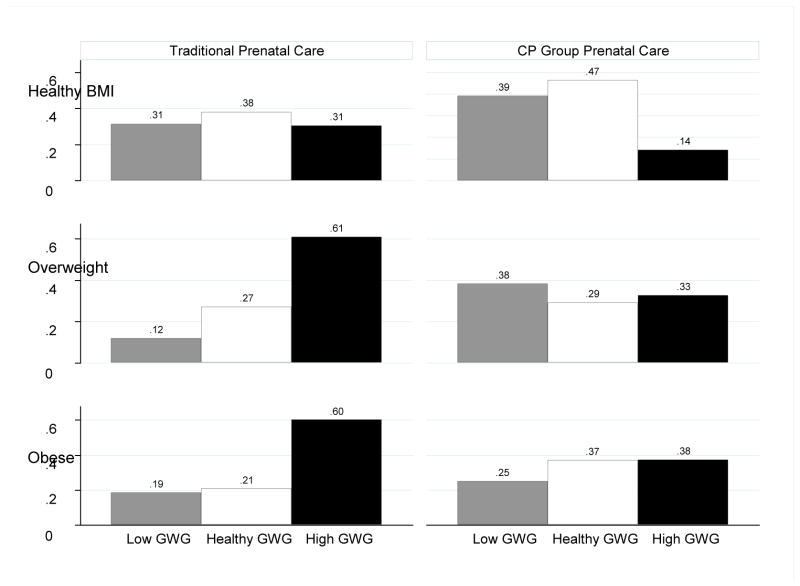
There was no evidence that the effect of prenatal care format on type of gestational weight gain varied according to gestational age for women who were of a healthy weight or were overweight before their pregnancy (Table 4). Women in CP were significantly less likely to have excessive weight gain compared to women in TC, and the effect was most pronounced for women who entered into pregnancy overweight or obese (Figure 2). Therefore, although overweight women enrolled in CP prenatal care were more likely than similar TC participants to have low gestational weight gain, this difference was primarily because women in the latter group were most likely to have excessive gestational weight gain. CP prenatal care had a relatively equalizing effect on gestational weight gain for overweight and obese women. In contrast, overweight and obese women in the TC group were much more likely to exceed the recommended gestational weight gain guidelines. Table 4 presents the various effect size metrics for all women combined, and then separately based on participants’ weights before pregnancy.
Table 4.
Summary of effect sizes indexing comparative effectiveness of two prenatal care models on excessive gestational weight gain, by pre-pregnancy body mass index (BMI) category
|
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Pre-pregnancy BMI Category | ||||||||
|
| ||||||||
| All | Healthy | Overweight | Obese | |||||
|
| ||||||||
| CP | TC | CP | TC | CP | TC | CP | TC | |
| Predicted probability | ||||||||
| Predicted probability that mother will gain excessive weight during pregnancy. | .26 | .48 | .14 | .31 | .33 | .61 | .38 | .60 |
|
| ||||||||
| Risk ratio | ||||||||
| CP reduced the risk of excessive weight gain during pregnancy to ____% of what it would have been in the traditional model of prenatal care. | 54 | 45 | 54 | 63 | ||||
| Absolute risk difference | ||||||||
| CP reduced the risk of excessive weight gain during pregnancy by ____ percentage points compared to the traditional model of prenatal care. | 22 | 17 | 28 | 22 | ||||
| Number needed to treat | ||||||||
| You would need to provide ___ women with CP prenatal care (rather than traditional prenatal care) in order to prevent one case of excessive weight gain during pregnancy. | 5 | 6 | 4 | 5 | ||||
CP = Centering Pregnancy (group) prenatal care; TC = traditional care (individual).
Figure 2.
Predicted probabilities of type of gestational weight gain (GWG), by format of prenatal care and pre-pregnancy body mass index category.
Note. GWG – gestational weight gain. Predicted probabilities estimated from multinomial logistic regression models shown in Model II, Table 3.
Overall, post-hoc analyses indicated no adverse effects of low gestational weight gain on newborn birth weight outcomes. Although CP participants had infants with lower total birth weights than traditional care participants (b = −247.09, p = .004, 95% CI [−415.48, −78.69], B = −.28), results indicated no significant differences between groups on the odds of low birth weight (b = −1.30, p = .12, 95% CI [−.34, 2.94], OR = 3.67). Overweight CP participants had infants with lower total birth weights than overweight TC participants (b = −439.92, p = .02, 95% CI [−794.01, −85.82], B = −.43), but total birth weights were still within healthy ranges for both prenatal care groups (3,197 grams for infants of CP mothers versus 3,327 grams for infants of TC mothers).
DISCUSSION
This non-randomized study used propensity score matching to compare gestational weight gain for women in CenteringPregnancy (CP) group prenatal care and women enrolled in traditional individual care (TC). After controlling for over 20 relevant background characteristics, women enrolled in CP prenatal care were significantly less likely than matched TC participants to gain weight in excess of the IOM clinical guidelines (predicted probability .26 vs .48). The effect of prenatal care format on gestational weight gain did not vary according to gestational age at delivery, but it did vary by pre-pregnancy BMI. Among women with healthy pre-pregnancy BMIs, CP prenatal care was associated with slightly higher probabilities of low weight gain (predicted probability .39 vs .31) and healthy weight gain (predicted probability .47 vs .38). Among overweight women, CP was associated with a significantly higher probability of low gestational weight gain (predicted probability .38 vs .12). Notably, regardless of whether women entered into pregnancy healthy, overweight, or obese, women enrolled in CP were significantly less likely to have excessive weight gain, especially those who were overweight or obese before pregnancy. Additional post-hoc analyses provided no evidence of adverse effects on newborn health outcomes (total birth weights and low birth weights).
Evidence suggests that intervening on maternal weight gain during pregnancy holds promise for interrupting the intergenerational cycle of the obesity epidemic (27). It is believed that adiposity in offspring develops at least in part during the critical window of development in utero (28). Consequently, reversing the obesity epidemic must focus on modulating maternal factors to promote healthy in utero and post-natal growth. Having a practical, effective, and sustainable gestational weight gain intervention would have significant implications for public health. Indeed, excessive gestational weight gain has been linked to numerous detrimental health outcomes, including preterm birth, postpartum weight retention, maternal obesity, and childhood obesity (8,29), each of which is associated with increased healthcare expenditures. Existing behavioral interventions have had modest success in restricting excessive gestational weight gain, and few efforts have been effective enough to increase the probability of women gaining within their recommended target range (6,30).
This study compared the effectiveness of two prenatal care delivery models to reduce excessive gestational weight gain, using a sample of predominantly African American women attending a community health center in the urban South. Compared to women who received traditional care, the CP group had fewer mothers who exceeded the recommended gestational weight gain, especially among those who were overweight or obese before pregnancy. The increased probability of overweight women in CP gaining below their recommended target ranges did not appear to jeopardize infant weight outcomes. This finding mirrors the conclusion of an AHRQ review, wherein strong, consistent evidence was cited of an association between weight gain below the IOM guidelines and low birth weight, but only for underweight and normal weight women (8).
African-American women are at increased risk of entering into pregnancy overweight and gaining additional weight during their childbearing years (31,32,33,34). These women are at high-risk for continued excessive weight gain during each pregnancy. The present study is unique due to the predominantly African American sample, and demonstrates that CP group prenatal care was successful in reducing excessive weight gain during pregnancy in this population.
Interpretation of these results must be tempered by study limitations. Patients were not randomly assigned to CP and TC, but appropriate statistical controls and matching procedures were used to provide a fair and meaningful comparison between the two different prenatal care models. Another limitation is the reliance on retrospective chart reviews. Recording errors could have occurred when providers documented patient height and weight at entry into prenatal care, gestational weight gain during pregnancy, and other patient characteristics. However, such errors are unlikely to vary systematically between CP and TC patients. Our computation of total gestational weight gain used maternal weight measured at the last prenatal visit, not at delivery. Although this measure is often used in the literature, some women may have been misclassified as having gained less than they actually did. The reliance on retrospective data also limited our collection of antepartum data, which may have been used to estimate the propensity scores. Consequently, some variables that may have improved the matching may have been omitted (e.g., transportation or work schedules). Finally, although the predominantly African American sample is a notable strength of the study, our results may not generalize to dissimilar populations. Nonetheless, the IOM has made explicit calls for “research to aid care providers and communities in assisting women – especially low-income and minority women – to meet the new [maternal weight gain] guidelines” (1, p.3).
Taken together, these data suggest that in populations similar to the one studied here, CP group prenatal care may be effective in decreasing the proportion of mothers with excessive gestational weight gain. As noted in a recent systematic review (35), however, little research exists that explores the mechanisms underlying any beneficial effects associated with group prenatal care. Because our study relied on a retrospective chart review, we did not collect process data on delivery of prenatal care, or extensive antepartum data that might permit empirical examination of the underlying mechanisms behind any observed effects. The logic model of CP prenatal care (9) implies that cognitive restructuring may occur during group-delivered prenatal care sessions, whereby group problem-solving, social support, peer influence, and increased educational time may change how expectant mothers think about and manage their nutrition, diet, and exercise during pregnancy. These cognitive shifts, along with promotion of healthy group norms, may then encourage healthy maternal behaviors. We are unaware of any research that has empirically examined the causal pathways by which CP may reduce excessive gestational weight gain. Such work would help inform the development of a structured theoretical framework for understanding the beneficial effects of group prenatal care.
Acknowledgments
Data used in this study were collected with support from contract #19199-GR1030830 from the Tennessee Governor’s Office of Children’s Care Coordination and the Tennessee Department of Health. S.B. Gesell was supported by NICHD/NIH grant K23HD064700. The secondary data analysis project described was supported by CTSA award No. UL1TR000445 from the National Center for Advancing Translational Sciences. The findings and conclusions of this study are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies or their employees. The authors would like to thank Karen Potvin Klein, MA, ELS (Translational Science Institute, Wake Forest University Health Sciences) for her editorial comments.
Contributor Information
Emily E. TANNER-SMITH, Email: e.tanner-smith@vanderbilt.edu, Vanderbilt University, Peabody Research Institute, Box 0181 GPC, 230 Appleton Place, Nashville, TN 37203, 615-322-6304 (P); 615-322-0293 (F).
Katarzyna T. STEINKA-FRY, Email: k.steinka-fry@vanderbilt.edu, Vanderbilt University, Peabody Research Institute, Box 0181 GPC, 230 Appleton Place, Nashville, TN 37203, 615-322-6304 (P); 615-322-0293 (F).
Sabina B. GESELL, Email: sgesell@wakehealth.edu, Department of Social Sciences and Health Policy, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, 336-713-8738 (P); 336-716-7554 (F)
References
- 1.Institute of Medicine. Weight Gain During Pregnancy; Reexamining the Guidelines. Washington, D.C: National Research Council; 2009. Committee to Reexamine IOM Pregnancy Weight Guidelines. [Google Scholar]
- 2.Dietz PM, Callaghan WM, Sharma AJ. High pregnancy weight gain and risk of excessive fetal growth. American Journal of Obstetrics & Gynecology. 2009;201(1):e51–e56. doi: 10.1016/j.ajog.2009.04.051. [DOI] [PubMed] [Google Scholar]
- 3.Gunderson EP, Abrams B, Selvin S. The relative importance of gestational gain and maternal characteristics associated with the risk of becoming overweight after pregnancy. International Journal of Obesity and Related Metabolic Disorders. 2000;24(12):1660–1668. doi: 10.1038/sj.ijo.0801456. [DOI] [PubMed] [Google Scholar]
- 4.Wrotniak BH, Shults J, Butts S, Stettler N. Gestational weight gain and risk of overweight in the offspring at age 7 y in a multicenter, multiethnic cohort study. The American Journal of Clinical Nutrition. 2008;87(6):1818–1824. doi: 10.1093/ajcn/87.6.1818. [DOI] [PubMed] [Google Scholar]
- 5.Mamun AA, O’Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation. 2009;119(13):1720–1727. doi: 10.1161/CIRCULATIONAHA.108.813436. [DOI] [PubMed] [Google Scholar]
- 6.Streuling I, Beyerlein A, von Kries R. Can gestational weight gain be modified by increasing physical activity and diet counseling? A meta-analysis of interventional trials. The American Journal of Clinical Nutrition. 2010;92(4):678–687. doi: 10.3945/ajcn.2010.29363. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine, Food and Nutrition Board. Nutrition During Pregnancy: Part I: Weight Gain, Part II: Nutrient Supplements. Washington, DC: National Academy Press; 1990. [Google Scholar]
- 8.Viswanathan M, Siega-Riz AM, Moos MK, Deierlein A, Mumford S, Knaack J, et al. Outcomes of maternal weight gain. Evidence Report/Technology Assessment. 2008:1–223. [PMC free article] [PubMed] [Google Scholar]
- 9.Rising SS. Centering Pregnancy: An interdisciplinary model of empowerment. Journal of Nurse-Midwifery. 1998;43(1):46–54. doi: 10.1016/s0091-2182(97)00117-1. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy HP, Farrell T, Paden R, Hill MS, Jolivet RR, Cooper BA, et al. A randomized clinical trial of group prenatal care in two military settings. Military Medicine. 2011;176(10):1169–1177. doi: 10.7205/milmed-d-10-00394. [DOI] [PubMed] [Google Scholar]
- 11.Strychar IM, Chabot C, Champagne F, Ghadirian P, Leduc L, Lemonnier M, et al. Psychosocial and lifestyle factors associated with insufficient and excessive maternal weight gain during pregnancy. Journal of the American Dietetic Association. 2000;100(3):353–356. doi: 10.1016/S0002-8223(00)00107-3. [DOI] [PubMed] [Google Scholar]
- 12.Brawarsky P, Stotland NE, Jackson RA, Fuentes-Afflick E, Escobar GJ, Rubashkin N, et al. Pre-pregnancy and pregnancy-related factors and the risk of excessive or inadequate gestational weight gain. International Journal of Gynaecology and Obstetrics. 2005;91(2):125–131. doi: 10.1016/j.ijgo.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Jain NJ, Denk CE, Kruse LK, Dandolu V. Maternal obesity: can pregnancy weight gain modify risk of selected adverse pregnancy outcomes? American Journal of Perinatology. 2007;24(5):291–298. doi: 10.1055/s-2007-981432. [DOI] [PubMed] [Google Scholar]
- 14.Klima C, Norr K, Vonderheid S, Handler A. Introduction of CenteringPregnancy in a public health clinic. Journal of Midwifery & Women’s Health. 2009;54(1):27–33. doi: 10.1016/j.jmwh.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Trudnak T. Doctoral dissertation. 2011. A comparison of Latina women in CenteringPregnancy and individual prenatal care. Available at: ProQuest Dissertations and Theses database (UMI Number: 3450358) [Google Scholar]
- 16.Ruiz-Mirazo E, Lopez-Yarto M, McDonald SD. Group prenatal care versus individual prenatal care: A systematic review and meta-analysis. Journal of Obstetrics and Gynaecology Canada. 2012;34(3):223–229. doi: 10.1016/S1701-2163(16)35182-9. [DOI] [PubMed] [Google Scholar]
- 17.Tanner-Smith EE, Steinka-Fry KT, Lipsey MW. A multi-site evaluation of the CenteringPregnancy® programs in Tennessee. Nashville, TN: Peabody Research Institute, Vanderbilt University; 2012. [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo S, Fraser MW. Propensity score analysis: Statistical methods and applications. Thousand Oaks, CA: Sage; 2010. [Google Scholar]
- 20.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 21.Rubin DB. Using propensity scores to help design observational studies: Application to the tobacco litigation. Health Services & Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 22.Steiner PM, Cook D. Matching and propensity scores. In: Little TD, editor. The Oxford handbook of quantitative methods. New York: Oxford University Press; 2012. [Google Scholar]
- 23.Hirano K, Imbens GW. Estimation of causal effects using propensity score weighting: An application to data on right heart catheterization. Health Services & Outcomes Research Methodology. 2001;2:259–278. [Google Scholar]
- 24.Rosenbaum PR. Covariance adjustment in randomized experiments and observational studies. Statistical Science. 2002;17(3):286–304. [Google Scholar]
- 25.Long JS. Regression models for categorical and limited dependent variables. Thousand Oaks, CA: Sage; 1997. [Google Scholar]
- 26.Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Chichester, UK: John Wiley & Sons; 2008. pp. 243–296. [Google Scholar]
- 27.Nader PR, Huang TT, Gahagan S, Kumanyika S, Hammond RA, Christoffel KK. Next steps in obesity prevention: altering early life systems to support healthy parents, infants, and toddlers. Childhood Obesity. 2012;8(3):195–204. doi: 10.1089/chi.2012.0004. [DOI] [PubMed] [Google Scholar]
- 28.Lawlor DA. The Society for Social Medicine John Pemberton Lecture 2011. Developmental overnutrition- an old hypothesis with new importance? International Journal of Epidemiology. 2013;42(1):7–29. doi: 10.1093/ije/dys209. [DOI] [PubMed] [Google Scholar]
- 29.Davis EM, Babineau DC, Wang X, Zyzanski S, Abrams B, Bodnar LM, Horwitz RI. Short inter-pregnancy intervals, parity, excessive pregnancy weight gain and risk of maternal obesity. Maternal and Child Health Journal. 2013 doi: 10.1007/s10995-013-1272-3. online first. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oteng-Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Medicine. 2012;10:47. doi: 10.1186/1741-7015-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 32.Siega-Riz AM, Evenson KR, Dole N. Pregnancy-related weight gain- a link to obesity? Nutrition Reviews. 2004;62(s2):S105–S111. doi: 10.1111/j.1753-4887.2004.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 33.Davis EM, Zyzanski SJ, Olson CM, Stange KC, Horwitz RI. Racial, ethnic, and socioeconomic differences in the incidence of obesity related to childbirth. American Journal of Public Health. 2009;99(2):294–299. doi: 10.2105/AJPH.2007.132373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Annals of Behavioral Medicine. 2003;26(2):149–159. doi: 10.1207/S15324796ABM2602_07. [DOI] [PubMed] [Google Scholar]
- 35.Sheeder J, Yorga KW, Kabir-Greher K. A review of prenatal group care literature: The need for a structured theoretical framework and systematic evaluation. Maternal and Child Health Journal. 2012;16:177–187. doi: 10.1007/s10995-010-0709-1. [DOI] [PubMed] [Google Scholar]