Abstract
With the recent progress in identifying disease-causing genes in humans and in animal models, there are more and more opportunities for using retinal gene transfer to learn more about retinal physiology and also to develop therapies for blinding disorders. Success in preclinical studies for one form of inherited blindness have led to testing in human clinical trials. This paves the way to consider a number of other retinal diseases as ultimate gene therapy targets in human studies. The information presented here is designed to assist scientists and clinicians to use gene transfer to probe the biology of the retina and/or to move appropriate gene-based treatment studies from the bench to the clinic.
1. Introduction
Because of its ease of access, its benign immunologic response to gene transfer, and the ability to perform noninvasive functional and structural studies, the mammalian eye is an ideal target for gene transfer. Gene transfer has been used to learn about varied topics such as the development and differentiation of the retina and the nature of the suppressive immune response in this tissue. Gene augmentation strategies whereby a wild type copy of a gene is delivered have also been used successfully in proof-of-concept gene therapy studies in small and large animal models of more than a dozen different conditions (Acland et al., 2001, 2005; Alexander et al., 2007; Ali et al., 2000; Allocca et al., 2008, 2011; Andrieu-Soler et al., 2007; Batten et al., 2005; Bennett et al., 1996; Bennicelli et al., 2008; Boye et al., 2010; Cai et al., 2009, 2010; Carvalho et al., 2011; Conley and Naash, 2010; Dejneka et al., 2004; Gargiulo et al., 2009; Georgiadis et al., 2010; Ho et al., 2002; Janssen et al., 2008; Kjellstrom et al., 2007; Kong et al., 2008; Kumar-Singh and Chamberlain, 1996; Mancuso et al., 2009; Mao et al., 2011; Michalakis et al., 2010; Mihelec et al., 2011; Min et al., 2005; Narfstrom et al., 2003a, b, c; Pang et al., 2006, 2008, 2010a, b, 2011; Pawlyk et al., 2005; Sarra et al., 2001; Simons et al., 2011; Sun et al., 2011; Surace et al., 2005; Takahashi et al., 1999; Tan et al., 2009; Williams et al., 2006; Zeng et al., 2004; Zou et al., 2011). In addition, there has been success with strategies aimed at rescuing disease due to toxic gain-of-function mutations (Chadderton et al., 2009; LaVail et al., 2000; Lewin et al., 1998; Millington-Ward et al., 2011; Mussolino et al., 2011; O’Reilly et al., 2007; Palfi et al., 2006; Tam et al., 2008, 2010).
The expansion of the “toolkit” of vectors that can be used to deliver nucleic acids to retinal cells in recent years will enhance these opportunities. There are now a number of promising physicochemical (nonviral) reagents as well as recombinant virus vectors available for studies of the retina. New recombinant viral vectors contain modifications of capsids, envelopes, and surface proteins designed to achieve the desired transduction parameters. A large number of these will continue to be useful to evaluate a variety of biochemical, cell biological, developmental, immunologic, physiologic, and therapeutic parameters involving wild-type and mutant retinal proteins. Some of them may also be useful in generating new animal models via somatic gene transfer.
At this point in time, the vast majority of vectors that have been tested have a very limited ability to diffuse across tissue interfaces. Thus, to deliver nucleic acids to the outer retina (photoreceptors and retinal pigment epithelium (RPE) cells), it is necessary to carry out a subretinal injection. Expertise in delivering vectors to the outer retina is not widely available, particularly for large animal models and humans. Therefore, we describe the procedures for carrying out subretinal injections in small and large animal models and ultimately in humans. All of the studies described in this chapter assume that the investigator obtains the appropriate institutional and federal approvals (including rDNA approvals) before carrying out such studies. They also assume that the investigator has an appropriately trained assistant. The investigator should also be sure to use the minimum number of animals/subjects to obtain statistically significant results, all instruments that come in contact with the eye should be sterile, investigators should have the appropriate qualifications, rigorous safety studies in large animal models should be carried out before testing retinal gene transfer in humans, informed consent should be obtained from human subjects enrolling in clinical trials, and animals/subjects should be appropriately anesthetized and be given the appropriate postoperative care.
2. Nucleic Acid Delivery to the Outer Retina in the Mouse
In this section, we provide procedures for delivering nucleic acids to the outer retina of the mouse (Bennett et al., 1996; Liang et al., 2000). The surgical approach to subretinal injection depends mainly on the size of the eye. In the mouse, the relative volume of the vitreous space occupied by the crystalline lens is large (Fig. 13.1). Thus, it is difficult to introduce a cannula using an anterior approach as there is a high likelihood of damaging the lens and causing damage that will lead to a cataract and/or inflammation. Therefore, a posterior approach is used, in which an incision is made across the posterior sclera and choroid, and a cannula is placed in the subretinal space. An assistant pushes the plunger of the injection syringe when signaled by the surgeon. The injection is not performed by direct visualization as the pupil is rotated away from the surgeon in order to expose the posterior part of the globe. Accuracy of the injection should be assessed after the procedure.
Figure 13.1.
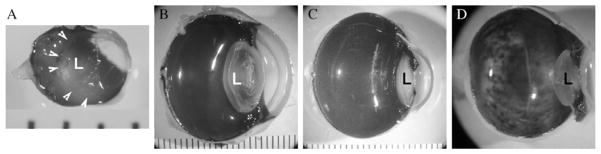
The space occupied by the lens relative to the vitreous cavity differs across species and dictates the surgical approach. In the mouse (A), the lens (L) occupies the majority of the cavity and so injections are usually carried out with a trans-choroidal approach. In larger animals ((B) dog, (C) monkey) and (D) humans, the lens is much smaller relative to the rest of the eye and so injections can be carried out from an anterior approach under direction visualization. Arrowheads in (A) indicate the borders of the lens. Distance between each bar in (A)–(C) is 1mm.
2.1. Surgical procedures in the mouse
2.1.1. Required materials
Devices and materials of the injection apparatus
Dissecting microscope (15×) (e.g., Nikon SMZU microscope, Optical Apparatus, Ardmore, PA)
Heating pad
Fiber optic light source
10μl Hamilton syringe with 33-gauge blunt needle (e.g., Hamilton #801RN and Hamilton #79633, Baxter Scientific Products, Edison, NJ)
Additional materials
Indirect ophthalmoscope with 78 or 90D lens; ideally equipped with a blue filter (e.g., Keeler Vintage)
Vector of interest, stored on wet ice, purified, and in a sterile container (e.g., sterile 1.5ml Eppendorf tube)
Vannas iridotomy scissors (e.g., catalog #RS-5610, Roboz Surgical, Rockville, MD)
Jeweler’s forceps (e.g., Dumont #5, Roboz Surgical)
Hand rest (e.g., foam block, towel)
Other reagents
Dilating agents: 1.0% (w/v) tropicamide, topical (Alcon, Fort Worth, TX)
Analgesic: for example, Meloxicam 5mg/kg SC once at the time of surgery; acetaminophen (1.6mg/ml) in drinking water for 3 days prior to and 2–3 days following surgery
Anesthetic: (subject to approval of Institutional Animal Care and Use Committee and to controlled substance approvals) Ketamine/Xylazine (100mg/ kg/10mg/kg IM) or isoflurane 1–5% (decreased following initial dose depending on the degree of sedation); topical—proparacaine HCl 0.5%
Povidone-iodine 5% (Betadine 5%, Escalon Ophthalmics, Skillman, NJ)
Wratten 47B gelatin excitation filter (Kodak, Rochester, NY)
PredG ointment (prednisolone acetate–gentamicin, 0.3%/0.6%, Allergan Pharmaceuticals, Irvine, CA)
Phosphate-buffered saline (PBS)
Disinfectant: 10% bleach (i.e., 0.525g sodium hypochlorite/100ml) or cidex
Disposables
Personal protective apparel (gloves, mask, gowns, booties)
Surgical tape
30-gauge 0.5-in. needles
2.1.2. Injection
Starting 3 days prior to injection, the animal is treated with acetaminophen in drinking water. Just prior to surgery, the animal is anesthetized and the pupils are dilated with 0.5% tropicamide. The anesthetized animal is positioned under the microscope with surgical tape. All procedures are done aseptically using sterile instruments, surgical fields, and solutions. One drop of betadine solution is placed in the fornix and ocular adnexa. A conjunctival incision is made parallel to the base of the cornea, just anterior to the equator of the eye. The eye is rotated by grasping the conjunctival flap near the cornea. The topical anesthetic, ophthetic (proparacaine HCl 0.5%), is applied (1 drop OU). A sclerotomy is made by advancing a 27-gauge needle so that the bevel just enters the vitreous cavity. A 33-gauge needle connected to a Hamilton syringe is inserted through the sclerotomy 2mm posterior to the temporal limbus. The cannula is then advanced tangential to the curvature of the globe to the subretinal space in the posterior pole. One microliter of transfection solution (e.g., purified recombinant adeno-associated virus) is injected into the subretinal space with the assistant pushing the plunger. (There is a Hamilton syringe under development that can be operated by a single person, if an assistant is unavailable.) The needle/plunger is held in place for 5s following injection in order to minimize reflux from the injection site. A successful subretinal injection raises a dome-shaped retinal detachment (bleb, Fig. 13.2), apparent immediately after injection when viewed with an indirect ophthalmoscope. The detachment covers only a small fraction (~1/5) of the retina. The solution is not drained but is resorbed within a few hours by the retina.
Figure 13.2.
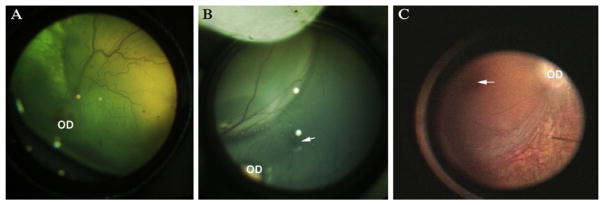
Appearance of the “bleb” immediately following subretinal injection in (A) dog, (B) non-human primate (NHP), and (C) human. OD, optic disc; arrow indicates the fovea in the NHP and the human. Panel (C) was taken from an intraoperative video recording.
Each retina undergoes only one injection. A localized retinal detachment indicates a successful injection. With practice, an investigator can expect to achieve accurate subretinal injections in ~80% of the animals.
The eyes are dressed with PredG ointment immediately following the surgery so that the cornea does not dessicate. The animals are attended and are kept warm through recovery on a heating pad. Animals are provided appropriate postoperative analgesia (meloxicam and acetaminophen). Animals are monitored for potential (although rare) postoperative complications including infection. The investigators will be prepared to treat a rodent or terminate the experiment for any particular animals that appears to be in distress. In our experience, the infection rate and mortality rate for this procedure is low.
2.2. Determination of retinal health/transduction outcome post surgery
Indirect ophthalmoscopy is useful for assessing retinal health serially over time and can be carried out without anesthesia. Expression of certain reporter genes (e.g., green fluorescent protein (GFP)) can be appreciated through indirect ophthalmoscopy if the ophthalmoscope is equipped with a cobalt blue filter (Fig. 13.2; Bennett et al., 1997). If not, a Wratten filter can be taped over the light source in order to excite the GFP at the appropriate wavelength. Additional imaging will depend upon availability of specialized equipment. The following noninvasive imaging protocols are provided as examples. In all of these, the retinas are first dilated with tropicamide: (1) animals injected with vectors carrying lucifererase can be evaluated with an In Vivo Imaging System (IVIS; XENOGEN, Caliper Life Sciences, Hopkinton, MA) after i.p. injection of the luciferase substrate, luciferin (150mg/kg); (2) the retina can be evaluated through optical coherence tomography (OCT) in order to determine the thicknesses of the various retinal cell layers. Animals are positioned in front of the OCT probe (e.g., Spectral Domain Imaging System, Bioptigen, Inc., Durham, NC) and the retina is brought into focus. Images are collected; (3) fundus photographs may be obtained if an appropriate camera is available (e.g., Micron III fundus camera, Phoenix Research Laboratories, Inc., Pleasanton, CA). For this, the animals are held under the photographic lens so that the retina can be viewed through the fundus camera. Photographs are taken. The procedure is quick (generally 1min per eye for a trained investigator) and does not require anesthesia. Sterile eye lubricant (saline or Artificial Tears) can be applied to the corneas topically as needed. After any of these procedures, the corneas can be dressed with 1cm of PredG ointment to alleviate dryness.
The investigator should make sure that the animals do not become hypothermic during any imaging procedures incorporating anesthesia as there is a tendency for this to induce a (temporary) cataract which will limit visualization of the retina.
3. Nucleic Acid Delivery to the Outer Retina in the Dog
3.1. Surgical procedures in the dog
In this section, we provide procedures for delivering nucleic acids to the outer retina of the dog. The same procedures would be effective in other similar-sized animals (e.g., cats, rabbits). In these large animals, the relative volume of the vitreous space occupied by the crystalline lens is small (Fig. 13.1). This allows instruments to be introduced under direct visualization with an anterior approach (Acland et al., 2001, 2005; Amado et al., 2010; Bennicelli et al., 2008). A transchoroidal approach is possible, but it requires manipulations through the choriocapillaris layer that do not allow direct visualization. It is advisable to avoid introduction of potentially immunogenic material in this region of high blood flow (vascularity). Further, the injection procedure cannot be visualized using a transchoroidal approach. In contrast, an anterior approach allows one to assess accuracy of the injection during and immediately after the procedure. There is no need in the dog to carry out a vitrectomy prior to performing the injection as long as an equivalent amount of fluid (as that to be injected) is removed from the anterior segment. The injection is achieved by delivery through a small retinotomy with a 39-gauge cannula. As with the mouse, a localized retinal detachment is generated, raising a “bleb” (Fig. 13.2). Most, if not all the volume, of injected material is trapped between the outer retina and RPE in this scenario. Thus, cells expressing the transgene are in this region of the retina (Fig. 13.3). There is negligible escape of material back through the retinotomy site into the vitreous as evidenced by the fact that the size of the bleb does not change once it is formed. It is possible to use an even smaller diameter (i.e., 41-gauge) cannula for subretinal injections, but it is more difficult to maneuver this flexible device through the semisolid vitreous.
Figure 13.3.
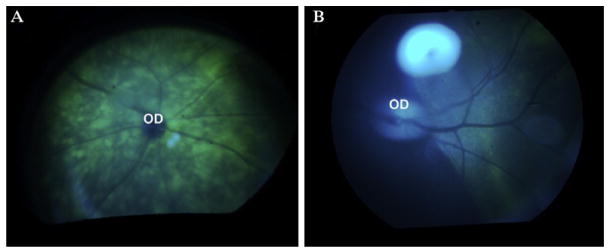
Green fluorescent protein (GFP) is visible through illumination with blue light with an ophthalmoscope in animals after subretinal injection of a recombinant adeno-associated virus vector delivering the gene encoding GFP. The GFP is below the layer of the inner retinal vessels, which appear dark. (A) Mouse; (B) non-human primate; OD, optic disc.
3.1.1. Required materials
Devices and materials of the microinjection apparatus
Operating microscope (e.g., Zeiss operating microscope)
Heating pad
Innorex 25/39-gauge subretinal injection syringe (Surmodics, Edina, MN). This unit is not FDA approved, but contains all of the elements needed for subretinal injection in 1 unit. Alternatively, the reagents listed below for human surgery (Section 5.1.1) can be used.
Hand rest (e.g., foam block, towel)
Additional materials
Indirect ophthalmoscope with 20 or 28D lens; ideally equipped with a blue filter
Vector of interest, stored on wet ice, purified, and in a sterile container (e.g., sterile 1.5ml Eppendorf tube)
Other reagents
Sedation: acepromazine, 0.1–0.3mg/kg IM then atropine 0.03mg/kg IM
Anesthetic (subject to approval of Institutional Animal Care and Use Committee and to controlled substance approvals): Induction (after aseptic preparation of the site of venipuncture) will be with propofol (2mg/kg IV). This will be followed by intubation and ventilation with 2.5% isofluorane.
Povidone-iodine 5% (Betadine 5%, Escalon Ophthalmics)
Eye drops:
1.0% (w/v) tropicamide (Alcon)
Mydfin (phenylephrine hydrochloride) 2.5%
Flurbiprofen, 0.3% ophthalmic solution
Ciprofloxacin, 0.3% ophthalmic solution
Analgesic: topical—proparacaine HCl 0.5%
Artificial Tears: Systane lubricant, Alcon
PredG ointment (prednisolone acetate–gentamicin, 0.3%/0.6%, Allergan Pharmaceuticals)
Gonak/Goniosol solution (hydrocellulose; Akorn, Inc., Lake Forest, IL)
Wescote scissors
Bishop forceps
Steven’s scissors
Hemostat
Lid speculum
2.5% fluorescein in PBS
Wratten 47B gelatin excitation filter (Kodak)
Phosphate-buffered saline
Disinfectant: 10% bleach (i.e., 0.525g sodium hypochlorite/100ml)
Disposables
Corneal contact lens (e.g., flat Machemer lens)
Surgical tape
Hi Temperature Ophthalmic Cautery
1cc syringe with 27-gauge needle (e.g., tuberculin (tb) syringe with removable needle)
30-gauge needle
5cc syringe with 1⅝-in. 25-gauge needle
3.1.2. Injection
All procedures are done aseptically using sterile instruments, surgical fields, and solutions. Anesthesia in the dog is achieved after pre-anesthesia with pre-acepromazine, 0.1–0.3mg/kg IM and then atropine, 0.03mg/kg IM. Induction (after aseptic preparation of the site of venipuncture) will be with propofol. This will be followed by intubation and ventilation with 2.5% isofluorane. This will be decreased to maintain anesthesia. It is estimated that the dog will be intubated a maximum of 20min. Although isoflurane anesthesia is our preferred method, it is possible to perform the procedure using IV ketamine and diazepam.
Just prior to surgery (i.e., during the time that acepromazine is administered), the pupils are dilated with mydriacyl (tropicamide) 1% and mydfin (phenylephrine hydrochloride) 2.5%, and flurbiprofen, proparacaine, and ciprofloxacin drops are applied to the cornea. After the animal has been anesthetized, it is positioned chin down in the operating field and one drop of betadine solution is placed in the fornix and ocular adnexa. Five cc of sterile saline or local anesthetic solution is injected retro-orbitally with a 25-gauge needle to proptose the eye and prevent the nicatating membrane from covering the surgical field. Proptosis will prevent Bell’s phenomenon, which prevents visualization in the dog, due to rotation of the eye from the straight ahead position. The fluid injected retro-orbitally prior to the procedure is resorbed within 1h.
A speculum is used to hold the eyelids open. A drop of proparacaine may be added for topical anesthesia. A radial conjunctival incision is performed to expose the sclera incision site. This site is lightly treated with Cautery for hemostasis. The anterior chamber is tapped with a 30-gauge needle to remove ~0.1μl of aqueous humor. It is advisable to bend the tip of the 30-gauge needle 30° in order to avoid damaging the lens or iris. The anterior chamber tap is carried out to make space for the volume of vector solution that will be injected under the retina. This fluid can be stored frozen and used later for other studies. A sclerotomy is then made by penetration of a 27-gauge needle into the geometric center of the globe. The site is ~3mm posterior to the limbus in a quadrant accessible to the dominant (injecting) hand.
The cornea is kept moist through application of sterile PBS. The contact lens is coupled to the cornea with Goniosol. Visualization is achieved via coaxial illumination through an operating microscope. A Surmodics 30-gauge subretinal injection syringe is inserted through the sclerotomy site. Alternative devices may be used (e.g., the devices that are used in human surgery). The cannula is then advanced through the vitreous. The speculum can be removed at this point since the corneal contact lens holds the eyelids open. The cannula is advanced to the point where the retina is indented and draped over the tip. It may be easiest for visualization to perform the injection in the tapetal (superior) portion of the retina rather than the nontapetal region. Under microscopic control, 25–400μl of the vector (which may contain 2.5% fluorescein for visualization) is injected into the subretinal space when the assistant is told to push the plunger on the syringe. This raises a dome-shaped retinal detachment (bleb; Fig. 13.2). The plunger is held in place for 5s following injection in order to overcome any impedance through the small gauge cannula which delays egress of the fluid. The solution is resorbed within a few hours by the retina. The retinas can be examined with indirect ophthalmoscopy to verify location of the retinal detachment immediately after the injection has taken place. Photographs may be taken with a fundus camera. A subconjunctival injection of 0.15ml of kenalog solution (40mg/ml) is delivered. The cornea is dressed with PredG ointment and the animal is awoken.
3.2. Determination of transduction outcome
Similar to studies in mice, indirect ophthalmoscopy is useful for assessing canine retinal health serially over time after gene transfer. Ophthalmoscopy can be carried out (after dilating the pupil) without anesthesia. If the subretinal injection has been targeted for the tapetal retina, there will likely be a change in the reflectivity of this layer in the region that had been injected. This is likely due to injection-induced alterations in the crystalline structure of molecules in the tapetum and forms a convenient marker for identifying the region of retina that has been exposed to the vector. Additional imaging can include OCT and fundus photography. Again, these procedures can be carried out without anesthesia. Sterile eye lubricant (saline or Artificial Tears) can be applied to the corneas topically as needed. After any of these procedures, the corneas can be dressed with 1cm of PredG ointment to alleviate dryness.
4. Nucleic Acid Delivery to the Outer Retina in the Non-Human Primate
4.1. Surgical procedures in the non-human primate
In this section, we provide procedures for delivering nucleic acids to the outer retina of the non-human primate (NHP). The procedure is similar to that used for dogs (Section 3) except that care is taken to avoid damage to the fovea. The fovea is a structure that is present only in primates (NHP and humans, Fig. 13.3). As with the dog, the instruments to be introduced under direct visualization with an anterior approach (and without vitrectomy) and accuracy of the injection can be assessed during and immediately after the procedure (Amado et al., 2010; Bennett et al., 1999; Lebherz et al., 2005a, b; Vandenberghe et al., 2011).
4.1.1. Required materials
Devices and materials of the injection
Operating microscope (e.g., Zeiss operating microscope)
Heating pad
Innorx 30-gauge subretinal injection syringe (Surmodics). This unit is not FDA approved, but contains all of the elements needed for subretinal injection in one unit. Alternatively, the devices listed below for human surgery (Section 5.1.1) can be used.
Additional materials
Indirect ophthalmoscope with 23D lens; ideally equipped with a blue filter
Vector of interest, stored on wet ice, purified, and in a sterile container (e.g., sterile 1.5ml Eppendorf tube)
Hand rest (e.g., foam block, towel)
Other reagents
Anesthetic (subject to approval of Institutional Animal Care and Use Committee and to controlled substance approvals): Induction (after aseptic preparation of the site of venipuncture) will be with propofol. This will be followed by intubation and ventilation with 2.5% isofluorane. As an alternative, Dexmedetomidine (0.05–0.1mg/kg IM) may be used.
Povidone-iodine 5% (Betadine 5%, Escalon Ophthalmics)
Eye drops:
1.0% (w/v) tropicamide (Alcon)
Mydfin (phenylephrine hydrochloride) 2.5%
Flurbiprofen, 0.3% ophthalmic solution
Ciprofloxacin, 0.3% ophthalmic solution
Analgesic: topical—proparacaine HCl 0.5%
Artificial Tears: Systane lubricant, Alcon
PredG ointment (prednisolone acetate–gentamicin, 0.3%/0.6%, Allergan Pharmaceuticals)
Gonak/Goniosol solution (hydrocellulose; Akorn, Inc.)
Analgesic: Flunixin meglumine (1.0mg/kg IM once a day)
Wescote scissors
Bishop forceps
Steven’s scissors
Hemostat
Phosphate-buffered saline
Lid speculum
Disinfectant: 10% bleach (i.e., 0.525g sodium hypochlorite/100ml)
Mold to immobilize the head (e.g., foam pillow with space carved out to rest the back of the head)
2.5% fluorescein in PBS
Disposables
Corneal contact lens (e.g., flat Machemer lens)
Specialized protective apparel for the investigators (face shields, biohazard suits)
Hi Temperature Cautery
1cc syringe with 27-gauge needle (e.g., tb syringe with removable needle)
30-gauge needle
5cc syringe with 1.5-in. 25-gauge needle
4.1.2. Injection
All procedures are done aseptically using sterile instruments, surgical fields, and solutions. Additional care should be taken to minimize the possibility of body contact with fluids from the NHP due to the dangers imposed by Simian B virus. For example, face shields and biohazard suits should be worn by the investigators. Anesthesia in the NHP can be achieved with Dexmedetomidine.
The pupils are dilated with mydriacyl (tropicamide) 1% and mydfin (phenylephrine hydrochloride) 2.5%, and flurbiprofen, proparacaine, and ciprofloxacin drops are applied to the cornea. The animal is positioned in the foam pillow so that its chin is facing up in the operating field. One drop of betadine solution is placed in the fornix and ocular adnexa. A drop of proparacaine is used for topical anesthesia. The remainder of the surgical procedure is carried out similarly as described above, for the dog (Section 3.1.2). After the subretinal injection has been completed, flunixin meglumine (1.0mg/kg IM once a day) is delivered for analgesia. The cornea is dressed with PredG ointment and the animal is awoken.
4.2. Determination of transduction outcome
Similar to studies in dogs, indirect ophthalmoscopy is useful for assessing retinal health serially over time in the NHP. Ophthalmoscopy (and other imaging procedures) in the NHP should be carried out under anesthesia due to the challenges of having the animal cooperate. Additional imaging can include OCT and fundus photography. Pupil dilation will be required prior to all of these procedures. Sterile eye lubricant (saline or Artificial Tears) can be applied to the corneas topically as needed. After any of these procedures, the corneas can be dressed with 1cm of PredG ointment to alleviate dryness.
5. Nucleic Acid Delivery to the Outer Retina in the Human
5.1. Surgical procedures in the human
In this section, we provide procedures for delivering nucleic acids to the outer retina in human subjects (Maguire et al., 2008, 2009). The procedure is similar to that used for NHP (Section 4; Fig. 13.2) except that special procedures are implemented to provide the surgeon with more control and to ensure the maximum amount with respect to the surgical procedure. These procedures include a three-port approach with a full vitrectomy and protection of the fovea during the procedure mediated by the dense liquid, Perfluoron. There is a wealth of experience with three port pars plana vitrectomy in human retinal surgery and the details of this procedure are not described here. The injection approach has been developed for other human subretinal applications. As with the other large animals, accuracy of the injection can be assessed both during (Fig. 13.2) and immediately after the procedure. Further, additional maneuvers such as fluid–gas exchange and laserpexy can be employed with the pars plana approach in order to manipulate the sub-retinal bleb or to manage potential complications.
5.1.1. Required materials
Devices and materials for the injection
Operating microscope (e.g., Zeiss operating microscope)
Good manufacturing processes (GMP) vector, stored in a cooler and delivered to the operating room in a sterile vessel (e.g., capped syringe)
Other reagents
Anesthetic (subject to the surgeon’s preferences and IRB approval): For example, induction (after aseptic preparation of the site of venipuncture) can be with propofol. This can be followed by intubation and ventilation with 2.5% isofluorane.
Indirect ophthalmoscope with 23D lens
Povidone-iodine 5% (Betadine 5%, Escalon Ophthalmics)
Eye drops:
1.0% (w/v) tropicamide (Alcon)
Mydfin (phenylephrine hydrochloride) 2.5%
Flurbiprofen, 0.3% ophthalmic solution
Ciprofloxacin, 0.3% ophthalmic solution
Analgesic: topical—proparacaine HCl 0.5%
Artificial Tears: Systane lubricant, Alcon
PredG ointment (prednisolone acetate–gentamicin, 0.3%/0.6%, Allergan Pharmaceuticals)
Gonak/Goniosol solution (hydrocellulose; Akorn, Inc.)
Wescote scissors
Bishop forceps
Steven’s scissors
Hemostat
Phosphate-buffered saline
Lid speculum
Perfluorooctane liquid (Perfluoron, Alcon)
Disposables
Corneal contact lens (e.g., flat Machemer lens)
Surgical gowns, masks, gloves, drapes, etc.
Hi Temperature Cautery
Suture
5.1.2. Injection
All procedures are done using standard surgical procedures (i.e., sterile instruments, surgical fields, supplies, and solutions). It may be advisable to provide a short course of oral steroids just prior to and following the procedure to minimize inflammation due to the surgical procedure (Maguire et al., 2008, 2009).
General anesthesia or monitored sedation anesthetic technique can be used according to the surgeon’s/patient’s preferences. The pupil is dilated with mydriacyl (tropicamide) 1% and mydfin (phenylephrine hydrochloride) 2.5%, and flurbiprofen, proparacaine, and ciprofloxacin drops are applied to the cornea. The subject is positioned supine in the operating field. One drop of betadine solution is placed in the fornix and ocular adnexa.
The eye is stabilized with a retrobulbar injection of 4.0ml marcaine (0.25%) and 1.0ml triamcinolone acetonide (40mg/ml). Standard techniques for vitreo-retinal surgery are used. The surgery can be recorded intraopera-tively with a video device, if that is available. A three port pars plana vitrectomy is performed with removal of posterior cortical vitreous. If epiretinal membranes are observed, they should be removed (Maguire et al., 2009).
The fovea should be buttressed from hydrodynamic stress during injection with perfluorooctane (PFO) liquid (Perfluoron, Alcon), which is heavier than water. A 21-guage silicone-tipped cannula is used to inject a small amount (~0.2ml) of PFO liquid over the macula. This will coalesce into a small bubble over the fovea. The subretinal injection cannula is placed no closer than 3000μm from the foveal center. Injection in eyes with advanced degeneration is typically easier if the tip is placed in the vicinity of the papillomacular bundle between the fovea and disc, as the retina is thicker in this area. Even in eyes with advanced retinal degeneration, the retina in this area is usually thick enough to allow for successful placement of the cannula tip and the injection into the subretinal space. A Bausch & Lomb Storz 39-gauge translocation cannula (San Dimas, CA) is used. The cannula tip is positioned so as to avoid direct injury to retinal arterioles. After lowering infusion pressure, the surgeon asks the assistant to inject a small amount of material to be sure that the injection is going subretinally. When subretinal delivery is observed, the remainder of the injection solution is injected into the subretinal space. Under microscopic control, 100–400μl of the GMP grade vector is injected into the subretinal space when the assistant is told to push the plunger on the syringe. This creates a localized dome-shaped retinal detachment. The cannula/plunger is held in place for 5s after the injection to minimize reflux of the injection material. The PFO liquid is then aspirated. A 50% fluid–air exchange is then performed prior to closure of incisions, carefully avoiding draining through the retinotomy created for the subretinal injection. Air exchange is principally done to compartmentalize the subretinal injection so that the vector does not come into contact with anterior uveal structures and remains central to the area of the retina–RPE.
The subject is recovered from anesthesia but is positioned in the postoperative period to orient the eye so that the desired area of retinal exposure is in the most dependent position while the subretinal injection fluid is resorbed and the retina reattaches. The sclera incisions are closed.
5.2. Determination of transduction outcome
As in the other species, indirect ophthalmoscopy is useful for assessing retinal health serially over time in humans. It is often difficult to appreciate the borders of the original injection site following subretinal injection in the human. Usually the retinotomy site can be identified as a small atrophic region. Additional imaging can include OCT, autofluorescence imaging, fundus photography, and adaptive optics scanning laser ophthalmoscopy, depending upon the data desired, equipment availability and expertise of the personnel. Pupil dilation will be required prior to all of these procedures. Sterile eye lubricant (Artificial Tears) can be applied to the corneas topically as needed. After any of these procedures, the corneas can be dressed with 1cm of PredG ointment to alleviate dryness.
Acknowledgments
We are grateful for support from NIH (Pioneer Award 1DP1OD008267-01 and 1R24EY019861-01A1), the University of Pennsylvania, Institute for Translational Medicine and Experimental Therapeutics (ITMAT), Choroideremia Research Foundation, Foundation Fighting Blindness, the Center for Cellular and Molecular Therapeutics at The Children’s Hospital of Philadelphia, Research to Prevent Blindness, the Grousbeck Family Foundation, Paul and Evanina Mackall Foundation Trust, and the F.M. Kirby Center for Molecular Ophthalmology.
References
- Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- Acland GM, Aguirre GD, Bennett J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JJ, Umino Y, Everhart D, et al. Restoration of cone vision in a mouse model of achromatopsia. Nat Med. 2007;13:685–687. doi: 10.1038/nm1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali R, Sarra GM, Stephens C, et al. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat Genet. 2000;25:306–310. doi: 10.1038/77068. [DOI] [PubMed] [Google Scholar]
- Allocca M, Doria M, Petrillo M, et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest. 2008;118:1955–1964. doi: 10.1172/JCI34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allocca M, Manfredi A, Iodice C, et al. AAV-mediated gene replacement either alone or in combination with physical and pharmacological agents results in partial and transient protection from photoreceptor degeneration associated with {beta}PDE deficiency. Invest Ophthalmol Vis Sci. 2011;52(8):5713–5719. doi: 10.1167/iovs.10-6269. [DOI] [PubMed] [Google Scholar]
- Amado D, Mingozzi F, Hui D, et al. Safety and efficacy of subretinal re-administration of an AAV2 vector in large animal models: Implications for studies in humans. Sci Transl Med. 2010;2:21ra16. doi: 10.1126/scitranslmed.3000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrieu-Soler C, Halhal M, Boatright J, et al. Single-stranded oligonucleotide-mediated in vivo gene repair in the rd1 retina. Mol Vis. 2007;13:682–706. [PMC free article] [PubMed] [Google Scholar]
- Batten ML, Imanishi Y, Tu DC, et al. Pharmacological and rAAV gene therapy rescue of visual functions in a blind mouse model of Leber congenital amaurosis. PLoS Med. 2005;2:e333. doi: 10.1371/journal.pmed.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J, Tanabe T, Sun D, et al. Photoreceptor cell rescue in retinal degeneration (rd) mice by in vivo gene therapy. Nat Med. 1996;2:649–654. doi: 10.1038/nm0696-649. [DOI] [PubMed] [Google Scholar]
- Bennett J, Duan D, Engelhardt JF, et al. Real-time, noninvasive in vivo assessment of adeno-associated virus-mediated retinal transduction. Invest Ophthalmol Vis Sci. 1997;38:2857–2863. [PubMed] [Google Scholar]
- Bennett J, Maguire AM, Cideciyan AV, et al. Stable transgene expression in rod photoreceptors after recombinant adeno-associated virus-mediated gene transfer to monkey retina. Proc Natl Acad Sci USA. 1999;96:9920–9925. doi: 10.1073/pnas.96.17.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennicelli J, Wright JF, Komaromy A, et al. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye SE, Boye SL, Pang J, et al. Functional and behavioral restoration of vision by gene therapy in the guanylate cyclase-1 (GC1) knockout mouse. PLoS One. 2010;5:e11306. doi: 10.1371/journal.pone.0011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Nash Z, Conley SM, et al. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS One. 2009;4:e5290. doi: 10.1371/journal.pone.0005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Conley SM, Naash MI. Gene therapy in the retinal degeneration slow model of retinitis pigmentosa. Adv Exp Med Biol. 2010;664:611–619. doi: 10.1007/978-1-4419-1399-9_70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LS, Xu J, Pearson RA, et al. Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy. Hum Mol Genet. 2011;20(16):3161–3175. doi: 10.1093/hmg/ddr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton N, Millington-Ward S, Palfi A, et al. Improved retinal function in a mouse model of dominant retinitis pigmentosa following AAV-delivered gene therapy. Mol Ther. 2009;17:593–599. doi: 10.1038/mt.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley SM, Naash MI. Nanoparticles for retinal gene therapy. Prog Retin Eye Res. 2010;29:376–397. doi: 10.1016/j.preteyeres.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejneka N, Surace E, Aleman T, et al. Fetal virus-mediated delivery of the human RPE65 gene rescues vision in a murine model of congenital retinal blindness. Mol Ther. 2004;9:182–188. doi: 10.1016/j.ymthe.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Gargiulo A, Bonetti C, Montefusco S, et al. AAV-mediated tyrosinase gene transfer restores melanogenesis and retinal function in a model of oculo-cutaneous albinism type I (OCA1) Mol Ther. 2009;17:1347–1354. doi: 10.1038/mt.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadis A, Tschernutter M, Bainbridge JW, et al. AAV-mediated knockdown of peripherin-2 in vivo using miRNA-based hairpins. Gene Ther. 2010;17:486–493. doi: 10.1038/gt.2009.162. [DOI] [PubMed] [Google Scholar]
- Ho TT, Maguire AM, Aguirre GD, et al. Phenotypic rescue after adeno-associated virus-mediated delivery of 4-sulfatase to the retinal pigment epithelium of feline mucopolysaccharidosis VI. J Gene Med. 2002;4:613–621. doi: 10.1002/jgm.302. [DOI] [PubMed] [Google Scholar]
- Janssen A, Min SH, Molday LL, et al. Effect of late-stage therapy on disease progression in AAV-mediated rescue of photoreceptor cells in the retinoschisin-deficient mouse. Mol Ther. 2008;16:1010–1017. doi: 10.1038/mt.2008.57. [DOI] [PubMed] [Google Scholar]
- Kjellstrom S, Bush RA, Zeng Y, et al. Retinoschisin gene therapy and natural history in the Rs1h-KO mouse: Long-term rescue from retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:3837–3845. doi: 10.1167/iovs.07-0203. [DOI] [PubMed] [Google Scholar]
- Kong J, Kim SR, Binley K, et al. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008;15:1311–1320. doi: 10.1038/gt.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh R, Chamberlain JS. Encapsidated adenovirus minichromosomes allow delivery and expression of a 14 kb dystrophin cDNA to muscle cells. Hum Mol Genet. 1996;5:913–921. doi: 10.1093/hmg/5.7.913. [DOI] [PubMed] [Google Scholar]
- LaVail MM, Yasumura D, Matthes MT, et al. Ribozyme rescue of photoreceptor cells in P23H transgenic rats: Long-term survival and late stage therapy. Proc Natl Acad Sci USA. 2000;97:11488–11493. doi: 10.1073/pnas.210319397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebherz C, Auricchio A, Maguire AM, et al. Long-term inducible gene expression in the eye via adeno-associated virus gene transfer in nonhuman primates. Hum Gene Ther. 2005a;16:178–186. doi: 10.1089/hum.2005.16.178. [DOI] [PubMed] [Google Scholar]
- Lebherz C, Maguire A, Auricchio A, et al. Non-human primate models for retinal neovascularization using AAV2-mediated overexpression of vascular endothelial growth factor. Diabetes. 2005b;54:1141–1149. doi: 10.2337/diabetes.54.4.1141. [DOI] [PubMed] [Google Scholar]
- Lewin AS, Drenser KA, Hauswirth WW, et al. Ribozyme rescue of photoreceptor cells in a transgenic rat model of autosomal dominant retinitis pigmentosa. Nat Med. 1998;4:967–971. doi: 10.1038/nm0898-967. [DOI] [PubMed] [Google Scholar]
- Liang F-Q, Anand V, Maguire AM, et al. Intraocular delivery of recombinant virus. In: Rakoczy PE, editor. Methods in Molecular Medicine: Ocular Molecular Biology Protocols. Humana Press Inc; Totowa, NJ: 2000. pp. 125–139. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: A phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso K, Hauswirth WW, Li Q, et al. Gene therapy for red-green colour blindness in adult primates. Nature. 2009;461(7265):784–787. doi: 10.1038/nature08401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, James T, Jr, Schwein A, et al. AAV delivery of wild-type rhodopsin preserves retinal function in a mouse model of autosomal dominant retinitis pigmentosa. Hum Gene Ther. 2011;22:567–575. doi: 10.1089/hum.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis S, Muhlfriedel R, Tanimoto N, et al. Restoration of cone vision in the CNGA3−/− mouse model of congenital complete lack of cone photoreceptor function. Mol Ther. 2010;18:2057–2063. doi: 10.1038/mt.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihelec M, Pearson RA, Robbie SJ, et al. Long-term preservation of cones and improvement in visual function following gene therapy in a mouse model of Leber congenital amaurosis (LCA) caused by GC1 deficiency. Hum Gene Ther. 2011;20(16):3161–3175. doi: 10.1089/hum.2011.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millington-Ward S, Chadderton N, O’Reilly M, et al. Suppression and replacement gene therapy for autosomal dominant disease in a murine model of dominant retinitis pigmentosa. Mol Ther. 2011;19:642–649. doi: 10.1038/mt.2010.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SH, Molday LL, Seeliger MW, et al. Prolonged recovery of retinal structure/function after gene therapy in an Rs1h-deficient mouse model of x-linked juvenile retinoschisis. Mol Ther. 2005;12:644–651. doi: 10.1016/j.ymthe.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Mussolino C, Sanges D, Marrocco E, et al. Zinc-finger-based transcriptional repression of rhodopsin in a model of dominant retinitis pigmentosa. EMBO Mol Med. 2011;3:118–128. doi: 10.1002/emmm.201000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narfstrom K, Bragadottir R, Redmond TM, et al. Functional and structural evaluation after AAV. RPE65 gene transfer in the canine model of Leber’s congenital amaurosis. Adv Exp Med Biol. 2003a;533:423–430. doi: 10.1007/978-1-4615-0067-4_54. [DOI] [PubMed] [Google Scholar]
- Narfstrom K, Katz ML, Bragadottir R, et al. Functional and structural recovery of the retina after gene therapy in the RPE65 null mutation dog. Invest Ophthalmol Vis Sci. 2003b;44:1663–1672. doi: 10.1167/iovs.02-0595. [DOI] [PubMed] [Google Scholar]
- Narfstrom K, Katz ML, Ford M, et al. In vivo gene therapy in young and adult RPE65−/− dogs produces long-term visual improvement. J Hered. 2003c;94:31–37. doi: 10.1093/jhered/esg015. [DOI] [PubMed] [Google Scholar]
- O’Reilly M, Palfi A, Chadderton N, et al. RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am J Hum Genet. 2007;81:127–135. doi: 10.1086/519025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfi A, Ader M, Kiang AS, et al. RNAi-based suppression and replacement of rds-peripherin in retinal organotypic culture. Hum Mutat. 2006;27:260–268. doi: 10.1002/humu.20287. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Chang B, Kumar A, et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther. 2006;13:565–572. doi: 10.1016/j.ymthe.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Boye SL, Kumar A, et al. AAV-mediated gene therapy for retinal degeneration in the rd10 mouse containing a recessive PDEbeta mutation. Invest Ophthalmol Vis Sci. 2008;49:4278–4283. doi: 10.1167/iovs.07-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J, Boye SE, Lei B, et al. Self-complementary AAV-mediated gene therapy restores cone function and prevents cone degeneration in two models of Rpe65 deficiency. Gene Ther. 2010a;17:815–826. doi: 10.1038/gt.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Alexander J, Lei B, et al. Achromatopsia as a potential candidate for gene therapy. Adv Exp Med Biol. 2010b;664:639–646. doi: 10.1007/978-1-4419-1399-9_73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Dai X, Boye SE, et al. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Mol Ther. 2011;19:234–242. doi: 10.1038/mt.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlyk BS, Smith AJ, Buch PK, et al. Gene replacement therapy rescues photoreceptor degeneration in a murine model of Leber congenital amaurosis lacking RPGRIP. Invest Ophthalmol Vis Sci. 2005;46:3039–3045. doi: 10.1167/iovs.05-0371. [DOI] [PubMed] [Google Scholar]
- Sarra GM, Stephens C, de Alwis M, et al. Gene replacement therapy in the retinal degeneration slow (rds) mouse: The effect on retinal degeneration following partial transduction of the retina. Hum Mol Genet. 2001;10:2353–2361. doi: 10.1093/hmg/10.21.2353. [DOI] [PubMed] [Google Scholar]
- Simons DL, Boye SL, Hauswirth WW, et al. Gene therapy prevents photoreceptor death and preserves retinal function in a Bardet-Biedl syndrome mouse model. Proc Natl Acad Sci USA. 2011;108:6276–6281. doi: 10.1073/pnas.1019222108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Pawlyk B, Xu X, et al. Gene therapy with a promoter targeting both rods and cones rescues retinal degeneration caused by AIPL1 mutations. Gene Ther. 2011;17:117–131. doi: 10.1038/gt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Surace EM, Domenici L, Cortese K, et al. Amelioration of both functional and morphological abnormalities in the retina of a mouse model of ocular albinism following AAV-mediated gene transfer. Mol Ther. 2005;12:652–658. doi: 10.1016/j.ymthe.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Miyoshi H, Verma IM, et al. Rescue from photoreceptor degeneration in the rd mouse by human immunodeficiency virus vector-mediated gene transfer. J Virol. 1999;73:7812–7816. doi: 10.1128/jvi.73.9.7812-7816.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam LC, Kiang AS, Kennan A, et al. Therapeutic benefit derived from RNAi-mediated ablation of IMPDH1 transcripts in a murine model of autosomal dominant retinitis pigmentosa (RP10) Hum Mol Genet. 2008;17:2084–2100. doi: 10.1093/hmg/ddn107. [DOI] [PubMed] [Google Scholar]
- Tam LC, Kiang AS, Chadderton N, et al. Protection of Photoreceptors in a Mouse Model of RP10. Adv Exp Med Biol. 2010;664:559–565. doi: 10.1007/978-1-4419-1399-9_64. [DOI] [PubMed] [Google Scholar]
- Tan M, Smith A, Pawlyk B, et al. Gene therapy for retinitis pigmentosa and Leber congenital amaurosis caused by defects in AIPL1: Effective rescue of mouse models of partial and complete Aipl1 deficiency. Hum Mol Genet. 2009;18:2099–2114. doi: 10.1093/hmg/ddp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe L, Bell P, Maguire A, et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci Transl Med. 2011;3:88ra54. doi: 10.1126/scitranslmed.3002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ML, Coleman JE, Haire SE, et al. Lentiviral expression of retinal guanylate cyclase-1 (RetGC1) restores vision in an avian model of childhood blindness. PLoS Med. 2006;3:e201. doi: 10.1371/journal.pmed.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Takada Y, Kjellstrom S, et al. RS-1 gene delivery to an adult Rs1h knockout mouse model restores ERG b-wave with reversal of the electronegative waveform of X-linked retinoschisis. Invest Ophthalmol Vis Sci. 2004;45:3279–3285. doi: 10.1167/iovs.04-0576. [DOI] [PubMed] [Google Scholar]
- Zou J, Luo L, Shen Z, et al. Whirlin replacement restores the formation of the USH2 protein complex in whirlin knockout photoreceptors. Invest Ophthalmol Vis Sci. 2011;52:2343–2351. doi: 10.1167/iovs.10-6141. [DOI] [PMC free article] [PubMed] [Google Scholar]


