Abstract
Objectives
A variety of biological, biochemical, and biophysical markers implicated in the pathophysiology of pre-eclampsia during the last two decades have instigated the growing interest in this study to include both βhCG and lipid profile studies in the early second trimester as early predictors of pregnancy-induced hypertension. Early identification of at-risk women may help in taking timely preventive and curative management to prevent or delay complications associated with pregnancy-induced hypertension.
Method
A prospective study was performed on 120 patients attending the outpatient department of the Obstetrics and Gynaecology of the Maharaja Agrasen Hospital. All the patients were screened for serum βhCG and serum lipid profile in their early second trimester (14–20 weeks) and followed up till their delivery. Comparative studies of serum βhCG and serum lipid profile were performed between those who remain normotensive (group I) and those who developed pregnancy-induced hypertension (group II).
Results
TG, total cholesterol, VLDL, and LDL values for those women who developed PIH (group II) were significantly higher than those who remain normotensive (group I), with p value of <0.05 which is statistically significant. HDL and βhCG values for group II were not higher than those in group I with p value >0.05 which is statistically insignificant.
Conclusion
Maternal lipid profile in second trimester is very good noninvasive test which can be used for prediction of pregnancy-induced hypertension before its clinical onset. However, there is no correlation between maternal serum βhCG and pregnancy-induced hypertension.
Keywords: Pregnancy-induced hypertension, βHCG, TG, Total cholesterol, VLDL, LDL
Introduction
Pregnancy is nature’s precious gift which has to be nurtured during its entire 9 months of duration to achieve good maternal and fetal outcome. There are several complications which may impair a favorable pregnancy outcome. Pregnancy-induced hypertension is one of the commonest medical problems encountered during pregnancy. Cunningham et al. [1] describe hypertension in pregnancy as a major cause of maternal and perinatal morbidity, complicating 5–10 % of all pregnancies worldwide whereas pregnancy-induced hypertension has been identified in 3.9 % of all pregnancies.
Early diagnosis, close antenatal surveillance, and timely intervention are key to the management of pregnancy-induced hypertension in pregnancy. During the last two decades, a variety of biological, biochemical, and biophysical markers implicated in the pathophysiology of pre-eclampsia have been proposed to predict its development. Currently, there are no reliable, economic, and reproducible screening tests that are available, and moreover, the pathophysiologic mechanism of pregnancy-induced hypertension is still not clearly known.
The most current hypothesis regarding the pathophysiologic mechanism of pregnancy-induced hypertension points to early placental abnormalities [2, 3]. In pathogenesis of pre-eclampsia, endothelial cell activation or dysfunction is believed to be the central theme [4]. Human placenta synthesizes steroids, proteins, and various glycoprotein hormones.
Since it is postulated that pregnancy-induced hypertension is a likely trophoblastic disorder, it may be essential for understanding of this disease to investigate the pathologic and secretory reaction of placenta. Twin and molar pregnancy that are frequently associated with elevated βhCG level also carry an increased risk of pre-eclampsia.
Correlation between serum lipid levels and gestational hypertension and pre-eclampsia has been suggested by Van den Elzen et al. [5] and Sattar et al. [6]. An abnormal lipid profile is strongly associated with atherosclerotic cardiovascular disease and has a direct effect on endothelial dysfunction [4]. The most important feature in pre-eclampsia is due to a vasospastic phenomenon in kidneys, uterus, placenta, and brain. Altered lipid synthesis leading to a decrease in PGI2:TXA2 ratio is also suggested to be an important pathway of pathogenesis in pre-eclampsia [7]. If alterations in circulating lipids are of pathognomonic importance in the development of pre-eclampsia, then changes in lipid parameter should be demonstrable much before the clinical onset of disease.
Uncontrolled pregnancy-induced hypertension can lead to maternal and fetal complications and increased risk of hypertension and diabetes in the fetus, in the later stages of life as proposed by Yagnik et al. [8] and Susane [9]. In view of these findings, this study was undertaken, in which both βhCG and lipid profile are studied in the early second trimester as early predictors of pregnancy-induced hypertension. Early identification of women at risk of pregnancy-induced hypertension is done which may help in taking timely preventive management to prevent or delay these complications.
Methods
This was a prospective study, conducted on 120 patients. The study population included women with singleton pregnancy visiting antenatal outpatient department of the Obstetrics and Gynaecology of Maharaja Agrasen Hospital, Punjabi Bagh, New Delhi from March 2010 to March 2011.
All patients were screened for serum βhCG and serum lipid profile in their early second trimester (14–20 weeks) by taking blood sample after 12 h of fasting. These values were recorded, and patients were followed up till their delivery. Comparative study of serum βhCG and serum lipid profile were done between those who remain normotensive (group I) and those who developed pregnancy-induced hypertension (group II).
Exclusion criteria include the following:
hypertension diagnosed before 20 weeks of gestation
diabetes mellitus
multiple pregnancy
molar pregnancy
hypothyroidism and
ultrasound-proven fetal congenital malformations
Informed consent was taken from the patient, and a fixed protocol was followed as per the proforma. Patients were called on a predetermined date after 12 h of fasting, and blood sample was collected for serum βhCG and serum lipid profile in two different tubes. Serum βhCG was determined by EIA (enzyme immune assay) using AIA 360 spectral equipment. Serum lipid profile was done using a fully automated machine-Beckmann coultier CX-5 Pro and Olympus AV-400 by means of enzyme calorimetric method. Total lipid was calculated as 250 + serum triglyceride + serum cholesterol. VLDL was calculated as serum triglyceride divided by 5.
Statistical Analysis
Means ± SD of all parameters of interest were calculated for both groups, and the difference of means between the two groups was tested by t test. Chi square test was used to find out significant correlation (Figs. 1, 2, 3, 4, 5, 6).
Fig. 1.
Distribution of cases according to BMI
Fig. 2.
Correlation between PIH with serum TG
Fig. 3.
Correlation between PIH and serum Cholesterol
Fig. 4.
Correlation between PIH and serum VLDL
Fig. 5.
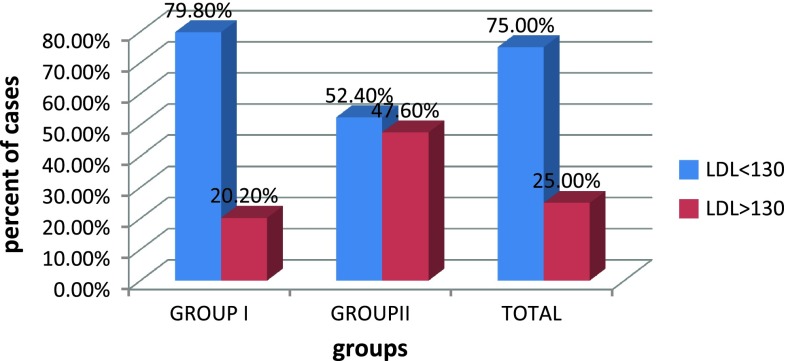
Correlation between PIH and serum LDL
Fig. 6.
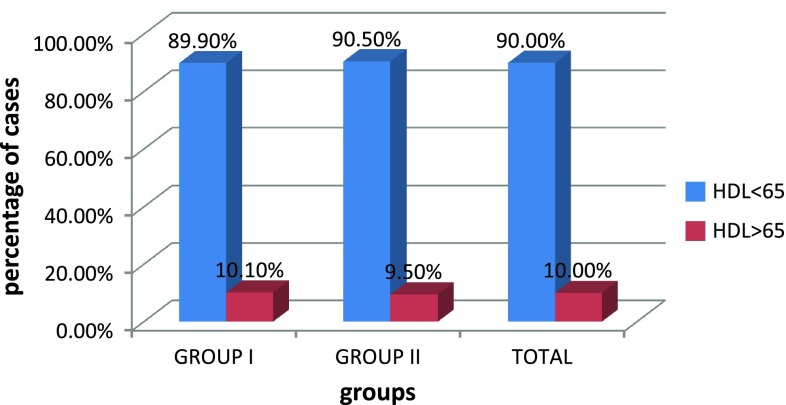
Correlation between PIH and serum HDL
Results
Out of these cases, 21 cases developed pregnancy-induced hypertension (group II), and 99 cases remained normotensive (group I).The prevalence of pregnancy-induced hypertension in our study was 17.5 % as shown in Table 1. In their study of 164 cases, Vidyabati et al. [10] found prevalence rate of pregnancy-induced hypertension as 17.68 %.
Table 1.
Prevelance of PIH
| No. of cases | Percent | |
|---|---|---|
| Group I (those who remain normotensive) | 99 | 82.5 |
| Group II (those who develop pregnancy-induced hypertension) | 21 | 17.5 |
| Total | 120 | 100 |
We did not observe any rise in βhCG (mIU/ml) levels in women who developed pregnancy-induced hypertension in this study.
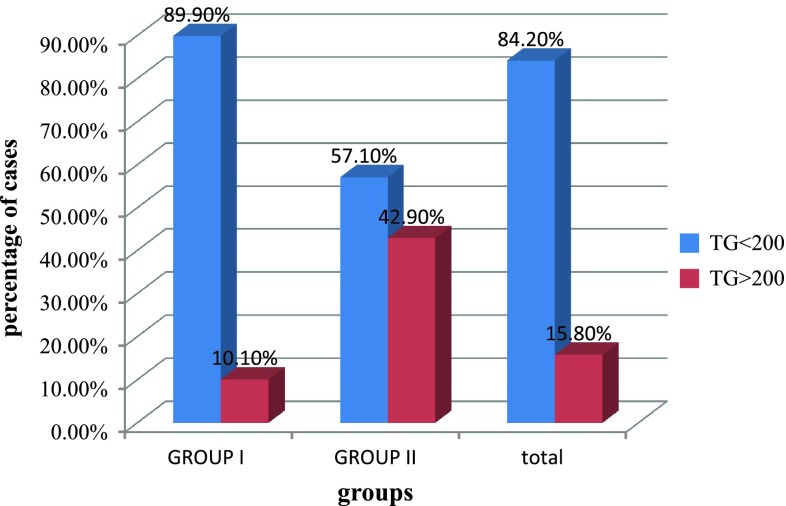
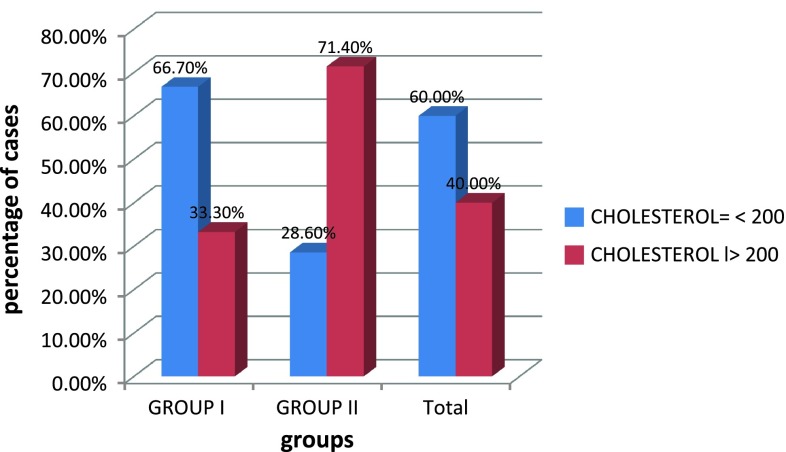
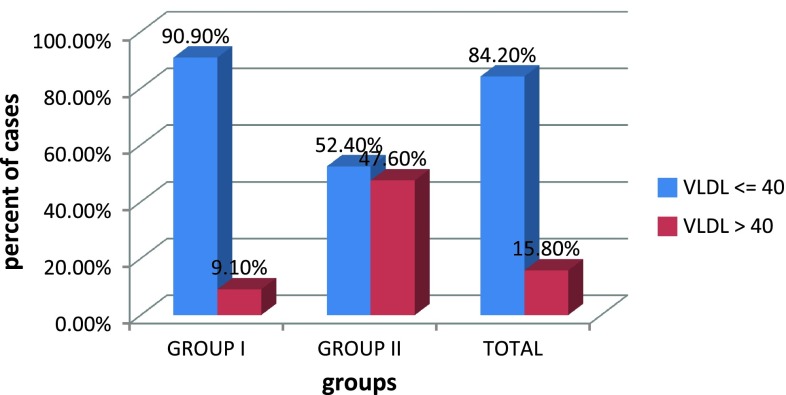
In our study, 42.9 % of PIH cases had TG level of greater than 200 mg/dl, 71.4 % of cases with PIH had elevated cholesterol level >200 mg/dl, 47.6 % of group II cases had VLDL value greater than 40 mg/dl and 47.6 % of cases in group II have LDL value greater than 130 mg/dl,. This showed that TG, cholesterol, VLDL, and LDL values for those women who developed PIH were significantly higher than in the cases of those who remain normotensive, with p value <0.05 as shown in Table 6.
Table 6.
Comparison of means ± SD of serum lipid profile between both the groups
| Variable | Group I | Group II | p value |
|---|---|---|---|
| TG | 139.56 ± 46.89 | 167.24 ± 50.68 | 0.017 |
| Total chol | 188.07 ± 30.29 | 204.14 ± 40.13 | 0.04 |
| LDL | 113.8 ± 23.4 | 121.9 ± 34.3 | 0.186 |
| VLDL | 27.86 ± 9.14 | 34.42 ± 10.03 | 0.004 |
| HDL | 50.07 ± 13.37 | 51.86 ± 10.97 | 0.56 |
We observed no statistical significance between HDL value and pregnancy-induced hypertension as shown in Table 6 as the p value is 0.936. We have 19 cases (90.5 %) out of the 21 in group II with HDL <65; and 89 cases (89.90 %) out of the 99 in group I with HDL <65. Thus, the results are similar in both groups. The expected inverse relationship between pre-eclampsia risk and HDL concentration was not evident.
Discussion
In our study, 51.7 % of cases were in the age group of 25–29 years as shown in Table 2. There were only three cases aged more than 35 years, and they remained normotensive till delivery. As the number of cases with age >35 years was less, and hence, it is difficult to comment on the relation between elderly patients and PIH risk (Table 3).
Table 2.
Distribution of cases according to age
| Age group | Total | Pearson chi square | ||||
|---|---|---|---|---|---|---|
| 20–24 | 25–29 | 30–34 | 35–39 | p value | ||
| Group I | ||||||
| Count | 24 | 53 | 19 | 3 | 99 | 0.624 |
| % Within group | 24.20 | 53.53 | 19.20 | 3.00 | 100.00 | |
| Group II | ||||||
| Count | 7 | 9 | 5 | 0 | 21 | |
| % Within group | 33.30 | 42.80 | 23.80 | 0.00 | 100.00 | |
| Total | ||||||
| Count | 31 | 62 | 24 | 3 | 120 | |
| % Within group | 25.80 | 51.50 | 20.00 | 2.50 | 100.00 | |
Table 3.
Distribution of cases according to parity
| Parity | Total no. of cases | Group 1 | Group 2 |
|---|---|---|---|
| G1 | 59 | 47 | 12 |
| G2 | 36 | 30 | 6 |
| G3 | 13 | 12 | 1 |
| G4 | 7 | 6 | 1 |
| G5 | 1 | 1 | 0 |
| G6 | 3 | 3 | 0 |
| G7 | 0 | 0 | 0 |
| G8 | 1 | 0 | 1 |
| Total | 120 | 99 | 21 |
Out of the 120 cases of our study, 59 (57.84 %) cases were primigravida and 61 (50.8 %) were multigravida. Twelve (57.14 %) cases out of the 21 with PIH were primigravida which suggests that PIH is more common in primigravida cases, and this is similar to that reported by many studies.
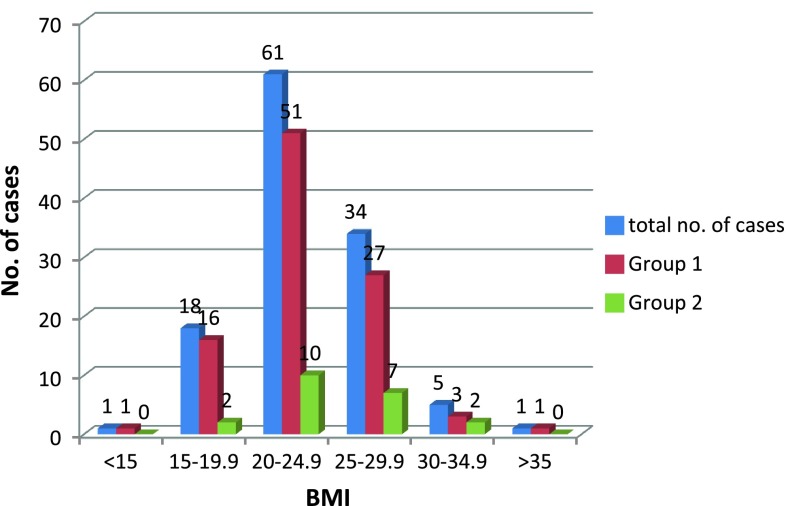
The maximum numbers of cases in both groups were having BMI of 20–25 kg/m [2] as shown in Table 4. In this study, we have only six cases with BMI >30 kg/m [2], out of which two developed PIH and four cases remained normotensive. Due to less number of cases with the elevated BMI, it is difficult to comment on the relation of pregnancy-induced hypertension risk in patients with BMI >30 kg/m [2].
Table 4.
Distribution of cases according to BMI
| BMI (kg/m2) | Total no. of cases | Group 1 | Group 2 |
|---|---|---|---|
| <15 | 1 | 1 | 0 |
| 15–19.9 | 18 | 16 | 2 |
| 20–24.9 | 61 | 51 | 10 |
| 25–29.9 | 34 | 27 | 7 |
| 30–34.9 | 5 | 3 | 2 |
| >35 | 1 | 1 | 0 |
| Total | 120 | 99 | 21 |
Comparison of blood pressures in second trimester (14–20 weeks) between both the groups did not vary significantly. Blood pressures of group II cases at the time of delivery were significantly higher in comparison to those of group I cases. This is true for both systolic and diastolic Blood pressures as shown in Table 5. These results are similar to the finding of Vidyabati et al. [10].
Table 5.
Comparison of means ± SD of systolic and diastolic blood pressure between both the groups
| BP | Group I | Group II | p value |
|---|---|---|---|
| At 14–20 weeks (mmHg) | |||
| Diastolic | 72.24 ± 5.75 | 72.67 ± 4.95 | 0.754 |
| Systolic | 112.20 ± 4.49 | 111.43 ± 3.59 | 0.46 |
| At delivery (mmHg) | |||
| Diastolic | 79.49 ± 4.60 | 92.57 ± 4.06 | 0 |
| Systolic | 121.37 ± 5.187 | 140.48 ± 1.78 | 0 |
Morssink et al. [11] and Pouta et al. [12] did not find any correlation between serum βhCG and pregnancy-induced hypertension in their study. Also, Morssink et al. [13] evaluated cases with pre-eclampsia and demonstrated that significantly rise of serum βhCG was only associated with severe pre-eclampsia. In our study also, we did not find any correlation between serum βhCG and pregnancy-induced hypertension. The divergent points of maternal serum βhCG with wide range of cutoff values may be responsible for this result. Also, the small-sized sample could be a contributing factor.
In this study, we observed an association between maternal early pregnancy dyslipidemia and the subsequent risk of pre-eclampsia. Pregnant women who subsequently developed pre-eclampsia had higher total cholesterol, TG, VLDL, and LDL cholesterol levels compared with pregnant women who remained normotensive as depicted in Table 6. This finding is similar to that of many other studies.
Lorentzen et al. [14] concluded that serum-free fatty acids and triglyceride are increased before 20 weeks of gestation in women who later develop pre-eclampsia. Clausen et al. [15] concluded that hypertriglyceridemic dyslipidemia before 20 weeks of gestation is associated with the risk of developing early onset pre-eclampsia.
Cekmen et al. [16] have shown that plasma triglyceride and LDL levels were significantly higher in pre-eclamptic subjects than in controls, whereas the plasma HDL levels were significantly lower in pre-eclamptic cases than in control group. De et al. [17] concluded that there is significant rise in triglyceride and VLDL levels and a fall in HDL levels in pre-eclamptic patients. Vidyabati et al. [10] concluded that total cholesterol; VLDL, and LDL in women who subsequently developed PIH were significantly higher than in normotensive patients.
In our study, we had 19 cases (90.5 %) out of the 21 in group II with HDL <65 and 89 cases (89.90 %) out of the 99 in group I with HDL <65. Thus, the results are similar in both the groups. The expected inverse relationship between pre-eclampsia risk and HDL concentration was not evident in our study as depicted in Table 6. Vidyabati et al. [10] had similar finding.
Our results, when taken together with those of earlier studies, indicate that dyslipidemia particularly hypertriglyceridemia and elevated lipoprotein precede the clinical manifestations of pre-eclampsia and thus may be of etiologic and pathophysiologic importance in pregnancy-induced hypertension. The association between dyslipidemia and the risk of pre-eclampsia is biologically plausible and is compatible with what is known about the pathophysiology of pre-eclampsia.
In the literature, at least three hypotheses for association between dyslipidemia and pre-eclampsia have been postulated. First, investigators have noted that elevated plasma lipids and lipoproteins may induce endothelial dysfunction secondary to oxidative stress [18]. Free radicals can be generated by different enzymatic processes [19]. They are extremely reactive and interact with polyunsaturated fatty acids to produce lipid peroxides with a much longer half life. Lipid peroxides are normally present in lipoproteins and seem to contribute to vascular tone regulation through stimulation of the arachidonic acid enzymatic pathways [20]. When oxidative stress reaches a certain level, cellular damage occurs, including structural damage in cellular membranes in mitochondrial and nuclear DNAs and impairment of enzymatic functions at multiple levels. Oxidative stress can have an affect mainly on vessel endothelium and on many tissues and organs both locally and systematically. During these processes, other molecules involved in vasodilatation such as nitric oxide (NO) are inhibited by high lipid peroxide concentration [4]. All these circumstances might be the contributory factors for the etiopathogenesis of hypertension in pre-eclampsia.
It is also noted that dyslipidemia may impair trophoblast invasion thus contributing to a cascade of pathophysiologic events that lead to the development of pre-eclampsia. In this disorder, there is underperfusion, ischemia, and hypoxia in the placenta, which is then thought to release a variety of mediators in the maternal circulation including hormones, cytokines, and reactive oxygen species such as NO, superoxide anion, and hydrogen peroxide, which cause endothelial dysfunction and permanent systemic vasoconstriction leading to pre-eclampsia.
The second mechanism is the pathologic process of pre-eclampsia via dysregulation of lipoprotein lipase resulting in dyslipidemic lipid profile [21]. Serum from pre-eclamptic women had both, a higher ratio of free fatty acids to albumin and increased uptake of free fatty acids, which are further esterified to triglyceride [22].
A third possible mechanism may be via the metabolic syndrome. Metabolic characteristics of insulin-resistance syndrome namely, hyperinsulinemia and hyperuricaemia, are also present in pre-eclampsia [23]. Moreover, women with a history of pre-eclampsia, compared with their BMI matched counterparts without such a history, have higher circulating concentrations of fasting insulin, lipid, and inflammatory and co agulation factors, many years after delivery [24].
Thus, estimation of maternal lipid profile in the early second trimester will bring about early recognition and better management of patients at risk of pregnancy-induced hypertension, before the clinical syndrome and complications of PIH appear. Also early treatment of such cases is essential for a better feto-maternal outcome.
Conclusion
Maternal lipid profile in the early second trimester is very good and effective noninvasive test, which is a reproducible, economical, and reliable technique which can be used for prediction of pregnancy-induced hypertension, before its clinical onset.
However, we could not find any correlation between maternal serum βhCG and pregnancy-induced hypertension. The divergent points of maternal serum βhCG with wide range of cutoff values may be responsible for this result. Also, the small-sized sample could be a contributing factor. Further detailed study with a larger-sized sample is suggested in this regard.
Acknowledgments
Conflict of interest
None declared.
References
- 1.Cunningham FG, Leveno KJ, Bloom SL, et al. Pregnancy hypertension. In: Kenneth J, et al., editors. Williams obsterics. 23. New York: McGraw-Hill; 2010. p. 706. [Google Scholar]
- 2.Robertson WB, Khong TY, Brosens I, et al. The placental bed biopsy: review from three European centres. Am J Obstet Gynaecol. 1986;155:401–412. doi: 10.1016/0002-9378(86)90843-4. [DOI] [PubMed] [Google Scholar]
- 3.Liu DF, Dickerman LH, Redline RW. Pathologic findings in pregnancies with unexplained increases in midtrimester maternal serum human chorionic gonadotropin levels. Am J Clin Pathol. 1999;111:209–215. doi: 10.1093/ajcp/111.2.209. [DOI] [PubMed] [Google Scholar]
- 4.Gratacos E. Lipid mediated endothelial dysfunction: a common factor to preeclampsia and chronic vascular disease. Eur J Obstet Gynaecol Reprod Biol. 2000;92:63–66. doi: 10.1016/S0301-2115(00)00427-9. [DOI] [PubMed] [Google Scholar]
- 5.Van den Elzen HJ, Wladimiroff JW, Cohen-Overbeek TE, et al. Serum lipids in early pregnancy and risk of preeclampsia. Br J Obstet Gynaecol. 1999;103(2):117–122. doi: 10.1111/j.1471-0528.1996.tb09661.x. [DOI] [PubMed] [Google Scholar]
- 6.Sattar N, Bendomir A, Berry C, et al. Lipoprotein subfraction concentrations in preeclampsia: pathogenic parallels to atherosclerosis. Obstet Gynaecol. 1997;89:403–408. doi: 10.1016/S0029-7844(96)00514-5. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Walsh SW, Kay HH. Placental lipid peroxides and thromboxane are increased and prostacyclin is decreased in women with preeclampsia. Am J Obstet Gynaecol. 1992;167:946–949. doi: 10.1016/S0002-9378(12)80017-2. [DOI] [PubMed] [Google Scholar]
- 8.Yagnik CS, Fall CHD, Coyaji KJ, et al. Neonatal anthropometry: the thin-fat Indian baby. Int J Obes Relat Metab Disord. 2003;27:173–180. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- 9.Ozanne SE, Fernandez-Twinn D, Hales CN. Fetal growth and adult disease. Semin Perinatol. 2004;28(1):81–87. doi: 10.1053/j.semperi.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Vidyabati RK, Davina H, Singh NK, et al. Serum βhCG levels and lipid profile in early second trimester as predictors of pregnancy induced hypertension. J Obstet Gynecol India. 2010;60(1):44–50. doi: 10.1007/s13224-010-0008-1. [DOI] [Google Scholar]
- 11.Morssink LP, Heringa MP, Beekhuis JR, et al. The association between hypertensive disorder of pregnancy and abnormal second trimester maternal serum levels of MShCG and alpha fetoprotein. Obstet Gynaecol. 1997;89:666–670. doi: 10.1016/S0029-7844(97)00088-4. [DOI] [PubMed] [Google Scholar]
- 12.Pouta AM, Hartikainen AL, Vuolteenaho OJ, et al. Midtrimester N-terminal proatrial natriuretic peptide, free beta MShCG, and alpha fetoprotein in predicting preeclampsia. Obstet Gynaecol. 1998;91:940–944. doi: 10.1016/S0029-7844(98)00112-4. [DOI] [PubMed] [Google Scholar]
- 13.Morssink LP, Heringa MP, Beekhuis JR, et al. The HELP syndrome: its association with unexplained elevation of MSAFP and MShCG in the second trimester. Prenat Diag. 1997;17:601–606. doi: 10.1002/(SICI)1097-0223(199707)17:7<601::AID-PD115>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 14.Lorentzen B, Endressen MJ, Clausen T, et al. Fasting serum free fatty acids and triglycerides are increased before 20 weeks of gestation in women who later develop preeclampsia. Hypertens Pregnancy. 1994;13:103–109. doi: 10.3109/10641959409084177. [DOI] [Google Scholar]
- 15.Clausen T, Djurovic S, Henriksen T. Dyslipidemia in early second trimester is a feature of women with early onset preeclampsia. Br J Obstet Gynaecol. 2001;108:1081–1087. doi: 10.1016/S0306-5456(01)00247-9. [DOI] [PubMed] [Google Scholar]
- 16.Cekmen MB, Erbagci AB, Balat A, et al. Plasma lipid and lipoprotein concentrations in pregnancy induced hypertension. Clin Biochem. 2003;36:575–578. doi: 10.1016/S0009-9120(03)00099-7. [DOI] [PubMed] [Google Scholar]
- 17.De J, Mukhopadhyay A, Saha PK. Study of serum lipid profile in pregnancy induced hypertension. Indian J Clin Biochem. 2006;21(2):165–168. doi: 10.1007/BF02912935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorentzen B, Henriksen T. Plasma lipids and vascular dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:33–39. doi: 10.1055/s-2007-1016250. [DOI] [PubMed] [Google Scholar]
- 19.Freenan BA, Crapo JD. Biology of disease. Free radicals and tissue injury. Lab Invest. 1982;47:412–426. [PubMed] [Google Scholar]
- 20.Bruckdorfer KR. Antioxidant, lipoprotein oxidation and arterial function. Lipids. 1996;31:83–85. doi: 10.1007/BF02637056. [DOI] [PubMed] [Google Scholar]
- 21.Sattar N, Greer IA, Louden J, et al. Lipoprotein subfraction changes in normal pregnancy: threshold effect of plasma triglyceride on appearance of small dense low density lipoproteins. J Clin Endocrinol Metab. 1997;82:2483–2491. doi: 10.1210/jcem.82.8.4126. [DOI] [PubMed] [Google Scholar]
- 22.Lorentzen B, Drevon C, Endresen M, et al. Fatty acid pattern of esterified and free fatty acids in sera of women with normal and preeclamptic pregnancy. Br J Obstet Gynaecol. 1995;102:530–537. doi: 10.1111/j.1471-0528.1995.tb11355.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaaja R, Tikkanen MJ, Viinikka L, et al. Serum lipoprotein, insulin, and urinary prostanoid metabolites in normal and hypertensive pregnant women. Obstet Gynaecol. 1995;85:353–356. doi: 10.1016/0029-7844(94)00380-V. [DOI] [PubMed] [Google Scholar]
- 24.Sattar N, Greer A. Pregnancy complications and maternal cardiovascular risk opportunities for intervention and screening. BMJ. 2002;325:157–160. doi: 10.1136/bmj.325.7356.157. [DOI] [PMC free article] [PubMed] [Google Scholar]