Abstract
Objective
To evaluate role of serum estradiol levels in predicting likelihood of pregnancy in women undergoing GnRH-a protocol in IVF-ET cycles.
Design
A 3-year retrospective analysis of estradiol levels on down-regulated day 2, day 6, and day of hCG trigger and subsequent clinical pregnancy rates.
Setting
A university hospital tertiary referral centre.
Population or Sample
Women undergoing IVF treatment.
Methods
Hormonal assessment on the down-regulated day 2, day 6, and day of hCG trigger.
Main Outcome Measure(s)
Comparison of hormonal profile, antral follicular count on day 2, endometrial thickness on day of trigger, and number of oocytes retrieved between pregnant and the non-pregnant group. The prediction of IVF success was based on the quantitative levels of estradiol on a specific day in down-regulated cycle.
Result(s)
The overall pregnancy rate was 32.25 % (50/160). Estradiol level on down-regulated day 2 was 31.9 ± 12.6 and on the day of trigger was 1,996.46 ± 1,252.36 in pregnant women, which was significantly higher as compared to estradiol levels in non-pregnant women (27.6 ± 12.3 and 1,525.1 ± 1,116.42, respectively). It was found to be a significant prognostic marker for successful IVF treatment. Estradiol levels on down-regulated day 6 were found to be non-significant between the two groups.
Conclusion(s)
Estradiol level on down-regulated day 2 of menstrual cycle and on the day of trigger was found to have a significant impact on the success of IVF-ET.
Keywords: Estradiol, Day 2, Day 6, Day of hCG trigger, IVF outcome
Introduction
Infertility affects millions of couples worldwide. Childlessness is a life crisis and impaired fertility has been reported to affect 10–15 % of couples [1].
According to the 2009 Assisted Reproductive Technology Report of CDC on ART success rate, clinical pregnancy was 36.9 % [2]. The success of IVF is age dependent; it’s success is 47.4 % for women under the age of 35 years, 38.7 % for women ages 35–37 years, 30.1 % for women ages 38–40 years, 20.3 % for women ages 41–42 years, and 10.7 % in women ages 43–44 years.
The prime concern in in vitro fertilization (IVF) cycle is to obtain sufficient number of mature oocytes, good quality embryos, and finally achieve a successful pregnancy. The high cost and relatively low implantation rates in IVF have given rise to the need to evaluate the predictors of success in these women. Prediction of successful IVF outcome has focused on clinical research for many years. Previous reports suggest the role of various parameters like levels of hormones [follicular stimulating hormone (FSH), estradiol, inhibin A, and inhibin B], number of ovarian antral follicles, anti-mullerian hormone, and the influence of woman’s age in predicting successful pregnancy in IVF cycles [3, 4, 5–10]. However, no consensus exists till date [11, 12].
The role of estradiol (E2) in IVF cycles is well known up to the fertilization stage, however its role beyond that stage remains controversial. On the day of hCG trigger (d-hCG), due to increased number of follicles, high estradiol levels lead to low endometrial receptivity and thus decreased pregnancy rates in IVF cycles [12, 13]. Different cutoffs of serum estradiol levels have been observed on day 4–6 and on the day of hCG trigger during the stimulation cycle by some researchers [14].
Based on the aforesaid facts, the aim of our study was to assess the role of serum estradiol levels on down-regulated day 2 of menstrual cycle, day 6 of ovarian stimulation, and day of hCG trigger in predicting the pregnancy rate in women undergoing controlled ovarian hyperstimulation in IVF-ET cycles.
Materials and Methods
Using a computerized database, record of 186 women for IVF-ET carried out from March 2008 to August 2011, at the IVF and Reproductive Biology Centre, Department of Obstetrics and Gynecology, Maulana Azad Medical College and associated Lok Nayak Hospital New Delhi, was studied. The exclusion criteria comprised any relevant systemic disease or endocrinal disorder, severe endometriosis or uterine and ovarian anomalies, no more than three previous failed IVF, no previous IVF cycle with a poor ovarian response or ovarian hyperstimulation syndrome. A total of 160 patients who successfully completed the IVF-ET procedure were recruited. Seventeen women who failed to respond to ovarian stimulation and nine women who had ovarian hyperstimulation syndrome were excluded from the study. Due to the retrospective study design based on routine practice, consultation with the hospital ethics committee was not required.
Informed written consent was obtained from all couples visiting our centre, followed by detailed history with general, physical, and gynecological evaluation along with all standard investigations for infertility. The male partner was also evaluated including semen analysis (based on WHO criteria, 1999) [13]. A total of 3–5 mL of venous blood was drawn from the antecubital vein from all women on the second day of their spontaneous menstrual cycle for estimation of serum FSH, luteinizing hormone (LH), E2, thyroid stimulating hormone, and progesterone (P4) levels. Patients were started on “conventional long protocol” in the same cycle by GnRH analog (Leuprolide acetate, Leupride, Sun Pharmaceutical Ind. Ltd, 0.5 mg intramuscularly daily). Down regulation was confirmed by day 2 baseline ovarian and endometrial assessment on transvaginal sonography (TVS) with serum FSH, LH, E2, and P4 estimation. Ovarian stimulation was done with daily injection of dosages ranging from 225 IU of recombinant gonadotropin (Recombinant-human follicle stimulating hormone, Folligraf, Bharat Serums and Vaccines Ltd, India). TVS was done on day 6 of ovarian stimulation to assess follicular growth and endometrial thickness along with serum E2 and P4 levels. According to individuals’ response, the doses of gonadotropins were adjusted for next 5 or 6 days. Serum E2 and P4 were repeated once three or more follicles were 16–18 mm on TVS.
Human chorionic gonadotropin (hCG, Inj. Fertigyn, Sun Pharmaceutical Ind. Ltd.) 10,000 IU was administered intramuscularly to simulate luteinizing hormone surge and to achieve final follicular maturation. Transvaginal oocyte retrieval was carried out under general anesthesia, 34–36 h after hCG administration. Grading of embryos was done on day 3 before embryo transfer. Luteal phase was supported by vaginal micronized progesterone (Tab Susten, Sun Pharmaceutical Ind. Ltd.) 600 mg daily, in two divided doses, starting immediately after the oocytes retrieval. Urine pregnancy test and serum β-hCG estimation were done 16 days after the embryo transfer to confirm pregnancy. The presence of fetal cardiac activity on TVS, two weeks post serum β-hCG estimation confirmed clinical pregnancy.
Statistical Analysis
Group A (pregnant group) and Group B (non-pregnant group) were compared with the help of SPSS 13.0 (Chicago, IL, USA) statistical software package. The descriptive statistics was represented as mean ± SD.
The groups were analyzed by using Independent Student t-test/Mann Whitney test wherever applicable, to compare the mean of estradiol levels in both the groups.
The change in the estradiol levels on day 2, day 6, day of hCG trigger, and successful IVF outcome was studied by applying repeated measure analysis followed by post receiver operating characteristic (ROC) comparison by Bonferroni method. ROC curve of the model was constructed and the area under curve was used for the assessment and effectiveness of the parameter to predict likelihood of the pregnancy. The significance was observed with p < 0.05.
Results
A total of 160 women consecutively underwent IVF-ET cycles from March 2008 to August 2011. The clinical pregnancy rate in this group was 31.25 % per embryo transfer. Of the total 160 women, 50 (50/160, 31.25 %) patients had a viable intrauterine pregnancy (group A), while 110 (110/160, 68.75 %) women failed to conceive (group B) after IVF-ET treatment.
Table 1 shows the comparison of different parameters between Group A and Group B. No significant difference was found in terms of mean age (in years) in women with viable pregnancies (32.28 ± 3.79, range 24–40 years, p = 0.42) as compared to the women in non-pregnant group (31.73 ± 4.62, range 20–43 years). However the mean infertility duration (in years) of the pregnant women (6.50 ± 3.66, range 01–18 years, p = 0.42) was slightly less as compared to the non-pregnant group of women (7.02 ± 4.33, range 09–13 years), but was found to be non-significant.
Table 1.
Comparison of different parameters between Group A (pregnant group) and Group B (pregnant group)
| S. no. | Parameters | Group A (pregnant women) | Group B (non-pregnant women) | |||
|---|---|---|---|---|---|---|
| (Numbers = 50) | Range | (Numbers = 110) | Range | p value | ||
| 1 | Age in years (mean ± SD) | 32.28 ± 3.79 | 24–40 | 31.73 ± 4.62 | 20–43 | 0.42 |
| 2 | Infertility duration in years (mean ± SD) | 6.50 ± 3.66 | 01–18 | 7.02 ± 4.33 | 09–13 | 0.42 |
| 3 | Basal FSH IU/mL (mean ± SD) | 5.13 ± 2.8 | 1.1–6.8 | 5.63 ± 6.9 | 1.4–7.9 | 0.51 |
| 4 | Basal LH IU/mL (mean ± SD) | 3.54 ± 3.2 | 0.3–15.6 | 3.36 ± 5.05 | 0.1–12.3 | 0.17 |
| 5 | Day 2 AFC (mean ± SD) | 7.40 ± 3.9 | 1–20 | 7.75 ± 4.1 | 01–23 | 0.23 |
| 6 | Day-2 estradiol pg/mL (mean ± SD) | 31.9 ± 12.6 | 5.8–49.7 | 27.6 ± 12.3 | 4.51–49.30 | 0.043a |
| 7 | Day-6 estradiol pg/mL (mean ± SD) | 408.6 ± 292.5 | 41.5–1,076.0 | 354.5 ± 276.2 | 19.50–1,001.0 | 0.273 |
| 8 | Day of hCG administration estradiol (mean ± SD) | 1,996.46 ± 1,252.36 | 429.73–6,510.0 | 1,525.1 ± 1,116.42 | 67.0–7,185.0 | 0.025a |
| 9 | Number of days of ovarian stimulation (mean ± SD) | 10.98 ± 2.5 | 7–12 | 10.75 ± 0.5 | 9–13 | 0.454 |
| 10 | Number of oocyte (s) retrieved (mean ± SD) | 8.64 ± 4.8 | 01–22 | 6.88 ± 4.8 | 01–23 | 0.34 |
| 11 | Number of Embryo Transferred (mean ± SD) | 2.22 ± 0.6 | 1–3 | 2.47 ± 0.7 | 1–3 | 0.12 |
aAll values are expressed in mean ± SD. p < 0.05 is significant
Basal serum FSH and LH levels showed no significant difference between the two groups. The level of serum FSH in group A was 5.13 ± 2.8 (range 1.1–6.8 mIU/mL) and in group B was 5.63 ± 6.9 (range 1.4–7.9 mIU/mL) p = 0.51. The level of serum LH in group A was 3.54 ± 3.2 (range 0.3–15.6 mIU/mL) and in group B was 3.36 ± 5.05 (range 0.1–12.3 mIU/mL) p = 0.17. The antral follicular count (AFC) was also non-significant in group A (7.40 ± 3.9 ranging 01–20 in both the ovaries) versus group B (7.75 ± 4.1 ranging 01–23 in both the ovaries). No significant differences in the number of days of stimulation, endometrial thickness, number of oocytes retrieved, and number of embryos replaced to the uterine cavity in both the groups (Table 1).
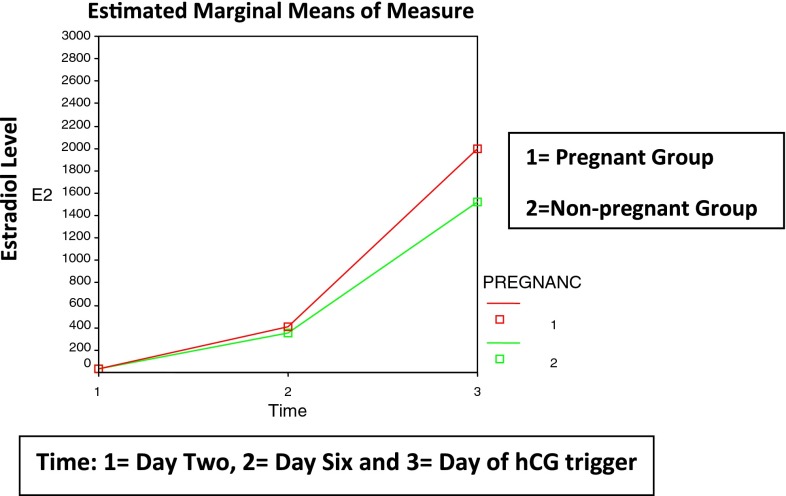
Independent Student t-test was performed to analyze the mean difference in the levels of estradiol at down-regulated day 2, day 6, and day of hCG trigger during ovarian stimulation between the group A and group B women who underwent IVF-ET cycle. There was a clear mean difference in the down-regulated day 2 (Table 2) estradiol levels between group A (ranged from 5.8–49.7 pg/mL, mean 31.9 ± 12.6) and group B (ranged from 4.51–49.30 pg/mL, mean 27.5 ± 12.3). Estradiol levels are found to be higher in group A (Fig. 1). This comparison was found to be statistically significant (p = 0.043).
Table 2.
Assessment of day-specific estradiol levels showing probability of pregnancy
| Estradiol level (pg/mL) | Day specific | Sensitivity (%) | Specificity (%) | Area under curve (%) |
|---|---|---|---|---|
| ≥31.2 | Down-regulated day 2 | 62.0 | 61.8 | 60.7 (p = 0.040a) |
| ≥1,400 | Day of hCG trigger | 60.0 | 59.0 | 63.4 (p = 0.007b) |
aStatistically significant
bStatistically highly significant
Fig. 1.
Comparison of estimated marginal mean estradiol levels between Group A and Group B
Similarly, there was a mean difference in the serum estradiol levels on the day of hCG trigger between group A (ranged from 429.73–6,510.0 pg/mL, mean 1,996.4 ± 1,252.3) and group B (ranged from 67.0–7,185.0 pg/mL, mean 1,525.3 ± 1,116.4). The levels were found to be significantly higher in pregnant group (p = 0.025).
As far as day 6 estradiol levels were considered, between group A (ranged from 41.5–1,076.0 pg/mL mean 408.6 ± 292.5) and group B (ranged from 19.50–1,001.0 pg/mL, mean 354.5 ± 276.2), the mean difference was statistically non-significant (p = 0.273).
Antral follicular count on day 2 (p = 0.598), endometrial thickness on day of trigger (p = 0.683), and number of oocytes retrieved (p = 0.623) were non-significantly allied with the success of IVF.
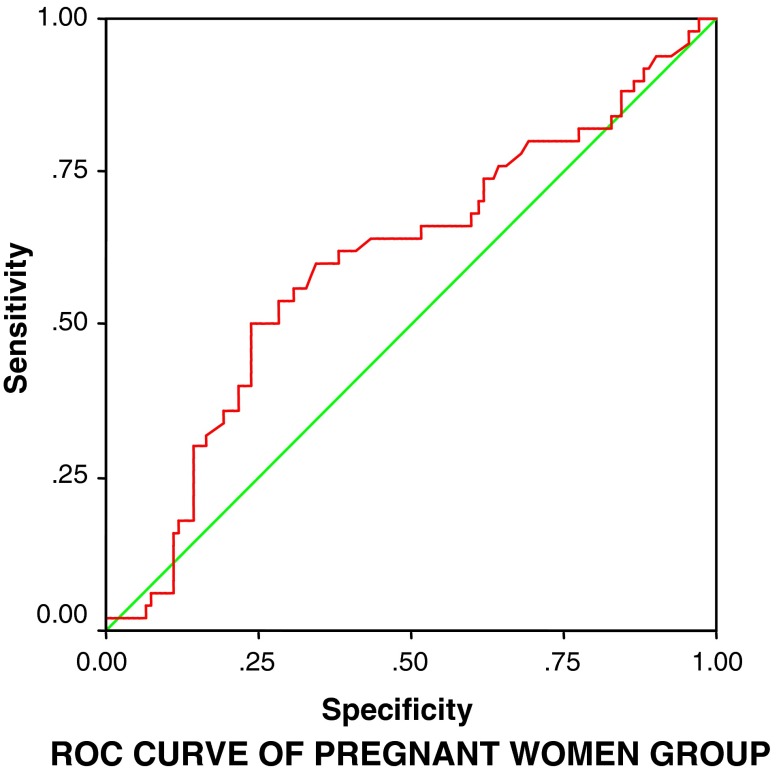
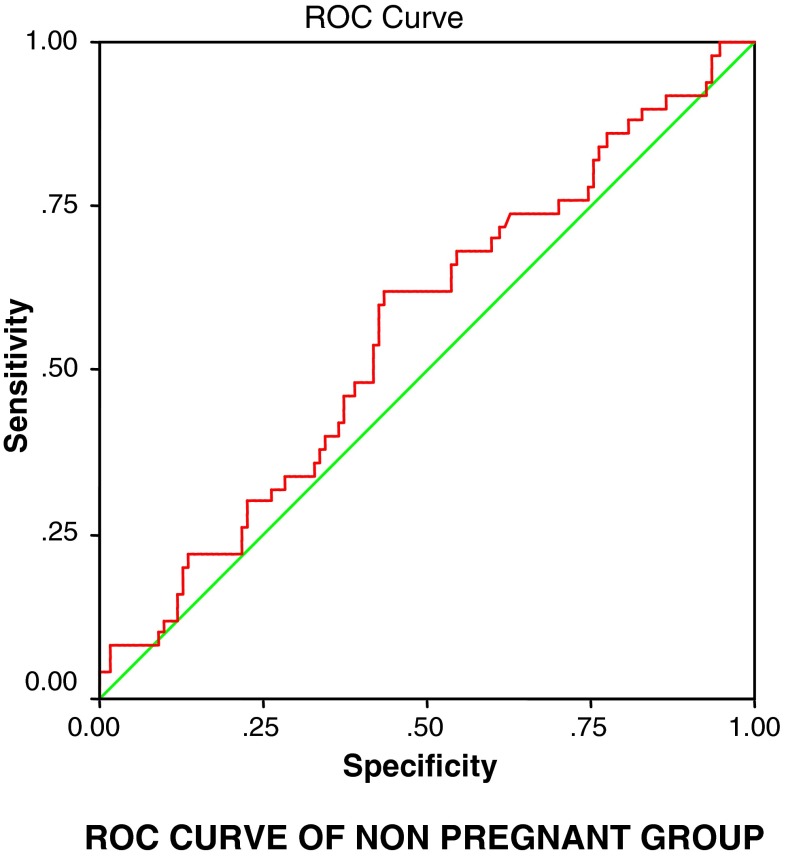
To justify the results derived from student t-test and forecasting the pregnancy outcome in IVF-ET cycle based on estradiol levels, the change of the estradiol levels on day 2, day of hCG trigger, and successful IVF outcome were studied by applying repeated measure analysis followed by post ROC comparison by Bonferroni method. ROC curve of the model was constructed and the area under curve was used for the assessment and effectiveness of the parameter to predict the likelihood of pregnancy. We observed that with an estradiol level ≥31.2 pg/mL on day 2 of down-regulated cycle, 61.8 % women can achieve pregnancy with p = 0.040 (area under curve 60.7 % and sensitivity 62.0 %; Fig. 2) and when estradiol levels are ≥1,400 pg/mL on the day of trigger, 59 % women can achieve pregnancy with p = 0.007 (area under curve 63.4 % and sensitivity 60.0 %; Fig. 3).
Fig. 2.
ROC curve of estradiol levels on day 2 and day of hCG trigger in Group A (pregnant group) forecasting the likelihood of the pregnancy in IVF procedure
Fig. 3.
ROC curve of estradiol levels on day 2 and day of hCG trigger in Group B (non-pregnant group) forecasting the likelihood of the pregnancy in IVF procedure
Discussion and Conclusion
Achievement of a successful pregnancy is the mainstay of an IVF program. The pregnancy rates in IVF-ET treatment are nearly 30–35 % [2]. Early identification of factors which can predict the IVF outcome is, therefore, of great value. For a long time, clinical research has been focused on the evaluation of predictors like FSH [3], estradiol [4], inhibin A [5], inhibin B [6], age [7], and antral follicles count [8]. Pregnancy success in IVF-ET cycle depends on endometrial receptivity [14], which is related to embryo quality and implantation ability. It is well established that certain level of estradiol per follicle is required to obtain pregnancy [15]. We thus attempted to explore the efficacy of E2 levels as a marker for clinical pregnancy.
Serum estradiol was used to monitor controlled ovarian hyperstimulation. Both low (500 pg/mL) and high (>4,000 pg/mL) serum estradiol concentrations, on the day of hCG administration, resulted in undesirably low and high oocyte yields, respectively, and thus, diminished their probabilities to achieve pregnancy. Elevated or diminished levels of estradiol could produce impaired maturation of oocyte cytoplasm and decrease the probability of obtaining pregnancy [16].
Optimal level of estradiol on down-regulated day 2 and day of hCG trigger is more likely to obtain a good number of oocytes and better embryos than with inferior levels of estradiol. Thus, the early appearance of estrogen within the follicle allows the follicle to respond to relatively low concentrations of FSH. The dominance of estradiol and FSH in follicular fluid is essential for sustained accumulation in granulosa cells, continued follicular growth, and estradiol production [17].
The present study suggested that mean estradiol levels at down-regulated day 2 of menstruation ≥31.2 pg/mL and day of hCG trigger ≥1,400 pg/mL are associated with a higher pregnancy rate (61.8 and 59.0 % respectively). Mean difference of E2 levels at day 6 showed no statistical significance between groups A (pregnant group) and group B (non-pregnant group).
The role of patient’s age in predicting performance in ART is well established. Thus, fecundity in natural and stimulated cycles declines with maternal age. Ovarian reserve diminishes with age and as a result the spontaneous fecundity rate also declines which in turn has an impact on the IVF outcome. Older women have less number of oocytes and thus lower implantation rates. But its predictive value is limited if considered as an isolated factor [18].
Antral follicular count on day 2 (p = 0.598), endometrial thickness on day of trigger (p = 0.683), and number of oocytes retrieved (p = 0.628) were non-significantly allied with the success of IVF in our study.
Anifandis et al. [15] suggested that the success of pregnancies in IVF-ET cycles was maximum when peak estradiol levels at the time of hCG administration are between 1,001 and 2,000 pg/mL; this is a significant prognostic marker. In our study, the range was found to be between 900 and 1,900 pg/mL.
According to Theocharis et al. [16], controlled ovarian stimulation for IVF cycles is usually monitored by serum estradiol levels. In our study, number of oocytes, total embryos obtained, and number of high grade embryos, were significantly better for patients with estradiol level above 90th percentile at hCG administration for the three age groups: group A (age < 35 years, E2 > 4,233 pg/mL), group B (age 35–38 years, E2 > 3,904 pg/mL), and group C (age > 38 years, E2 > 3,897 pg/mL) [19].
The number of oocytes retrieved ranged from 1–22 oocytes among pregnant women with an average of eight oocytes retrieved. Among the eight oocytes, 80–90 % got fertilized of which 50 % developed normally to “A” grade embryos. A total of 1–3 embryos were transferred per cycle.
To obtain pregnancy in an IVF cycle, the need for good quality embryos and the technique of embryo transfer are very crucial. Thus, patients with good embryos but difficult or bad transfer may hamper the probability of achieving pregnancy [20, 21]. Good interaction between the embryos and the endometrium is another crucial factor in order to favor the implantation process [22, 23]. When we estimated the probability of pregnancy in our study, it was not possible to know what happened during embryo transfer or appraisal of interaction between embryo and endometrium. But we assumed the same consequences as the same technique was applied by the same surgeon in all women.
The present study shows that it is possible to predict the probability of achieving pregnancy among patients undergoing IVF program. Estradiol levels on down-regulated day 2 and on the day of hCG trigger had a significant impact on the success of IVF-ET.
These data provide valuable information for better understanding of the correlation between parameters related to IVF cycles, and may help clinicians in counseling patients regarding the cycle response and expected pregnancy rate.
From this study we ascertain that, if the estimated estradiol level is ≥31.2 pg/mL on down-regulated day 2 cycle, 61.8 % women can achieve pregnancy (p = 0.040). Further assumption is, if estradiol level is ≥1,400 pg/mL on the day of hCG trigger, 59.0 % women can achieve pregnancy (p = 0.007 statistically significant).
Further studies with larger sample size are needed to confirm this model of prediction for achieving pregnancy in IVF-ET cycles.
Acknowledgments
The authors deeply acknowledge the patients for giving consent for the study. Technical assistance from staff of IVF and Reproductive Biology Centre and Department of Biochemistry and Biotechnology is highly acknowledged.
Conflict of interest
None.
References
- 1.Evers JL. Female subfertility. Lancet. 2002;360:151–159. doi: 10.1016/S0140-6736(02)09417-5. [DOI] [PubMed] [Google Scholar]
- 2.2009 Assisted Reproductive Technology, Success Rates, National Summary and Fertility Clinic Reports. National Centers for Chronic Disease Prevention and Health Promotion, 2011.
- 3.Scott RT, Toner JP, Muasher SJ, et al. Follicle-stimulating hormone levels on cycle day 3 are predictive of in vitro fertilization outcome. Fertil Steril. 1989;4:651–654. doi: 10.1016/s0015-0282(16)60615-5. [DOI] [PubMed] [Google Scholar]
- 4.Licciardi FL, Liu HC, Rosemwaks Z. Day 3 estradiol serum concentrations as prognosticators of ovarian stimulation response and pregnancy outcome in patients undergoing in vitro fertilization. Fertil Steril. 1995;5:991–994. doi: 10.1016/s0015-0282(16)57916-3. [DOI] [PubMed] [Google Scholar]
- 5.Yanushpolsky EH, Hurwitz S, Tikh E, et al. Predictive usefulness of cycle day 10 follicle-stimulating hormone level in a clomiphene citrate challenge test for in vitro fertilization in women younger than 40 years of age. Fertil Steril. 2003;1:111–115. doi: 10.1016/S0015-0282(03)00499-0. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann GE, Danforth DR, Seifer DB. Inhibin-B: The physiologic basis of the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril. 1998;3:474–477. doi: 10.1016/S0015-0282(97)00531-1. [DOI] [PubMed] [Google Scholar]
- 7.Tomas C, Nuojua-Huttunen S, Martinaken H. Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotrophins in in vitro fertilization. Hum Reprod. 1997;2:220–223. doi: 10.1093/humrep/12.2.220. [DOI] [PubMed] [Google Scholar]
- 8.Hendriks DJ, Mol BW, Bancsi LF, et al. Antral follicle count in the prediction of poor ovarian response and pregnancy after in vitro fertilization: a meta-analysis and comparison with basal follicle-stimulating hormone level. Fertil Steril. 2005;2:291–301. doi: 10.1016/j.fertnstert.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Ficicioglu C, Kutlu T, et al. Early follicular antimullerian hormone as an indicator of ovarian reserve. Fertil Steril. 2006;3:592–596. doi: 10.1016/j.fertnstert.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Hull MG, Fleming CF, Hughes AO, et al. The age related decline in female fecundity: a quantitative controlled study of implanting capacity and survival of individual embryos after in vitro fertilization. Fertil Steril. 1996;4:783–790. doi: 10.1016/s0015-0282(16)58214-4. [DOI] [PubMed] [Google Scholar]
- 11.Scott RT, Hofmann GE, Oehninger S, et al. Intercycle variability of day 3 follicle-stimulating hormone levels and its effect on stimulation quality in in vitro fertilization. Fertil Steril. 1990;2:297–302. doi: 10.1016/s0015-0282(16)53707-8. [DOI] [PubMed] [Google Scholar]
- 12.Ocal P, Aydin S, Cepni J, et al. Follicular fluid concentrations of vascular endothelial growth factor, inhibin A, inhibin B in IVF cycles: are they markers for ovarian response and pregnancy outcome. Eur J Obstet Gynecol Reprod Biol. 2004;2:194–199. doi: 10.1016/j.ejogrb.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 13.Khalaf Y, Taylor A, Braude P. Low serum E2 concentrations after five days of controlled ovarian hyperstimulation for in vitro fertilization are associated with poor outcome. Fertil Steril. 2000;74:63–66. doi: 10.1016/S0015-0282(00)00569-0. [DOI] [PubMed] [Google Scholar]
- 14.Devroey P, Bourrgain C, Macklon NS, et al. Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab. 2004;15:84–90. doi: 10.1016/j.tem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Anifandis G, Koutselini E, Louridas K, et al. Estradiol and leptin as conditional prognostic IVF markers. Reproduction. 2005;129:531–534. doi: 10.1530/rep.1.00567. [DOI] [PubMed] [Google Scholar]
- 16.Papageorgiou T, Guibert J, Goffinet F, et al. Percentile curves of serum estradiol levels during controlled ovarian stimulation in 905 cycles stimulated with recombinant FSH show that high estradiol is not detrimental to IVF outcome. Hum Reprod. 2002;17:2846–2850. doi: 10.1093/humrep/17.11.2846. [DOI] [PubMed] [Google Scholar]
- 17.Speroff L, Glass RH, Kase AG. Clinical gynecologic endocrinology and infertility. Maryland: Williams & Wilkins; 1994. Regulation of menstrual cycle; pp. 141–183. [Google Scholar]
- 18.Tesarik J, Mendoza C. Nongenomic. Effects of 17 beta-estradiol on maturing human oocytes: relationship to oocyte developmental potential. J Clin Endocrinol Metab. 1995;80:1438–1443. doi: 10.1210/jcem.80.4.7714121. [DOI] [PubMed] [Google Scholar]
- 19.Weghofer A, Margreiter M, Fauster Y, et al. Age-specific FSH levels as a tool for appropriate patient counseling in assisted reproduction. Hum Reprod. 2005;20(9):2448–2452. doi: 10.1093/humrep/dei076. [DOI] [PubMed] [Google Scholar]
- 20.Coroleu B, Barri PN, Carreras O, et al. The influence of the depth of embryo replacement into the uterine cavity on implantation rates after IVF: a controlled, ultrasound-guided study. Hum Reprod. 2002;17(2):341–346. doi: 10.1093/humrep/17.2.341. [DOI] [PubMed] [Google Scholar]
- 21.Tomas C, Tikkinen K, Tuomivaara L, et al. The degree of difficulty of embryo transfer is an independent factor for predicting pregnancy. Hum Reprod. 2002;17(10):2632–2635. doi: 10.1093/humrep/17.10.2632. [DOI] [PubMed] [Google Scholar]
- 22.Edgar DH. Estrogen and human implantation. Hum Reprod. 1995;10:2–4. doi: 10.1093/humrep/10.1.2. [DOI] [PubMed] [Google Scholar]
- 23.Makker A, Singh MM. Endometrial receptivity: clinical assessment in relation to fertility, infertility, and antifertility. Med Res Rev. 2006;26(6):699–746. doi: 10.1002/med.20061. [DOI] [PubMed] [Google Scholar]