In this series, a clinician extemporaneously discusses the diagnostic approach (regular text) to sequentially presented clinical information (bold). Additional commentary on the diagnostic reasoning process (italics) is integrated throughout the discussion.
Clinical Information
A 22-year-old woman presents to the emergency department with abdominal pain for the last 48 h. Her pain is diffuse in location, aching in nature, 9/10 in severity, and does not localize. She has no clear aggravating or alleviating factors.
Clinician
The importance of understanding the reason for presentation cannot be overstated, as the patient’s chief complaint is often critical to determining the final diagnosis. In a young woman with abdominal pain, common maladies must be considered first: cholecystitis, appendicitis, and gynecologic sources—including complications of pregnancy. The young age of the patient effectively excludes diagnoses usually seen in an older population, such as ischemic bowel, diverticulitis, and bowel obstruction.
Diagnostic Reasoning
Expert clinicians start collecting important information as soon as the patient encounter begins. Clinicians compare the data obtained about their patient to their illness scripts for various disorders. Illness scripts are collections of data, such as characteristic symptoms, epidemiologic factors, risk factors, exam findings, or test results that summarize a clinician’s knowledge about a disorder—like a small chapter about a disorder.1Clinicians create a differential diagnosis by including disorders that match the patient presentation and excluding disorders that do not.
While this mental exercise is helpful to exclude some diagnoses, clinicians should keep in mind that patients “don’t always read the textbook” and atypical presentations can be seen. Another possibility is that the clinician’s illness script may be incomplete (for example, due to lack of experience) or may even be inaccurate (for example, caring for unique patient populations). Even though it is highly unlikely that the described patient will have diseases commonly seen in older adults, occasionally younger patients can present with these disorders (i.e. young man with ischemic bowel after using a vasoactive substance such as cocaine). It is helpful to continue to reevaluate the diagnostic possibilities as new information becomes available, and sometimes that includes reconsidering possibilities that we initially thought were highly unlikely.
Clinical Information
She was diagnosed with major depressive disorder 3 years prior and takes no medications. In the past 2 years, she reports recurrent episodes of severe abdominal pain. Elsewhere, she has undergone cholecystectomy, appendectomy, and total abdominal hysterectomy (without salping-oophorectomy); all in an attempt to localize and eliminate her recurrent abdominal pain (records and pathological diagnosis were not available). She had uncomplicated cystitis 1 week prior and is now completing a course of trimethoprim-sulfamethoxazole. She takes no other medications, herbal supplements, or over-the-counter medications. She has received analgesics and opioid medications from multiple providers for the past 3 years.
Clinician
Although she may not get regular medical care, it seems that this patient has interfaced with the healthcare system frequently over a relatively short period of time. Her pain is significant enough to require opioids, but she does not have a clear diagnosis. The history of cryptic abdominal pain leading to multiple surgeries in a young patient may fit the illness script of acute intermittent porphyria or familial Mediterranean fever. However, both are rare disorders, and we should not jump to these entities without more information and without considering more common illnesses. Given her history of mood disorder, one may also consider somatoform disorder, or other illnesses with psychiatric overtones. It is also important to consider domestic abuse in a young woman with non-specific complaints who recurrently presents to the emergency department.
Diagnostic Reasoning
Sir Arthur Conan Doyle’s fictional detective Sherlock Holmes compared the human brain to an attic. He described the brain as a location where we keep information that we have learned in the past and may use in the future.2Since there is a finite amount of space in our “brain attic,” we must decide what information to keep and what to discard. While in school, postgraduate training, and also in everyday practice, we learn or hear about a myriad of conditions. Some are common and we develop robust illness scripts for these conditions. Many are uncommon and without frequent use, our illness scripts of these diseases dwindle with time.
The clinician is able to recognize a pattern of symptoms and pieces of information that may be consistent with two uncommon diseases, familial Mediterranean fever and acute intermittent porphyria. The clinician likely has limited experience with these disorders, but he used the information available in his brain attic and compared the illness scripts he remembers for these two conditions with the information that is being presented about this patient. Even when he recognizes that the presentation may be consistent with these diagnoses, he takes a step back and analyzes the probability of seeing these disorders. He recognizes that he is more likely to encounter a common disorder or an uncommon presentation of a common disorder than one of these rare illnesses.
The clinician mentions several diagnostic possibilities, but does not mention others. Inflammatory bowel disease, irritable bowel syndrome, serositis, vasculitis, and recurrent partial bowel obstructions induced by adhesions from previous surgeries are a few other diagnostic possibilities. The clinician seems to be waiting for more information instead of generating a more comprehensive differential diagnosis.
Clinical Information
She lives with her boyfriend and has been in a monogamous relationship for 3 years. She is unemployed, has 1–2 alcoholic beverages weekly, and has smoked a half pack of cigarettes per day for the last 7 years. She reports no illicit drug use. She achieved menarche at age 13, has never been pregnant, and has never been treated for a sexually transmitted infection. As she is adopted, her family history is unknown. On review of systems, she has had no menses or vaginal complaints since her hysterectomy. She reports decreased appetite during episodes of abdominal pain and nausea without vomiting. She denies fever, chills, diarrhea, or other systemic symptoms.
On physical examination, her blood pressure was 112/76 mmHg, heart rate was 114 beats per minute, respiratory rate was 16 breaths per minute, and her temperature was 98.4 °F (36.8° Celsius). She was in moderate distress due to abdominal pain. Her abdomen was soft and non-distended, but moderately tender throughout. She had no succussion splash. Bowel sounds were present but hypoactive. No rebound tenderness or guarding was present. Scars from the hysterectomy (transverse incision) and laparoscopic interventions were well healed.
Clinician
The description of bowel sounds as hypoactive is generally unhelpful, since the frequency and volume of bowel sounds may be a function of when the patient last ate. More helpful is the distinction between bowel sounds that are absent versus abnormal bowel sounds (e.g. tinkles and rushes). Absent bowel sounds may imply ileus, constipation, or bowel rupture. Abnormal sounds may imply intestinal obstruction, colitis, or irritable bowel syndrome.
Diagnostic Reasoning
Understanding the significance of physical exam findings can be challenging, since many of the maneuvers or findings have limited sensitivity and specificity. Here the clinician tries to put into context which findings are more likely to be helpful in determining the diagnosis in this patient.
Clinical Information
Initial laboratory values include sodium 132 mmol/L, potassium 4.3 mmol/L, chloride 102 mmol/L, bicarbonate 23 mmol/L, urea nitrogen 22 mg/dL, creatinine 0.9 mg/dL. White Blood Count 7,900 cells/cm, hemoglobin 13 g/dL, platelets 164,000 per cm.
Total protein 7.5 g/dL, albumin 3.9 g/dL, total bilirubin 0.4 mg/dL, aspartate aminotransferase 18
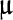 /L, alanine aminotransferase 23
/L, alanine aminotransferase 23
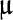 /L, alkaline phosphatase 60
/L, alkaline phosphatase 60
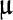 /L. The prothrombin and activated partial thromboplastin times were within normal limits. Amylase, lipase, and lactic acid were within normal limits. Urinalysis: specific gravity 1.030, pH 6.5, 1+ blood, 1+ leukocyte esterase, 1+ nitrite, 10-20 WBC per HPF, 0-2 RBC per HPF, trace bacteria.
/L. The prothrombin and activated partial thromboplastin times were within normal limits. Amylase, lipase, and lactic acid were within normal limits. Urinalysis: specific gravity 1.030, pH 6.5, 1+ blood, 1+ leukocyte esterase, 1+ nitrite, 10-20 WBC per HPF, 0-2 RBC per HPF, trace bacteria.
An upright abdominal x-ray showed a normal bowel gas pattern. A contrast-enhanced computed tomographic exam of the abdomen and pelvis showed no acute process (gallbladder, appendix, and uterus were absent).
Clinician
The initial laboratory results appear fairly nonspecific. A young woman with serum sodium of 132 mmol/L is unusual, but the significance in this patient is unclear. Her urine appears concentrated and urinalysis is consistent with possible urinary tract infection, for which she is in the midst of treatment.
Infection may be less likely, given her normal white blood cell count. While we would ideally like to make a diagnosis based on history, physical exam and laboratory analysis, often imaging is necessary. Here, the imaging is also nonspecific, which should alert us that perhaps additional evaluation and testing may be needed.
Chronic adrenal insufficiency can often present with atypical abdominal pain and hyponatremia, although typically with hyperkalemia and hypoglycemia, which are not seen here. Acute intermittent porphyria remains high on the differential, given patient’s demographics, abdominal pain, and lack of specific laboratory or radiographic findings. At this point, I might review the medications that traditionally precipitate porphyric attacks to see if trimethoprim/ sulfamethoxazole is on that list. I mention trimethoprim/ sulfamethoxazole because she was just recently taking this medication for a presumed urinary tract infection (UTI). With rare disorders, I always recommend reviewing lists of medications that may cause relapse, as most providers do not see these disorders often enough to remember all of them.
Diagnostic Reasoning
The clinician recognizes some mild abnormalities in the blood tests and urinalysis, yet is not ready to make a diagnosis based on this information since the presentation is not explained by these findings. Although it could be tempting to diagnose this patient with a UTI, the clinician recognizes that the patient’s current symptoms are not consistent with his illness script for a UTI. The nonspecific imaging findings alert him to move from common illness scripts to a more rare set of disorders. He realizes that further specialized testing will likely be needed to make the diagnosis. He uses the low sodium as an additional clue that may help in the diagnostic evaluation, and includes adrenal insufficiency as an additional diagnostic possibility.
He goes back to his brain attic and contrasts the available information (symptoms, multiple surgeries for recurrent abdominal pain, nonspecific findings on exam and routine tests), with the illness script he has for acute intermittent porphyria. He remembers that there are medications that can precipitate a crisis. Because he does not frequently see patients with acute intermittent porphyria, he does not remember the list of medications that can cause a crisis. That would occupy too much space in his brain attic—space that could be used to record other key information on many other disorders or other useful information that is pertinent for his work or life; however, he does remember that there is a list of medications and appropriately asks for that information.
Clinical Information
Due to the severity of her unrelenting abdominal pain, she was hospitalized. A urinary catheter was inserted. Given the extensive workup and surgeries without elimination of her symptoms, the clinician caring for this patient revisited her room after the initial encounter. The urine in the collecting bag appeared reddish-brown. Upon further questioning, the patient is aware her father is a first-generation Finnish immigrant, but does not know any further family history.
Clinician
We did something wrong, but it gave us the right answer. Placing a urinary catheter in this patient was probably inappropriate. A simple UTI is not a reason for a catheter and could very well have made things worse. If she did not have a UTI, placing a Foley might cause one. However, it did give a great clue in solving this mystery. At this point, the differential diagnosis is broad and includes somatoform disorder, domestic abuse, Addison’s disease, and acute intermittent porphyria (AIP). The discolored urine and northern European heritage necessitate that we rule out AIP.
Diagnostic Reasoning
The suspicion of acute intermittent porphyria as the underlying diagnosis is now even higher; however, the clinician appropriately keeps a cautious approach to this diagnosis. He recognizes the patient’s discolored urine and northern European heritage are consistent with AIP, but he avoids premature closure by keeping other disorders on his differential diagnosis.
Clinical Information
Her high levels of urine porphobilinogen (184 mg/L; normal 0-4 mg/L) confirmed the diagnosis of an acute porphyria. She received hemin with complete resolution of her symptoms. Four months after the initial presentation, she was doing well without further attacks of abdominal pain. Although confirmatory testing was not performed, AIP is the most likely final diagnosis, given the patient’s heritage and the increased prevalence of AIP compared to the other acute porphyrias (variegate porphyria and hereditary coproporphyria).
Discussion
Medicine is a discipline of lifelong learning. As clinicians, we are exposed to massive amounts of information, some old and some new. The number of existing medical disorders and the complexities of the evaluation, diagnosis, and management of these illnesses, threatens our ability to keep up with knowledge even in our areas of expertise.3
Over the years, from our educational and clinical experiences, we develop illness scripts: descriptions of symptoms, risk factors and epidemiology, exam findings, and test abnormalities that give us a summary of a disease presentation. Our illness scripts evolve over time, being modified as we learn new things about a disease (e.g. atypical presentations, new risk factors, new tests).4 For obvious reasons, the illness scripts we have for common disorders in our areas of practice will include much more information than those for illnesses we do not routinely encounter. Since our brains have a finite amount of storage space, we cannot keep complete illness scripts about all diseases we encounter.5
However, clinicians who are experts in diagnosing challenging cases will often have a brain attic that keeps information on the illness scripts of a large number of entities.6 The amount of information for each disease may not be exhaustive, but often includes key pieces of information that can help suggest a diagnostic possibility. For example, key pieces of information about AIP might include young person, recurrent abdominal pain, nondiagnostic laparotomies, hyponatremia, dark urine, and attacks that can be triggered by medications. The clinician does not need to be an expert in this area, but having enough information in his brain attic to suggest this diagnosis can trigger further reading and appropriate consultation with a specialist or further testing. With uncommon disorders many times patients remain undiagnosed for long periods of time until the disease “self-declares” with worsening symptoms, or until a clinician recognizes that some of the features could be explained by a rare disorder after comparing with an illness script he/she may have heard about years ago.
The concept of a brain attic is extensively discussed in the book “Mastermind: how to think like Sherlock Holmes” by Maria Konnikova.7 The author makes the point that Sherlock Holmes maintains a brain attic full of well-organized and helpful pieces of information that he utilizes when solving cases; however he does not use valuable space in this attic to record information that he finds not useful in his investigations. In “A Study in Scarlet” Holmes tells Watson:
“I consider that a man’s brain originally is like a little empty attic, and you have to stock it with such furniture as you choose. A fool takes in all the lumber of every sort that he comes across, so that the knowledge which might be useful to him gets crowded out, or at best is jumbled up with a lot of other things, so that he has a difficulty in laying his hands upon it. Now the skillful workman is very careful indeed as to what he takes into his brain-attic”2
A hallmark of the master diagnostician is familiarity with a large number of disorders, but extensive knowledge about every medical disorder is not possible or practical. These minutiae would clutter our brain attics and make useful information less easily retrievable. Instead, clinicians should develop detailed illness scripts for common conditions in their practice and maintain small illness scripts for disorders that, although uncommon, may still be encountered in their scope of practice—keeping this information in their brain attics for future use. A well-organized illness-script repertoire can allow a clinician to make a diagnosis that has gone unrecognized by others—a true hallmark of the master diagnostician.
Clinical Teaching Points
Eight enzymes catalyze the conversion of glycine and succinyl-CoA to heme in humans. A deficiency or inhibition in any of these enzymes leads to systemic syndromes called porphyrias which manifest with either acute neurovisceral attacks, skin lesions, or both. Acute neurovisceral attacks are caused primarily by three porphyrias: acute intermittent porphyria, variegate porphyria and hereditary coproporphyria.8
Attacks of acute porphyria usually cause severe abdominal pain. Other manifestations include: psychiatric symptoms ranging from depressed mood to frank psychosis; nausea, vomiting, and constipation; dark urine; and signs of increased sympathetic activity, including tachycardia, sweating, and hypertension.8 Autonomic instability secondary to neurotoxic injury results in up to 30 % mortality of each attack that requires hospitalization, making early recognition and diagnosis critical.9
Acute intermittent porphyria is the most common porphyria in adults and results from a deficiency in porphobilinogen deaminase, the third enzyme in the heme biosynthesis cascade. AIP classically presents in young women of Scandinavian or northern European descent. During times of increased heme synthesis, the biosynthetic pathway may bottle-neck at the third enzyme, resulting in accumulation of both porphobilinogen and aminolevulinic acid. These heme precursors induce axonal injury to visceral nerves, leading to the characteristic abdominal pain and autonomic instability seen in an AIP attack. Attacks can be precipitated by systemic hypoglycemia or medications (glucocorticoids, sulfa drugs, anti-epileptics).10
The goal of acute porphyria management is reversing the heme biosynthesis pathway by downregulating ALA-synthase. Since hypoglycemia induces ALA-synthase, large doses of IV dextrose (300–500 g per day) have been used. More recently, a heme analog, hemin, has been developed to replete the regulatory heme pool and thereby downregulate ALA-synthase. The standard dose for IV hemin is 3-4 mg/kg per day, given for 3–4 days. The high mortality rate in the acute setting necessitates immediate treatment, but long-term sequelae of untreated AIP can include chronic kidney disease, hypertension, and hepatocellular carcinoma.10
Acknowledgements
Contributor
Dr. Yvette Cua-Ramirez for providing the video of the live session.
Funders
None.
Prior Presentations
The case presented here was presented as a Clinical Vignette Unknown at the Southern Society of General Internal Medicine, New Orleans, 21–23 February 2013. The clinical information and case discussion closely reflect the topics discussed.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Disclosures
The opinions expressed in this article are those of the authors alone and do not reflect the views of the Department of Veterans Affairs.
REFERENCES
- 1.Charlin B, Boshuizen HP, Custers EJ, Feltovich PJ. Scripts and clinical reasoning. Med Educ. 2007;41:1178–1184. doi: 10.1111/j.1365-2923.2007.02924.x. [DOI] [PubMed] [Google Scholar]
- 2.Doyle AC. A Study In Scarlet. Philadelphia: J.B. Lippincott; 1890. [Google Scholar]
- 3.Alper BS, Hand JA, Elliott SG. How much effort is needed to keep up with the literature relevant for primary care? J Med Libr Assoc. 2004;92(4):429–437. [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt HG, Rikcers RM. How expertise develops in medicine: knowledge encapsulation and illness script formation. Med Educ. 2007;41:1133–1139. doi: 10.1111/j.1365-2923.2007.02915.x. [DOI] [PubMed] [Google Scholar]
- 5.Van Merriënboer JJ, Sweller J. Cognitive load theory in health professional education: design principles and strategies. Med Educ. 2010;44(1):85–93. doi: 10.1111/j.1365-2923.2009.03498.x. [DOI] [PubMed] [Google Scholar]
- 6.Mylopoulos M, Lohfeld L, Norman GR, Dhaliwal G, Eva KW. Renowned physicians' perceptions of expert diagnostic practice. Acad Med. 2012;87(10):1413–1417. doi: 10.1097/ACM.0b013e31826735fc. [DOI] [PubMed] [Google Scholar]
- 7.Konnikova M. Mastermind: How to Think Like Sherlock Holmes. New York: Penguin Group; 2013. [Google Scholar]
- 8.Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375(9718):924–937. doi: 10.1016/S0140-6736(09)61925-5. [DOI] [PubMed] [Google Scholar]
- 9.Jeans JB, Savik K, Gross CR, et al. Mortality in patients with acute intermittent porphyria requiring hospitalization: a United States case series. Am J Med Genet. 1996;65(4):269–273. doi: 10.1002/(SICI)1096-8628(19961111)65:4<269::AID-AJMG4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.Anderson KE, Bloomer JR, Bonkovsky HL, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142(6):439–450. doi: 10.7326/0003-4819-142-6-200503150-00010. [DOI] [PubMed] [Google Scholar]


