ABSTRACT
BACKGROUND
Randomized studies have shown optimal medical therapy to be as efficacious as revascularization in stable ischemic heart disease (IHD). It is not known if these efficacy results are reflected by real-world effectiveness.
OBJECTIVE
To evaluate the comparative effectiveness of routine medical therapy versus revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) in stable IHD.
DESIGN
Observational cohort study.
PATIENTS
Stable IHD patients from 1 October 2008 to 30 September 2011, identified using a Registry of all angiography patients in Ontario, Canada.
INTERVENTION
Revascularization, defined as PCI/CABG within 90 days after index angiography.
MAIN MEASURES
Death, myocardial infarction (MI) or repeat PCI/CABG. Revascularization was compared to medical therapy using a) multivariable Cox-proportional hazard models with therapy strategy treated as a time-varying covariate; and b) a propensity score matched analysis. Post-angiography medication use was determined.
KEY RESULTS
We identified 39,131 stable IHD patients, of whom 15,139 were treated medically, and 23,992 were revascularized (PCI = 15,604; CABG = 8,388). Mean follow-up was 2.5 years. Revascularization was associated with fewer deaths (HR 0.76; 95 % CI 0.68–0.84; p < 0.001) ,MIs (HR 0.78; 95 % CI 0.72–0.85; p < 0.001) and repeat PCI/CABG (HR 0.59; 95 % CI 0.50–0.70; p < 0.001) than medical therapy. In the propensity-matched analysis of 12,362 well–matched pairs of revascularized and medical therapy patients, fewer deaths (8.6 % vs 12.7 %; HR 0.75; 95 % CI 0.69–0.81; p < 0.001) , MIs (11.7 % vs 14.4 %; HR 0.84; 95 % CI 0.77–0.93 p < 0.001) and repeat PCI/CABG ( 17.4 % vs 24.1 %;HR 0.67; 95 % 0.63–0.71; p < 0.001) occurred in revascularized patients, over the 4.1 years of follow-up.
The revascularization patients had higher uptake of clopidogrel (70.3 % vs 27.2 %; p < 0.001), β-blockers (78.2 % vs 76.7 %; p = 0.010), and statins (94.7 % vs 91.5 %, p < 0.001) in the 1-year post-angiogram.
CONCLUSIONS
Stable IHD patients treated with revascularization had improved risk-adjusted outcomes in clinical practice, potentially due to under-treatment of medical therapy patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-014-2813-1) contains supplementary material, which is available to authorized users.
KEY WORDS: stable ischemic heart disease, angioplasty, medical therapy, coronary artery bypass grafting, comparative effectiveness
INTRODUCTION
The alternative treatment options for patients with chronic stable ischemic heart disease (IHD) are medical therapy alone or in combination with revascularization by either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI).1 Multiple randomized controlled trials (RCT), including the landmark Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) and Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Study, have compared the efficacy of revascularization versus optimal contemporary medical therapy.2–4 Although these studies have consistently shown no difference, they have been criticized due to restrictive recruitment strategies and unrealistic levels of medication compliance and lifestyle modification.5 Thus, there is substantial uncertainty as to the generalizability of the RCT findings to routine clinical practice.
Accordingly, we conducted a comparative effectiveness study of routine medical therapy versus revascularization in patients with stable IHD, using a population-based clinical registry in Ontario, Canada. Our objectives were to determine if the efficacy results in the RCTs were reflected by real-world effectiveness, and to provide insights as to the nature of any discrepancies between real world and clinical trial results.
METHODS
This study was approved by the Institutional Research Ethics Board at Sunnybrook Health Sciences Centre.
Data Sources
Our analyses were conducted using data from the Cardiac Care Network (CCN) of Ontario, Canada. CCN is a network of the 18 hospitals providing cardiac services in Ontario.6,7 CCN maintains a prospective clinical registry of all individuals who undergo cardiac angiography, PCI, or CABG in the province.7 The accuracy of the anatomical and clinical data in the CCN registry has been validated through selected chart audits and angiographic core lab over-reading.8
Data from the CCN registry were linked using encrypted unique identifiers to population-based administrative databases containing information on all Ontario residents. Ontario has more than 13 million residents, all of whom have universal access to physician and hospital services through a single-payer publicly funded healthcare program, administered by the Ministry of Health and Long Term Care of Ontario (MOHLTC). These databases are available at the Institute for Clinical Evaluative Sciences (ICES). The Canadian Institute for Health Information discharge abstract database (CIHI-DAD) contains data on all hospitalizations. The National Ambulatory Care Reporting Service (NACRS) database contains data for hospital-based ambulatory care, including emergency department visits. Death was ascertained using the Ontario Registered Persons Database (RPDB). The Ontario Drug Benefit database (ODB) has comprehensive drug utilization information on patients over 65 years, for whom full drug coverage is provided for by the MOHLTC.9
Study Population
Our cohort consisted of patients with an index angiogram for the indication of stable IHD, as entered by the procedural physician in the CCN registry, from 1 October 2008 to 30 September 2011. Inclusion criteria were obstructive coronary artery, defined as stenosis greater than 70 % in severity (or greater than 50 % in the left main artery).2 We excluded patients whose indications for angiography,in the CCN registry were myocardial infarction (MI), acute coronary syndrome (ACS), or valvular heart disease. We also excluded patients with a hospitalization for a MI/ACS in the 90 days prior to the index angiogram, in order to identify a stable cohort. We categorized patients broadly into two treatment strategies: 1) those with an initial medical strategy, versus 2) an initial revascularization strategy (either PCI or CABG) within 90 days of their index angiogram. This definition is similar to that used previously in the literature, and is accurate, given wait-times for elective coronary revascularization in Ontario (median and 90th percentile of 2 and 20 days for PCI and 14 and 50 days for CABG respectively).7,8 Patients with multiple angiograms in the accrual time period were categorized based on their first angiogram.
Outcomes
Our primary outcome was all-cause mortality, based on the RPDB. Secondary outcomes were hospitalization for non-fatal MI and repeat revascularization with either PCI or CABG. We defined MI using a validated algorithm based on the most responsible diagnosis (using International Classification of Disease (ICD) Version 10 codes I21,I22, and I25.2) in the CIHI-DAD and NACRS.10 Repeat PCI/CABG was ascertained in the CCN registry. Maximum follow-up was until 31 December 2012. In patients greater than 65 years of age (for whom drug utilization data are available in the ODB), we compared medication use prior to the index angiogram, to that within the first year post angiogram. Medication classes included thienopyridines (clopidogrel), angiotension converting enzyme (ACE) inhibitors, angiotension receptor blockers (ARB), β-blockers, statins, calcium channel blockers (CCB) and long acting nitrates. Aspirin was not included, as it is available over the counter and therefore not accurately recorded in the ODB database.
Statistical Analysis
Differences in baseline characteristics were compared using χ2 test for categorical variables, and ANOVA for continuous variables. Unadjusted Kaplan-Meier curves comparing death, MI and repeat PCI/CABG were developed for medical therapy and each of the revascularization modalities. We conducted two adjusted analyses: a) multivariable Cox-proportional hazard models with therapy strategy treated as a time-varying covariate, and b) propensity score matched analyses. Each analysis is described separately below.
Time-Varying Treatment Status Models
We developed Cox-proportional models to model the hazard of the occurrence of our primary and secondary outcomes. Treatment strategy was treated as a time-varying covariate. All patients were initially considered non-revascularized (medical therapy only) until the time of revascularization, at which point they were considered to have switched to the revascularization arm of the study. Such an approach mitigates the potential for immortal time/survivorship bias.11 We used a robust ‘sandwich-type’ variance estimator to account for the clustering of patients within hospital. Candidate variables for risk adjustment included demographics, comorbidities/disease severity, physician characteristics, and hospital factors. The significance of an interaction between age and treatment strategy was tested. Continuous variables were centered using median values.
As a sensitivity analysis, we repeated our models with PCI, CABG and medical therapy as three separate groups in a traditional non-time–varying Cox model in which patients were classified according to the choice of therapy in the initial 90 days post angiogram. We also repeated the analyses excluding all patients with a previous MI.
Propensity Matched Analyses
A propensity-matched analysis of patients who were equally likely to be treated with medical therapy versus revascularization was conducted to further account for measured confounding.12 We fitted a multivariable logistic regression to model the use of revascularization within 90 days of the index angiogram, using our baseline characteristics. This model was used to calculate a propensity score, which was the expected probability of being revascularized, conditional on the covariates in the model.12 We then created a propensity-score–matched cohort by matching each revascularized patient with a medical therapy patient (a 1:1 match).12 A nearest-neighbour–matching algorithm was used to match patients on the basis of the logit of their propensity score.12 A match was made if the difference in the logits of the propensity scores was less than 0.3 of the standard deviation of the scores (known as the caliper width).13 This process was repeated until matches had been attempted for all revascularized patients; the matching was done without replacement, and thus each matched pair was unique.12 To assess the degree of the balance between the two matched groups, a standardized difference was computed for each explanatory factor, with a standardized difference of less than 0.1 indicating a good balance in the matched cohort.14 Kaplan-Meier curves were estimated for the matched pairs and the stratified log-rank test was used to test for equality of the estimated survival curves.15 In addition, hazard ratios were calculated for death, MI and repeat PCI/CABG using a Cox model with robust variance estimators to account for the matched nature of the sample.
To account for possible immortal time/survivorship bias, we recreating the propensity-match pairs after excluding any patients who died in the first 90 days.11 As a subgroup analysis, we repeated the propensity match in a restricted cohort of patients who would have been eligible for the COURAGE study. We performed this subgroup to provide insight as to the impact of the restrictive RCT enrollment criteria on any discrepancies we observed in effectiveness between RCT and clinical practice. The restricted cohort excluded patients with significant left-main artery disease, Canadian Cardiovascular Society (CCS) 4 angina, CCS 0–2 angina without positive stress testing, patients with left ventricular ejection fraction (LVEF) < 30, % and those with revascularization in the 6 months prior to the index angiogram.2
SAS Version 9.3 (SAS Institute Inc, Cary, North Carolina) was used for all analyses; p values of < 0.05 were significant.
RESULTS
Study Population
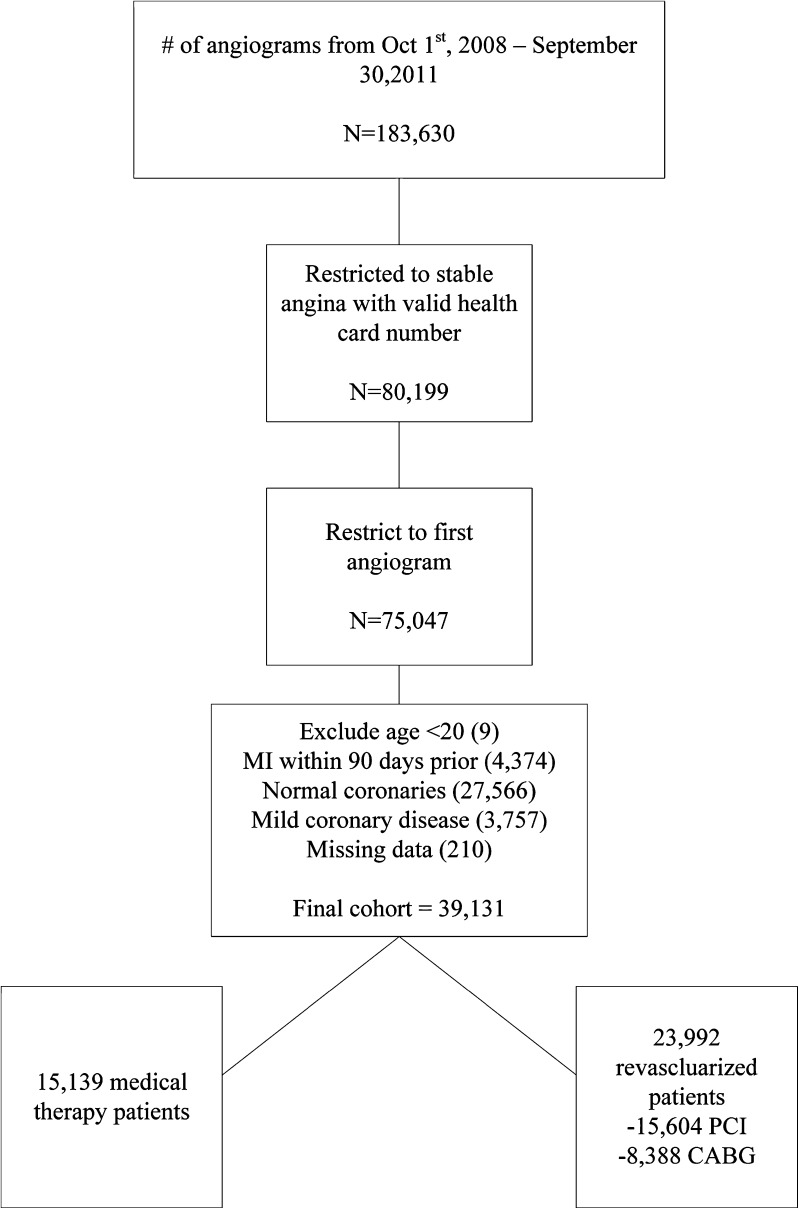
As illustrated in Fig. 1, there were a total of 183,630 angiograms performed from 1 October 2008 to 30 September 2011. Our final cohort consisted of 39,131 patients, of whom 15,139 were treated medically, and 23,992 revascularized (15,604 with PCI and 8,388 with CABG). The median time to PCI was 0 days (mean 8.1;IQR 0–13), and the median to CABG was 28 days (mean 31.3; IQR 8–49).
Figure 1.
Study population.
The baseline characteristics of the final cohort are shown in Table 1, with medication use for the 20,663 patients over the age of 65 years found in Table 2. The revascularization patients had higher uptake of cardio-protective medications such as β-blockers (78.2 % vs 76.7 %; p = 0.010), and statins (94.7 % vs 91.5 %, p < 0.001) in the 1-year post angiogram. Medical therapy patients had higher rates of anti-ischemic medications such as CCB and long acting nitrates, as well as slightly higher use of ACE/ARB (82.0 % vs 80.4 %; p = 0.004). As expected given the need for dual anti-platelets after PCI, revascularization patients had a higher use of clopidogrel (70.3 % vs 27.2 %; p < 0.001).
Table 1.
Baseline Characteristics of Full Cohort
| Covariates | Total (n = 39,131) |
Medical therapy (n = 15,139) | Revascularized patients (n = 23,992) | p value | PCI (n = 15,604) | CABG (n = 8,388) |
p value |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Mean age, yrs | 66.0 (10.3) | 67.0 (10.2) | 65.3 (10.3) | < 0.001 | 65.0 (10.7) | 66.0 ( 9.7) | < 0.001 |
| Male gender | 75.3 | 74.3 | 75.9 | < 0.001 | 72.9 | 81.3 | < 0.001 |
| Rural | 14.5 | 14.4 | 14.5 | 0.69 | 14.6 | 14.5 | 0.92 |
| Income* | < 0.001 | < 0.001 | |||||
| 1 | 18.6 | 19.6 | 17.9 | 18.4 | 17.1 | ||
| 2 | 20.4 | 20.4 | 20.4 | 20.4 | 20.6 | ||
| 3 | 20.2 | 20.3 | 20.2 | 20.3 | 19.9 | ||
| 4 | 20.7 | 20.2 | 21.0 | 20.8 | 21.3 | ||
| 5 | 20.1 | 19.4 | 20.5 | 20.2 | 21.1 | ||
| Medical comorbidities | |||||||
| PVD | 9.4 | 11.5 | 8.1 | < 0.001 | 7.6 | 9.0 | < 0.001 |
| Previous MI | 28.1 | 35.8 | 23.3 | < 0.001 | 24.8 | 20.4 | < 0.001 |
| COPD | 6.9 | 8.6 | 5.8 | < 0.001 | 6.1 | 5.1 | < 0.001 |
| Malignancy | 3.4 | 4.0 | 3.0 | < 0.001 | 3.0 | 3.0 | < 0.001 |
| Mean Charlson score | 0.9 ( 1.3) | 1.1 (1.5) | 0.8 ( 1.2) | < 0.001 | 0.7 (1.3) | 0.8 (1.2) | < 0.001 |
| Cardiac risk factors | |||||||
| Diabetes | 44.0 | 48.0 | 41.4 | < 0.001 | 40.5 | 43.1 | < 0.001 |
| Hypertension | 86.7 | 89.5 | 84.9 | < 0.001 | 84.9 | 85.1 | < 0.001 |
| Hyperlipidemia | 80.8 | 83.0 | 79.5 | < 0.001 | 79.9 | 78.7 | < 0.001 |
| History smoking | 31.6 | 33.2 | 30.5 | < 0.001 | 30.3 | 31.0 | < 0.001 |
| Cardiac status/testing | |||||||
| Native stenosis† | |||||||
| LM | 13.0 | 10.8 | 14.4 | < 0.001 | 5.5 | 31.0 | < 0.001 |
| Prox LAD | 33.2 | 28.7 | 36.0 | < 0.001 | 29.7 | 47.6 | < 0.001 |
| Mid/distal LAD | 49.6 | 47.5 | 51.0 | < 0.001 | 46.0 | 60.3 | < 0.001 |
| Circumflex | 51.5 | 50.9 | 51.9 | 0.07 | 43.6 | 67.2 | < 0.001 |
| RCA | 60.8 | 60.5 | 60.9 | 0.43 | 54.7 | 72.6 | < 0.001 |
| Had previous CABG | < 0.001 | < 0.001 | |||||
| Yes | 18.0 | 30.1 | 10.4 | 15.1 | 1.6 | ||
| No | 81.9 | 69.9 | 89.5 | 84.9 | 98.2 | ||
| LV function | < 0.001 | < 0.001 | |||||
| ≤ 34 % | 5.4 | 8.4 | 3.5 | 3.3 | 3.9 | ||
| 35–49 % | 12.9 | 16.0 | 11.0 | 10.6 | 11.7 | ||
| ≥ 50 % | 48.6 | 46.5 | 50.0 | 49.7 | 50.5 | ||
| NA | 33.0 | 29.1 | 35.5 | 36.4 | 33.8 | ||
| Exercise ECG risk | < 0.001 | < 0.001 | |||||
| High risk | 27.7 | 21.3 | 31.7 | 28.8 | 37.1 | ||
| Low risk | 22.4 | 22.9 | 22.2 | 23.0 | 20.7 | ||
| Uninterpretable | 4.9 | 4.8 | 4.9 | 5.3 | 4.2 | ||
| NA | 45.0 | 51.1 | 41.2 | 42.9 | 38.0 | ||
| Functional imaging risk | < 0.001 | < 0.001 | |||||
| High risk | 32.0 | 31.2 | 32.6 | 31.1 | 35.3 | ||
| Low risk | 23.0 | 25.1 | 21.7 | 23.2 | 19.1 | ||
| Unknown/NA | 44.9 | 43.7 | 45.7 | 45.7 | 45.7 | ||
| CCS class | < 0.001 | < 0.001 | |||||
| 0 | 16.5 | 22.0 | 13.0 | 11.3 | 16.2 | ||
| 1 | 14.0 | 15.9 | 12.8 | 12.3 | 13.8 | ||
| 2 | 38.3 | 35.5 | 40.0 | 40.8 | 38.5 | ||
| 3 | 28.5 | 24.2 | 31.1 | 32.3 | 29.0 | ||
| 4 | 2.8 | 2.4 | 3.0 | 3.3 | 2.5 | ||
| Physician-level factors: Referral physician | |||||||
| Specialty | 0.014 | < 0.001 | |||||
| Cardiology | 41.3 | 40.5 | 41.9 | 42.1 | 41.6 | ||
| Internal medicine | 9.4 | 9.3 | 9.6 | 9.1 | 10.4 | ||
| GP/FP | 25.7 | 26.1 | 25.5 | 25.6 | 25.3 | ||
| Hospital-level factors | |||||||
| Mean Cath volume | 4,092.3 | 4,031.3 | 4,130.9 | < 0.001 | 4,120.9 | 4,149.5 | < 0.001 |
| Hospital type | < 0.001 | < 0.001 | |||||
| Cath only | 13.2 | 13.6 | 13.0 | 13.4 | 12.2 | ||
| PCI and Cath only | 6.1 | 7.2 | 5.4 | 5.0 | 6.1 | ||
| CABG, PCI and Cath | 80.8 | 79.3 | 81.7 | 81.7 | 81.8 | ||
All covariates are presented as percentages, with the exception of mean values
CABG coronary artery bypass grafting; COPD chronic obstructive pulmonary disease; MI myocardial infarction; PCI percutaneous coronary intervention, PVD peripheral vascular disease, LM left main, LAD left anterior descending, RCA right coronary artery, LV left ventricular, ECG electrocardiogram, CCS Canadian Cardiovascular Society, Cath catheterization, GP/FP general practitioner/family practitioner
*Income quintile: 1 = lowest, 5 = highest
†LM if ≥ 50 % stenosis, Prox LAD if ≥ 70 % stenosis, Mid/distal LAD if ≥ 70 % stenosis, Circumflex if ≥ 70 % stenosis, RCA if ≥ 70 % stenosis
Table 2.
Medication Use
| Medication | Total (n = 20,663) | Medical therapy patients (n = 8,630) | Revascularized patients (n = 12,033) | p value |
|---|---|---|---|---|
| Medications within 3 months pre-index, % | ||||
| ACE or ARB | 68.0 | 71.1 | 65.8 | < 0.001 |
| Thienopyridines (clopidogrel) | 16.8 | 17.1 | 16.6 | 0.34 |
| β blockers | 63.2 | 63.5 | 63.0 | 0.46 |
| Calcium channel blockers | 38.4 | 38.5 | 38.3 | 0.81 |
| Nitrates | 48.1 | 41.0 | 53.3 | < 0.001 |
| Statins | 75.3 | 76.7 | 74.2 | < 0.001 |
| Medications within 3 months post-index, % | ||||
| ACE or ARB | 71.0 | 72.9 | 69.7 | < 0.001 |
| Thienopyridines (clopidogrel) | 47.6 | 20.2 | 67.3 | < 0.001 |
| β blockers | 69.4 | 67.1 | 71.0 | < 0.001 |
| Calcium channel blockers | 38.3 | 41.2 | 36.3 | < 0.001 |
| Nitrates | 31.9 | 34.8 | 29.9 | < 0.001 |
| Statins | 84.7 | 82.1 | 86.7 | < 0.001 |
| Medications within 12 months post-index, % | ||||
| ACE or ARB | 81.1 | 82.0 | 80.4 | 0.004 |
| Thienopyridines (clopidogrel) | 52.3 | 27.2 | 70.3 | < 0.001 |
| β blockers | 77.6 | 76.7 | 78.2 | 0.010 |
| Calcium channel blockers | 45.5 | 48.1 | 43.7 | < 0.001 |
| Nitrates | 43.6 | 47.1 | 41.2 | < 0.001 |
| Statins | 93.4 | 91.5 | 94.7 | < 0.001 |
ACE angiotension converting enzyme inhibitors; ARB angiotension receptor blockers
Unadjusted Outcomes
Over a median follow-up of 2.5 years (maximum 4.1 years), 7.6 % of CABG and 6.8 % of PCI patients died, compared to 13.4 % of medical therapy patients (p < 0.001) (Online Appendix Fig. 1a). Additionally, 6.1 % of CABG patients had a MI, compared to 11.4 % for PCI and 15.3 % for medical therapy (p < 0.001) (Online Appendix Fig. 1b). For repeat revascularization, 4.8 % of CABG patients had a repeat procedure, compared to 23.0 % of medical patients and 21.7 % of PCI patients (p < 0.001) (Online Appendix Fig. 1c).
Time-Varying Multivariable Cox-Models
In time-varying multivariable models, revascularization was associated with fewer deaths (Hazard ratio (HR) 0.76; 95 % CI 0.68–0.84; p < 0.001), MIs (HR 0.78; 95 % CI 0.72–0.85; p < 0.001) and repeat PCI/CABG (HR 0.59; 95 % CI 0.50–0.70; p < 0.001), compared to medical therapy (Online Appendix Table 1). There was a significant interaction between age and treatment strategy on mortality (p = 0.0373), with the magnitude of benefit of revascularization decreasing with advancing age.
In sensitivity analyses, there was a consistent reduction in death associated with both PCI and CABG compared to medical therapy (PCI: HR 0.73; 95 % CI 0.65–0.81; p < 0.001 and CABG: HR 0.70; 95 % CI 0.62–0.79; p < 0.001, respectively). There was no difference in non-fatal MI between PCI and medical therapy (HR 0.98, 95 % CI 0.90–1.07; p = 0.642). In contrast, CABG was associated with a lower hazard of non-fatal MI (HR 0.42; 95 % CI 0.36–0.48; p < 0.001). Both PCI and CABG were associated with a reduction in repeat revascularization compared to medical therapy (PCI: HR 0.90; 95 % CI 0.85–0.95; p < 0.001 and CABG: HR 0.10; 95 % CI 0.08–0.11; p < 0.001, respectively). When all patients with a previous MI were excluded, revascularization continued to be associated with a reduction in mortality (HR 0.69; 95 % CI 0.62–0.77; p < 0.001) and repeat MI (HR 0.73; 95 % CI 0.66–0.82).
Propensity-Matched Analyses
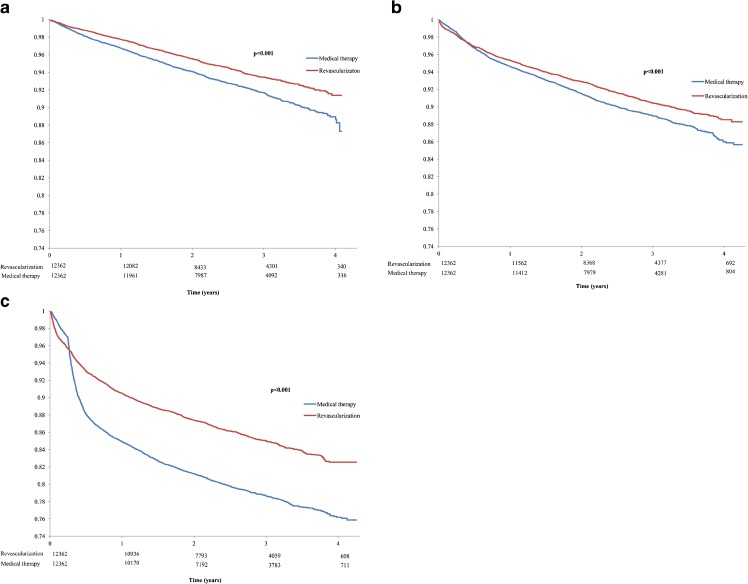
We were able to identify 12,362 pairs of well-matched medical therapy and revascularization patients (Table 3), with standardized differences of less than 0.1 for all covariates. The use of medications in the propensity-matched patients above 65 years (n = 13,535) is found in Online Appendix Table 2, and is consistent with the overall cohort. As seen in Fig. 2a, over the follow-up period, 12.7 % of the medical therapy patients died, compared to 8.6 % of the revascularization patients (HR 0.75; 95 % CI 0.69–0.81; p < 0.001). There were similar benefits seen with MI, with 14.3 % of the medical therapy patients having a subsequent MI compared to 11.7 % of the revascularization patients (HR 0.84; 95 % CI 0.77–0.93; p < 0.001) (Fig. 2b). For repeat PCI/CABG, 17.4 % of the revascularization patients required a subsequent procedure compared to 24.1 % of the medical patients (HR 0.67; 95 % 0.63–0.71; p < 0.001) (Fig. 2c).
Table 3.
Baseline Characteristics of the Propensity Matched Cohort
| Medical therapy patients | Revascularized patients | |
|---|---|---|
| n = 12,362 | n = 12,362 | |
| Treatment | ||
| CABG | 0.0 | 25.0 |
| PCI | 0.0 | 75.0 |
| Medical therapy | 100.0 | 0.0 |
| Demographics | ||
| Mean age, yrs | 66.47(10.27) | 66.55 (10.19) |
| Male gender | 74.2 | 73.6 |
| Rural | 14.5 | 14.5 |
| Income* | ||
| 1 | 19.4 | 19.4 |
| 2 | 20.4 | 21.0 |
| 3 | 20.3 | 20.0 |
| 4 | 20.2 | 20.3 |
| 5 | 19.6 | 19.3 |
| Medical comorbidities | ||
| PVD | 10.3 | 10.2 |
| Previous MI | 31.6 | 31.4 |
| COPD | 7.6 | 7.7 |
| Malignancy | 3.6 | 3.6 |
| Mean Charlson score | 0.94 (1.35) | 0.94 (1.39) |
| Cardiac risk factors | ||
| Diabetes | 46.4 | 46.1 |
| Hypertension | 88.3 | 89.2 |
| Hyperlipidemia | 81.8 | 82.2 |
| History smoking | 32.2 | 32.0 |
| Cardiac status/testing | ||
| Native stenosis† | ||
| LM | 10.8 | 10.4 |
| Prox LAD | 28.3 | 27.6 |
| Mid/distal LAD | 47.9 | 48.0 |
| Circumflex | 50.1 | 49.7 |
| RCA | 59.5 | 59.5 |
| Had previous CABG | ||
| Yes | 20.2 | 19.1 |
| No | 79.7 | 80.9 |
| Unknown | 0.1 | 0.0 |
| LV function | ||
| ≤ 34 % | 6.1 | 5.9 |
| 35–49 % | 14.7 | 14.7 |
| ≥ 50 % | 48.8 | 49.2 |
| NA | 30.5 | 30.2 |
| Exercise ECG risk | ||
| High risk | 23.7 | 22.6 |
| Low risk | 23.4 | 23.7 |
| Uninterpretable | 4.9 | 4.8 |
| NA | 48.0 | 48.9 |
| Functional imaging risk | ||
| High risk | 31.8 | 31.8 |
| Low risk | 24.4 | 25.3 |
| Unknown/NA | 43.8 | 42.9 |
| CCS class | ||
| 0 | 18.9 | 19.6 |
| 1 | 15.5 | 15.6 |
| 2 | 37.6 | 36.9 |
| 3 | 25.6 | 25.5 |
| 4 | 2.5 | 2.4 |
| Physician-level factors: Referral Physician | ||
| Specialty | ||
| Cardiology | 40.3 | 42.1 |
| Internal medicine | 9.5 | 9.6 |
| GP/FP | 26.8 | 24.7 |
| Hospital-level factors | ||
| Mean Annual Cath volume | 4,059.82 | 4,068.44 |
| Hospital type | ||
| Cath only | 13.1 | 14.3 |
| PCI and Cath only | 7.0 | 5.9 |
| CABG, PCI and Cath | 79.9 | 79.9 |
All covariates are presented as percentages, with the exception of mean values
CABG coronary artery bypass grafting; COPD chronic obstructive pulmonary disease; MI myocardial infarction; PCI percutaneous coronary intervention, PVD peripheral vascular disease; LM left main, LAD left anterior descending, RCA right coronary artery, LV left ventricular, ECG electrocardiogram, CCS Canadian Cardiovascular Society, Cath catheterization, GP/FP general practitioner/family practitioner. *Income quintile: 1 = lowest, 5 = highest; † LM if ≥ 50 % stenosis, Prox LAD if ≥ 70 % stenosis, Mid/distal LAD if ≥ 70 % stenosis, Circumflex if ≥ 70 % stenosis, RCA if ≥ 70 % stenosis
Figure 2.
a Propensity matched KM curves for survival. b Propensity matched cohort KM curves for Myocardial Infarction. c Propensity matched cohort KM curves for Repeat Revascularization.
When restricted to patients who survived at least 90 days, we found a similar benefit associated with revascularization in survival (HR 0.77; 95 % CI 0.67–0.87; p < 0.001), non-fatal MI (HR 0.88; 95 % CI 0.79–0.97; p = 0.01) and repeat PCI/CABG (0.67; 95 % CI 0.63–0.72; p < 0.001). In the 4,838 propensity-matched pairs of medical therapy and revascularized patients who would have met the eligibility criteria for COURAGE (22.6 % of original cohort: Online Appendix Figure 2, Online Appendix Table 3), there continued to be a statistically significant benefit for mortality, MI and repeat PCI/CABG associated with revascularization (Online Appendix Fig. 3a-c).
DISCUSSION
In this population-based analysis of stable IHD after coronary angiography, we found that patients treated with revascularization had improved risk-adjusted outcomes compared to patients treated medically. This is in contrast to the efficacy results from RCTs. Our results were robust to multiple sensitivity analyses, accounting for survivorship bias, and also when restricted to a population comparable to that enrolled in the COURAGE trial. Our study suggests that we cannot be complacent in applying RCT results regarding the efficacy of optimal medical therapy to clinical practice—rather, it is critical to consider the underlying reasons for the discrepancies between our findings and that of RCTs.
There are several potential explanations for our findings. First, ours was an observational study, and thus prone to confounding. To account for this, we performed multiple statistical methods for risk adjustment; however, none can account for unmeasured variables. Although we cannot discount that residual confounding may persist, it is reassuring that our results remain consistent across the different methods. Second, the differences may be driven by the restrictive nature of RCT populations. When we restricted our cohort to a group comparable to that enrolled in the COURAGE trial, although the magnitude of the differences was less, we continued to see an improvement in outcomes associated with revascularization.
Third, translation of the efficacy results from clinical trials requires that both revascularized and medical patients received optimal management.2 Unfortunately, multiple studies have shown that evidence-based, guideline recommended therapies are underutilized in stable IHD.16–18 Indeed, Borden and colleagues found relatively little impact on these practice patterns even after the publication of the COURAGE trial.19 Reassuringly, both groups of patients in our cohort achieved relatively high levels of medication use post angiography. The only medication in which there were large differences in uptake between groups was clopidogrel; however, in stable IHD, dual anti-platelet therapy with both aspirin and clopidogrel is not associated with a mortality benefit and instead is indicated primarily after a PCI.20 Nonetheless revascularized patients had higher levels of some cardio-protective medications such as statins, which are associated with a survival benefit in stable IHD.21 Although this difference in the use of cardio-protective medications may be one of the mediators of the improved outcomes seen in revascularized patients, given the small absolute differences in medication use, it is unlikely that this is the sole explanation.
Importantly, this finding suggests that revascularization may simply be a marker of better overall quality of care. Previous studies have suggested that patients receiving routine medical therapy in routine clinical practice are undertreated compared to patients who are revascularized.22 Further research is needed to determine if other performance markers of improved quality of care are more consistently achieved in revascularized patients, when compared to routine medical therapy patients.23 For example, are revascularized patients followed up more frequently, or are life style modifications, such as increased physical activity and smoking cessation more emphasized, when compared to medical therapy patients? If confirmed, this has important implications for clinical practice, and provides a focus for quality improvement efforts. Previous investigators have found similar results regarding the improved real-world effectiveness of PCI and CABG compared to medical therapy; our work reinforces these real world discrepancies with RCT findings in a more contemporary post-COURAGE cohort.24,25
It is important to consider the role of observational studies such as ours, compared to RCTs. Large, multi-centre RCTs represent the highest level of evidence for determining the efficacy of an intervention.1 As such, we are not suggesting that our contrasting results should take precedence over RCTs, or that revascularization should be the modality of choice in stable IHD. Instead, the goal of comparative effectiveness studies is to understand the reason for any differences observed between these two types of studies. If the discrepancies are purely due to issues such as selection bias, then we can be reassured. However, it is important to confirm if the reasons are secondary to the type of care received in clinical practice versus RCTs; for example, can we be doing better in terms of how routine medical therapy patients are cared for in the real world? This identifies potential actionable areas to improve clinical practice, such that the efficacy of optimal medical therapy seen in RCTs can be realized.
Our results must be interpreted in the context of several limitations that merit discussion. First, we only included patients post angiography, which constitutes a more selected population than the general population of patients with stable IHD. However, this is similar to patients who were enrolled in the RCTs in this area.2,4 Second, we used clinical registry and administrative databases that may lack information on potentially important confounders such as concomitant valve disease. Finally, the observational nature of our study precludes any conclusions about causality. It is important that our results not be interpreted such that the observed benefits are due to revascularization alone. Rather, further research is needed to elucidate the underlying reasons for the differences that we observed, with the goal of ultimately more closely approximating the results seen in trials such as COURAGE and BARI-2D.
In conclusion, we found that stable IHD patients treated with revascularization had improved risk-adjusted outcomes in clinical practice, potentially due to under-treatment of the medical therapy patients. This may provide a focus for quality improvement to optimize real world medical therapy.
Electronic supplementary material
(PDF 513 kb)
Acknowledgements
Contributors
The authors acknowledge that the clinical registry data used in this publication are from the Cardiac Care Network of Ontario and its member hospitals. The Cardiac Care Network of Ontario serves as an advisory body to the MOHLTC, and is dedicated to improving the quality, efficiency, access and equity of adult cardiovascular services in Ontario, Canada. The Cardiac Care Network of Ontario is funded by the Ontario Ministry of Health and Long-Term Care (MOHLTC). This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the MOHLTC. The funding organizations did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
Funders
This study is funded through an operating grant from the Canadian Institute of Heath Research (CIHR), and in part by research funding from the Schulich Heart Centre at Sunnybrook Health Sciences Centre and the Sunnybrook Research Institute. The opinions, results, and conclusions reported in this article are those of the authors. The funding sources had no role in the study’s design, conduct or reporting.
Dr H. Wijeysundera is supported by a Distinguished Clinical Scientist Award from the Heart and Stroke Foundation of Canada. Dr Tu is supported by a Career Investigator award from the Heart and Stroke Foundation and a Canada Research Chair in Health Services Research. Dr. Ko is supported by a Clinician Scientist Phase II personnel award from the Heart and Stroke Foundation, Ontario Provincial Office. Dr Austin is supported in part by a Career Investigator Award from the Heart and Stroke Foundation. Dr D. Wijeysundera is supported by a Clinician Scientist Salary award from CIHR, and a Merit Award from the Department of Anesthesia at the University of Toronto.
Conflicts of Interest
The authors have no relevant conflicts of interest.
REFERENCES
- 1.Fraker TD, Jr, Fihn SD, Gibbons RJ, Abrams J, Chatterjee K, Daley J, et al. 2007 chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol. 2007;50(23):2264–74. doi: 10.1016/j.jacc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 3.Trikalinos TA, Alsheikh-Ali AA, Tatsioni A, Nallamothu BK, Kent DM. Percutaneous coronary interventions for non-acute coronary artery disease: a quantitative 20-year synopsis and a network meta-analysis. Lancet. 2009;373(9667):911–8. doi: 10.1016/S0140-6736(09)60319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Group BDS, Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360(24):2503–15. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kereiakes DJ, Teirstein PS, Sarembock IJ, Holmes DR, Jr, Krucoff MW, O’Neill WW, et al. The Truth and Consequences of the COURAGE Trial. J Am Coll Cardiol. 2007;50(16):1598–603. doi: 10.1016/j.jacc.2007.07.063. [DOI] [PubMed] [Google Scholar]
- 6.Cardiac Care Network of Ontario. [http://www.ccn.on.ca/index.php]. Accessed 6 February 2013.
- 7.Cardiac Care Network (CCN) Annual Report 2010/2011. 2011.
- 8.Tu JV, Ko DT, Guo H, Richards JA, Walton N, Natarajan MK, et al. Determinants of variations in coronary revascularization practices. CMAJ. 2011. [DOI] [PMC free article] [PubMed]
- 9.Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;10(2):67–71. [PubMed] [Google Scholar]
- 10.Ko DT, Newman AM, Alter DA, Austin PC, Chiu M, Cox JL, et al. Secular trends in acute coronary syndrome hospitalization from 1994 to 2005. Can J Cardiol. 2010;26(3):129–34. doi: 10.1016/S0828-282X(10)70350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 12.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2010. [DOI] [PMC free article] [PubMed]
- 14.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin PC. Comparing paired vs non-paired statistical methods of analyses when making inferences about absolute risk reductions in propensity-score matched samples. Stat Med. 2011;30(11):1292–301. doi: 10.1002/sim.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stafford RS, Radley DC. The underutilization of cardiac medications of proven benefit, 1990 to 2002. J Am Coll Cardiol. 2003;41(1):56–61. doi: 10.1016/S0735-1097(02)02670-0. [DOI] [PubMed] [Google Scholar]
- 17.Wijeysundera HC, Machado M, Farahati F, Wang X, Witteman W, van der Velde G, et al. Association of temporal trends in risk factors and treatment uptake with coronary heart disease mortality, 1994–2005. JAMA. 2010;303(18):1841–7. doi: 10.1001/jama.2010.580. [DOI] [PubMed] [Google Scholar]
- 18.Wijeysundera HC, Mitsakakis N, Witteman W, Paulden M, van der Velde G, Tu JV, et al. Achieving quality indicator benchmarks and potential impact on coronary heart disease mortality. Can J Cardiol. 2011;27(6):756–62. doi: 10.1016/j.cjca.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Borden WB, Redberg RF, Mushlin AI, Dai D, Kaltenbach LA, Spertus JA. Patterns and intensity of medical therapy in patients undergoing percutaneous coronary intervention. JAMA. 2011;305(18):1882–9. doi: 10.1001/jama.2011.601. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706–17. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 21.Opie LH, Commerford PJ, Gersh BJ. Controversies in stable coronary artery disease. [Review] [114 refs] Lancet. 2006;367(9504):69–78. doi: 10.1016/S0140-6736(06)67927-0. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg BA, Steg PG, Bhatt DL, Fonarow GC, Zeymer U, Cannon CP, et al. Comparisons of guideline-recommended therapies in patients with documented coronary artery disease having percutaneous coronary intervention versus coronary artery bypass grafting versus medical therapy only (from the REACH International Registry) Am J Cardiol. 2007;99(9):1212–5. doi: 10.1016/j.amjcard.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 23.American College of Cardiology, American Heart Association, Physician Consortium for Performance ImprovementClinical Performance Measures: Chronic Stable Coronary Artery Disease. . 2003.
- 24.Hannan EL, Samadashvili Z, Cozzens K, Walford G, Jacobs AK, Holmes DR, Jr, et al. Comparative outcomes for patients who do and do not undergo percutaneous coronary intervention for stable coronary artery disease in New York. Circulation. 2012;125(15):1870–9. doi: 10.1161/CIRCULATIONAHA.111.071811. [DOI] [PubMed] [Google Scholar]
- 25.Muhlbaier LH, Pryor DB, Rankin JS, Smith LR, Mark DB, Jones RH, et al. Observational comparison of event-free survival with medical and surgical therapy in patients with coronary artery disease. 20 years of follow-up. Circulation. 1992;86(5 Suppl):II198–204. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 513 kb)