Abstract
Autophagy (or “self-eating”) is the process by which cellular contents are recycled to support downstream metabolism. An explosion in research in the past decade has implicated its role in both health and disease and established the importance of the autophagic response during periods of stress and nutrient deprivation. Atherosclerosis is a state where chronic exposure to cellular stressors promotes disease progression and alterations in autophagy is predicted to be consequential. Recent reports linking macrophage autophagy to lipid metabolism, blunted inflammatory signaling, and an overall suppression of proatherogenic processes support this notion. We review this data and provide a framework for understanding the role of macrophage autophagy in the pathogenesis of atherosclerosis, one of the most formidable diseases of our time.
Keywords: Autophagy, Macrophage, Lipid metabolism, Inflammation, Atherosclerosis
The pathogenesis of atherosclerosis
In the United States, cardiovascular diseases cause nearly one-third of all deaths with atherosclerotic vascular disease comprising the vast majority of the underlying pathology [1]. Studies focusing on the initiation of atherosclerosis both in human and animal models suggest that accumulation of lipoproteins in the vasculature is a pivotal early event [2]. These initial steps progress into an indolent growth phase of the arterial intima (normally a small region between endothelium and smooth muscle cells) with gradual lipid accumulation, recruitment of various cell types including inflammatory cells, and deposition of extracellular matrix. Intimal growth and an increased inflammatory response are two main factors for disease progression [3]. Of the several contributing cell types, macrophages play a critical role: accumulated lipids are largely engulfed by macrophages leading to the secretion of proinflammatory cytokines and further macrophage recruitment, thus contributing to robust increases in atherosclerotic plaque size and complexity. These steps have been the subject of several recent reviews [3–5].
The macrophage: professional phagocyte of the atherosclerotic plaque
Macrophages are immune cells derived from the differentiation of monocyte precursors upon migration into tissues. They are primarily responsible for the phagocytosis of extracellular pathogens and cellular debris, antigen presentation and activation of the adaptive immune system, and the secretion of either pro- or anti-inflammatory cytokines depending on their activation state [6]. Although parsing the precise subsets of macrophages located in atherosclerotic plaques and delineating their functional significance is an active area of research [7], the contribution of macrophages to atherosclerotic progression, cytokine production, and the maintenance of vessel-wall inflammation is well established [5, 8]. Monocyte recruitment to the vessel intima is initiated by the secretion of chemokines from endothelial cells which are activated by excess lipoprotein accumulation [9, 10]. Indeed, efforts to lower circulating monocytes or prevent their interactions with the endothelium via chemokine/chemokine receptor blockade profoundly reduces atherosclerotic plaque burden [11–13].
Upon differentiation in the subendothelial space, mature macrophages encounter vast amounts of native and modified ApoB-containing lipoproteins. Uptake of these particles by receptor-mediated endocytic pathways, intracellular lipid hydrolysis and metabolism, and repackaging/storage into membrane-bound neutral lipid stores constitute the basic steps of classical foam cell formation [14]. Uptake of modified lipoproteins and their internalization also leads to activation of downstream inflammatory pathways, secretion of proinflammatory cytokines, and the establishment of a positive feedback loop for further monocyte recruitment and intimal migration [10, 15]. An overview of the involvement of the macrophage in atherogenesis is shown in Figure 1.
Figure 1. The Role of Monocytes/Macrophages in Atherosclerotic Progression.
Atherosclerosis starts with the accumulation of lipid molecules/lipoproteins in the intima, which under normal circumstances is a small region below the endothelial layer (1). Activation of the endothelium leads to cytokine secretion, recruitment of circulating monocytes, and differentiation into macrophages (2). Macrophages uptake the accumulated lipids by receptor- mediated endocytosis. This includes both native and modified lipids such as oxidized LDL which are internalized by scavenger receptors (3). Lipid-exposed macrophages exacerbate the oxidative environment accelerating the lipid modification and uptake process. Macrophages also secrete inflammatory cytokines which lead to further monocyte entry to the region and local macrophage proliferation (4). Macrophage accumulation along with increased extracellular matrix production are major contributors to intimal expansion (4,5). When lipid overload exceeds the metabolic capacity of macrophages, intracellular lipid accumulation occurs leading to macrophage foam cell formation (6). Foam cell apoptosis and inefficient clearance further activate inflammation, intimal growth, and necrotic core formation, thereby contributing to increased lesion complexity and risk of rupture.
Besides inflammatory signaling, several other pathways are also activated in macrophages which work in concert to wreak havoc on the plaque. Generation of reactive oxygen intermediates, myeloperoxidase-induced reactive nitrogen species, and secretion of cathepsins and matrix metalloproteinases exacerbate the toxic environment in the subendothelial space resulting in a vicious cycle of lipoprotein oxidative modification, enhanced lipoprotein uptake, and increased inflammatory signaling [16–19]. Prominent contributors to the toxic plaque environment include several stress inducers (dead or apoptotic cell debris, hypoxia, elevated reactive oxygen species (ROS), and accumulated lipids). Under normal circumstances, most cells have compensatory mechanisms to handle such insults. The induction of autophagy is one such response and many of the toxic intermediates that are also found in the atherosclerotic plaque have been associated with concomitant increases in macrophage autophagy [20]. Until recently, a role for autophagy in macrophages of the atherosclerotic plaque was undefined. We briefly review the process of autophagy in Box 1 and detail a current view of its link to the pathogenesis of atherosclerosis below.
Box 1. Autophagy, the process of degrading intracellular macromolecules and organelles.
Autophagy (in Greek self [auto]-eating [phagy]) is a highly evolutionarily conserved catabolic process to degrade and recycle cytoplasmic contents via a lysosomal route for use in downstream metabolism. Several different types of autophagy have been defined in mammals based on the nature of the cargo and the method of delivery to lysosomes (i.e. macroautophagy, microautophagy, and chaperone-mediated autophagy) [21, 22]. Macroautophagy, or the bulk degradation of macromolecules and organelles, is the most extensively studied form and is the focus of this review. The other types of autophagy have not been directly addressed in the context of macrophages and atherosclerosis [23, 24].
The autophagy process starts with the formation of double-membrane vesicles called autophagosomes which sequester cytoplasmic material. The completion of vesicle formation and targeting to the lysosomal compartment generates single-membrane autolysosomes. Exposure to the numerous hydrolases and highly acidic environment of the lysosome results in denaturation and degradation of the cargo into amino acids, fatty acids, carbohydrates, and nucleotides which are shuttled to the cytosol for use in cellular function. A series of >30 specialized proteins (mostly coded by AuTophaGy related (ATG) genes) partake in the nucleation and elongation of autophagosome vesicles and eventual fusion with the lysosomal compartment. These events are depicted in Figure I with details of the molecular machinery provided in several excellent reviews [20–22, 25].
In mammals, autophagy is the major degradation pathway besides the ubiquitin-proteasome system. Autophagic degradation is primarily used for the removal of larger cellular structures such as damaged organelles, long-lived proteins, and protein aggregates. Accordingly, stress factors such as nutrient and growth factor deprivation, ROS, DNA damage, hypoxia, dysfunctional organelles, protein aggregates, and intracellular pathogens are potent activators of autophagy, enabling cells to withstand such insults and enhance cell survival [20]. Autophagy is regulated by several upstream signaling pathways with one of the most extensively studied being the nutrient-sensing mammalian target of rapamycin (mTOR) kinase. In settings of nutrient-excess, mTOR signaling is activated leading to inhibition of autophagy, while in starvation, the reverse is true and autophagy is active [26]. Other autophagy-regulating pathways include AMP-activated protein kinase (AMPK) and sirtuin1 which also coordinate cellular energy status with autophagy, several Beclin-1-interacting proteins which regulate Beclin-1 protein complex formation and the initiation of autophagy, p53 which can link autophagy to the DNA damage response, and NF-κB which integrates various proinflammatory signaling pathways with autophagy [20, 27–30]. Indeed, the regulation and function of autophagy in the immune system and particularly its role in innate immunity and macrophage biology has been a subject of intense interest in the past few years. Macrophage autophagy has been implicated in diverse roles such as viral and microbial degradation, antibacterial ROS generation, antigen presentation, and modulation of proinflammatory signaling [30–33]. Despite the clear associations between autophagy, innate immunity, and atherosclerosis, it was not until recently that macrophage autophagy was directly assessed in the context of atherosclerosis.
Box 1, Figure I. Overview of Autophagy.
Autophagy begins with the formation of a double-membrane vesicle (autophagosome) which engulfs the adjacent cytoplasm including proteins and organelles. More than 30 proteins serve as the machinery to promote autophagosome nucleation/formation, vesicle elongation, and eventual docking/fusion with the lysosome. Certain autophagy proteins have been studied extensively and served as targets for mouse models of autophagy deficiency; these include Beclin-1 (involved in vesicle nucleation) and ATG5 and ATG7 (involved in vesicle elongation). Although autophagy is a bulk degradation process, chaperone proteins such as p62/SQSTM1 can selectively shuttle polyubiquitinated proteins and organelles for degradation (so-called selective autophagy). Autophagy is capable of being induced in response to various cellular stresses, the classic one being nutrient deprivation. Accordingly, mTOR, a major nutrient sensing kinase in cells, is a critical regulator of autophagy. Upon formation of the vesicle, autophagosomes fuse with lysosomes to generate autolysosomes, exposing the internal contents to lysosomal hydrolases for degradation into the basic metabolites needed for cellular function.
Macrophage autophagy: a nexus for lipid metabolism and inflammatory signaling in atherosclerosis
The uptake of lipoproteins after initial accumulation in the arterial intima, degradation through the endosomal-lysosomal system, intracellular lipid trafficking, lipid storage and foam cell formation, the interface with inflammatory signaling, and release of lipids via cholesterol efflux, collectively define some of the critical events in the life of an atherosclerotic macrophage (Figure 2, left). Efficient lipoprotein-derived cholesterol metabolism and efflux are considered atheroprotective mechanisms while increased accumulation and aberrant lipid handling is atherogenic [34]. As an important component of the cellular prodegradative response, autophagy would be an ideal pathway to be stimulated in the atherosclerotic environment. Indeed, limited early evidence indicated that autophagy occurs in the atherosclerotic plaque. Double-membrane vesicles resembling autophagosomes are seen in several cell-types including macrophages by transmission electron microscopy [35, 36]. Several markers of autophagy including the coat protein LC3 are readily detectable in atherosclerotic plaques by immunoblot and immunohistochemistry [37]. However, a detailed understanding of autophagy’s role in atherosclerotic macrophages was only possible by characterizing tissue-specific mouse models of autophagy-deficiency.
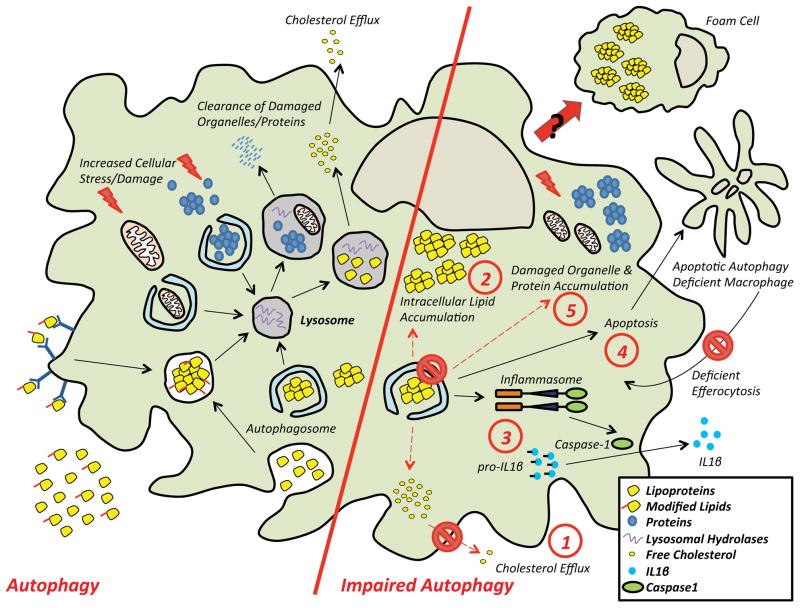
Figure 2. Role of Autophagy in Atherosclerotic Macrophages and the Consequences of Impaired Autophagy.
(Left side) Macrophages located in the atherosclerotic plaque are subject to an environment of lipid excess. The autophagy-lysosomal system plays a central role in the response to such overload. The increased burden of cytoplasmic lipid droplets as well as damaged organelles and proteins are carried to the lysosomes by autophagy. The ensuing degradation not only prevents the accumulation of cytotoxic cargo, but also serves as an important mechanism for cholesterol efflux.
(Right side) An impairment in autophagy results in several known consequences. (1) Cholesterol efflux decreases with a (2) concomitant accumulation of intracellular lipids. (3) Inflammasomes are hyperactivated leading to increased IL-1β secretion. (4) Cells are prone to apoptotic death while their clearance by efferocytosis is less efficient. (5) Inefficient degradation leads to the accumulation of damaged organelles and proteins. Taken together, these events exacerbate macrophage dysfunction and plaque progression.
To date, two reports have directly investigated the consequence of macrophage-specific autophagy deficiency in atherosclerosis [38, 39]. ATG5, a critical component of the protein complex responsible for autophagosome elongation [25], was targeted in the myeloid lineage using a Cre-LoxP approach. On a proatherogenic background (either apolipoprotein E-null or low density lipoprotein receptor (LDLR)-null), these mice develop markedly increased atherosclerotic plaques both at the level of the aortic root and in all areas of the aorta [38, 39]. Although the mechanisms underlying the enhanced plaque formation are likely multifactorial, evidence has been provided for the following: 1) defects in lipophagy and cholesterol efflux, 2) increased foam cell formation, 3) hyperactivation of the inflammasome and elevated interleukin 1 beta (IL-1β) production, 4) enhanced macrophage apoptosis with concomitant defects in efferocytic uptake, and 5) accumulation of cytotoxic organelles and proteins. These proposed mechanisms are summarized in (Figure 2, right) and will be discussed below.
Foam Cells, Lipophagy, and Cholesterol Efflux
The notion that autophagy can degrade intracellular lipid droplets and contribute to cellular lipid metabolism, so-called lipophagy, was first demonstrated in the liver. In settings of nutrient deprivation, lipophagy can aid in the lipolysis of hepatic triglyceride stores. Consequently, mice with autophagy-deficient hepatocytes have increased triglyceride stores and hepatosteatosis [40]. Similarly, the accumulation of membrane-bound lipid droplets (composed of cholesteryl esters and triglycerides) within macrophages and smooth muscle cells is a pathognomonic feature of atherosclerosis. The contribution of these lipid-laden “foam cells” is a major reason for the inflammatory milieu and plaque progression [34]. Excess lipid storage in foam cells is often balanced by mechanisms of lipid removal called cellular cholesterol efflux. Induction of cholesterol efflux from macrophages leads to increased net cholesterol transfer from peripheral tissues to the liver, a process known as reverse cholesterol transport [41]. For years, the primary efflux mechanism was believed to be the hydrolysis of cholesteryl esters by cytoplasmic hydrolases, trafficking of free cholesterol to the plasma membrane, and delivery of free cholesterol to the periphery via ATP-binding cassette transporters (ABCA1 or ABCG1) [42].
The concept of lipophagy in hepatocytes raised the possibility whether an autophagic mechanism was functional in macrophages. Indeed, there is now evidence that the autophagy-lysosomal system is an important contributor to the efflux of cholesterol. The disruption of autophagy in cultured macrophages either chemically (chloroquine) or genetically (ATG5-deficiency) abrogates cholesterol efflux to apoloprotein AI (ApoA-I). Lipid-loaded macrophages show enhanced markers of autophagy, and lipid droplets can be co-localized with lysosomal markers over time. Furthermore, inhibitors of lysosomal acid lipase, the enzyme responsible for hydrolysis of cholesteryl esters into free cholesterol, also diminish cholesterol efflux, suggesting that autophagic lipid delivery and hydrolysis in the lysosome are critical steps. The delivery of free cholesterol for peripheral efflux still appears to be an ABCA1-dependent process although the details are unknown. Finally, lipophagy-mediated efflux is clearly important physiologically as macrophage-specific ATG5-null mice also have defects in reverse cholesterol transport (i.e. cholesterol delivery to the liver for excretion) in vivo [43]. Although lipid-loading of macrophages induces lipophagy as a likely counterregulatory mechanism, the triggers for this process are not currently known. The involvement of mTOR and associated signaling pathways is likely supported by the recent observation that the Wip1 phosphatase regulates macrophage lipophagy, cholesterol efflux, and atherogenesis via modulation of ataxia telangiectasia mutated (ATM)-mTOR signaling [44]. The links between lipophagy and cholesterol efflux are summarized in Figure 3A.
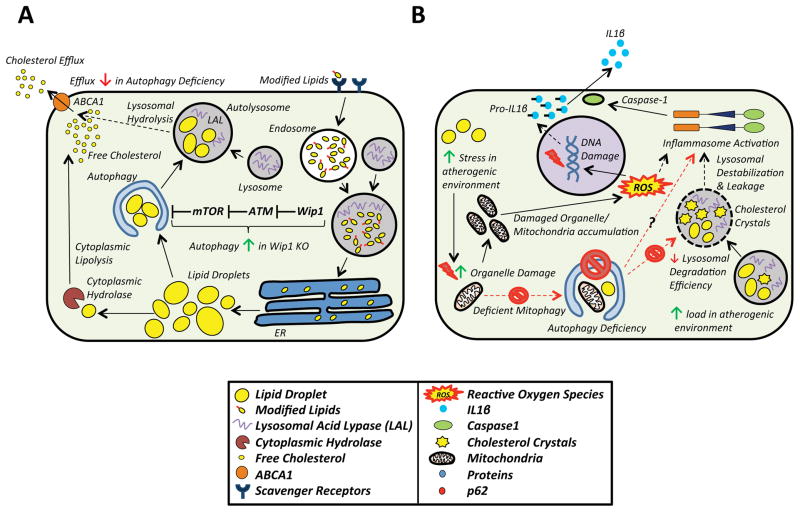
Figure 3. Mechanisms That Link Macrophage Autophagy to Atherosclerosis.
A) Although lipolysis by cytoplasmic hydrolyses were thought to be the only mechanism for cholesterol efflux, the role of autophagy in cholesterol efflux is now appreciated. Neutral lipid stores, a prominent feature of foam cells, are engulfed by autophagosomes via lipophagy, shuttled to lysosomes, hydrolyzed by lysosomal acid lipase, and the freed cholesterol is effluxed to the periphery via an ABCA1-dependent mechanism. In autophagy deficiency, the capacity of macrophages to efflux cholesterol efflux diminishes significantly. Pathways which regulate autophagy also have an impact on cholesterol efflux. Wip1 phosphatase, which activates mTOR in an ATM-dependent manner, is such an example. Wip1 KOs show decreased mTOR activation, increased lipophagy, and cholesterol efflux.
B) Deficient autophagy also induces inflammasome activation and IL-1β secretion. The mechanistic link between autophagy deficiency and increased inflammasome activation is not clear. Possibilities include the accumulation of damaged mitochondria due to inefficient mitophagy with subsequent increases in ROS. ROS can induce inflammasome activation either directly or indirectly via its ability to cause DNA damage and secondary inflammatory signaling. Autophagy deficiency also reduces lysosomal degradation efficiency. The accumulation of intralysosomal lipids and cholesterol crystals is accelerated with autophagy deficiency, which may cause lysosomal membrane destabilization, lysosomal leakage, and inflammasome activation.
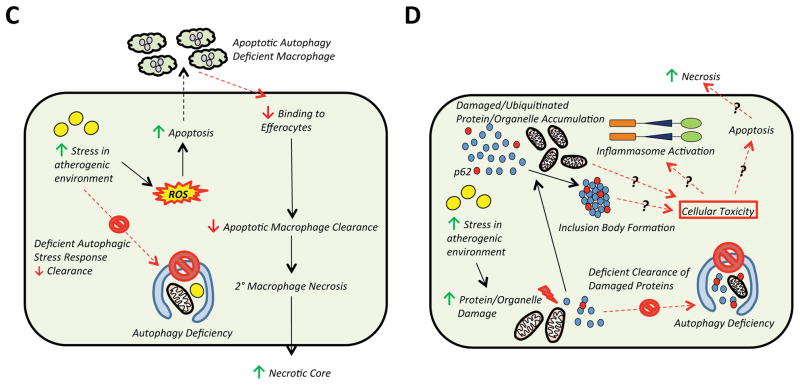
C) Defects in autophagy lead to increased ROS and apoptotic cell death. Clearance of apoptotic autophagy-deficient macrophages by efferocytosis is also abrogated through unclear mechanisms. The accumulation of apoptotic and dying cells causes secondary necrosis which increases the necrotic core and lesion size and complexity.
D) Although not specifically examined in atherosclerotic models, it is known that autophagy deficiency is also linked to the accumulation of damaged proteins/organelles and p62/SQSTM1-enriched inclusion body formation. Levels of p62/SQSTM1 are known to be markedly elevated in atherosclerotic plaques, suggesting that this aggregate formation is not only a marker of autophagy deficiency but might mediate cellular toxicity, inflammasome activation, and apoptosis as observed in other disease conditions where protein aggregation occurs.
Autophagy, the Inflammasome, and IL-1β production
The intense inflammatory response that characterizes the atherosclerotic plaque is largely orchestrated by macrophages where IL-1β serves as the prototype proatherogenic cytokine [3, 5, 45]. The triggers for this inflammation have been proposed to be related to aberrant lipid modification and metabolism but the details remain unclear. Until recently, cholesterol crystals, a commonly encountered form of cholesterol found in the atherosclerotic plaque had been thought to be an inert byproduct of dying macrophages and the necrotic core. However, emerging evidence supports a role for certain types of crystalline molecules as Damage-Associated Molecular Patterns (DAMPs), capable of activating the NLRP3 inflammasome, a complex of proteins that mediate the production of mature IL-1β and IL-18 [46]. Cholesterol crystals have now been similarly shown to function as DAMPs by activating the macrophage inflammasome and IL-1β production [47]. Accordingly, transplantation of bone marrow from mice deficient in various components of the NLRP3 inflammasome into proatherogenic LDLR-null mice is atheroprotective [47]. This mechanism might also have broader relevance to atherosclerosis as recent data suggest that oxidized LDL is poor hydrolyzed and precipitates as inflammasome-inducing cholesterol crystals in macrophage lysosomes [48].
Earlier work on Crohn’s Disease and ATG16L1, another autophagy gene required for autophagosome formation, had shown that ATG16L1-deficient macrophages developed selectively increased IL-1β and IL-18 levels upon Toll-like receptor (TLR) activation, suggestive of inflammasome activation [49]. Additionally, defective mitochondrial autophagy (mitophagy) leads to increased superoxide/ROS production, a potent activator of the inflammasome [50, 51]. The autophagy-inflammasome link appears to play an important role in atherosclerosis as well. ATG5-null macrophages have synergistically increased inflammasome activation and IL-1β production upon exposure to the TLR4 agonist lipopolysaccharide (LPS) and cholesterol crystals [38]. Also, mice with macrophage-selective autophagy deficiency have excessive serum as well as aortic IL-1β levels when fed a Western Diet [38].
Although the exact reasons for inflammasome activation in the plaque are not known, there are two plausible mechanisms. First, inefficient mitophagy of damaged mitochondria results in increased superoxide levels, a potent inducer of inflammasome activation. In support of this, markers of protein oxidation and superoxide production are concomitantly elevated in autophagy-deficient macrophages and atherosclerotic plaques [38]. A second mechanism could be related to the increased burden dysfunctional lysosomes in autophagy-deficient macrophages. Accumulation of poorly digestible lipids such as cholesterol crystals in lysosomes has been shown to destabilize the lysosomal membrane and activate the inflammasome [47, 52, 53]. Defects in autophagic flux could undermine the replenishment of the lysosomal pool and integrity of lysosomes, thus exacerbating the phenotype. The links between autophagy, inflammasomes, and IL-1β production are summarized in Figure 3B.
Autophagy, oxidative stress, and efferocytosis
Although the interface of autophagic and apoptotic pathways is complex and an active area of investigation, cellular fate primarily lies in the decision to activate autophagy as a pro-survival mechanism or apoptosis as a cell death response [54]. A major regulatory node occurs at the Beclin-1 protein complex, where cross-talk with the Bcl-2 family of proteins determines the initiation of autophagy [55]. This cross-talk would be predicted to take a prominent role in the atherosclerotic plaque, where intense oxidative and endoplasmic reticulum stress leads to a vicious cycle of macrophage apoptosis and the clearance of apoptotic cells by efferocytosis [56, 57].
Indeed, upon exposure to pro-apoptotic stress stimuli (such as the oxysterol 7-ketocholesterol), autophagic flux is robustly induced in macrophages [36, 39]. This induction appears to play a protective role as disruption of macrophage autophagy either chemically (via lysosomal protease inhibitors) or genetically (ATG5-deficiency) leads to elevated NADPH-mediated oxidative stress and markedly increased apoptosis [39]. Intriguingly, apoptotic macrophages with a disrupted autophagy apparatus are not recognized and poorly cleared by neighboring efferocytes through as yet undetermined mechanisms. In agreement with these observations, the atherosclerotic lesions of mice with autophagy-deficient macrophages have increased apoptosis, necrotic cores, and overall lesion complexity [39]. These findings suggest that macrophage autophagy acts both to delay apoptotic cell death when repair is possible and to increase the clearance of apoptotic cells by neighboring macrophages, if repair is not possible. The links between autophagy, oxidative stress, and apoptosis are summarized in Figure 3C.
Autophagy and Clearance of Cytotoxic Protein Aggregates
The increased inflammasome activation and apoptotic signaling observed in the autophagy-deficient setting raises a potentially important and as yet unexplored process. Prolonged oxidative damage leads to protein misfolding and the accumulation of dysfunctional proteins in need of degradation [58]. Protein size and the propensity to aggregate dictate whether a proteosomal or an autophagy-lysosomal route of degradation is used. Large polyubiquitinated protein aggregates are often shuttled to the autophagosome via chaperone proteins such as p62/SQSTM1 [59, 60]. Diseases of protein aggregation have thus far been a focus of the neuroscience arena with protein aggregation being a hallmark of neurodegenerative disorders including Huntington’s, Parkinson’s, and Alzheimer’s disease [61]. Salient features of these protein aggregation diseases are the marked stimulation of IL-1β, the inflammasome complex, and pro-apoptotic pathways [61]. This supports the notion that unregulated protein aggregation has cytotoxic and deleterious outcomes. Although not studied directly in the context of atherosclerosis, increased protein aggregation may explain induced inflammasome activation and elevated atherosclerosis in autophagy-deficient settings. In this regard, the elevation of p62/SQSTM1 is a prominent feature of autophagy-deficient macrophages and atherosclerotic plaques [38]. The links between autophagy and the clearance of protein aggregates are summarized in Figure 3D.
Macrophage autophagy in atherosclerosis: Unanswered Questions
Based on the emerging data of the past two years, macrophage autophagy undoubtedly plays an essential role in atherosclerosis. Deletion of ATG5 in macrophages leads to atherosclerotic plaques that are larger, lipid-laden, hyperinflammatory, and replete with apoptotic cells/necrotic cores. Mechanistically, autophagy has now been implicated in mediating cholesterol efflux, suppressing inflammasome activation, and ameliorating apoptosis in atherosclerotic macrophages. However, these observations are clearly a limited assessment of the full range of functions autophagy would be predicted to have in atherosclerosis. Moving forward, it will be important to address several outstanding issues for a more complete mechanistic understanding.
First, the evaluation of autophagy has largely been limited to the characterization of macrophage-specific ATG5-null mice [38, 39, 43]. A frequently used mouse model in studies of autophagy, ATG5-deficiency reflects a complete absence of autophagy (i.e. a disruption in both basal as well as inducible autophagy). As discussed above, exposure to modified lipids and oxidative stress are potent inducers of autophagy [39, 43]. Such models cannot adequately parse the highly regulated “induced” versus the constitutive “basal” versions of autophagy. For example, phosphorylation of Beclin-1 is an important mechanism by which the initiation of autophagy is regulated [55]. Yet, Beclin-1 heterozygous mice, a model of autophagy haploinsufficiency and presumably a more direct evaluation of inducible autophagy, do not show hyperactivation of the inflammasome or enhanced atherosclerotic plaque formation [38]. A more complete evaluation of the Beclin-1 Heterozygous [62] and others such as tissue-specific ATG7- and ATG16L1-deficient [63, 64], and ATG16L1 hypomorphic [65] mice in the context of atherosclerosis will be insightful.
Second, various components of the autophagy machinery have recently been associated with pathways other than autophagosome formation. Toll-like receptor-mediated phagocytosis of microorganisms usurps several autophagy proteins including LC3, ATG5, ATG7, and Beclin-1 in a process independent of autophagy [49]. This so-called LC3-associated phagocytosis (LAP) has also been linked to the clearance of necrotic and apoptotic cells [66]. Although it is currently not known whether LAP is relevant in atherosclerosis, focusing on the interplay between phagocytosis and autophagy in atherosclerotic macrophages is necessary.
Third, plaque macrophages are subject to an ever-increasing intracellular accumulation of lipids as well as damaged organelles and misfolded/aggregated proteins. It is becoming increasingly evident that through the use of adapter proteins, cells retain the capacity to undergo “selective” rather than bulk autophagy [67, 68]. The selective handling mitochondria (mitophagy), peroxisomes (pexophagy), lipids (lipophagy), aggregated proteins (aggrephagy), and microorganisms (xenophagy) have all been described [59, 69–73]. As discussed above, lipophagy is likely the initiating event in autophagy-mediated cholesterol efflux while a disruption in mitophagy contributes to hyperactivation of the inflammasome [38, 43]. The mechanisms that enable an atherosclerotic macrophage to induce and degrade cargo by selective autophagy are poorly understood. In this regard, the chaperone protein p62/SQSTM1 is worthy of mention. Through its ubiquitin-binding domain (UBD) and LC3-interacting region (LIR), p62/SQSTM1 can corral and shuttle polyubiquitinated proteins to autophagosomes for degradation [59, 60]. However, p62/SQSTM1 has also been shown to be important for PINK1/Parkin-mediated mitophagy [74]. The markedly elevated protein levels of p62/SQSTM1 in macrophages of the atherosclerotic plaque allude to the dysregulation of this pathway in atherosclerosis [38]. Investigation of the macrophage’s repertoire of ubiquitinated proteins and organelles and their links to adapter proteins such as p62/SQSTM1 will be highly informative (Figure 3D).
Finally, despite the overwhelming evidence that macrophage autophagy deficiency leads to increased atherosclerosis, there is surprisingly little information on the extent by which plaque progression perturbs the autophagic process. Ultimately, if autophagy is to be linked with disease pathogenesis, this needs to be ascertained. The marked increase of p62/SQSTM1 in plaque macrophages suggests a vast disruption in autophagy-mediated degradation [38]. How does one reconcile this with the observation that stress stimuli (such as lipid excess and ROS) induce markers of autophagy in plaque macrophages [39, 43]? Taken together, these data seem to indicate that the initiation and progression of autophagy remain intact during atherogenesis, yet the eventual degradation of the cargo via the lysosomal apparatus is stalled. Could a progressive lysosomal dysfunction in atherosclerotic macrophages underlie the defects in the autophagy-lysosomal process? Indeed, this concept has been proposed and several lines of evidence support it [75, 76]. Macrophages treated with modified lipids including oxidized- and aggregated-LDL develop engorged lysosomes with poor hydrolytic capacity [77, 78]. Cholesterol crystal-mediated activation of the inflammasome occurs by disruption of lysosomal membrane permeability [47, 79]. Recent data suggest that perturbed hydrolysis of oxidized-LDL-derived cholesteryl esters in lysosomes can even lead to in situ cholesterol crystal formation [48]. Experiments dissecting the relation between autophagic flux, autophagosome-lysosome fusion, and lysosomal function in atherosclerotic macrophages will begin to address these issues [80].
Harnessing Macrophage Autophagy as a Therapy for Atherosclerosis
Efforts to induce autophagy as a therapeutic strategy stem from recurrent observations that an enhanced prodegradative cellular response ameliorates many disease-defining pathophysiological processes. The available evidence supports a similar effect for autophagy in atherosclerosis. As is true in many efforts to modulate cellular processes, unregulated and exuberant induction of autophagy can be deleterious, directing a cell’s demise via the poorly understood type II programmed cell death or autosis [81]. Here, we discuss a limited set of approaches that either have demonstrated efficacy in animal models of atherosclerosis and/or are likely to provide future therapeutic insights (Table 1).
Table 1.
Methods of inducing autophagy in atherosclerosis by way of chemicals, biologics, or genetics.
| Treatment | Mechanism | Effect on Atherosclerosis | Refs |
|---|---|---|---|
| mTOR-dependent Compounds | |||
| Rapamycin | mTORC1 inhibition | Reduction | [83–85] |
| mTOR-independent Compounds | |||
| Lithium, Valproic Acid | Reduce inositol and IP3 levels | Reduction | [86, 87] |
| Verapamil, Dihydropyridines | Calcium channel blocker | Reduction | [88–90] |
| Trifluoperazine | Dopamine antagonist | Reduction | [91] |
| Peptide therapeutics | |||
| Tat-Beclin1 | GAPR-1 interaction | Unknown | [96] |
| Genetic manipulations | |||
| Beclin 1 | Autophagy initiation | Unknown | [94, 95] |
| TFEB | Autophagy-Lysosomal Biogenesis | Unknown | [97–99] |
Many pharmacological approaches for autophagy induction have been proposed [82]. These include agents that target upstream regulators of autophagy such as mTOR [83–85], inositol/IP3 [86, 87], calcium [88–90], and dopamine [91]. Although all pharmacologic strategies are plagued with non-specificity, the most straightforward approach is direct targeting of mTOR signaling (Box 1, Figure I) [92, 93]. Interestingly, many independent reports have established that the classic mTOR inhibitor rapamycin or its derivatives are atheroprotective with effects on plaque macrophage content [83–85]. Outstanding questions here are the degree to which this atheroprotection is specific for macrophage autophagy and the mTORC1- versus mTORC2-dependent signaling pathways. Atherosclerosis quantification in rapamycin-treated mice with a deficiency in macrophage autophagy (e.g. ATG5-null), mTORC1 (e.g. Raptor-null), or mTORC2 (e.g. Rictor-null) will be informative not only to ascertain specificity but to better understand the regulation of macrophage autophagy in the plaque.
Heterologous expression of Beclin-1, involved in initiation of autophagosome formation, has been shown to induce autophagy both in cell culture and in mice [94, 95]. This has led to a clever strategy of conjugating a region of Beclin-1 to the cell-penetrating HIV-Tat domain, creating an injectable autophagy-inducing peptide [96]. Tat-Beclin-1 has the ability to clear protein aggregates, reduce viral titers, and enhance microbial killing both in cell culture and in mice. Use of such biologics has the advantage of higher specificity, providing a therapeutic tool while allowing the interrogation of mechanisms in vivo.
Finally, an insightful mechanism of regulating the autophagy-lysosomal system deserves mention. Transcription factor EB (TFEB) is a recently discovered protein that mediates the transcriptional upregulation of nearly two-thirds of autophagy and lysosomal genes [97–99]. By inducing both autophagy and lysosomal function, it is the only known “master regulator” of lysosomal biogenesis and the cellular prodegradative response. Interestingly, it has an emerging role in lipid metabolism and its activation is regulated by many of the same autophagy-modulating factors including mTOR and certain triggers of cellular stress [100, 101]. Studies aimed at deciphering TFEB’s role in macrophages will likely uncover novel methods of autophagy induction to reduce atherosclerosis.
Concluding remarks and future perspectives
The field of autophagy has grown immensely in the past decade. It has been shown to be involved in the modulation of numerous pathophysiological processes and plays a prominent role in several human diseases. Surprisingly, its dysregulation in atherosclerosis and atherosclerotic macrophages has only been recently addressed. The emerging data support the notion that macrophage autophagy plays a prominent role in the pathogenesis of atherosclerosis and raise the exciting possibility that manipulation of autophagy can have far-reaching therapeutic benefit.
Box 2. Outstanding questions.
What are the predominant mechanisms that make macrophage autophagy atheroprotective?
Do other types of autophagy such as Chaperone-Mediated Autophagy have roles in plaque macrophages and atherosclerosis?
What are the functional differences between “basal” versus “inducible” macrophage autophagy in atherosclerosis?
How do the various types of selective autophagy (e.g. lipophagy vs mitophagy) in macrophages affect plaque progression?
What are the roles of selective autophagy chaperone proteins such as p62/SQSTM1 in atherosclerosis?
How does the autophagy-lysosomal system become dysfunctional in plaque macrophages, and is the defect at the level of autophagy, autophagosome-lysosome fusion, or the lysosome?
Can the stimulation of the macrophage autophagy-lysosomal system (such as Rapamycin’s inhibitory effect on mTOR signaling) be harnessed to ameliorate plaque progression?
Highlights.
Autophagy is a critical degradation process in macrophages.
Recent data link defects in macrophage autophagy to the progression of atherosclerosis.
Macrophage autophagy has roles in cholesterol homeostasis, oxidative stress, and inflammatory signaling.
Harnessing the autophagic response in macrophages holds immense therapeutic potential.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):143–52. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arteriosclerosis, thrombosis, and vascular biology. 1995;15(5):551–61. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–55. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2011;31(7):1506–16. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nature reviews Immunology. 2013;13(10):709–21. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature reviews Immunology. 2011;11(11):723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nature reviews Cardiology. 2010;7(2):77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JL, Newby AC. Macrophage heterogeneity in atherosclerotic plaques. Current opinion in lipidology. 2009;20(5):370–8. doi: 10.1097/MOL.0b013e3283309848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104(4):503–16. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 10.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends in cardiovascular medicine. 2008;18(6):228–32. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koenen RR, Weber C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nature reviews Drug discovery. 2010;9(2):141–53. doi: 10.1038/nrd3048. [DOI] [PubMed] [Google Scholar]
- 12.van Wanrooij EJ, et al. CXCR3 antagonist NBI-74330 attenuates atherosclerotic plaque formation in LDL receptor-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(2):251–7. doi: 10.1161/ATVBAHA.107.147827. [DOI] [PubMed] [Google Scholar]
- 13.Coller BS. Leukocytosis and ischemic vascular disease morbidity and mortality: is it time to intervene? Arteriosclerosis, thrombosis, and vascular biology. 2005;25(4):658–70. doi: 10.1161/01.ATV.0000156877.94472.a5. [DOI] [PubMed] [Google Scholar]
- 14.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arteriosclerosis, thrombosis, and vascular biology. 2006;26(8):1702–11. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 15.Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovascular research. 2008;79(3):360–76. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrell CN. Reactive oxygen species: finding the right balance. Circulation research. 2008;103(6):571–2. doi: 10.1161/CIRCRESAHA.108.184325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Podrez EA, et al. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. The Journal of clinical investigation. 1999;103(11):1547–60. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, et al. Lysosomal cysteine proteases in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2004;24(8):1359–66. doi: 10.1161/01.ATV.0000134530.27208.41. [DOI] [PubMed] [Google Scholar]
- 19.Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(12):2108–14. doi: 10.1161/ATVBAHA.108.173898. [DOI] [PubMed] [Google Scholar]
- 20.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Molecular cell. 2010;40(2):280–93. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Current opinion in cell biology. 2010;22(2):124–31. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nature cell biology. 2013;15(7):713–20. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaushik S, Cuervo AM. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends in cell biology. 2012;22(8):407–17. doi: 10.1016/j.tcb.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mijaljica D, Prescott M, Devenish RJ. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy. 2011;7(7):673–82. doi: 10.4161/auto.7.7.14733. [DOI] [PubMed] [Google Scholar]
- 25.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neufeld TP. TOR-dependent control of autophagy: biting the hand that feeds. Current opinion in cell biology. 2010;22(2):157–68. doi: 10.1016/j.ceb.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruderman NB, et al. AMPK and SIRT1: a long-standing partnership? American journal of physiology Endocrinology and metabolism. 2010;298(4):E751–60. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tasdemir E, et al. Regulation of autophagy by cytoplasmic p53. Nature cell biology. 2008;10(6):676–87. doi: 10.1038/ncb1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crighton D, et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126(1):121–34. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 30.Sumpter R, Jr, Levine B. Autophagy and innate immunity: triggering, targeting and tuning. Seminars in cell & developmental biology. 2010;21(7):699–711. doi: 10.1016/j.semcdb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nature reviews Immunology. 2007;7(10):767–77. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmid D, Munz C. Immune surveillance via self digestion. Autophagy. 2007;3(2):133–5. doi: 10.4161/auto.3591. [DOI] [PubMed] [Google Scholar]
- 33.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469(7330):323–35. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouimet M, Marcel YL. Regulation of lipid droplet cholesterol efflux from macrophage foam cells. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(3):575–81. doi: 10.1161/ATVBAHA.111.240705. [DOI] [PubMed] [Google Scholar]
- 35.Kockx MM, et al. Cell composition, replication, and apoptosis in atherosclerotic plaques after 6 months of cholesterol withdrawal. Circulation research. 1998;83(4):378–87. doi: 10.1161/01.res.83.4.378. [DOI] [PubMed] [Google Scholar]
- 36.Martinet W, De Meyer GR. Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circulation research. 2009;104(3):304–17. doi: 10.1161/CIRCRESAHA.108.188318. [DOI] [PubMed] [Google Scholar]
- 37.Martinet W, et al. 7-ketocholesterol induces protein ubiquitination, myelin figure formation, and light chain 3 processing in vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2004;24(12):2296–301. doi: 10.1161/01.ATV.0000146266.65820.a1. [DOI] [PubMed] [Google Scholar]
- 38.Razani B, et al. Autophagy links inflammasomes to atherosclerotic progression. Cell metabolism. 2012;15(4):534–44. doi: 10.1016/j.cmet.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao X, et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell metabolism. 2012;15(4):545–53. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh R, et al. Autophagy regulates lipid metabolism. Nature. 2009;458(7242):1131–5. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khera AV, Rader DJ. Future therapeutic directions in reverse cholesterol transport. Current atherosclerosis reports. 2010;12(1):73–81. doi: 10.1007/s11883-009-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghosh S, et al. Macrophage cholesteryl ester mobilization and atherosclerosis. Vascular pharmacology. 2010;52(1–2):1–10. doi: 10.1016/j.vph.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouimet M, et al. Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell metabolism. 2011;13(6):655–67. doi: 10.1016/j.cmet.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Guezennec X, et al. Wip1-dependent regulation of autophagy, obesity, and atherosclerosis. Cell metabolism. 2012;16(1):68–80. doi: 10.1016/j.cmet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Shibata N, Glass CK. Regulation of macrophage function in inflammation and atherosclerosis. Journal of lipid research. 2009;50(Suppl):S277–81. doi: 10.1194/jlr.R800063-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nature reviews Immunology. 2010;10 (3):210–5. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 47.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheedy FJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nature immunology. 2013;14(8):812–20. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanjuan MA, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450(7173):1253–7. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 50.Zhou R, et al. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–5. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 51.Naik E, V, Dixit M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208(3):417–20. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9(8):847–56. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masters SL, Latz E, O’Neill LA. The inflammasome in atherosclerosis and type 2 diabetes. Sci Transl Med. 2011;3(81):81ps17. doi: 10.1126/scitranslmed.3001902. [DOI] [PubMed] [Google Scholar]
- 54.Gump JM, Thorburn A. Autophagy and apoptosis: what is the connection? Trends in cell biology. 2011;21(7):387–92. doi: 10.1016/j.tcb.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang R, et al. The Beclin 1 network regulates autophagy and apoptosis. Cell death and differentiation. 2011;18(4):571–80. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nature reviews Immunology. 2010;10(1):36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Current biology : CB. 2001;11(19):R795–805. doi: 10.1016/s0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- 58.Doyle SM, Genest O, Wickner S. Protein rescue from aggregates by powerful molecular chaperone machines. Nature reviews Molecular cell biology. 2013;14(10):617–29. doi: 10.1038/nrm3660. [DOI] [PubMed] [Google Scholar]
- 59.Lamark T, Johansen T. Aggrephagy: selective disposal of protein aggregates by macroautophagy. International journal of cell biology. 2012;2012:736905. doi: 10.1155/2012/736905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Komatsu M, Kageyama S, Ichimura Y. p62/SQSTM1/A170: physiology and pathology. Pharmacological research : the official journal of the Italian Pharmacological Society. 2012;66(6):457–62. doi: 10.1016/j.phrs.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Masters SL, O’Neill LA. Disease-associated amyloid and misfolded protein aggregates activate the inflammasome. Trends in molecular medicine. 2011;17(5):276–82. doi: 10.1016/j.molmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 62.Liang XH, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402(6762):672–6. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 63.Hara T, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–9. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 64.Saitoh T, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456(7219):264–8. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 65.Cadwell K, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456(7219):259–63. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinez J, et al. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(42):17396–401. doi: 10.1073/pnas.1113421108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Kraft C, Peter M, Hofmann K. Selective autophagy: ubiquitin-mediated recognition and beyond. Nature cell biology. 2010;12(9):836–41. doi: 10.1038/ncb0910-836. [DOI] [PubMed] [Google Scholar]
- 68.Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7(3):279–96. doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manjithaya R, et al. Molecular mechanism and physiological role of pexophagy. FEBS letters. 2010;584(7):1367–73. doi: 10.1016/j.febslet.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120(2):159–62. doi: 10.1016/j.cell.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 71.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nature reviews Molecular cell biology. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu K, Czaja MJ. Regulation of lipid stores and metabolism by lipophagy. Cell death and differentiation. 2013;20(1):3–11. doi: 10.1038/cdd.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weidberg H, Shvets E, Elazar Z. Lipophagy: selective catabolism designed for lipids. Developmental cell. 2009;16(5):628–30. doi: 10.1016/j.devcel.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 74.Geisler S, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nature cell biology. 2010;12(2):119–31. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 75.Jerome WG. Advanced atherosclerotic foam cell formation has features of an acquired lysosomal storage disorder. Rejuvenation Res. 2006;9(2):245–55. doi: 10.1089/rej.2006.9.245. [DOI] [PubMed] [Google Scholar]
- 76.Jerome WG. Lysosomes, cholesterol and atherosclerosis. Clinical lipidology. 2010;5(6):853–865. doi: 10.2217/clp.10.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Griffin EE, et al. Aggregated LDL and lipid dispersions induce lysosomal cholesteryl ester accumulation in macrophage foam cells. J Lipid Res. 2005;46(10):2052–60. doi: 10.1194/jlr.M500059-JLR200. [DOI] [PubMed] [Google Scholar]
- 78.Yancey PG, Jerome WG. Lysosomal sequestration of free and esterified cholesterol from oxidized low density lipoprotein in macrophages of different species. Journal of lipid research. 1998;39(7):1349–61. [PubMed] [Google Scholar]
- 79.Rajamaki K, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5(7):e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robinet P, Ritchey B, Smith JD. Physiological difference in autophagic flux in macrophages from 2 mouse strains regulates cholesterol ester metabolism. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(5):903–10. doi: 10.1161/ATVBAHA.112.301041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y, et al. Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(51):20364–71. doi: 10.1073/pnas.1319661110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maiuri MC, et al. Macrophage autophagy in atherosclerosis. Mediators of inflammation. 2013;2013:584715. doi: 10.1155/2013/584715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mueller MA, et al. Prevention of atherosclerosis by the mTOR inhibitor everolimus in LDLR-/- mice despite severe hypercholesterolemia. Atherosclerosis. 2008;198(1):39–48. doi: 10.1016/j.atherosclerosis.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 84.Verheye S, et al. Selective clearance of macrophages in atherosclerotic plaques by autophagy. Journal of the American College of Cardiology. 2007;49(6):706–15. doi: 10.1016/j.jacc.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 85.Chen WQ, et al. Oral rapamycin attenuates inflammation and enhances stability of atherosclerotic plaques in rabbits independent of serum lipid levels. British journal of pharmacology. 2009;156(6):941–51. doi: 10.1111/j.1476-5381.2008.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi SE, et al. Atherosclerosis induced by a high-fat diet is alleviated by lithium chloride via reduction of VCAM expression in ApoE-deficient mice. Vascular pharmacology. 2010;53(5–6):264–72. doi: 10.1016/j.vph.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 87.Bowes AJ, et al. Valproate attenuates accelerated atherosclerosis in hyperglycemic apoE-deficient mice: evidence in support of a role for endoplasmic reticulum stress and glycogen synthase kinase-3 in lesion development and hepatic steatosis. The American journal of pathology. 2009;174(1):330–42. doi: 10.2353/ajpath.2009.080385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rouleau JL, et al. Verapamil suppresses atherosclerosis in cholesterol-fed rabbits. Journal of the American College of Cardiology. 1983;1(6):1453–60. doi: 10.1016/s0735-1097(83)80049-7. [DOI] [PubMed] [Google Scholar]
- 89.Pauletto P, et al. Antiatherogenic action of nitrendipine in hypercholesterolemic rabbits: changes in aortic macrophage accumulation and smooth muscle cell phenotype. Journal of vascular research. 1996;33(1):5–12. doi: 10.1159/000159126. [DOI] [PubMed] [Google Scholar]
- 90.Kyselovic J, et al. Calcium channel blocker inhibits Western-type diet-evoked atherosclerosis development in ApoE-deficient mice. The Journal of pharmacology and experimental therapeutics. 2005;315(1):320–8. doi: 10.1124/jpet.105.089847. [DOI] [PubMed] [Google Scholar]
- 91.Mohindroo A, Ahluwalia P. Effect of trifluoperazine on certain arterial wall lipid-metabolizing enzymes inducing atherosclerosis in rhesus monkeys. Lipids. 1997;32(8):867–72. doi: 10.1007/s11745-997-0111-3. [DOI] [PubMed] [Google Scholar]
- 92.Ravikumar B, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nature genetics. 2004;36(6):585–95. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 93.Berger Z, et al. Rapamycin alleviates toxicity of different aggregate-prone proteins. Human molecular genetics. 2006;15(3):433–42. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- 94.Spencer B, et al. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(43):13578–88. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu H, et al. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117(7):1782–93. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shoji-Kawata S, et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature. 2013;494(7436):201–6. doi: 10.1038/nature11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sardiello M, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325(5939):473–7. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 98.Settembre C, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332(6036):1429–33. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Settembre C, Ballabio A. TFEB regulates autophagy: an integrated coordination of cellular degradation and recycling processes. Autophagy. 2011;7(11):1379–81. doi: 10.4161/auto.7.11.17166. [DOI] [PubMed] [Google Scholar]
- 100.Settembre C, et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nature cell biology. 2013;15(6):647–58. doi: 10.1038/ncb2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Settembre C, et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. The EMBO journal. 2012;31(5):1095–108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]