Abstract
Carriers of the apolipoprotein E (APOE) ε4 allele, the major genetic risk for Alzheimer's disease (AD), harbor an increased load of β-amyloid (Aβ) plaque burden that is felt to be a major instigator of AD development. Data has suggested that lifestyle factors may reduce AD risk by directly mitigating Aβ pathology, which could be particularly beneficial in APOE ε4 carriers. We therefore examined the interaction between lifetime cognitive activity and the APOE ε4 allele in relation to brain Aβ burden. We obtained measures of lifetime cognitive activity in 118 cognitively normal human individuals (mean age: 76.13 ± 5.56 years, 70 women) using a validated questionnaire that included measures over early, middle, and current age epochs. Hierarchical regression models (adjusted for age, gender, and years of education) were conducted to examine effects of APOE ε4 carrier status, lifetime cognitive activity, and the interaction of the two factors with cortical Aβ deposition, quantified using [11C] Pittsburgh-compound-B (PIB)-PET. As expected, the ε4 carriers exhibited higher PIB retention compared with noncarriers. Lifetime cognitive activity moderated the APOE genotype effect such that cortical PIB retention was diminished in ε4 carriers that reported higher cognitive activity over the life course. The findings suggest that greater lifetime cognitive activity may forestall AD pathology, specifically in genetically susceptible individuals. The effect could imply that cognitive training promotes increased neural efficiency that might retard the lifelong neurally mediated deposition of Aβ.
Keywords: aging, Alzheimer's disease, APOE, β-amyloid, lifestyle activity, PIB-PET
Introduction
The β-amyloid (Aβ) protein, an Alzheimer's disease (AD) hallmark, can be found in aggregated forms in cognitively normal older adults. There it is proposed to incite a neurotoxic cascade that leads to neurodegeneration, cognitive decline, and eventually dementia (Jack et al., 2010). The lack of effective treatment necessitates the identification of factors that may forestall such AD pathology. The ε4 variant of the apolipoprotein E (APOE) allele, the major genetic risk factor for AD (Farrer et al., 1997), is related to higher brain Aβ deposition (Reiman et al., 2009), as well as faster cognitive decline (Packard et al., 2007) in older people. This risk-related polymorphism thus offers the potential to target individuals at higher risk for dementia as well as to identify lifestyle factors that could modify the pathogenic mechanisms underlying these risks.
Participation in cognitively stimulating activities (e.g., playing games, reading books) has been shown to protect against cognitive decline (Wilson et al., 2012) and AD (Wilson et al., 2002b). In fact, a recent analysis of modifiable risk factors emphasized that reducing cognitive inactivity throughout life could prevent millions of AD cases (Barnes and Yaffe, 2011). The APOE genotype may modify the relationships between lifestyle and dementia risk. There is evidence that cognitive engagement could be particularly protective against cognitive decline and dementia among APOE ε4 carriers (Carlson et al., 2008; Niti et al., 2008), yet reports are inconsistent (Woodard et al., 2012). The findings imply that the relationship between lifetime cognitive activity and genotype may be important in establishing dementia risk.
The biological mechanisms underlying these possible gene-lifestyle interactions are unknown. Existing research suggests that lifestyle activities, such as greater lifetime cognitive activity, as well as more long-term physical activity, may reduce brain Aβ burden (Liang et al., 2010; Landau et al., 2012). In the present study, we assessed whether associations of lifetime cognitive activity and brain Aβ deposition are modified by APOE status and whether higher cognitive engagement throughout life decreases Aβ burden, particularly in APOE ε4 carriers. Brain Aβ deposition was measured in cognitively normal older adults using [11C] Pittsburgh-compound-B (PIB) positron emission tomography (PET). In the same individuals we measured cognitive activity over the life course using a previously validated questionnaire (Wilson et al., 2003). We hypothesized that greater lifetime cognitive activity would mitigate effects of the APOE ε4 allele on Aβ burden. Follow-up analyses examined, whether the expected advantageous effect was similarly detectable for different life epochs.
Materials and Methods
Study participants
One-hundred-eighteen cognitively normal older human individuals from the Berkeley Aging Cohort, an ongoing longitudinal study, were investigated. Eligibility criteria included a Geriatric Depression Scale (Yesavage et al., 1982) score ≤10, Mini-mental state examination score (MMSE) ≥25, normal memory functions (all scores within −1.5 SD of age- and education-adjusted norms), and age between 60 and 90 years. The subjects were on average 76.13 years of age (SD = 5.56), 70 (59%) women, reported 16.86 (2.02) years of education and had a mean MMSE score of 28.71 (1.31). Each participant underwent standardized neuropsychological testing, and MRI and PIB-PET scanning. Subjects were free of serious neurological and psychiatric illnesses, except hyperlipidemia (46 cases, 39%) and diabetes mellitus (7 cases, 6%). Hypertension was present in 45 (38%) cases, as measured using blood pressure recordings of the brachial artery (available in 112 cases) and defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg. Written informed consent was obtained from each participant in accordance with the Institutional Review Boards of the University of California, Berkeley, and the Lawrence Berkeley National Laboratory (LBNL). The present sample included all 65 individuals from a previous publication (Landau et al., 2012) that only investigated associations of lifetime cognitive activity and cortical PIB retention.
Assessment of cognitive activity
From each participant, lifetime cognitive activity was obtained using a previously validated cognitive activity questionnaire (Wilson et al., 2003). In brief, a 25-item interview was used, in which the frequency of common cognitively demanding activities, such as reading books, newspapers, and magazines, writing letters, going to the library, and playing games, was recorded across age epochs at year 6, 12, 18, and 40 (retrospectively), and at the current age. Responses were provided on a 5-point frequency scale ranging from 1 (once a year or less) to 5 (every day or almost every day). For each participant, we calculated the mean of each age epoch and created four cognitive activity measures: lifetime cognitive activity (average over all age epochs), early (average over the age epochs 6, 12, and 18), middle life (average over the age epoch 40), and current (average over the current age epoch) life. Age epoch-related cognitive activity measures were correlated (intraclass correlation coefficient; ICC = 0.72, 95% CI: 0.63, 0.80). There were, however, significant differences across self-reported early life, middle life, and current cognitive activity, as examined using a repeated-measures ANOVA (F(2,234) = 38.13, p < 0.001).
Reliability of the scales was assessed in 75 participants who fully completed at least two cognitive activity interviews (mean interval 1.63 years, SD = 0.50). The ICC for lifetime cognitive activity was high (r = 0.91, 95% CI: 0.86, 0.94), replicating earlier reports (Wilson et al., 2003). Reliability was also good for early life (ICC: r = 0.92, 95% CI: 0.88, 0.95), middle life (ICC: r = 0.89, 95% CI: 0.83, 0.93), and current (ICC: r = 0.79, 95% CI: 0.67, 0.87) cognitive activity. Nonsignificant differences between first and second administration for early life (mean = 0.01, 95% CI: −0.08, 0.09), middle life (mean = 0.01, 95% CI: −0.07, 0.09), and current life (mean = 0.03, 95% CI: −0.07, 0.14) cognitive activity measures further supported temporal stability. There was no significant difference in the reproducibility of cognitive activity scores across age epochs, as indicated using a repeated-measures ANOVA (age epoch × administration: F(2,148) = 0.19, p = 0.82).
Assessment of covariates
Additional variables (covariates) were assessed to control for potential bias or confounding effects. Perceived memory function, previously associated with regional Aβ burden (Perrotin et al., 2012), was estimated by the average of two scales, participants' ratings of their memory ability in comparison with other age-matched people and their own memory ability 20 years ago, using a 4-point scale from 1 (better) to 4 (much worse). Depression was measured using the Geriatric Depression Scale. Current physical activity was quantified using the modified Minnesota leisure-time activities questionnaire (Taylor et al., 1978; Geffken et al., 2001). Participants indicated frequency (over the last 2 weeks and months per year) and duration (time spent per session) of 15 leisure-time physical activities and any extra physical activity. Frequency and duration information were multiplied using an activity-specific intensity code indicating calorie expenditure (Taylor et al., 1978) and summed to represent intensity of physical activity (total kilocalories of energy expended) during the last year. Finally, the socioeconomic status was estimated from the participant's self-reported professional background, based on the 1990 occupation classification systems of the U.S. Bureau of the Census (Hauser and Warren, 1997).
Acquisition and processing of the neuroimaging data
MRI.
The structural MRI scans were acquired at LBNL on a 1.5T Magnetom Avanto system (Siemens Medical Systems) using a 12-channel head-coil run in triple mode. High-resolution T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) scans were collected axially with the following measurement parameters: TR = 2110 ms, TE = 3.58 ms, flip angle = 15°, field-of-view = 256 × 256 mm, number of slices = 160 with a 50% gap, voxel size = 1 × 1 × 1 mm3.
PIB-PET.
The PIB-PET scans were collected at LBNL, with injection of ∼15 mCi of the [11C] PIB tracer. The PIB-PET images of 93 subjects were acquired using an ECAT EXACT HR (Siemens) PET scanner and 25 subjects were studied with a Siemens Biograph PET/CT scanner. Dynamic acquisition frames were obtained in the 3D acquisition mode over a 90 min measurement interval (4 ×15 s frames, 8 × 30 s frames, 9 × 60 s frames, 2 × 180 s frames, 8 × 300 s frames, and 3 × 600 s frames) after a 10 min transmission scan or CT. The data processing, described in detail previously (Wirth et al., 2013), used graphical analysis with a cerebellar gray reference region to construct distribution volume ratio (DVR) images (Logan et al., 1996).
The native-space PIB-PET (DVR) images were coregistered to the native-space MRI images for each participant. Global cortical PIB retention (representing cortical Aβ burden) was calculated by extracting a mean DVR value from frontal, temporal, parietal, and anterior/posterior cingulate ROIs using the automated FreeSurfer 5.1 processing stream (Fischl et al., 2002) and the Desikan-Killiany parcellation (Desikan et al., 2006). There was no significant effect of scanner type on PIB retention (t(116) = 0.59, p = 0.55).
Assessment of the APOE genotype
The APOE genotyping was performed using DNA obtained from blood samples and results were dichotomized into APOE ε4 allele carrier (ApoE4+) or noncarrier (ApoE4−) status. Thirty-three participants (28%) had a least one APOE ε4 allele, with two (2%) homozygous APOE ε4 carriers.
Statistical analysis
All statistical analyses were performed using SPSS. Statistical significance was defined as p < 0.05, two-tailed. Hierarchical regression models with ordinary least-squares estimation were performed. Main effects and interactions between APOE ε4 carrier status (dummy-coded independent variable: ApoE4+ = 1, ApoE4− = 0) and lifetime cognitive activity (continuous independent variables) on cortical PIB retention (continuous dependent variable) were examined. APOE ε4 carrier status and lifetime cognitive activity were entered at first level of the hierarchical regression model. The interaction between APOE ε4 carrier status and lifetime cognitive activity was entered at second level. At each level, the unique change in explained variance (R2) was evaluated using F-ratio statistics. For each regression model, standardized residuals (≥3 SD) and Mahalanobis Distance (≥20) were inspected. Unless reported otherwise, significant results remained unchanged when outliers and influential cases were removed from the original dataset and the analyses rerun. Analyses were adjusted for age, gender, and years of education as done previously (Wilson et al., 2003; Landau et al., 2012). Adjustments were done by using the standardized regression residuals of lifetime cognitive activity and cortical PIB retention after regression on age, gender, and years of education.
Simple slope analyses were conducted to interpret the directionality of significant interactive effects (Aiken and West, 1991; Cohen et al., 2003). First, the interaction was visualized and evaluated descriptively as follows: Observed cortical PIB retention (mean-centered) was graphed as a function of APOE carrier status and lifetime cognitive activity (mean-centered). In addition, predicted global and voxelwise cortical PIB retention scores were calculated for low (−1 SD of the mean) and high (+1 SD of the mean) lifetime cognitive activity using the unstandardized regression coefficients and graphed. Second, we formally examined whether the relationship between lifetime cognitive activity and PIB retention differed significantly from zero in APOE ε4 carriers. The APOE genotype variable was dummy-coded (ApoE4+ = 0, ApoE4− = 1), so that the regression coefficient of lifetime cognitive activity could be used to assess the relationship between lifetime cognitive activity and PIB retention within ε4 carriers in the hierarchical regression model with an interactive term. In follow-up hierarchical regression models and simple slopes analyses (each adjusted for age, gender, and years of education), we also assessed contributions of different age epochs, namely early, middle, and current life cognitive activity.
In a series of analyses, we investigated whether our results remained stable when controlling for perceived memory function, depression, current physical activity, or socioeconomic status. This was done by additional adjustments of cortical PIB retention and cognitive activity measures for these variables (one at a time) and submission of the residuals to hierarchical regression models. The covariates were not significantly associated with cortical PIB retention (all p > 0.05); nevertheless, the analyses were conducted to elaborate potential influences of these variables that could bias the associations we detected.
Results
The APOE ε4 carriers did not differ significantly from noncarriers in demographic characteristics, time intervals between neuroimaging and neuropsychological measurements, as well as reported cognitive activity (Table 1). Lifetime (and age epoch-related) cognitive activity measures were not significantly correlated with age, gender, years of education, subjective memory function, depression, current physical activity, and socioeconomic status (all p > 0.05, data not shown). Further, there were no significant associations between cognitive activity and vascular risk factors (i.e., diabetes, hyperlipidemia, and hypertension; all p > 0.05, data not shown) assessed using t tests over all life epochs.
Table 1.
Characteristics for APOE ϵ4 noncarriers and carriers groups
| APOE ϵ4 noncarriers | APOE ϵ4 carriers | p value | |
|---|---|---|---|
| Participants, no. (%) | 85 (72) | 33 (28) | |
| Age at PIB, years (SD) | 76.72 (5.80) | 74.59 (4.63) | 0.06 |
| Women, no. (%) | 50 (59) | 20 (61) | 1.00 |
| Education | 16.73 (1.86) | 17.21 (2.37) | 0.24 |
| MMSE, mean (SD) | 28.72 (1.33) | 28.70 (1.26) | 0.94 |
| Measurement characteristics | |||
| Δtime (NTS-MRI), years (SD) | 0.29 (0.17) | 0.30 (0.18) | 0.83 |
| Δtime (NTS-PIB), years (SD) | 0.34 (0.37) | 0.38 (0.32) | 0.57 |
| Δtime (MRI-PIB), years (SD) | 0.13 (0.33) | 0.14 (0.35) | 0.83 |
| Cognitive activity | |||
| Lifetime | 3.53 (0.56) | 3.51 (0.51) | 0.85 |
| Early life | 3.36 (0.73) | 3.38 (0.63) | 0.89 |
| Middle life | 3.68 (0.57) | 3.55 (0.47) | 0.23 |
| Current life | 3.88 (0.56) | 3.85 (0.64) | 0.76 |
p Values were estimated from parametric or nonparametric statistical comparisons.
Δ, Difference; NTS, neuropsychological test session.
The hierarchical regression model (adjusted for age, gender, and years of education) confirmed increased cortical PIB retention in ε4 carriers (p < 0.001; Table 2). Greater lifetime cognitive activity across all subjects was related to lower PIB retention (p < 0.001; Table 2). There was a significant interaction between APOE ε4 carrier status and lifetime cognitive activity (p < 0.05; Table 2). Simple slope analysis corroborated that higher lifetime cognitive activity mitigated cortical PIB retention specifically in ε4 carriers (B = −0.59, SE = 0.17, β = −0.59, p < 0.001; Fig. 1A,B).
Table 2.
Hierarchical regression models for APOE ϵ4 carrier status, lifetime cognitive activity, and cortical PIB retention
| Models | Unstandardized coefficients |
Standardized coefficients Beta | ΔR2 | Correlations |
||
|---|---|---|---|---|---|---|
| B | SE | Zero-order | Partial | |||
| Model adjusted for age, gender, and years of educationa | ||||||
| Main effect level | 0.21*** | |||||
| APOE ϵ4 carrier statusb | 0.82*** | 0.18 | 0.38*** | 0.38 | 0.39 | |
| CA, lifetime | −0.25** | 0.08 | −0.25** | −0.25 | −0.27 | |
| Interaction effect level | 0.04* | |||||
| ApoE4 × CA, lifetime | −0.45* | 0.19 | −0.22* | |||
| Model additionally adjusted for subjective memory functiona | ||||||
| Main effect level | 0.21*** | |||||
| APOE ϵ4 carrier statusb | 0.82*** | 0.18 | 0.38*** | 0.38 | 0.39 | |
| CA, lifetime | −0.24** | 0.08 | −0.24** | −0.25 | −0.26 | |
| Interaction effect level | 0.04* | |||||
| ApoE4 × CA, lifetime | −0.47* | 0.19 | −0.23* | |||
| Model additionally adjusted for depressiona | ||||||
| Main effect level | 0.20*** | |||||
| APOE ϵ4 carrier statusb | 0.81*** | 0.18 | 0.37*** | 0.38 | 0.38 | |
| CA, lifetime | −0.25** | 0.08 | −0.25** | −0.26 | −0.27 | |
| Interaction effect level | 0.04* | |||||
| ApoE4 × CA, lifetime | −0.44* | 0.19 | −0.22* | |||
| Model additionally adjusted for current physical activitya | ||||||
| Main effect level | 0.20*** | |||||
| APOE ϵ4 carrier statusb | 0.82*** | 0.18 | 0.38*** | 0.38 | 0.39 | |
| CA, lifetime | −0.23** | 0.08 | −0.23** | −0.24 | −0.25 | |
| Interaction effect level | 0.03+ | |||||
| ApoE4 × CA, lifetime | −0.37+ | 0.19 | −0.18+ | |||
| Model additionally adjusted for socioeconomic statusa | ||||||
| Main effect level | 0.20*** | |||||
| APOE ϵ4 carrier statusb | 0.82*** | 0.18 | 0.38*** | 0.38 | 0.39 | |
| CA, lifetime | −0.23** | 0.08 | −0.23** | −0.24 | −0.25 | |
| Interaction effect level | 0.02+ | |||||
| ApoE4 × CA, lifetime | −0.36+ | 0.20 | −0.17+ | |||
aDependent variable: cortical PIB retention.
bAPOE ϵ4 carrier status: dummy coded with carriers = 1, noncarriers = 0.
***p < 0.001,
**p < 0.01,
*p < 0.05,
+p < 0.1 (trend).
CA, Cognitive activity; Δ, difference.
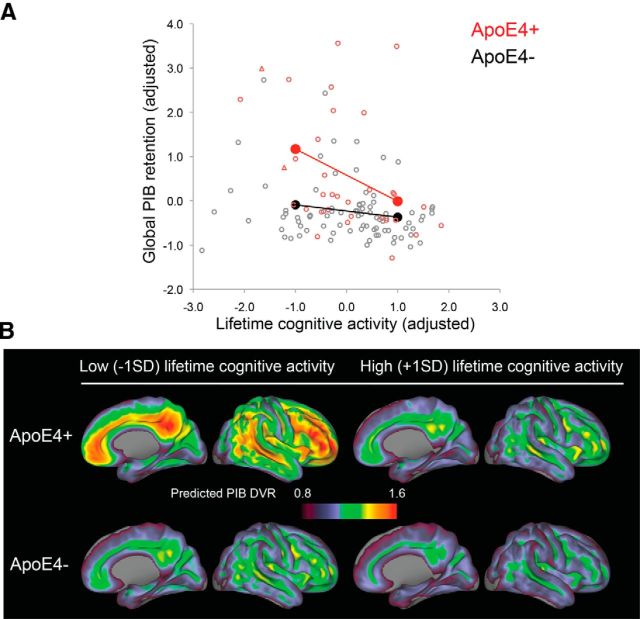
Figure 1.
Visualization of the significant interaction between APOE ε4 carrier status (ApoE4+, ApoE4−) and lifetime cognitive activity. A, B, Figures demonstrate that cortical PIB retention is reduced in ε4 carriers with greater lifetime cognitive activity. A, The graph conceptualizes APOE ε4 carrier status as moderator variable and shows predicted cortical PIB retention (solid circles) in ε4 carriers (red) and noncarriers (black) for low- and high-lifetime cognitive activity; lines demonstrate linear trends. In addition, observed cortical PIB retention is depicted for heterozygous ε4 carriers (red circles), homozygous ε4 carriers (red triangles) and noncarriers (gray circles). Variables are residuals after adjusting for age, gender, and years of education. B, Predicted voxelwise cortical PIB retention in ε4 carriers and noncarriers for low- and high-lifetime cognitive activity (adjusted).
In follow-up models, higher lifetime cognitive activity remained significantly associated with lower PIB retention in ε4 carriers when adjusted for perceived memory function (B = −0.60, SE = 0.17, β = −0.60, p < 0.001), depression (B = −0.58, SE = 0.17, β = −0.58, p < 0.001), current physical activity (B = −0.52, SE = 0.17, β = −0.52, p < 0.01), and socioeconomic status (B = −0.51, SE = 0.17, β = −0.51, p < 0.01). The interaction between genotype and cognitive activity was maintained after adjustment for perceived memory function (p < 0.05; Table 2) and depression (p < 0.05; Table 2), but was reduced to trend level for current physical activity (p = 0.055; Table 2) and socioeconomic status (p = 0.067; Table 2).
Follow-up hierarchical regression models (adjusted for age, gender, and years of education) explored the contribution of different age epochs. The APOE ε4 carrier status significantly interacted with early life (B = −0.42, SE = 0.20, β = −0.20, p < 0.05) and middle life (B = −0.65, SE = 0.20, β = −0.30, p < 0.01), but not current (B = −0.10, SE = 0.19, β = −0.06, p = 0.58) cognitive activity to modulate cortical PIB retention. Simple slope analyses corroborated that higher cognitive activity was significantly related to lower PIB retention in ε4 carriers for early life (B = −0.55, SE = 0.17, β = −0.55, p < 0.01) and middle life (B = −0.77, SE = 0.17, β = −0.77, p < 0.001) cognitive activity; the effect was not significant for current cognitive activity (B = −0.18, SE = 0.16, β = −0.18, p = 0.25). Accounting for the contribution of current cognitive activity, the gene-environment interaction was reduced to trend level for early life (B = −0.38, SE = 0.19, β = −0.19, p = 0.052) and remained significant for middle life (B = −0.50, SE = 0.18, β = −0.28, p < 0.01).
Adjustment for perceived memory function and depression did not change the results. Early life cognitive activity remained significantly associated with PIB retention in ε4 carriers accounting for current physical activity (B = −0.48, SE = 0.17, β = −0.48, p < 0.01) and socioeconomic status (B = −0.49, SE = 0.18, β = −0.49, p < 0.01); the interactions with APOE ε4 carrier status were reduced to trend level (B = −0.36, SE = 0.20, β = −0.17, p = 0.073 and B = −0.35, SE = 0.20, β = −0.17, p = 0.082, respectively). The other age epoch-related results remained unchanged (data not shown).
Discussion
Lifetime cognitive engagement appears to have potential for the prevention of AD (Wilson et al., 2002a,b; Barnes and Yaffe, 2011) and may benefit cognitive aging by mitigating AD pathogenic processes (Landau et al., 2012). The present findings demonstrate that associations between lifetime cognitive activity and AD pathogenesis are amplified in carriers of the APOE ε4 allele. The data suggest that lifetime cognitive activity could help to forestall the deleterious impact of the APOE ε4 allele on brain Aβ deposition.
The presence of at least one APOE ε4 allele was significantly related to higher brain Aβ deposition in our data, mirroring previous reports (Reiman et al., 2009; Vemuri et al., 2010; Head et al., 2012). Lifetime cognitive activity, however, interacted with the APOE ε4 carrier status to modulate Aβ pathology. Specifically, higher cognitive activity correlated with lower Aβ-plaque burden particularly in ε4 carriers. In a highly comparable way, self-reported long-term physical activity, as well as vascular risk factors, were more strongly related to Aβ deposition in APOE ε4 carriers (Head et al., 2012; Brown et al., 2013; Rodrigue et al., 2013). These findings indicate that individuals with genetic risk could be more susceptible to both protective factors and risk factors. There was no significant effect of lifetime cognitive activity in the noncarriers, which is in agreement with prior studies likewise reporting the absence of effects of lifestyle and vascular risk on cortical Aβ deposition in noncarriers (Head et al., 2012; Brown et al., 2013; Rodrigue et al., 2013). A lower proportion of individuals with Aβ among the noncarriers may have made it difficult to detect smaller effects of lifestyle, although the absolute number of carriers and noncarriers with high Aβ-plaque levels was comparable in the present study. There also could be beneficial effects of cognitive engagement in noncarriers using other biomarker outcomes. This is suggested by findings showing that cognitive activity or training can improve brain function and structure in older adults (Lövdén et al., 2010; Anguera et al., 2013). Nevertheless, our and other studies (Kivipelto et al., 2008; Head et al., 2012) imply that a healthy lifestyle could be particularly protective against AD in ε4 carriers.
Our data also imply that an active lifestyle over extended and potentially critical life periods (perhaps starting before Aβ accumulation) could protect against elevated brain Aβ burden and thus offset detrimental effects of the APOE polymorphism. Similarly, an earlier study found that long-term physical activity initiated during middle life lowered Aβ deposition specifically in APOE ε4 carriers (Head et al., 2012). Animal studies have further shown that both preventative and therapeutic exposure to enriched environments can mitigate Aβ pathology (in transgenic Aβ-expressing mice) or protect against Aβ-related synaptotoxic effects (in wild-type mice) in experimental compared with control animals (Lazarov et al., 2005; Costa et al., 2007; Herring et al., 2011; Li et al., 2013). A previous report that evaluated lifestyle factors has failed to detect beneficial effects of cognitive activity on Aβ burden (Vemuri et al., 2012). This might be explained by different study and sample characteristics. In contrast to our study, Vemuri et al. (2012) examined a mixed sample of cognitively normal individuals, as well as mild cognitive impairment patients. Moreover, the cognitive activity measure was constructed of education, occupation and current cognitive activity, whereas the present cognitive activity interview examined the frequency of cognitive activities over the life span (Wilson et al., 2003). It is also possible that effects of cognitive activity, APOE genotype, and Aβ might be influenced by the timing of the lifestyle events in relationship to the life course.
At least two biological mechanisms might underlie the observed effects. Rodent models have suggested that beneficial effects of enriched environments directly operate via Aβ-dependent pathways, such as alterations in Aβ catabolism (Lazarov et al., 2005; Costa et al., 2007). On the other hand, effects of a cognitively demanding lifestyle could also be mediated by neuronal factors. There is indication that higher neural activity promotes Aβ secretion in animals (Bero et al., 2011) and humans (Brody et al., 2008). Lifelong cognitive activity might thus nurture efficiency in neural systems (Jagust and Mormino, 2011; Gold et al., 2013), which could modulate neural activity and thereby attenuate Aβ secretion and accumulation, suggested to begin after the age of 50 (Kok et al., 2009). In young and middle-age APOE ε4 carriers, greater brain activation (Filippini et al., 2009) and lower resting state glucose metabolism (Reiman et al., 2004) has been reported in brain regions susceptible to Aβ burden. Such neuronal insufficiencies could make beneficial lifestyle factors specifically effective in APOE ε4 carriers in a manner similar to “cognitive training,” improving neural efficiency that could prevent otherwise elevated brain Aβ deposition in the genetically susceptible individuals.
Our findings converge with recent epidemiological reports, indicating greater effects of lifestyle factors in ε4 carriers. In particular, middle-life cognitive activity was protective in individuals with genetic susceptibility (Carlson et al., 2008). Another study indicated that nonbeneficial lifestyle behaviors, such as physical inactivity, high dietary fat intake, alcohol drinking, and smoking were associated with greater risk of dementia and AD especially among ε4 carriers (Kivipelto et al., 2008). Our study directly adds to this picture suggesting that lifelong engagement in cognitively stimulating activities could diminish Aβ aggregation particularly in APOE ε4 carriers. Cognitive activity could thereby be beneficial, especially when individuals engage over extended life periods, starting well before Aβ deposition emerges.
The present study is relatively small in sample size, thus the gene-environment relationship on AD pathology needs to be further investigated and replicated in larger cohorts. Our results are based on self-reports, some of which are retrospective. Though we have used a reliable and previously validated cognitive activity questionnaire, it is possible that the current cognitive status and health status of older individuals (in particular with genetic risk and Aβ pathology) could bias self-reported evaluations of past cognitive activity. This explanation does not seem likely because results remained stable after additional adjustments for subjective memory function and depression. However, it is still possible that an undetected bias influenced participants' responses and therefore the relationships we detected. Adjustment for current physical activity and socioeconomic status somewhat influenced the interactive effects between cognitive activity and APOE status. This might be attributable to statistical power, because associations between cognitive activity and brain Aβ burden within ε4 carriers were unaffected. The results seen here can best be confirmed by either a prospective study or randomized intervention.
The results are part of a growing literature indicating beneficial effects of lifestyle factors in reducing the risk of cognitive decline and AD development. The present findings suggest that cognitive activity could modify heightened Aβ-plaque accumulation in carriers of the APOE ε4 allele, a major genetic risk factor for AD. This importance of lifelong health behaviors, specifically in individuals with genetic susceptibility, has essential implications for public health approaches to AD risk reduction, in particular in the absence of effective treatments.
Footnotes
This research work was supported by NIH Grant AG034570, and the Swiss National Science Foundation Grant PA00P1-131515. We thank Grace Tang (UC Berkeley, behavioral data analysis support), Cindee Madison (UC Berkeley, neuroimaging data analysis support), and Jacob Vogel (UC Berkeley, data collection and discussion).
The authors declare no competing financial interests.
References
- Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Newbury Park, CA: Sage Publications; 1991. [Google Scholar]
- Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, Kong E, Larraburo Y, Rolle C, Johnston E, Gazzaley A. Video game training enhances cognitive control in older adults. Nature. 2013;501:97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DL, Magnoni S, Schwetye KE, Spinner ML, Esparza TJ, Stocchetti N, Zipfel GJ, Holtzman DM. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BM, Peiffer JJ, Taddei K, Lui JK, Laws SM, Gupta VB, Taddei T, Ward VK, Rodrigues MA, Burnham S, Rainey-Smith SR, Villemagne VL, Bush A, Ellis KA, Masters CL, Ames D, Macaulay SL, Szoeke C, Rowe CC, Martins RN. Physical activity and amyloid-beta plasma and brain levels: results from the Australian imaging, biomarkers and lifestyle study of ageing. Mol Psychiatry. 2013;18:875–881. doi: 10.1038/mp.2012.107. [DOI] [PubMed] [Google Scholar]
- Carlson MC, Helms MJ, Steffens DC, Burke JR, Potter GG, Plassman BL. Midlife activity predicts risk of dementia in older male twin pairs. Alzheimers Dement. 2008;4:324–331. doi: 10.1016/j.jalz.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. Ed 3. Mahwah, NJ: L. Erlbaum Associates; 2003. [Google Scholar]
- Costa DA, Cracchiolo JR, Bachstetter AD, Hughes TF, Bales KR, Paul SM, Mervis RF, Arendash GW, Potter H. Enrichment improves cognition in AD mice by amyloid-related and unrelated mechanisms. Neurobiol Aging. 2007;28:831–844. doi: 10.1016/j.neurobiolaging.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278:1349–1356. doi: 10.1001/jama.1997.03550160069041. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- Gold BT, Kim C, Johnson NF, Kryscio RJ, Smith CD. Lifelong bilingualism maintains neural efficiency for cognitive control in aging. J Neurosci. 2013;33:387–396. doi: 10.1523/JNEUROSCI.3837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser RM, Warren JR. Socioeconomic indexes for occupations: a review, update, and critique. Soc Methodol. 1997;27:177–298. doi: 10.1111/1467-9531.271028. [DOI] [Google Scholar]
- Head D, Bugg JM, Goate AM, Fagan AM, Mintun MA, Benzinger T, Holtzman DM, Morris JC. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch Neurol. 2012;69:636–643. doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A, Lewejohann L, Panzer AL, Donath A, Kröll O, Sachser N, Paulus W, Keyvani K. Preventive and therapeutic types of environmental enrichment counteract beta amyloid pathology by different molecular mechanisms. Neurobiol Dis. 2011;42:530–538. doi: 10.1016/j.nbd.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Mormino EC. Lifespan brain activity, beta-amyloid, and Alzheimer's disease. Trends Cogn Sci. 2011;15:520–526. doi: 10.1016/j.tics.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Rovio S, Ngandu T, Kåreholt I, Eskelinen M, Winblad B, Hachinski V, Cedazo-Minguez A, Soininen H, Tuomilehto J, Nissinen A. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med. 2008;12:2762–2771. doi: 10.1111/j.1582-4934.2008.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok E, Haikonen S, Luoto T, Huhtala H, Goebeler S, Haapasalo H, Karhunen PJ. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol. 2009;65:650–657. doi: 10.1002/ana.21696. [DOI] [PubMed] [Google Scholar]
- Landau SM, Marks SM, Mormino EC, Rabinovici GD, Oh H, O'Neil JP, Wilson RS, Jagust WJ. Association of lifetime cognitive engagement and low beta-amyloid deposition. Arch Neurol. 2012a;69:623–629. doi: 10.1001/archneurol.2011.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, Holtzman DM, Morris JC, Head D. Exercise and Alzheimer's disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68:311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jin M, Zhang D, Yang T, Koeglsperger T, Fu H, Selkoe DJ. Environmental novelty activates beta2-adrenergic signaling to prevent the impairment of hippocampal LTP by Abeta oligomers. Neuron. 2013;77:929–941. doi: 10.1016/j.neuron.2012.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Bodammer NC, Kühn S, Kaufmann J, Schütze H, Tempelmann C, Heinze HJ, Düzel E, Schmiedek F, Lindenberger U. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia. 2010;48:3878–3883. doi: 10.1016/j.neuropsychologia.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Niti M, Yap KB, Kua EH, Tan CH, Ng TP. Physical, social and productive leisure activities, cognitive decline and interaction with APOE-epsilon 4 genotype in Chinese older adults. Int Psychogeriatr. 2008;20:237–251. doi: 10.1017/S1041610207006655. [DOI] [PubMed] [Google Scholar]
- Packard CJ, Westendorp RG, Stott DJ, Caslake MJ, Murray HM, Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Jolles J, Perry IJ, Sweeney BJ, Twomey C, et al. Association between apolipoprotein E4 and cognitive decline in elderly adults. J Am Geriatr Soc. 2007;55:1777–1785. doi: 10.1111/j.1532-5415.2007.01415.x. [DOI] [PubMed] [Google Scholar]
- Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69:223–229. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB, Alexander GE, Klunk WE, Mathis CA, Price JC, Aizenstein HJ, DeKosky ST, Caselli RJ. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Sr, Diaz-Arrastia R, Park DC. Risk factors for beta-amyloid deposition in healthy aging: vascular and genetic effects. JAMA Neurol. 2013;70:600–606. doi: 10.1001/jamaneurol.2013.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Shaw LM, Trojanowski JQ, Aisen PS, Weiner M, Petersen RC, Jack CR., Jr Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol. 2010;67:308–316. doi: 10.1002/ana.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Roberts RO, Lowe VJ, Kantarci K, Senjem ML, Gunter JL, Boeve BF, Petersen RC, Jack CR., Jr Effect of lifestyle activities on Alzheimer disease biomarkers and cognition. Ann Neurol. 2012;72:730–738. doi: 10.1002/ana.23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002a;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Bienias JL, Aggarwal NT, Mendes De Leon CF, Morris MC, Schneider JA, Evans DA. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002b;59:1910–1914. doi: 10.1212/01.WNL.0000036905.59156.A1. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Boyle PA, Bennett DA. Influence of late-life cognitive activity on cognitive health. Neurology. 2012;78:1123–1129. doi: 10.1212/WNL.0b013e31824f8c03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Barnes L, Bennett D. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol. 2003;25:634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- Wirth M, Madison CM, Rabinovici GD, Oh H, Landau SM, Jagust WJ. Alzheimer's disease neurodegenerative biomarkers are associated with decreased cognitive function but not beta-amyloid in cognitively normal older individuals. J Neurosci. 2013;33:5553–5563. doi: 10.1523/JNEUROSCI.4409-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard JL, Sugarman MA, Nielson KA, Smith JC, Seidenberg M, Durgerian S, Butts A, Hantke N, Lancaster M, Matthews MA, Rao SM. Lifestyle and genetic contributions to cognitive decline and hippocampal structure and function in healthy aging. Curr Alzheimer Res. 2012;9:436–446. doi: 10.2174/156720512800492477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]