Abstract
The present study is designed to demonstrate the ovarian surface epithelial cells’ (OSE) estrogen receptor α (ERα) and progesterone receptor (PR) during pregnancy and estrous cycle in rat. Moreover, determination of the levels of plasma progesterone, estradiol, FSH and LH was also made. The levels of plasma progesterone, estradiol, FSH and LH concentrations were determined on days 7 (n = 5), 14 (n = 5), and 21 (n = 5) of pregnancy in three groups of rats and during the estrous cycle (n = 5) using an ELISA kit. Immunohistochemical method for PR and ERα expressions was also made on the ovary. During pregnancy, FSH and LH remained low except at term when LH levels began to increase from 16 ng/ml to 47 ng/ml. Progesterone levels significantly exceeded estradiol values in all pregnant rats with a peak value of 202 ng/ml on day 14. Elevated progesterone levels were associated negatively with LH and estradiol levels during pregnancy. The levels of estradiol surged significantly on day 21. Immunohistochemistry of the ovary showed low levels of OSE cells staining positive for ERα expression. ERα positive cells were absent on day 7 and 14 of pregnancy, only day 21 recorded a very low percentage of immunostaining (0.5%) within the nuclei of OSE cells. On the contrary, immunostaining of PR was not observed within the nuclei of OSE cells in all groups of study. In conclusion, these results may suggest that the progesterone effect during pregnancy seems to be overriding the positive effect of estrogens on OSE cells. High progesterone levels may have a direct negative effect on gonadotropin production and thereby it might inhibit events leading to both follicular development and OSE proliferation. Understanding the factors affecting OSE proliferation may help elucidating the mechanism(s) of assisted diseases such as ovarian cancer.
Keyword: OSE pregnancy rat steroid receptors gonadotropins
1. Introduction
The mammalian ovary is a highly dynamic organ with dramatically changing environment, it is covered by a single layer of epithelial tissue; the ovarian surface epithelium (OSE). OSE cells derived from mesodermal epithelium of gonadal ridges, are flattened mesothelium of peritoneum separated from underneath ovarian stroma by a basement membrane and a connective tissue layer, tunica albuginea (Leung and Choi, 2007). OSE occupies the entire ovarian lining, and varies in morphology from simple squamous to cuboidal to low pseudo stratified columnar (Auersperg et al., 2001). Despite their inconspicuous appearance, OSE participates in transporting and exchanging nutrients and other bioactive metabolites from the peritoneal cavity and ovary. At pre-ovulation, OSE in proximity to rupture site undergoes apoptotic cell death and the wound caused by ovulation is repaired by highly proliferating OSE cells from surroundings of the ruptured follicle (Murdoch and Van Kirk, 2002). OSE cell proliferation also occurs at post-ovulatory phase especially in post-menopausal woman due to ageing of ovary; epithelial line invaginates, producing crypts and glands, which eventually develop into cysts within the stromal compartment (Auersperg, 2013). It has been hypothesized that repeated cycles of ovulation-induced trauma and repair of the OSE at the site of ovulation contribute to malignancy, it makes the OSE susceptible to mitogenic factors and other genotoxic radicals. There are several studies that suggested that menstrual cycle can affect tumor growth through the high levels of reproductive hormones (Wood et al., 2005; Wood and Hrushesky, 2005). A host of tumorigenetic factors, viz. cytokeratin, desmoplakin, transforming growth factor-α (TGF-α) and receptors for estrogen, progesterone and epidermal growth factor (EGF) are expressed by OSE (Li et al., 2003).
Approximately 90% of human ovarian cancers (OCs) originate from OSE. Epithelial ovarian cancer (EOC) is the fifth leading cause of cancer deaths and the third among gynecological cancer worldwide (Ferlay et al., 2010). It is well known that pregnancy and use of oral contraceptives can reduce the risk of EOC development (Tiedemann, 2000; Clow et al., 2002). Additionally, it has been reported that EOC is absent in animal species where seasonal ovulation and multiple pregnancies take place. Suppression of ovulation and steroid production by the pre-ovulatory follicles are main significant alterations that occur within the ovary during pregnancy. OC is highly associated with high exposure to gonadotropins. This is evident because a research on the use of ovulation inducing treatments by infertile women showed a direct proportionality between the number of OC cases and the ovulation induction use (Rizzuto et al., 2013). At menopause, it is evident that gonadotropins are released in high quantities and hence causing the high risk of cancer in post-menopause females (Leung and Choi, 2007). Both the follicle stimulating (FSH) and luteinizing hormones (LH) are very important trophic factors especially for the survival and proliferation of the follicular somatic cells and aiding in the cyclic recruitment of the antral follicles (Zhang et al., 2013). On the other hand, theory states that OC is triggered by an increase of estrogen production that triggers OSE proliferation (Baby and Bartlewski, 2011). Steroid hormones have been implicated in the etiology of some EOC. Expression of estrogen (ER), progesterone (PR) and androgen (AR) receptors has been reported in human EOC (Akahira et al., 2002; Hamilton et al., 1981).
According to Fortune (1994) senescent ovary, like the one during pregnancy or postnatal period, maintains oocytes in “resting” or primordial follicles (Fortune, 1994). Growing follicles either ovulate to release oocytes for fertilization, or degenerate through into atresia. Differentiated follicles also provide steroidal hormones to maintain the ovarian cycle and prepare uterus for implant, and corpus luteum (CL) produces hormones for establishing and maintaining pregnancy. Gonadotropins and estrogens are vital for maintaining ovarian cycle until antral stage just before ovulation.
The objective of this study is to determine hormonal fluctuation namely steroids and gonadotropins during pregnancy. A second purpose of our investigations is to examine the expression of steroid receptors in OSE cells at different stages of pregnancy.
2. Materials and methods
2.1. Animals
Adult female rats weighing 250–300 g were obtained from the central animal house at the King Fahd Medical and Research Centre (KFMRC) and maintained under controlled conditions of temperature (23–27 °C) and lighting (lights on 05:00–19:00 h). Vaginal smears were examined daily and on the day of estrous. Pregnancy was induced in the animals by transferring 1 female rat to the cage of a single male rat and left overnight. Mating was verified the next morning by the presence of vaginal plug in the vaginal smear; this was designated as Day 1 of pregnancy. Rats were bled 1 ml by retro-orbital plexus puncture under light diethyl-ether anesthesia. Blood samples were centrifuged immediately after collection. Plasma was separated and stored at −20 °C until assayed for, estradiol, progesterone, LH and FSH hormones. All experimental procedures were approved by the animal ethics committee.
2.2. Sample collection
Ovaries from estrus and pregnant animals were collected. The ovaries were gently handled during collection to avoid cell loss by excessive handling as the OSE is a very fragile layer. The ovaries were classified according to the reproductive status of the rats: ovaries from estrus rats (n = 5) and ovaries from pregnant rats (n = 15). This last group was further sub-divided into three groups based on the stage of pregnancy, the three groups were as follows; ovaries in early gestation (day 7) (n = 5) and ovaries in mid gestation (day 14) (n = 5) and late gestation (day 21) (n = 5). Pregnancy was confirmed by abdominal palpation. Full term pregnancies range from 21–22 days. Ovarian tissue samples from pregnant animals and estrous animals were collected alongside material from.
2.3. Histological methods
Ovarian tissues were fixed in Bouin’s, dehydrated in graded alcohols and embedded in paraffin, and sectioned serially at 6 μm and mounted on Super Frost plus microscope slides (VWR International Ltd., Leicestershire, UK) and left to dry overnight at 37 °C.
2.4. PR and ERα immunohisthemistry
The effect of the reproductive stage (estrous or pregnancy) on PR and ERα expressions within OSE cells was investigated using immunohisthemistry (IHC) methods. Sections were de-waxed in xylene, and hydrated in descending grades of alcohol to distilled water, and followed by double washing with phosphate buffer saline (PBS). A heat-induced epitope retrieval method was performed (antigen retrieval) by microwaving (high power at 800 W) the sections in a 0.01 M citrate buffer (pH 6.0). Endogenous peroxidase activity was inactivated through incubation with 3 percent hydrogen peroxide in methanol. Sections were incubated with monoclonal mouse anti-human estrogen receptor α clone 1D5 (Dako Autostainer/Autostainer Plus-Code IS657) to detect ER and with monoclonal mouse anti-human progesterone receptor clone PgR 636 (Dako/FLEX Code IR068) to detect PR. Sections were incubated overnight with the primary antibody at 4 °C. The NovoLink polymer detection system (RE7 150-K, Novastra Laboratories Ltd., UK) was used for visualization. Sections were incubated with protein blk for 5 min, followed by an overnight incubation with the primary antibody (1:2000 at 4 °C). After being washed with PBS, a post-primary blk was performed for 30 min. Sections were incubated with the NovoLink polymer solution for 30 min, and then washed with PBS. Peroxidase activity was developed using a DAB solution for 5 min. Sections were counterstained using hematoxylin stain. Optimization of antibody was necessary to enhance the permeability to the tissue. Antibody incubation time and temperature were adjusted to overnight at 4 °C instead of 60 min at 37 °C. As a negative control, the blking solution was left on the sections instead of the primary antibody to insure the purity of the staining procedure. Samples of breast cancer were used as a positive control. At each run of IHC, a set of slides including sections from all groups of study plus negative and positive control were run together under the same experimental condition. Two sections from each ovary were selected randomly for the analysis.
2.5. Steroids and gonadotropins determination
Concentrations of progesterone, estradiol, FSH and LH in peripheral plasma were determined in all participants using ELISA kits. For estradiol determination, the Estradiol ELISA kit (catalog no. 582251; Cayman Chemical Co., Ann Arbor, MI) was used. For progesterone determination, the DHT ELISA kit form BioVendor (catalog no. RCAN-DHT-280R) was used. For FSH determination, the RAT FSH ELISA kit form BioVendor (catalog no. RSHAKRFS-010R) was used. For LH determination, the RAT LH ELISA kit form BioVendor (catalog no. RSHAKRLH-010SR) was used. For all hormones the standard protocols provided by the manufacturers were followed using undiluted, 1:50, or 1:150 dilutions.
2.6. Statistics
Student’s t test was used to test the significance of difference between two means. Analysis of variance was carried out when more than two means were compared and the significance of the difference between means was determined by Duncan’s multiple range tests. Differences were considered to be significant at P ⩽ 0.05. Analysis was done using Minitab version 15.
3. Results
3.1. Estradiol and Progesterone concentrations during the estrous cycle and pregnancy
During a rat’s estrous cycle and pregnancy period, levels of estradiol and progesterone fluctuated as shown in Fig. 1 (a and b). The concentrations of estradiol on day 7 of pregnancy (62 pg/ml) were similar to the levels measured at estrous (53 pg/ml). Then on day 14 of pregnancy a significant increase in the concentration of estradiol was observed (152 pg/ml). Further increased was noticed on day 21 (288 pg/ml), the level of estradiol surged significantly and progressively up to term. The estradiol peak during pregnancy was higher than its peak during estrous (Fig. 1-a).
Figure 1.
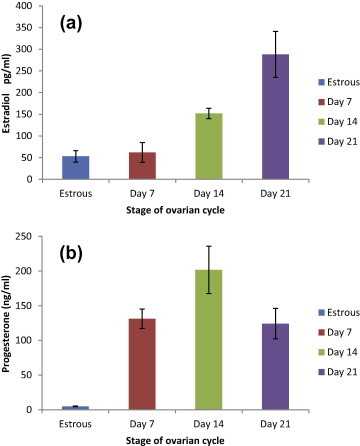
Histogram representing plasma concentrations of estradiol (a) and progesterone (b) at estrous cycle (n = 5), day 7 pregnancy (n = 5), day 14 pregnancy (n = 5) and day 21 pregnancy (n = 5). Different letters above the bars indicate statistically differences between groups among groups (P ⩽ 0.05). Values are means of concentrations ± MSE.
As measured by the radioimmunoassay technique, progesterone concentrations during all stages of pregnancy significantly exceeded estrous values. On day 7 of pregnancy, progesterone values increased sharply to 131 ng/ml, highest levels were recorded during the second week of pregnancy on day 14 (202 ng/ml) followed by a gradual decrease in the levels on day 21 (124 ng/ml) but values remain above estrous values (5 ng/ml). The concentrations of progesterone decrease gradually until parturition (Fig. 1-b).
3.2. FSH and LH concentrations during the estrous cycle and pregnancy
FSH and LH levels remained low throughout pregnancy compared to the estrous values (117 and 86 ng/ml) respectively. On day 7 of pregnancy, FSH concentration decreased significantly (78 ng/ml) onward, the levels remained steady low as recorded on day 14 (81 ng/ml). A slight increase in FSH concentration was recorded on day 21 (112 ng/ml) (Fig. 2-a).
Figure 2.
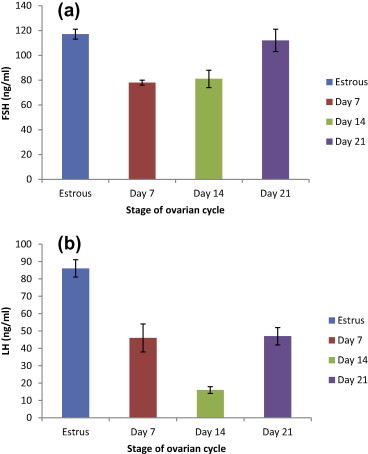
Histogram representing plasma concentrations of FSH (a) and LH (b) at estrous cycle (n = 5), day 7 pregnancy (n = 5), day 14 pregnancy (n = 5) and day 21 pregnancy (n = 5). Different letters above the bars indicate statistically differences between groups among groups (P ⩽ 0.05). Values are means of concentrations ± MSE.
During pregnancy, LH levels were significantly lower than the estrous levels (86 ng/ml). On day 7 of pregnancy, the value of LH hormone was 46 ng/ml followed with sharp decreasing on day 14 (16 ng/ml). An unexpected rise in LH value was reported on day 21 (47 ng/ml) which was continuous until parturition (Fig. 2-b).
3.3. Expression of Estrogen α receptor and progesterone receptor
The ovary of estrous rat showed low levels of ERα expression, 3.5% of OSE nuclei were stained with DAB brown color (Fig. 3). Generally the percentage of OSE cells staining positive for ERα was relatively low in the pregnant rats. ERα positive cells were absent on day 7 and 14 of pregnancy, only day 21 recorded a very low percentage of immunostaining (0.5%) within the nuclei of OSE cells (Fig. 4). On the contrary, immunostaining of PR was not observed within the nuclei of OSE cells in all groups of study, PR was absent in the rat OSE cells (Fig. 5).
Figure 3.

Histogram representing percentage of ER at estrous cycle (n = 5), day 7 pregnancy (n = 5), day 14 pregnancy (n = 5) and day 21 pregnancy (n = 5). Different letters above the bars indicate statistically differences between groups among groups (P ⩽ 0.05). Values are means of concentrations ± MSE.
Figure 4.
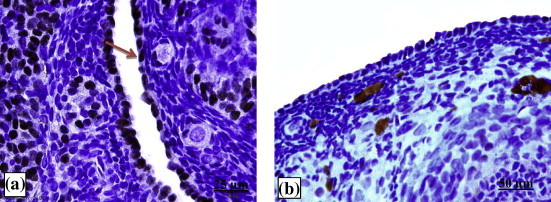
Photomicrographs showing ERα expression within OSE cells at estrous (a) and on day 14 of pregnancy. (b) Red arrow shows a positive staining (brown color).
Figure 5.

Photomicrographs showing PR expression within OSE cells at estrous (a) and on day 14 of pregnancy. (b) Red arrow shows a negative staining (blue color).
4. Discussion
Multiple pregnancies play a significant role in reducing chances of OC. Recent research shows that breastfeeding and pregnancy reduces the risk of getting OC. Pregnancy is one of the factors which suppress ovulation process. Ceaseless ovulation hypothesis insinuates that recurring monthly menstrual sequence of epithelium trauma and successive replacement of worn out tissues after ovulation increases the exposure of the OSE to various risk factors including genetic instabilities (Fathalla, 1971). On the other hand, theory states that OC is triggered by an increase of estrogen production and the proliferation of ovarian surface epithelium (Baby and Bartlewski, 2011).
The physiology of OSE cells is of interest since the majority of female OCs originates in these cells (Vanderhyden et al., 2003). Several studies have investigated the dynamic of follicular growth at pregnancy but none had focused on the role of pregnancy as a protective factor against OC. The current study was designed to determine hormonal fluctuation namely steroids and gonadotropins during pregnancy and to examine the expression of steroid receptors in OSE cells at different stages of pregnancy.
During pregnancy several factors, notably high steroidal hormones produced in feto-placental unit and CL, may retard follicle development, and suppress OSE proliferation. According to a recent “gonadotropin theory”, the expression of LH and FSH receptors on the surface of OSE cells is a direct response of gonadotropins (Choi et al., 2007). Most likely the hormone-stimulated receptors trigger the localized proliferation events. Besides, these receptors also initiate the oncogenic pathways. OSE cell differentiation was investigated in the presence of exogenously supplemented estrogens and progesterone (Ho, 2003; Laviolette et al., 2010). In the current study, determination of the levels of steroids and gonadotropins in the plasma of pregnant rats has been made. The pattern of hormone fluctuation during pregnancy reported here is consistent with previous studies (Kallen, 2004).
In this study, estradiol values were generally low except at the end of pregnancy. At the first week of pregnancy on day 7, the levels of estradiol rose then followed by a steady decline until the middle stage of pregnancy and then continued to rise gradually toward the end of term.
In this study, FSH and LH levels remained low throughout the pregnancy period except for some instances like on day 21. Low concentrations of plasma LH during pregnancy may contribute to the suppression of follicular maturation during this time. Gonadotropins like LH, FSH and human chorionic gonadotropin (hCG) are the other hormones regulating OSE proliferation and transformation into cancerous cells (Trabert et al., 2013; Yang et al., 2013). The levels of FSH and LH which are required for ovulation may be suppressed by the high concentration of progesterone. FSH is considered to be the most important hormone controlling folliculogenesis, especially at later stages of follicle growth (Chaffin and Vandevoort, 2013). According to theory, high level of FSH and LH is known to wear the epithelium, and hence responsible for OC. Several studies revealed that gonadotropins treatment may increase OSE proliferation in different animal models (Hilliard et al., 2013; Stewart et al., 2004). The results of this study suggest that pregnancy may protect from OC by decreasing the levels of gonadotropins throughout pregnancy and therefore reduce the exposure of OSE cells to FSH and LH. However, the role of gonadotropins on OSE cells is not fully characterized.
In conclusion, these results may suggest that progesterone effect during pregnancy seems to be overriding the positive effect of estrogens on OSE cells. High progesterone levels may have a direct negative effect on gonadotropin production and thereby it might inhibit events leading to both follicular development and OSE proliferation. Understanding the factors affecting OSE proliferation may help elucidating the mechanism(s) of assisted diseases such as ovarian cancer.
Acknowledgment
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. (504/247/1433). The authors, therefore, acknowledge with thanks DSR technical and financial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Akahira J., Suzuki T., Ito K., Kaneko C., Darnel A.D., Moriya T., Okamura K., Yaegashi N., Sasano H. Differential expression of progesterone receptor isoforms A and B in the normal ovary, and in benign, borderline, and malignant ovarian tumors. Jpn. J. Cancer Res. 2002;93:807–815. doi: 10.1111/j.1349-7006.2002.tb01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auersperg N. Ovarian surface epithelium as a source of ovarian cancers: unwarranted speculation or evidence-based hypothesis? Gynecol. Oncol. 2013;130:246–251. doi: 10.1016/j.ygyno.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Auersperg N., Wong A.S., Choi K.C., Kang S.K., Leung P.C. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr. Rev. 2001;22:255–288. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- Baby T.E., Bartlewski P.M. Circulating concentrations of ovarian steroids and follicle-stimulating hormone (FSH) in ewes with 3 or 4 waves of antral follicle emergence per estrous cycle. Reprod. Biol. 2011;11:19–36. doi: 10.1016/s1642-431x(12)60061-8. [DOI] [PubMed] [Google Scholar]
- Chaffin C.L., Vandevoort C.A. Follicle growth, ovulation, and luteal formation in primates and rodents: a comparative perspective. Exp. Biol. Med. (Maywood.) 2013;238:539–548. doi: 10.1177/1535370213489437. [DOI] [PubMed] [Google Scholar]
- Choi J.H., Wong A.S., Huang H.F., Leung P.C. Gonadotropins and ovarian cancer. Endocr. Rev. 2007;28:440–461. doi: 10.1210/er.2006-0036. [DOI] [PubMed] [Google Scholar]
- Clow O.L., Hurst P.R., Fleming J.S. Changes in the mouse ovarian surface epithelium with age and ovulation number. Mol. Cell Endocrinol. 2002;191:105–111. doi: 10.1016/s0303-7207(02)00061-8. [DOI] [PubMed] [Google Scholar]
- Fathalla M.F. Incessant ovulation–a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Fortune J.E. Ovarian follicular growth and development in mammals. Biol. Reprod. 1994;50:225–232. doi: 10.1095/biolreprod50.2.225. [DOI] [PubMed] [Google Scholar]
- Hamilton T.C., Davies P., Griffiths K. Androgen and oestrogen binding in cytosols of human ovarian tumours. J. Endocrinol. 1981;90:421–431. doi: 10.1677/joe.0.0900421. [DOI] [PubMed] [Google Scholar]
- Hilliard T.S., Modi D.A., Burdette J.E. Gonadotropins activate oncogenic pathways to enhance proliferation in normal mouse ovarian surface epithelium. Int. J. Mol. Sci. 2013;14:4762–4782. doi: 10.3390/ijms14034762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S.M. Estrogen, progesterone and epithelial ovarian cancer. Reprod. Biol. Endocrinol. 2003;1:73. doi: 10.1186/1477-7827-1-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen C.B. Steroid hormone synthesis in pregnancy. Obstet. Gynecol. Clin. North Am. 2004;31:795–816. doi: 10.1016/j.ogc.2004.08.009. x. [DOI] [PubMed] [Google Scholar]
- Laviolette L.A., Garson K., Macdonald E.A., Senterman M.K., Courville K., Crane C.A., Vanderhyden B.C. 17beta-estradiol accelerates tumor onset and decreases survival in a transgenic mouse model of ovarian cancer. Endocrinology. 2010;151:929–938. doi: 10.1210/en.2009-0602. [DOI] [PubMed] [Google Scholar]
- Leung P.C., Choi J.H. Endocrine signaling in ovarian surface epithelium and cancer. Hum. Reprod. Update. 2007;13:143–162. doi: 10.1093/humupd/dml002. [DOI] [PubMed] [Google Scholar]
- Li A.J., Baldwin R.L., Karlan B.Y. Estrogen and progesterone receptor subtype expression in normal and malignant ovarian epithelial cell cultures. Am. J. Obstet. Gynecol. 2003;189:22–27. doi: 10.1067/mob.2003.328. [DOI] [PubMed] [Google Scholar]
- Murdoch W.J., Van Kirk E.A. Steroid hormonal regulation of proliferative, p53 tumor suppressor, and apoptotic responses of sheep ovarian surface epithelial cells. Mol. Cell Endocrinol. 2002;186:61–67. doi: 10.1016/s0303-7207(01)00675-x. [DOI] [PubMed] [Google Scholar]
- Rizzuto I., Behrens R.F., Smith L.A. Risk of ovarian cancer in women treated with ovarian stimulating drugs for infertility. Cochrane Database Syst. Rev. 2013;8 doi: 10.1002/14651858.CD008215.pub2. CD008215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S.L., Querec T.D., Gruver B.N., O’Hare B., Babb J.S., Patriotis C. Gonadotropin and steroid hormones stimulate proliferation of the rat ovarian surface epithelium. J. Cell Physiol. 2004;198:119–124. doi: 10.1002/jcp.10401. [DOI] [PubMed] [Google Scholar]
- Tiedemann D. Oncology today: ovarian cancer. RN. 2000;63:36–41. [PubMed] [Google Scholar]
- Trabert B., Lamb E.J., Scoccia B., Moghissi K.S., Westhoff C.L., Niwa S., Brinton L.A. Ovulation-inducing drugs and ovarian cancer risk: results from an extended follow-up of a large US infertility cohort. Fertil. Steril. 2013;100:1660–1666. doi: 10.1016/j.fertnstert.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhyden B.C., Shaw T.J., Ethier J.F. Animal models of ovarian cancer. Reprod. Biol. Endocrinol. 2003;1:67. doi: 10.1186/1477-7827-1-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P.A., Bove K., You S., Chambers A., Hrushesky W.J. Cancer growth and spread are saltatory and phase-locked to the reproductive cycle through mediators of angiogenesis. Mol. Cancer Ther. 2005;4:10651075. doi: 10.1158/1535-7163.MCT-05-0028. [DOI] [PubMed] [Google Scholar]
- Wood P.A., Hrushesky W.J. Sex cycle modulates cancer growth. Breast Cancer Res. Treat. 2005;91:95–102. doi: 10.1007/s10549-005-8269-6. [DOI] [PubMed] [Google Scholar]
- Yang H.P., Anderson W.F., Rosenberg P.S., Trabert B., Gierach G.L., Wentzensen N., Cronin K.A., Sherman M.E. Ovarian cancer incidence trends in relation to changing patterns of menopausal hormone therapy use in the United States. J. Clin. Oncol. 2013;31:2146–2151. doi: 10.1200/JCO.2012.45.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Guo L., Zhu B., Feng Y., Yu S., An N., Wang X. Effects of 3,5,3′-Triiodothyronine (T3) and Follicle Stimulating Hormone on Apoptosis and Proliferation of Rat Ovarian Granulosa Cells. Chin. J. Physiol. 2013;56 doi: 10.4077/CJP.2013.BAB186. [DOI] [PubMed] [Google Scholar]



