Abstract
This study deals with the analysis of the integument of the red palm weevil Rhynchophorus ferrugineus of both sexes using the GC–MS technique. The results of the study revealed many promising compounds. These include aspidofractinine-3-methanol (kopsinyl alcohol) which was found in the acetone extract of the sternum of females, and 3-buten-2-ol (32-B) which was found in the extracts of sternum and tergum of males. This compound the aggregation pheromone was secreted by males. Additionally, compounds with methoxy groups were found. These may be responsible for insects’ resistance. This study, through separation and identification of these compounds, aims to open a new possibility for their future medical and therapeutic usage.
Keywords: GC–MS, Rhynchophorus ferrugineus, Aspidofractinine-3-methanol, Kopsinyl alcohol, 3-Buten-2-ol, Methoxy, Medical, Pheromone
1. Introduction
Insects, and substances extracted from them, have been used as medicinal resources by human cultures all over the world. Besides being sources of medicines, these organisms have also played mystical and magical roles in the treatment of several illnesses in a range of cultures. Chemical studies are needed to discover which biologically active compounds are actually present within insect bodies. The therapeutic potential of insects represents a significant contribution to the debate on biodiversity conservation, as well as opening perspectives for the economic and cultural valorization of animals traditionally regarded as useless. Their use needs to be at a sustainable level to avoid overexploitation. Sun et al. (2007, 2009) said that the polysaccharides extracted from insects have evident immunomodulatory activities. Many international researchers have studied anti-bacterial peptides isolated from insects of Diptera, Lepidoptera and Hymenoptera as they relate to gene expression and regulation, peptide characters, and biological activities (Kimbrell 1991; Laszlo 2000; Pullet and Stoklin 2005). The objectives of the present study are to: (1) Analyze components of the integument of insects using the technique GC–MS, (2) Present active compounds that may have significant medical, therapeutic benefits, or be indicative of sexual pheromone installation.
2. Methodology
2.1. Source of adult insects
Adult insects were obtained from the laboratory colony of insects Physiology at the Biology Department, Faculty of Science and Humanities, Salman bin Abdel Aziz University, Al-Kharj, KSA. Rearing of adult red palm weevil was according to Al-Rajhy et al. (2005).
2.2. Preparation of the insects for the study using Gas Chromatography–Mass Spectrometry (GC–MS)
2.2.1. Extraction of specimens
Adult insects were anesthetized and transferred to anatomy dishes, autopsied, and the tergum and sternum of abdominal segments of both sexes were separated (20 females and 20 males). The samples were then ground separately using three solvents acetone, chloroform, and diethyl-ether in a Pyrex homogenizer. Specimens were then centrifuged at 4000 rpm for 10 min. The supernatant was then obtained for injection into GC–MS.
2.2.1.1. Analysis of samples using GC–MS
Chemical analysis of extracts of adult male and female insects’ abdominal segments, extracted with different solvents (12 specimens) was performed using Gas Chromatography–Mass Spectrometry (GC–MS). GC–MS detection and identification was conducted through sample vaporization, where molecules were separated due to differences in molecular weight. Once the molecules were separated, they were ionized within the GC–MS. And the chemical compounds were identified, based upon the relative intensity of the ions. GC–MS ion trap configuration then allowed Mass Spec/Mass Spec (MS/MS) analysis of samples, which served to reduce false positives in high background areas. GC–MS parameters included: Shemadzo, 5050 GC–MS 2 supplied samples model 20S-Aoc, and automatic injector 20i-Aoc characteristic fragmentation split/splitless by column separation Rtx-30 m, 0.25 mm ID, detector ETP, Helium gas at 1.5 ml/min, temperature 50–310 °C for oven at high 10 m/min and 250 °C for injector, 280 °C for detector were injected with 1 μl of each extract separately.
3. Results
3.1. Gas chromatography analysis (GC–MS) for integument extract of adult red palm weevil
GC–MS analysis of the tergums and sternums of both male and female red palm weevil organic extracts revealed the presence of various compounds belonging to various chemical groups (Fig. 1). The type of compounds differed according to the insects’ sex and the solvent used. Table 1 shows the number of compounds belonging to various chemical groups detected in the tergums and Table 2 shows the number of compounds detected in the sternums of both sexes. From both tables, it can be concluded that
-
a)
The most prevailing chemical groups in both the sternum and tergum in both sexes are the hydrocarbons and the alcohols.
-
b)
Ketones are equally distributed in both sexes.
-
c)
The number of hydrocarbons and alcohols present in males was approximately two-fold that in the females.
Figure 1.
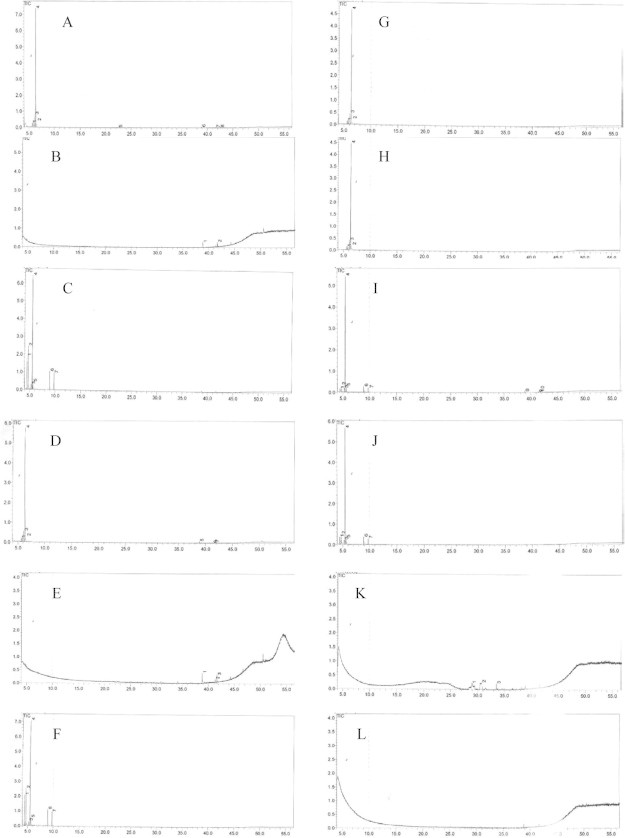
Total chromatograph (GC–MS) analysis for R. ferrugineus from integument extracts of individual females and males, (A–C) female sternum, (D–F) female tergum, (G–I) male sternum, (J–L) male tergum.
Table 1.
Chemical groups detected in the tergum of males and females of Rhynchophorus ferrugineus.
| Extraction solvent | Male |
Female |
||||
|---|---|---|---|---|---|---|
| Chemical group | Acetone | Chloroform | Di. Ether | Acetone | Chloroform | Di. Ether |
| (1) Hydrocarbons | 2 | 3 | – | 9 | 7 | 7 |
| (2) Carboxylic acid | 2 | 9 | – | – | 2 | 4 |
| (3) Alcohols | 6 | – | – | 3 | 2 | 15 |
| (4) KETONES | 2 | 1 | – | 3 | 4 | 2 |
| (5) Aldehydes | – | – | – | – | 1 | 3 |
| (6) Esters | 1 | 3 | – | – | – | 4 |
| (7) Amines | – | – | – | 1 | – | 1 |
| (8) Benzoic acid | – | 1 | – | – | – | – |
| (9) Others | – | – | – | 2 | – | – |
Table 2.
Chemical groups detected in the sternum of males and females of Rhynchophorus ferrugineus.
| Extraction solvent | Male |
Female |
||||
|---|---|---|---|---|---|---|
| Chemical group | Acetone | Chloroform | Di. Ether | Acetone | Chloroform | Di. Ether |
| (1) Hydrocarbons | 16 | 18 | 16 | 22 | 10 | 4 |
| (2) Carboxylic acid | 1 | 3 | – | 3 | – | – |
| (3) Alcohols | 1 | – | 18 | 1 | – | 12 |
| (4) Ketones | 3 | – | 1 | 6 | – | 2 |
| (5) Aldehydes | – | – | 1 | – | – | – |
| (6) Esters | – | 2 | 2 | – | – | – |
| (7) Amines | 1 | – | 2 | 1 | – | 1 |
4. Discussion
Of all compounds detected, two compounds seemed to be of interest. These are depicted in Table 3. The first is the kopsinyl alcohol which is also known as aspidofractinine-3-methanol (2alpha, 3beta, 5alpha). This was detected in the acetone extract of the female insect sternum. This compound is chemically related to the indole alkaloid kopsiflorine that was isolated from Kopsia dasyrachis and found to be an effective anticancer drug in those types of cancer that are resistant to vincristine (Kam et al., 1998). Thus, it will be of great interest to isolate the insects’ kopsinyl alcohol and test it against different types of cancers whether resistant or non-resistant types. The second compound is also an alcohol manley 3-buten-2-ol which is also synonymous with 2-methyl-buten-2-ol and 3-hydroxy-1-butene and 2-methyl-2-propenol or methyl vinyl carbinol. This compound was detected in the acetone extract of the male tergum and the ether extract of the male sternum. A similar compound has been described as the sexual male pheromone (the aggregation pheromone) in the male bark beetle Ips typographus (3-methyl-3-buten-1-ol) (Birgersson et al., 1988). This same compound has also been described by Zhang et al. (2000) as one of sex pheromones in the Chinese larch bark beetle Ips cembrae. Thus, it is possible that 3-buten-2-ol detected in this study is the male sex pheromone in the male red palm weevil. With regard to the location of the gland secreting the sex aggregating pheromone one can recall Spiegel et al.’s (2004) report who identified the male sex pheromone glands of Lutzomyia cruzi (sandfly) at the fourth abdominal tergite and the work of Hollander et al. (1981) who reported that the sex pheromone producing gland in the female gypsy moth Lymantria dispar is located on the dorsal and ventral aspects of the intersegmental membrane. In this study, the male sex aggregating pheromone secreting gland seemed to be in the sternum/tergum site. On a broader basis, the results of this study directly strengthen the beliefs that insects just like plants can/may provide useful medicinal compounds as has been previously suggested by Costa-Neto (2005).
Table 3.
Important chemicals compounds in R. ferrugineus which are separated with GC–MS.
| Compound Name | Structure formula | Other name | Gender |
|---|---|---|---|
| Aspidofractinine-3-methanol, (2alph, 3beta., 5alpha.) | 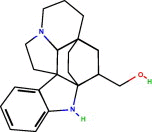 |
Kopsinyl alcohol | Sternum female acetone extract |
| 3-buten-2-ol | Methylvinylcarbinol
|
Sternum ether extract and tergum acetone extract male | |
2-methyl-2-propenol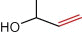
|
|||
3-Hydroxy-1-butene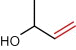
|
Acknowledgment
We extend our sincere thanks to the Deanship of Scientific Research, the University for its Support to this research which is part of the project no. 37/H/1432 H, which was supported by the Deanship.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Rajhy D.H., Hussin H.I., Al-Shawaf A.A. Insecticidal activity of carbaryl and its mixture with piperonylbutoxide against the red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) and their effects on acetylcholinesterase activity. J. Pak. Biol. Sci. 2005;8(5):679–682. [Google Scholar]
- Birgersson G., Fredrik S., Gunnar B., Jan L. Individual variation in aggregation pheromone content of the bark beetle, Ips typographus. J. Chem. Ecol. 1988;14(9):1737–1761. doi: 10.1007/BF01014641. [DOI] [PubMed] [Google Scholar]
- Costa-Neto E.M. Entomotherapy, or the medicinal use of insects. J. Ethnobiol. 2005;25(1):93–114. [Google Scholar]
- Hollander, A.L., Chih-Ming, Y., Charles, P.S., 1981. Location, morphology and histology of sex pheromone glands of the female gypsy moth, Lymantria dispar (L.).
- Kam T.S., Subramaniam G., Kooi-Mow S., Yoganathan K., Toyoshima T.K.M., Mun-Chual R., Masahiko H., Kanki K. Reversal of multidrug resistance (MDG) by Aspidofractinin-type indole alkaloids. Bioorg. Med. Chem. Lett. 1998;8:2769–2772. doi: 10.1016/s0960-894x(98)00486-7. [DOI] [PubMed] [Google Scholar]
- Kimbrell D.A. Insect antibacterial protein: not just for insects and against bacteria. Bioassays. 1991;13(12):657–663. doi: 10.1002/bies.950131207. [DOI] [PubMed] [Google Scholar]
- Laszlo O., Jr. Antibacterial peptides isolated from insects. J. Pept. Sci. 2000;6:497–551. doi: 10.1002/1099-1387(200010)6:10<497::AID-PSC277>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Pullet P., Stoklin R. Insect antibacterial peptides: structures, properties and gene regulation. Protein Pep. Lett. 2005;12:3–11. doi: 10.2174/0929866053406011. [DOI] [PubMed] [Google Scholar]
- Sun L., Feng Y., He Z., Ma T., Zhang X. Studies on alkaline solution extraction of polysaccharide from silkworm pupa and its immunomodulating activities. Forest Res. 2007;20(6):782–786. [Google Scholar]
- Sun L., Feng Y., He Z., Chen X.M. Study on extraction, analysis of water-soluble polysaccharide from cockroaches and its immunologic activities. Forest Res. 2009;22(2):256–261. [Google Scholar]
- Spiegel, C.N., Reginaldo, P.B., Maurilio, J.S., 2004. Ultrastructural cytochemistry of the sex pheromone glands of Lutzomyia cruzi male sand flies (Diptera: Psychodidae: Phlebotominae). [DOI] [PubMed]
- Zhang Q., Göran B., Fredrik S., Guo- Fa C. Pheromone components in the larch bark beetle, Ips cembrae from China: quantitative variation among attack phases and individuals. J. Chem. Ecol. 2000;26(4):841–858. [Google Scholar]



