Abstract
Black cumin seed oil (BCSO) was tested for its inhibitory effect against some pathogenic bacteria (Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 8739, Listeria monocytogenes Scott A and Salmonella enteritidis PT4) in Domiati cheese during cold storage. Physical, chemical and sensorial changes in cheese during storage were recorded. Pasteurized milk was inoculated before renneting with a mixed culture of bacteria at ca. 4 log CFU mL−1. In vitro and in situ supplementation with BCSO showed antimicrobial impact on the growth of S. aureus, E. coli, L. monocytogenes and S. enteritidis inoculated into media and cheese samples. Supplementing of cheese with BCSO (0.1% or 0.2%, w/w) significantly reduced the counts of the inoculated pathogens by ca. 1.3 log and 1.5 log CFU g−1 after 21 days of storage. In addition, BCSO controlled the development of titratable acidity, limited the changes in ripening indices, flavor components and kept considerable physicochemical and sensorial properties of cheese.
Keywords: Soft cheese, Black cumin oil, Food borne pathogens, Antibacterial
1. Introduction
Soft cheese is one of the most appreciated cheeses in Middle Eastern countries. The cheese is a pickled cheese (salt 2–15%), although it may be sold fresh. This type of cheese is produced either by enzymatic or acidic coagulation of fresh milk (buffalos’ or cows’ milk) or reconstituted skim milk powder with oils (Abou-Donia, 1986). It also has been made with or without the addition of starter cultures to cheese milk. Starter cultures govern the flavor and texture of the cheese, and help to suppress the growth of spoilage bacteria.
Spices are being sought for their medicinal value as antioxidants and as antimicrobials (Frankel et al., 1996; El-Ghorab et al., 2010). Black cumin seeds have a strong and hot peppery taste and have been used in coffee, tea, salads and breads. Seeds are also used as a natural remedy for asthma, hypertension, diabetes, inflammation, cough, bronchitis and influenza (Ramadan, 2007). Studies were conducted on biological properties of the essential oils and thymoquinone of black cumin on antioxidant activity (Burits and Bucar, 2000; Luther et al., 2007; Lutterodt et al., 2010) and as antimicrobial (Hanafy and Hatern, 1991). Black cumin oil is rich in linoleic and oleic acids as well as sterols and tocols (Ramadan et al., 2003; Ramadan and Moersel, 2002; Ramadan, 2007).
Cold-pressing technology for oil production involves no heat treatment or chemical treatments (solvent extraction). The consumption of new and improved products such as cold-pressed oils may improve human health and may prevent certain diseases (Lutterodt et al., 2010). Common spices and flavors added to cheeses include red and green peppers, black peppercorns, horseradish, thyme, cloves, cumin, caraway, parsley, tarragon, nutmeg, basil, onion/garlic, and sundried tomatoes. Other flavors include liquid and natural smoke, soot/ashes, and nuts like almonds and walnuts. Levels of the additives are typically less than 1% of the curd (Hayaloglu and Farkye, 2011).
Foodborne pathogens are considered as major concerns of food safety (Buzby and Roberts, 1996). The infection risk is high because the infective dose of Escherichia coli O157:H7 is low such as 10–20 CFU/g (Bolton et al., 1996). Plant extracts rich in bioactive compounds may serve as natural antimicrobial agents (Luther et al., 2007). Listeria monocytogenes, Salmonella enteritidis and E. coli have the ability to survive and grow up to 16 days in raw and pasteurized milk kept at 4 °C (Mahgoub et al., 2011, 2013). Hence, the aims of this contribution were to: (1) delineate the potential of BCSO as antibacterial and antioxidant agent during manufacture and storage of cheese prepared with starter cultures containing contaminating bacteria as well as artificially inoculated bacterial pathogens (Staphylococcus aureus ATCC 6538, E. coli ATCC 8739, L. monocytogenes Scott A and S. enteritidis PT4), and (2) evaluate the physicochemical and sensory attributes of cheese supplemented with BCSO. The obtained results will be utilized to develop novel cheese rich in bioactive molecules with a desirable shelf life.
2. Material and methods
2.1. Materials
BCSO was obtained from local market in Zagazig (Egypt). Standards used for sterol characterization were purchased from Supelco (Bellefonte, PA, USA). Standards used for tocopherols (α-, β-, γ- and δ-tocopherol) were purchased from Merck (Darmstadt, Germany). Bacterial strains of S. aureus ATCC 6538 and E. coli ATCC 8739 were from Egyptian Culture Collection (MERCIN, Ain Shams University, Cairo, Egypt). Salmonella enterica subsp. enterica seriovar Enteritidis PT4 and L. monocytogenes ScottA were kindly obtained from Prof. George-John Nychas (Agriculture University of Athens, Greece). Starter cultures including Lactobacillus bulgaricus, Streptococcus thermophilus and Lactobacillus lactis susbp. lactis, were obtained from Chr-Hansen’s Laboratories (Copenhagen, Denmark). Raw bovine milk was collected from a private farm located in Sharkiah governorate (Egypt) and handled within 1 h after milking. Powder animal rennet was obtained from Chr-Hansen’s Laboratories, (Copenhagen, Denmark). Rennet was diluted with distilled water to a standard rennet solution before use.
2.2. Methods
2.2.1. Analysis of BCSO
Chromatographic analysis of BCSO including gas chromatography analysis of fatty acid methyl esters (FAME) and sterols as well as normal phase high performance liquid chromatography (NP-HPLC) separation, identification and quantification of tocopherols was carried out according to Ramadan et al. (2010).
2.2.2. Phenolic compounds in BCSO
Aliquots of BCSO were dissolved in n-hexane (5 mL) and mixed with 10 mL methanol–water (80:20, v/v) in a glass tube for two min in a vortex. After centrifugation at 3000 rpm for 10 min, the hydroalcoholic extracts were separated from the lipid phase using a Pasteur pipet then combined and concentrated in vacuo at 30 °C until a syrup consistency was reached. The lipidic residue was re-dissolved in 10 mL methanol–water (80:20, v/v) and the extraction was repeated twice. Hydroalcoholic extracts were re-dissolved in acetonitrile (15 mL) and the mixture was washed three times with n-hexane (15 mL each). Purified phenols in acetonitrile were concentrated in vacuo at 30 °C then dissolved in methanol for further analysis. Aliquots of phenolic extracts were evaporated to dryness under nitrogen. The residue was re-dissolved in 0.2 mL water and diluted (1:30) Folin–Ciocalteu’s phenol reagent (1 mL) was added. After 3 min, 7.5% sodium carbonate (0.8 mL) was added. After 30 min, the absorbance was measured at 765 nm using a UV-260 visible recording spectrophotometer (Shimadzu, Kyoto, Japan). Gallic acid was used for the calibration and the results of triplicate analyses are expressed as parts per million of gallic acid.
2.2.3. Minimal inhibitory concentration (MIC) test
The agar disk diffusion method was conducted for the determination of antimicrobial activities of BCSO against L. monocytogenes, S. enteritidis, S. aureus and E. coli. Briefly, 0.1 mL from 8 log CFU/mL bacterial suspension was spread onto Mueller Hinton Agar (MHA) plates. Filter paper disks (6 mm in diameter) impregnated with 10 μL of the undiluted oil were placed on the inoculated plates. The plates, after remaining at room temperature in laminar flow for 2 h, were incubated at 37 °C for 24 h. The diameters of the inhibition zones were measured in millimeters. All tests were performed in triplicate.
2.2.4. Preparation of inoculum
L. monocytogenes, S. enteritidis, S. aureus and E. coli strains were maintained frozen in broth until use. Prior to use, the cultures were activated by three successive transfers in tryptic soy broth (Difco Laboratories) at 37 °C for 24 h. Cells were harvested by centrifugation (10000g for 10 min at 4 °C), washed three times and re-suspended in Ringer’s solution (Lab M, Bury, UK). The resulting pellet was washed once with Ringer’s solution (LAB, Merck) to remove residual organic material, re-centrifuged, and then re-suspended in Ringer’s to a final volume of 10 mL. A final inoculum was prepared by serially diluting in Ringer’s solution to reach a final level of 5 log CFU/mL. Aliquot of 0.1 mL of each pathogen was inoculated into pasteurized milk before manufacturing cheese in the second experiment, so the final count of each becomes ca. 4 Log CFU/g in cheese samples.
2.2.5. Manufacture of Domiati soft cheese
Fresh milk was separated into skim milk and cream, whereas cream was used to standardize the percentage of milk fat. Milk containing 4% fat was used in the preparation of Domiati cheese containing L. Bulgaricus (LB), Lactobacillus lactis susbp. Lactis (LL) and S. thermophilus (ST) as well as rennet at level of 1%. The first experiment was divided into three batches and served as control experiment. The first batch was divided into control; cheese supplemented with 0.1% (w/w) BCSO and cheese supplemented with 0.2% (w/w) BCSO. All milk treatments were pasteurized at 80 °C for 30 min, cooled and adjusted to 40 °C, then calcium chloride and sodium chloride were added at levels of 0.02% and 4% (w/w), respectively, then inoculated with 2% of mixed (1:1) LB, LL and ST starter culture and rennet before renneting in the control experiments. The BCSO was added to supplemented Domiati cheese at concentrations of 0.1% and 0.2% before renneting. Different cheeses were made from milk treatments by the conventional method of making Domiati cheese (Fahmi and Sharara, 1950). Cheese samples were packed in plastic containers with formerly boiled whey and stored in refrigerator at 4 °C for 42 days. At intervals of (0, 15, 30 and 42 days of storage), samples were analyzed for total bacterial count (TBC), Enterobacteriaceae count (EC) and lactic acid bacteria (LAB) count as well as physicochemical and sensory properties.
The second experiment was divided also into three batches. The first batch was artificially inoculated with mixture culture from L. monocytogenes, S. enteritidis, S. aureus and E. coli manufactured with starter cultures (mixture of LB, LL and ST) without oil and served as the control. The second batch was artificially inoculated with mixture culture from L. monocytogenes, S. enteritidis, S. aureus and E. coli manufactured with starter cultures (mixture of LB, LL and ST) and supplemented with 0.1% BCSO. The third batch was also artificially inoculated with mixture culture from L. monocytogenes, S. enteritidis, S. aureus and E. coli manufactured with starter cultures (mixture of LB, LL and ST) and supplemented with 0.2% BCSO. All milk treatments before adding traditional starter culture or inoculation with the pathogens were pasteurized at 80 °C for 30 min, cooled, adjusted to 40 °C, calcium chloride and sodium chloride were added at the levels of 0.02% and 4% (w/w), respectively, and inoculated with 2% of mixed (1:1) starter culture and rennet as well as inoculation with the bacterial pathogens before renneting. Cheese samples were packed in plastic containers with formerly boiled whey and stored in refrigerator for 42 days. At intervals period (0, 7, 15, 21, 28, 35 and 42 days of storage), samples were analyzed for L. monocytogenes, S. enteritidis, S. aureus and E. coli count during storage. The whole experiment was run in triplicate.
2.2.6. Microbiological analysis
Three samples were microbiologically examined for each treatment after 0, 7, 15, 21, 28, 35 and 42 days of repining. Twenty-five grams of cheese samples were added aseptically to 225 mL of sterile peptone saline diluents (1.0 g peptone, 8.5 g sodium chloride in 1 L distilled water) and homogenized in a stomacher. Total bacterial count (TBC) was determined on plate count agar (PCA, Merck, Germany) after incubation at 30 °C for 48 h. For LAB enumerations, 1 mL sample was inoculated into 10 mL of molten de Man Rogosa Sharpe agar (MRS, Biolife 401728). After solidifying 10 mL overlay of the same molten medium was added. The incubation was carried out at 37 °C for 72 h (Dave and Shah, 1996). Total coliform was counted on MacConky Agar (DM141D, UK) with a double layer of the same medium incubated at 37 °C. S. enteritidis strain PT4 was counted on Xylose Lysine Deoxycholate agar (XLD) agar (Merck, 1.05287) after incubation for 24 h at 37 °C. S. aureus was counted on Baired Parker agar (Biolife, 401116) supplemented with egg yolk incubated at 37 °C for 48 h. S. aureus colonies were further tested for positive coagulase reaction (Bactident Coagulase Biolife). E. coli strain was counted on Tryptone Bile X-Glucuronide agar (TBX agar, LAB HAL003) incubated at 37 °C for 24 h. L. monocytogenes was enumerated on polymyxinPolymyxin-aAcriflavin-Llithium Chloride-Ceftazidime-Aesculin-Mannitol agar (PALCAM, Biolife 401604) after incubation for 48 h at 35 °C. The lowest detection limit was 1 or 2 log CFU g−1. All plates were examined for typical colony types and morphological characteristics associated to each culture medium. Presumptive colonies of the above bacteria were verified by confirmation tests. For estimated counts below the limit of detection, the most-probable-number (MPN) technique was used. Serial dilutions of three Frather or buffered peptone water tubes of three successive dilutions were incubated at 37 °C for 48 h. Buffered peptone water was used as a nonselective medium for recovering sub-lethally injured bacteria and for minimizing the underestimation of counts. All plates were examined for typical colony types and morphological characteristics associated to each culture medium.
2.2.7. Physicochemical analysis
Cheeses were analyzed in duplicate for moisture, fat, salt and titratable acidity using the methods of AOAC (2002). Total nitrogen (TN) content was determined by the Kjeldahl method (Butikofer and Fuchs, 1997). Total volatile fatty acids (TVFA) were estimated according to Kosikowski (1978).
2.2.8. Sensory evaluation
Low fat cheese samples were sensorial evaluated during storage for 0, 2, and 4 weeks according to Papas et al. (1996). The evaluation was carried out by the score panel of the staff members of Food Science Department, Faculty of Agriculture, Zagazig University (Egypt).
2.3. Statistical analysis
Data were statistically analyzed using ANOVA variance analysis through the general linear model (GLM) procedure of the statistical analysis system software (SAS version 9.1, SAS Institute, Inc., 2003). Least significant differences were used to separate means at p < 0.05. The model included treatment, storage time, and their interaction as fixed effects. Differences between effects were assessed by the Duncan test (P < 0.05).
3. Results and discussion
3.1. Bioactive lipids and fatty acids of cold-pressed BCSO
In BCSO the levels of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) were 17.1%, 24.1% and 58.8%, respectively (Table 1). The major fatty acid (FA) in BCSO was linoleic acid at a level of 55.3% of total FA followed by oleic acid at a concentration of 24.1% and palmitic acid at 12.5%, respectively. BCSO contained about 17.1 g of SFA per 100 g of total FA, which is lower than that of 30.8% of total FA in the cold-pressed cardamom oil and comparable to that of 13.8% and 15.9% of total FA found in the cold-pressed milk thistle and roasted pumpkin oils, respectively (Parry et al., 2006). The SFA levels were higher than those of 7.4–9.7% of total FA in the cold-pressed parsley, onion, hemp, mullein and cranberry seed oils (Parker et al., 2003). BCSO contained a significant level of MUFA (24.1/100 g total fatty acids) which is comparable to the cold pressed hemp, cranberry, blueberry, onion and milk thistle seed oils but was much lower than that of 81% and 82% in the cold-pressed carrot and parsley seed oils (Parker et al., 2003). The PUFA of BCSO content was comparable to that in the cold-pressed cranberry (67.6%), onion (64–65%), milk thistle (61%) and blueberry (69%) seed oils, but lower than that in the cold-pressed red raspberry, marionberry, hemp, boysenberry and mullein seed oils with a PUFA content of 73–86% of total FA (Parker et al., 2003; Parry et al., 2006).
Table 1.
Levels of fatty acids (as a percentage of total fatty acids), tocopherols (mg/kg oil) and phytosterols (mg/kg oil) in cold-pressed BCSO.
| SFA (%) | 17.17 |
| MUFA (%) | 24.1 |
| PUFA (%) | 58.73 |
| α-Tocopherol (mg/kg oil) | 0.26 |
| β-Tocopherol (mg/kg oil) | 0.03 |
| γ-Tocopherol (mg/kg oil) | 0.24 |
| δ-Tocopherol (mg/kg oil) | 5 |
| Campesterol (mg/kg oil) | 0.23 |
| Stigmasterol (mg/kg oil) | 0.32 |
| Lanosterol (mg/kg oil) | 0.12 |
| β-Sitosterol (mg/kg oil) | 1.19 |
| Δ5-Avenasterol (mg/kg oil) | 1.06 |
| Δ7-Avenasterol (mg/kg oil) | 0.84 |
| Campesterol (mg/kg oil) | 0.23 |
| Stigmasterol (mg/kg oil) | 0.32 |
| Lanosterol (mg/kg oil) | 0.12 |
BCSO is characterized by high levels of unsaponifiables (17.9 g/kg oil). Levels of tocopherols in BCSO are shown in Table 1. α-Tocopherol constituted ca. 45% of the total tocopherols in BCSO. γ- and δ-Tocopherols were measured in low levels. γ- and γ-Tocopherols proved to be the major tocopherols in vegetable oils. α-Tocopherol occurred in highest concentrations in camelina, linseed, cold-pressed rapeseed and corn oil (Schwartz et al., 2008). α-Tocopherol is the most active antioxidant of tocopherol isomers, while β-tocopherol has 25–50% of the antioxidative activity of α-tocopherol, and the γ-isomer 10–35% (Kallio et al., 2002).
Six phytosterol compounds were detected in BCSO, wherein the sterol marker was β-sitosterol which comprised ca. 31.6% of the total sterols (Table 1). The next major components were Δ5- and Δ7-avenasterol and these three major components accounted for more than 90% of total sterols. Other components, e.g., lanosterol and campesterol were found in lower levels. Among the different sterols, sitosterol has been most intensively investigated with respect to its physiological effects in man.
BCSO oil was characterized by higher levels of phenolics (3.5 g/kg). Thus, BCSO may be used in different food products to provide nutrition and health benefits. Phenolic compounds have been reported to be present in vegetable oils, which is very important for the oxidative stability of the PUFA. Additionally, oils rich in antioxidants may play a role in reducing the risk of chronic diseases. Polyphenols, are considered as powerful active compounds expressing strong antioxidant activities. This activity is mainly due to their redox potential, which can play an important role in adsorbing and neutralizing free radicals, quenching reactive oxygen species, and chelating metals (Bettaieb et al., 2010). Lutterodt et al. (2010) evaluated the total phenolic contents (TPC) of BCSO and found that their TPC values varied from 1.02 to 1.40 mg gallic acid equivalents (GAE) per gram of oil. In another study the level of TPC in the BCSO was 3.53 mg GAE/g oil, which is greater than that detected in Akron wheat bran (2.29–3.24 mg GAE/g bran) (Parry et al., 2006). The TPC values of BCSO were comparable to that of the cold-pressed roasted pumpkin and marionberry seed oils, but was higher than that of 1.73–2.0 mg GAE/g oil for the cold-pressed red raspberry, blueberry and boysenberry seed oils, and that of 1.8–3.4 mg GAE/g oil for the cold-pressed parsley, onion, cardamom, mullein and milk thistle seed oils (Parry et al., 2006).
3.2. Antibacterial activity of BCSO
BCSO showed the best antibacterial activity against S. aureus with an inhibition zone of 12.4 mm closely followed by E. coli and L. monocytogens and S. enteritidis with inhibition zones of 10.5, 10.1 and 9.22 mm, respectively. The minimum inhibitory concentration (MIC) against the studied bacteria was about 100–200 μg/mL. In a preliminary experiment, the concentrations 0.1% and 0.2 % (w/w) of BCSO gave a maximum inhibitory effect on bacterial pathogens while an increase in concentration did not produce any significant changes (p < 0.05) and gave unacceptable sensory attributions in cheese. Therefore, these concentrations (0.1% and 0.2%) were selected in the subsequent experiments. In another preliminary experiment, the traditional starter culture was less effective against pathogens than cheese made from rennet only supplemented with 0.1% or 0.2% oil. These concentrations did not produce any significant changes (p < 0.05) in the total number of traditional starter cultures.
3.3. Total bacteria and pathogenic bacteria in Domiati cheese supplemented with BCSO
The results presented in Fig. 1 show the changes in the levels of total bacterial count (TBC) and lactic acid bacteria (LAB) in cheese supplemented with BCSO at 0.1% or 0.2% (w/w) and preserved for 42 days. It can be observed that TBC increased in all samples parallel with LAB. These parallel increases in the TBC and LAB are due to the fact that LAB population is the major constituent of total bacteria which in turn is the major of inoculated starter culture (El-Soda, 1993). Adding the BCSO (0.1% or 0.2% w/w) to cheese did not produce any significant changes (p < 0.05) on the total bacteria and lactic acid bacteria during cold storage. There was no difference between the actions of the two concentrations of BCSO. Hence, the antibacterial action of BCSO against these two groups of bacteria showed the least effect because the inoculated inoculums from LAB were very high (about 6 Log CFU g−1) after preparing cheese. This consistency in the bacterial count is apparently due to the antimicrobial action of bioactive compounds in BSCO which mainly contain phenolic compounds causing bacteriostatic and antiproliferative actions against bacterial growth. BCSO was characterized by high level of phenolics which are considered as powerful active compounds expressing strong antimicrobial activities. Bacteriostatic and antiproliferative actions of natural extracts are closely linked with their phenolic content (Ahn et al., 2004; Luther et al., 2007). This activity is mainly due to their redox potential, which can play an important role in adsorbing and neutralizing free radicals and chelating metals, especially iron and copper cations (Bettaieb et al., 2010).
Figure 1.
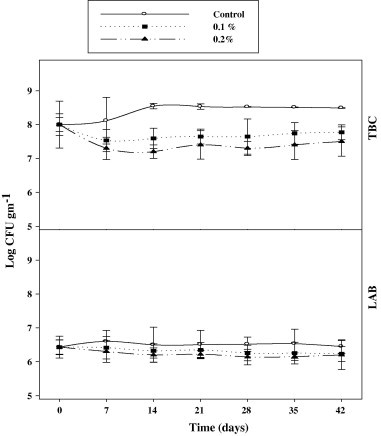
Total bacterial count (TBC) and lactic acid bacteria (LAB) count in soft cheese (control) and cheeses supplemented with 0.1% or 0.2% (w/w) BSCO during preservation at 4 °C for 42 day.
The data in Fig. 2 show the changes in the level of S. aureus, E. coli, L. monocytogenes Scott A and S. enteritidis in cheese supplemented with BCSO during 42 days of storage. Pasteurized milk before renneting with starter culture was inoculated with a mixed culture of the bacteria at ca. 3.95 log CFU ml−1. In non-treated cheese, the four microorganisms grew or survived with time to reach maximum level after 14 days, with a higher rate of growth in case of L. monocytogenes which reached a maximum of 4.65 log CFU g−1 against 4.30 log in case of S. aureus after 7 days of storage, referring to higher viability in the first case being psychrotrophic bacteria. Supplementing cheese with BCSO considerably reduced the counts of the four pathogens. S. aureus, E. coli and S. enteritidis were reduced to the level of ca. 2.6 log after 21 days of storage, i.e. with corresponding magnitudes of reduction equivalent to ca. 1.30 to 1.50 log. The level of S. enteritidis was nearly at the level of the starting inoculating level until the end of storage in the control, referring to a bacteriostatic effect of salt in cheese. Supplementation with different concentrations of oil (0.1% or 0.2%) did not exert a significant reducing effect against the proliferation of L. monocytogenes and S. aureus while supplementation with these concentrations exerted a significant reducing effect against the proliferation of S. enteritidis and E. coli. Thus, the noticed antimicrobial action of the BCSO might be exclusively due to the bioactive compounds in the oil. The antimicrobial action of BCSO is originating from the phenolic compounds in BCSO which are considered as powerful active compounds expressing strong antimicrobial activities. From the results of the activities against pathogenic bacteria, it can be stated that BCSO has general antibacterial activity against pathogenic bacteria. This endowed potentiality can participate in keeping a good hygienic quality of cheese during storage under cold conditions.
Figure 2.
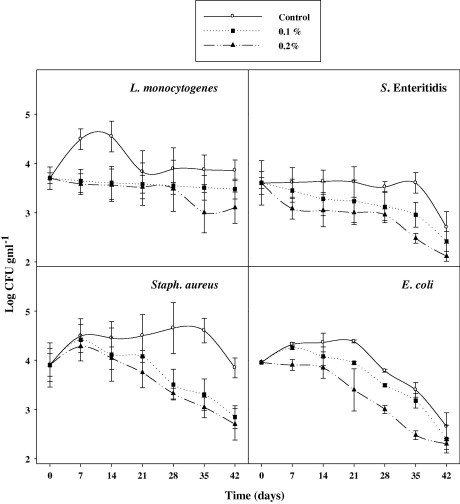
Changes in the counts of pathogens (L. monocytogenes, S. enteritidis, S. aureus and E. coli) inoculated into soft cheese (control) and cheeses supplemented with 0.1% or 0.2% (w/w) BSCO during preservation at 4 °C for 42 day.
3.4. Physico-chemical properties of cheese supplemented with BCSO
The effect of supplementation with BCSO on the properties of cheese is presented in Tables 2–4. The moisture contents of cheeses supplemented with BCSO were slightly higher than control cheese. Also, significant (p < 0.05) differences in moisture contents were found between all cheese treatments. Generally, the results reveal that there was a gradual loss in the moisture content of all cheeses during storage. This might be due to the shrinkage of the curd as a result of acid development which helps to expel the whey from the cheese mass. Data in Table 2 describe the effect of adding different concentrations of BCSO to milk on the chemical composition of cheese. The titratable acidity, total solids, and fat contents were nearly same as for control. Fat values of BCSO-supplemented cheese (0.1% and 0.2%, w/w) after 30 days of storage were 21.62% and 22.2%, respectively. On the other hand, with the progressive of maturation period, the titratable acidity of cheese samples was increased while pH values were decreased. This might be due to the continuous fermentation of lactose to lactic acid as well as the gradual increase of degradation products in the resultant cheese. Also, total solids and fat values had the same trend like acidity. Fat contents of BCSO-supplemented cheese significantly (p < 0.05) increased as ripening period reached maximum values at the end of ripening period. This probably attributed to the decrease in solids-non fat content as a result of protein degradation and its partial loss in whey during ripening.
Table 2.
Physico-chemical properties of Domiati soft cheese supplemented with different concentrations of cold pressed BCSO.
| Treatment | Moisture (%) |
Fat (%) |
Total Protein (%) |
Acidity (%) |
Yield (%) |
Curd tension (mg/100 mg) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | 15 days | 30 days | Fresh | 15 days | 30 days | Fresh | 15 days | 30 days | Fresh | 15 days | 30 days | Fresh | 15 days | 30 days | Fresh | 15 days | 30 days | |
| Control | 61.13 | 58.43 | 55.29 | 17.55 | 19.85 | 21.90 | 15.63 | 15.00 | 14.04 | 0.54 | 0.58 | 0.72 | 30.13 | 27.31 | 25.65 | 32.60 | 34.4 | 35.5 |
| 0.1% Oil | 61.31 | 58.48 | 55.41 | 17.62 | 20.00 | 21.62 | 15.50 | 14.93 | 13.91 | 0.52 | 0.56 | 0.68 | 27.41 | 24.51 | 23.32 | 32.50 | 33.1 | 33.9 |
| 0.2% Oil | 61.42 | 58.61 | 55.50 | 17.70 | 20.50 | 22.20 | 15.38 | 14.80 | 13.78 | 0.50 | 0.53 | 0.66 | 27.41 | 24.43 | 22.93 | 32.10 | 32.4 | 33.3 |
| LSD | 0.7421 | 0.6939 | 0.8796 | 0.09102 | 0.0987 | 0.0292 | 0.0436 | 0.0014 | 0.0514 | 0.1979 | 0.0216 | 0.0867 | 0.1022 | 0.712 | 0.8535 | 0.0484 | <.0001 | <.0001 |
Table 3.
Ripening indices and flavor components (mean n = 3) of Domiati soft cheese supplemented with different concentrations of BCSO.
| Treatment | TN%a |
SN/TN%b |
TVFAc (0.1 N NaOH/100 mg) |
Di-acetyl (μg/100 mg) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | 15 days | 30 days | Fresh | 15 days | 30 days | Fresh | 15 days | 30 days | Fresh | 15 days | 30 days | |
| Control | 2.40 | 2.3 | 2.15 | 9.7 | 19.47 | 28.5 | 8.6 | 16.5 | 19.7 | 46.5 | 56.0 | 64.0 |
| 0.1% Oil | 2.45 | 2.36 | 2.21 | 12.8 | 23.2 | 31.1 | 8.4 | 17.3 | 20.9 | 42.0 | 46.0 | 54.0 |
| 0.2% Oil | 2.42 | 2.34 | 2.17 | 10.2 | 19.9 | 27.9 | 8.5 | 17.9 | 22.8 | 42.0 | 47.0 | 56.0 |
| LSD | 0.932 | 0.973 | 0.958 | 0.0054 | 0.00002 | 0.0026 | 0.0335 | 0.0079 | 0.0004 | 0.0022 | 0.0195 | 0.0075 |
TN = total nitrogen.
SN/TN = soluble nitrogen/total nitrogen.
TVFA = Total volatile fatty acids.
Table 4.
Sensory characteristics (mean n = 3) of Domiati soft cheese supplemented with different concentrations of cold pressed BCSO.
| Treatments | Flavor (50) |
Body & texture (40) |
Appearance (10) |
Total (100) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | 15 days | 30 days | Fresh | 15 days | 30 days | Fresh | 15 days | 30 days | Fresh | 15 days | 30 days | |
| Control | 44 | 46 | 47 | 36 | 35 | 34 | 9.0 | 8.0 | 7.6 | 89 | 89.5 | 88.6 |
| 0.1% Oil | 44 | 45 | 46 | 36 | 35 | 34.2 | 8.4 | 7.9 | 9 | 88.4 | 87.9 | 89.2 |
| 0.2% Oil | 43 | 47 | 48 | 34 | 34 | 33.5 | 7.2 | 6.8 | 6.4 | 84.2 | 87.7 | 87.9 |
| LSD | 0.0004 | 0.0004 | 0.0006 | 0.1882 | 0.2181 | 0.3607 | 0.0287 | 0.0015 | 0.0618 | 0.4696 | 0.0454 | 0.1312 |
Each value is the mean of three replicates.
The change of cheese yield over lactation followed the same trends of fat, protein and total solids in milk. Contents of milk fat, protein and total solids were highly positively correlated with cheese yield. The average yield of fresh cheese from milk was 15–16/100 kg. The obtained results showed that, fortification of low fat milk with BCSO significantly (P < 0.05) increased cheese yield which was parallel to the concentration added oil (Table 2). The yield of all cheeses significantly (P < 0.05) decreased during storage period which might be due to the loss of moisture. Similar results were reported for traditional Domiati cheese by Badawi and Kebary (1998).
Curd tension of cheese curd was determined to test the hardness of soft cheese curd fortified with BCSO. The results in Table 2 show that addition of BCSO to cheese significantly (P < 0.05) decreased the hardness of cheese curd (curd tension). However, increasing the fortification ratio to 0.2% lowered the hardness of cheese curd. Reduction in hardness was proportional to the concentration of BSCO. The reduction of cheese curd hardness as a result of adding BSCO might be due to their interference with interaction and fusion of casein micelles. Hardness of all cheese curds significantly (P < 0.05) decreased as storage period proceeded (Badawi and Kebary, 1998).
3.5. Ripening indices of Domiati cheese supplemented with BCSO
Table 3 represents the changes in nitrogen fractions and TVFA contents of soft cheese as a result of supplementation with BSCO. Control cheese and various samples possessed approximately the same TN and TVFA contents. A slight increase in SN values was noticed for treatments (0.1% and 0.2% oil) compared with other treatments. Values of SN/TN were 31 and 28 for samples supplemented with 0.1% and 0.2% BCSO at the end of ripening period, respectively. On the other hand, TN, SN/TN and TVFA contents in all cheese samples increased with the progress of ripening period. Protein content was decreased during pickling as a result of protein degradation leading to the formation of water soluble compound, wherein some of which was lost in the pickling solution leading to an increase in nitrogen content in whey (Talib et al., 2009). The same results clearly indicated that TVFA content in all cheese treatments gradually increased during storage period. It could be attributed to proteolytic and lipolytic action of starter cultures during manufacture and storage.
The results in Table 3 showed a slight increase in SN of cheese treatments in comparison with control at the end of the storage period (15 days). Besides, a gradual increase was noticed in the SN in all cheeses up till the end the storage period. Statistical analysis proved that variation in different cheese treatments and the storage period was not significantly affecting (p > 0.05) the SN levels.
Some flavor compounds of cheese treatments were assessed by the determination of some volatile compounds e.g. acetaldehyde, di-acetyl and TVFA which have been reported as flavor compounds in cheese. It is evident from Table 3 that, increasing the fat content in cheese treatments significantly increased these flavor compounds especially at higher BCSO concentration. This may be due to the ability of lactic organisms to hydrolysis free fatty acids to acetaldehyde and di-acetyl.
3.6. Sensory evaluation of Domiati cheese supplemented with BCSO
Flavor is the sensation produced by a material taken in the mouth, perceived principally by the senses of taste and smell, and also by the general pain, tactile, and temperature receptors in the mouth. Flavor also denotes the sum of the characteristics of the material which produces that sensation. Data shown in Table 4 indicate that sensory evaluation of cheese behaved the same trend in all cheese treatments, as gradual enhancement was noticed during the storage. However, continuous production of lactic acid and other organic acids lead to fragile cheese showing a gradual decrease in body and texture and appearance scores recorded for all cheese treatments up till the end of storage period. There were no clear differences between control and cheese samples in appearance value. Similar to appearance, the cheese supplemented with BCSO showed body and texture scores very close to control cheese. Body and texture results of the control, 0.1% and 0.2% BCSO-supplemented cheese after 30 days of ripening were 32, 31 and 32, respectively. The sample supplemented with 0.2% BCSO had the highest flavor scores either fresh or during ripening. The flavor scores of 0.1% and 0.2% BCSO-supplemented cheese after 30 days of maturation were 47 and 48, respectively. In all cheese treatments, the sensory evaluation scores gradually increased during ripening period. The total scores of organoleptic properties of 0.2% BCSO-supplemented cheese at the beginning of ripening period and after 15 and 30 days were 86.2, 87.8 and 88.4, respectively.
4. Conclusions
Cold pressed BCSO could be nutritionally considered as a non-conventional enhancer for soft cheese. It can be stated that BCSO has general antibacterial activity against pathogenic bacteria. This endowed potentiality can participate in keeping a good hygienic quality of soft cheese during storage.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abou-Donia S.A. Egyptian Domiati soft white pickled cheese. Journal of Dairy Science and Technology. 1986;21:167–195. [Google Scholar]
- Ahn J., Grun I.U., Mustapha A. Antimicrobial and antioxidant activities of natural extracts in vitro and in ground beef. Journal of Food Protection. 2004;67:148–155. doi: 10.4315/0362-028x-67.1.148. [DOI] [PubMed] [Google Scholar]
- AOAC . 17th ed. Association of Official Analytical Chemists, International Inc; Arlington, Virginia, USA: 2002. Official methods of analysis. [Google Scholar]
- Badawi R.M., Kebary K.M.K. Influence of fat replacers on the quality of low fat Tallaga cheese. Proceedings of 7th Egyptian Conference Dairy Science Technology. 1998:347–361. [Google Scholar]
- Bettaieb I., Bourgou S., Wannes W.A., Hamrouni I., Limam F., Marzouk B. Essential oils, phenolics, and antioxidant activities of different parts of cumin (Cuminum cyminum L.) Journal of Agricultural and Food Chemistry. 2010;58:10410–10418. doi: 10.1021/jf102248j. [DOI] [PubMed] [Google Scholar]
- Bolton F.J., Crozier L., Willamson J.K. Isolation of Escherichia coli O157:H7 from raw meat products. Letters in Applied Microbiology. 1996;23(5):317–321. doi: 10.1111/j.1472-765x.1996.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Burits M., Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytotherapy Research. 2000;14:323–328. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Butikofer U., Fuchs D. Development of free amino acids in Appenzeller, Emmentaler, Gruyere, Raclette, Sbrinz and Tilsiter cheese. Le Lait. 1997;77:91–100. [Google Scholar]
- J.C. Buzby, T. Roberts, C.T.J. Lin. J.M. MacDonald, Bacterial foodborne disease: medical costs and productivity losses, Economic Research Service/USDA. AER-741 (1996).
- Dave R.I., Shah N.P. Evaluation of media for selective enumeration of Streptococcus thermophilus, Lactobacillus delbrueckii spp. bulgaricus, Lactobacillus acidophilus and Bifidobacterium spp. Journal of Dairy Science. 1996;79:1529–1537. doi: 10.3168/jds.S0022-0302(96)76513-X. [DOI] [PubMed] [Google Scholar]
- El-Ghorab A.H., Nauman M., Anjum F.M., Hussin S., Nadeem M. A comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum) Journal of Agricultural and Food Chemistry. 2010;58:8231–8237. doi: 10.1021/jf101202x. [DOI] [PubMed] [Google Scholar]
- El-Soda A.M. The role of lactic acid bacteria in accelerated cheese ripening. FEMS Microbiology Reviews. 1993;12:239–251. [Google Scholar]
- Fahmi A.H., Sharara H.A. Egyptian Domiati cheese. Journal of Dairy Research. 1950;17:312–320. [Google Scholar]
- Frankel E., Huang S., Prior E. Evaluation of antioxidant activity of rosemary extracts, carnosol, and carnisic acid in bulk vegetable oils, and fish oil and their emulsions. Journal of the Science and Food Agriculture. 1996;72:201–208. [Google Scholar]
- Hanafy M.S.M., Hatern M.E. Studies on the antimicrobial activity of Nigella sativa seed (black cumin) Journal of Ethnopharmacology. 1991;34:275–278. doi: 10.1016/0378-8741(91)90047-h. [DOI] [PubMed] [Google Scholar]
- Hayaloglu A.A., Farkye N.Y. Cheese with added herbs, spices and condiments. Encyclopedia of Dairy Science (Second Edition) 2011:783–789. [Google Scholar]
- Kallio H., Yang B., Peippo P., Tahvonen R., Pan R. Triacylglycerols, glycerophospholipids, tocopherols and tocotrienols in berries and seeds of two subspecies (ssp. sinensis and mongolica) of Sea buckthorn (Hippophaë rhamnoides) Journal of Agricultural and Food Chemistry. 2002;50:3004–3009. doi: 10.1021/jf011556o. [DOI] [PubMed] [Google Scholar]
- Kosikowski F.V. Cheese and fermented milk foods. second ed. Cornell Univ., Inthacu; New York, U.S.A: 1978. [Google Scholar]
- Luther M., Parry J., Moore J., Meng J., Zhang Y., Cheng Z., Yu L. Inhibitory effect of Chardonnay and black raspberry seed extracts on lipid oxidation in fish oil and their radical scavenging and antimicrobial properties. Food Chemistry. 2007;104:1065–1073. [Google Scholar]
- Lutterodt H., Luther M., Slavin M., Yin J.-J., Parry J., Gao J.-M., Yu L. Fatty acid profile, thymoquinone content, oxidative stability, and antioxidant properties of cold-pressed black cumin seed oils. LWT-Food Science and Technology. 2010;43:1409–1413. [Google Scholar]
- Mahgoub S.A., Osman A.O., Sitohy M.Z. Inhibition of growth of pathogenic bacteria in raw milk by legume protein esters. Journal of Food Protection. 2011;74(9):1475–1481. doi: 10.4315/0362-028X.JFP-11-065. [DOI] [PubMed] [Google Scholar]
- Mahgoub S.A., Sitohy M.Z., Osman A.O. Counteracting recontamination of pasteurized milk by methylated soybean protein. Food Bioprocess Technology. 2013;6:101–109. [Google Scholar]
- Papas C.P., Kondly E., Voustsinas L.P., Malletou H. Effect of starter level, raining time and aging on the physicochemical, organoleptic and rheological properties of feta cheese. Journal of the Society of Dairy Technology. 1996;49:73. [Google Scholar]
- Parker T.D., Adams D.A., Zhou K., Harris M., Yu L. Fatty acid composition and oxidative stability of cold-pressed edible seed oils. Journal of Food Science. 2003;68:1240–1243. [Google Scholar]
- Parry J., Su L., Moore J., Cheng Z., Luther M., Rao J.N., Wang J.-Y., Yu L. Chemical compositions, antioxidant capacities, and antiproliferative activities of selected fruit seed flours. Journal of Agricultural and Food Chemistry. 2006;54:3773–3778. doi: 10.1021/jf060325k. [DOI] [PubMed] [Google Scholar]
- Ramadan M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L): an overview. International Journal of Food Science and Technology. 2007;42:1208–1218. [Google Scholar]
- Ramadan M.F., Moersel J.-T. Characterization of phospholipid composition of black cumin (Nigella sative L.) seed oil. Nahrung/Food. 2002;46:240–244. doi: 10.1002/1521-3803(20020701)46:4<240::AID-FOOD240>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Ramadan M.F., Kroh L.W., Moersel J.-T. Radical scavenging activity of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.) and niger (Guizotia abyssinica Cass.) crude seed oils and oil Fractions. Journal of Agricultural and Food Chemistry. 2003;51:6961–6969. doi: 10.1021/jf0346713. [DOI] [PubMed] [Google Scholar]
- Ramadan M.F., Kinni S.G., Seshagiri M., Mörsel J.-T. Fat-soluble bioactives, fatty acid profile and radical scavenging activity of Semecarpus anacardium seed oil. Journal of American Oil Chemists Society. 2010;87:885–894. [Google Scholar]
- Schwartz H., Ollilainen V., Piironen V., Lampi A.-M. Tocopherol, tocotrienol and plant sterol contents of vegetable oils and industrial fats. Journal of Food Composition and Analysis. 2008;21:152–161. [Google Scholar]
- Talib M.A., Abubakar M.M., Jideani I.A., Hassan A. Use of Jiben seeds extract to manufacture soft white cheese. American Journal of Applied Science. 2009;6:551–554. [Google Scholar]



