Abstract
Excessive renal efferent sympathetic nerve activity contributes to hypertension in many circumstances. While both hemodynamic and tubular effects likely participate, most evidence supports a major role for α-adrenergic receptors in mediating the direct epithelial stimulation of sodium retention. Recently, it was reported, however, that norepinephrine activates the thiazide-sensitive transporter, NCC, by stimulating β-adrenergic receptors. Here, we confirmed this effect and developed an acute adrenergic stimulation model to study the signaling cascade. The results show that norepinephrine increases the abundance of phosphorylated NCC rapidly (161% increase), an effect largely dependent on β-adrenergic receptors. This effect is not mediated by activation of angiotensin II receptors. We used immunodissected mouse distal convoluted tubule (DCT) to show that DCT cells are especially enriched for β1-adrenergic receptors, and that the effects of adrenergic stimulation can occur ex vivo (79% increase), suggesting they are direct. As two protein kinases, Ste20p-related Proline Alanine-rich kinase (SPAK) and Oxidative stress responsive 1 (OxSR1), phosphorylate and activate NCC, we examined their roles in norepinephrine effects. Surprisingly, norepinephrine did not affect SPAK abundance or its localization in the DCT; instead, we observed a striking activation of OxSR1. We confirmed that SPAK is not required for NCC activation, using SPAK knockout mice. Together, the data provide strong support for a signaling system involving β1- receptors in the DCT that activates NCC, at least in part via OxSR1. The results have implications regarding device- and drug-based treatment of hypertension.
Keywords: sympathetic nervous system, ion transport, diuretics, hypertension, renal, solute carrier family 12, member 3
Introduction
Excessive renal sympathetic nerve activity (RSNA) has been proposed to be a final common pathway for the genesis of hypertension,1 the largest global contributor to premature death.2 The sympathetic nervous system appears to be especially important in individuals with an exaggerated response to dietary NaCl (‘salt-sensitive hypertensives’), whether this results from genetic3 or acquired factors.4, 5 Norepinephrine infusion in animals reduces urinary sodium excretion and increases blood pressure,6–8 but these effects may not reflect direct stimulation of salt reabsorption by renal tubules. Renal nerves also stimulate renin secretion, which increases sodium reabsorption and raises blood pressure via the actions of angiotensin II and aldosterone. Additional effects of the sympathetic nervous system on the renal vasculature may also contribute, by altering renal hemodynamics. Where direct stimulation of Na+ reabsorption by tubules has been invoked, it is generally accepted that α-adrenergic receptors mediate these effects.1
More recently, Mu and colleagues8 reported that chronic adrenergic activation, by norepinephrine (NE) or isoproterenol, causes salt-sensitive hypertension by activating the thiazide-sensitive Na-Cl cotransporter (NCC) in the kidney; they postulated that this effect was mediated by glucocorticoid-dependent effects on WNK4. WNK effects are mediated primarily by activation of two kinases, SPAK (STE20/SPS1-related proline- and alanine-rich kinase, STK39) and OxSR1 (Oxidative Stress Response Kinase, OxSR1, often termed OSR11). These findings have been controversial, as some aspects were not corroborated by another group,7 and other mediators, such as angiotensin II, were felt likely to have contributed.9 Interestingly, however, a little-cited study published more than 30 years ago, using micropuncture techniques, reported that acute isoproterenol infusion stimulated salt transport along the DCT.10 Additionally, the work of Morel on adenylyl cyclase activities in microdissected nephron segments showed that the early distal tubule (corresponding to the DCT) of the rat is isoproterenol responsive.11 If confirmed, these results would provide novel mechanistic insights linking salt and the adrenergic nervous system that are likely to have therapeutic implications.
During the past 10 years, interest in the renal sympathetic control has surged, as technical advances have made renal denervation popular for treating resistant hypertension.12 Substantial effects of renal nerve ablation on arterial pressure have been observed in many studies, using a variety of experimental models;1 early success in clinical trials,13 led to wide adoption of the technique in Europe, and high anticipation for Food and Drug Administration approval in the U.S. The recent announcement that the SYMPLICITY HTN-3 trial14 did not reach its primary efficacy endpoint, when compared with sham treatment, was disappointing, but it suggests that maximal medical treatment of humans, as achieved in this trial, may interrupt many of the pathways activated by renal nerves.
Although excessive activity of NCC is known to cause hypertension in patients with the Mendelian disease Familial Hyperkalemic Hypertension (FHHt or pseudohypoaldosteronism type 2),15–17 many investigators suggest that such monogenic syndromes are not relevant for typical hypertension.18 In common forms of hypertension, however, a role for NCC is suggested by the substantial clinical efficacy of NCC inhibitors, thiazide diuretics, to treat hypertension. In fact, it is often forgotten that these drugs appear to be effective only when hypertension is present; they have little or no effect on the arterial pressure in normotensive individuals, but reduce it substantially in hypertensive ones.19–21 Therefore, it would be appealing to find a link between sympathetic overactivity and activated NCC. Here, we confirmed that NCC is activated in salt-sensitive hypertension resulting from adrenergic stimulation. As sympathetic nerve effects are typically rapid, we developed an acute model of adrenergic stimulation, permitting us to study signaling mechanisms in vivo in the absence of chronic compensation. This allowed us to confirm the involvement of β-adrenergic receptors, and establish that activation is mediated by an atypical signaling pathway in the DCT.
Methods
See online supplement for detailed methods.
Results
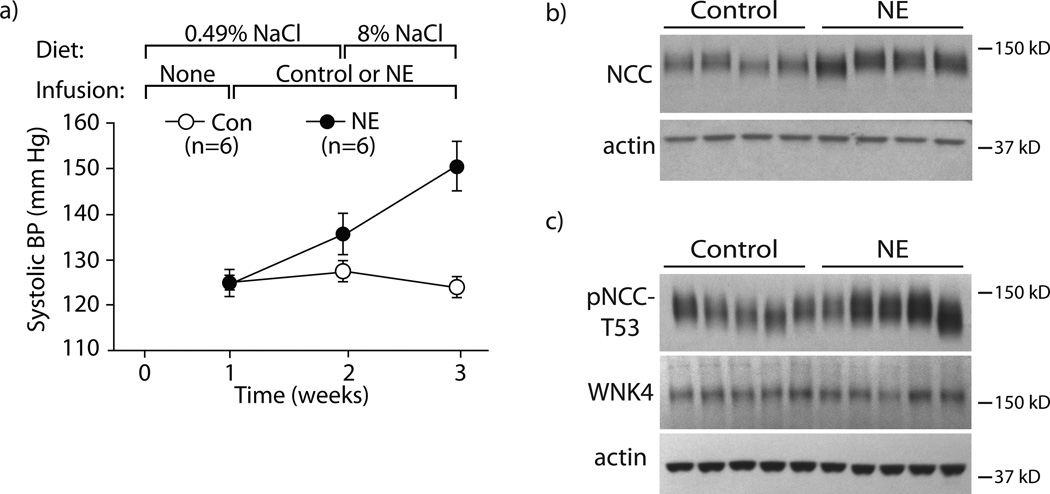
To confirm the effects of chronic adrenergic stimulation on blood pressure, we infused mice with NE via osmotic minipump for two weeks according to the protocol previously described.8 NE infusion increased systolic blood pressure (SBP) slightly, while mice consumed a normal salt diet (0.49% NaCl); when the mice consumed a high salt diet (8% NaCl) with continued NE infusion, however, the systolic pressure increased substantially (Fig 1a). In vehicle-infused mice, high salt intake did not affect pressure. NE also increased the abundance of NCC and phosphorylated (activated) NCC (pNCC) (Fig 1b, c, Supplemental Fig 1a, b). These results are similar to those reported previously by others,8 although we did not detect changes in the abundance of the NCC regulatory kinase WNK4, at least at the protein level (Fig 1c, Supplemental Fig 1c).
Figure 1. Confirmation that chronic norepinephrine (NE) infusion causes salt-sensitive hypertension and increases NCC and pNCC.
Panel A: NE infusion caused salt-induced hypertension. During week one, all animals were maintained on a normal salt diet without infusion. NE or control infusion was started during week two while the normal salt diet was continued. During week three, both groups were switched to a high salt diet. n=6 per group. Differences were determined by two-way ANOVA with repeated measures, where p-values are 0.0018 for Time, 0.0073 for Treatment (NE vs Control), and 0.0119 for Interaction. Panel B: NE infusion caused an increase in NCC abundance compared with control mice. n=4 per group. p<0.05 by unpaired t-test. Panel C: NE infusion caused an increase in pNCC-T53 abundance compared with control mice (p<0.05 by unpaired t-test); however, WNK4 abundance remained unchanged. n=5 per group. Representative images are shown. See online supplement for densiometry.
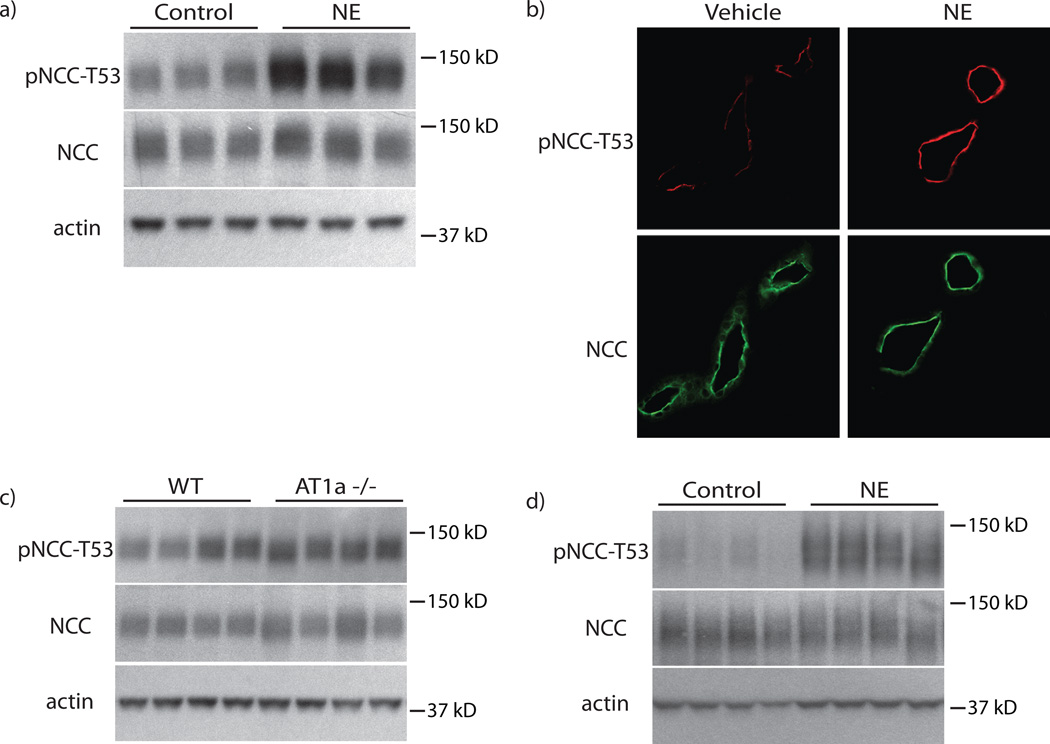
While these results confirm that chronic NE administration increased blood pressure and activated NCC, the effects may not have been direct. To develop an in vivo approach that limits compensatory changes, we determined NE effects 30 minutes after administration. Indeed, pNCC was increased at this time point; in contrast, and as expected, total NCC abundance remained unchanged (Fig 2a, b, Supplemental Fig 2a–e). To determine whether NE activation of NCC requires angiotensin II, a known NCC activator,22, 23 angiotensin II receptor type 1a (AT1A) knockout mice were treated with NE. These animals have normal NCC and pNCC abundance at baseline (Fig 2c, Supplemental Fig 2f, g) and the effect on pNCC 30 minutes after NE treatment was preserved (Fig 2d, Supplemental Fig 2h, i).
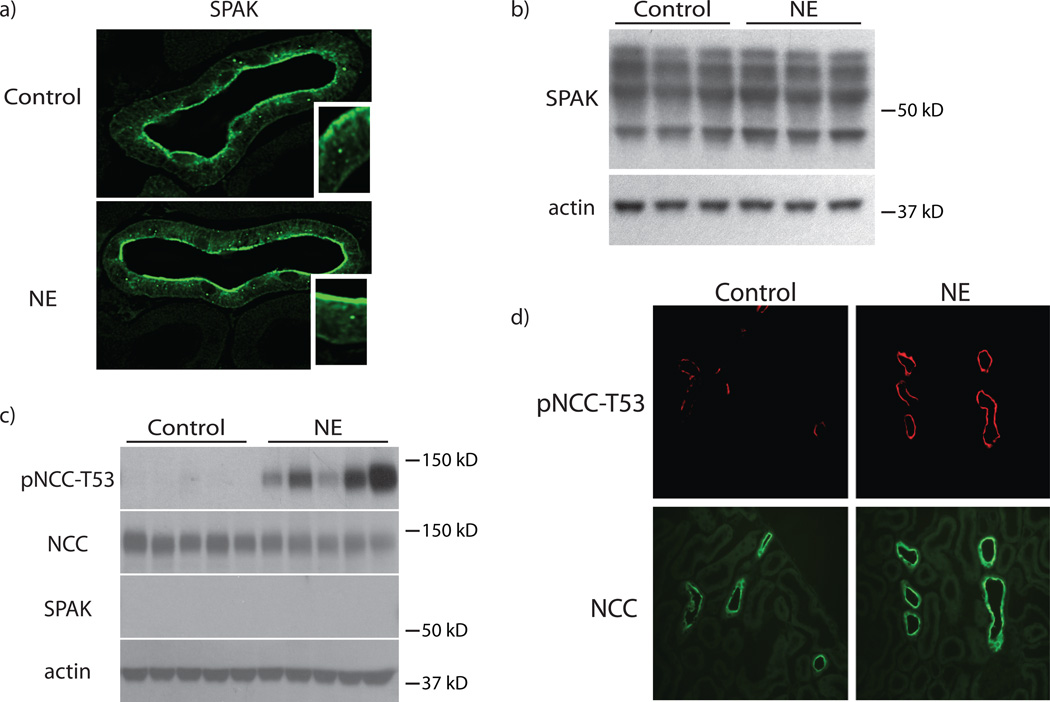
Figure 2. NE rapidly increases pNCC independent of angiotensin II signaling.
Panel A: Treatment with NE for 30 minutes increased pNCC-T53 abundance, but did not alter total NCC. n=8 per group. p<0.05 by unpaired t-test. Panel B: Increased pNCC-T53 abundance could also be observed by immunofluorescence staining. Staining was performed on 3 mice per group. Panel C: Genetic deletion of the angiotensin II receptor type 1a (AT1a) does not alter NCC or pNCC-T53 abundance compared with wild-type (WT) controls. n=7 per group. Panel D: Treatment with NE for 30 minutes in AT1a−/− mice increased pNCC-T53 compared with control animals. p<0.05 by unpaired t-test. Total NCC abundance remained unchanged. n=5 per group. Representative images are shown. See online supplement for densiometry.
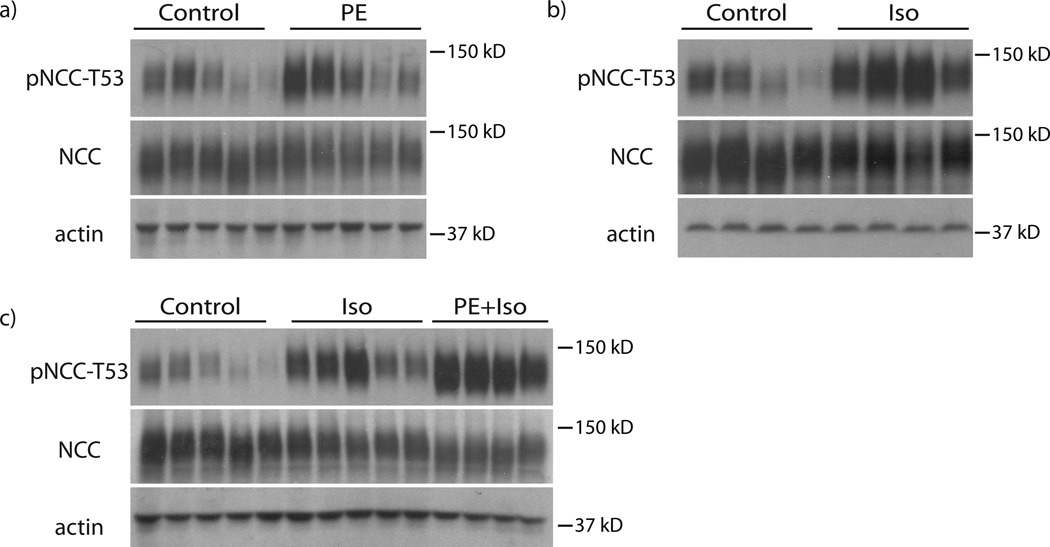
To test whether α or β receptors were responsible for rapid NCC activation, we used the α1-specific agonist phenylephrine and the β1/β2-specific agonist isoproterenol. Stimulation of α1 receptors with phenylephrine did not increase pNCC significantly (Fig 3a, Supplemental Fig 3a, b), whereas, isoproterenol-mediated stimulation of β receptors did (Fig 3b, Supplemental Fig 3c, d). Simultaneous stimulation of α1- and β- adrenergic receptors with both phenylephrine and isoproterenol increased pNCC more than either agonist alone, suggesting a potential interaction of receptor subtypes (Fig 3c, Supplemental Fig 3e, f).
Figure 3. β-receptors mediate the effects of NE on NCC.
Panel A: Treatment with the α-receptor agonist, phenylephrine (PE), for 30 minutes did not significantly increase pNCC-T53. Total NCC abundance also remained unchanged. n=5 per group. Panel B: Treatment with the β-receptors agonist, isoproterenol (Iso), for 30 minutes significantly increased pNCC-T53 abundance. p<0.05 by unpaired t-test. Total NCC abundance remained unchanged. n=4 per group. Panel C: Treatment with both agonists together increased pNCC-T53 abundance greater than either agonist alone. p<0.016 by unpaired t-test. Total NCC abundance remained unchanged. n= 5 for the Control and Iso groups and 4 for the Iso+PE group. See online supplement for densiometry.
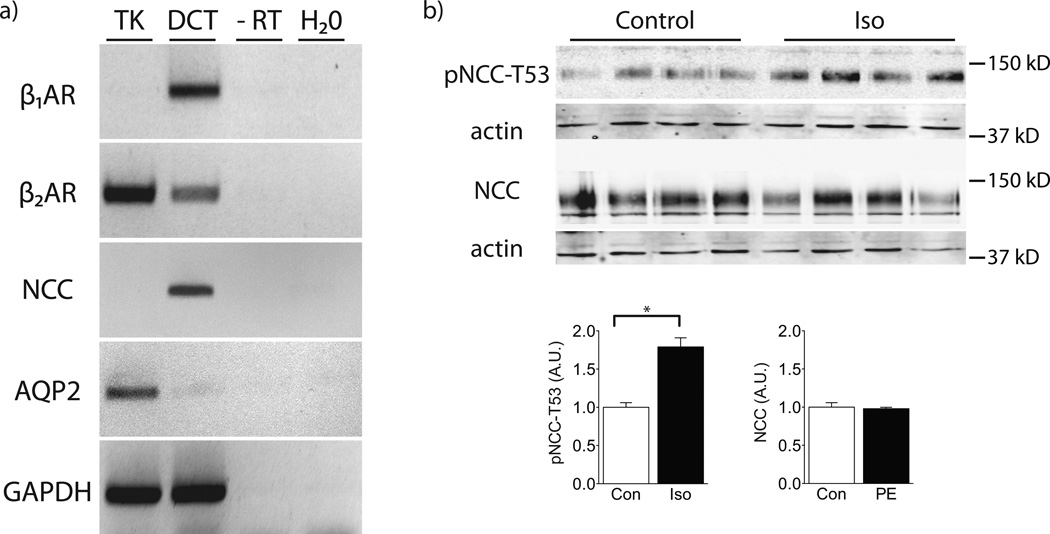
Although a role for adrenergic receptors in modulating renal renin release is well documented, the role and nature of adrenergic receptors on distal nephron cells is not as clear. We first tested whether DCT cells express β-adrenergic receptors by isolating DCT cells from kidney cortex of mice expressing green fluorescent protein only in the DCT using the COPAS platform.24 RNA isolated from whole kidney and DCT was then used for RT-PCR. While both β1 and β2 receptors were detected (Fig 4a), β1 receptors were highly enriched in DCT cells. In contrast, β2 receptors were not, consistent with results of an unbiased genetic screen of the same cells.25. When COPAS-sorted DCT cells were exposed, ex vivo, to isoproterenol, pNCC increased after 10 minutes (Fig 4b).
Figure 4. DCT-specific RT-PCR for β-adrenergic receptor subtypes.
Panel A: RT-PCR on COPAS-sorted DCT cells indicated that both β1- and β2-adrenergic receptors are expressed in the DCT, but the β1-receptor subtype is highly enriched. The template used for RT-PCR was either 5 ng cDNA produced from RNA extracted from total kidney (TK) or COPAS-sorted DCT cells (DCT). In place of template cDNA, negative control reactions contained either the product from a cDNA reaction using DCT RNA that lacked reverse transcriptase (-RT) or water (H20). β1AR, β1-adrenergic receptor; β2AR, β2-adrenergic receptor; NCC, Na+-Cl− cotransporter; AQP2, aquaporin-2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Panel B: Ex vivo treatment of COPAS-sorted DCT cells with isoproterenol (Iso) for 10 minutes increased pNCC-T53 abundance, but did not alter total NCC. n=4 per group. Graphs depict mean ± s.e.m. * p<0.05 by unpaired t-test.
Mu et al. suggested that the WNK kinase pathway is involved in chronic adrenergic effects on NCC.8 WNKs signal NCC predominantly via SPAK, the major serine/threonine kinase that phosphorylates and activates NCC. To test whether adrenergic agonists activate SPAK, we examined effects of NE on SPAK abundance and cellular localization. NE administration did not affect SPAK localization within DCT cells, and did not increase SPAK abundance, at least within 30 minutes (Fig 5a, b, Supplemental Fig 4a). It also did not cause an isoform switch, which corresponds to SPAK activation.26, 27 These results, however, do not indicate whether SPAK is necessary for NE to stimulate NCC. Thus, we tested effects of NE in SPAK−/− mice. Although pNCC abundance at baseline is very low in these mice,26, 27 pNCC increased robustly (Fig 5c,d, Supplemental Fig 4b). In fact, the proportional increase in pNCC abundance was larger in SPAK−/− mice than in wild type mice (Supplemental Fig 4c).
Figure 5. SPAK is not essential for the rapid effects of NE on NCC.
Panel A: Treatment with NE for 30 minutes did not alter SPAK cellular localization within the DCT in wild-type animals. Staining was performed on 3 mice per group. Panel B: Treatment with NE for 30 minutes did not alter SPAK abundance in wild-type animals. n=3 per group. Panel C: Treatment with NE for 30 minutes in SPAK−/− animals significantly increased pNCC-T53 abundance. p<0.05 by unpaired t-test. Total NCC protein remained unchanged. n=5 per group. Panel D: Increased pNCC-T53 abundance in SPAK−/− animals could also be observed by immunofluorescence staining. Staining was performed on 3 mice per group. See online supplement for densiometry.
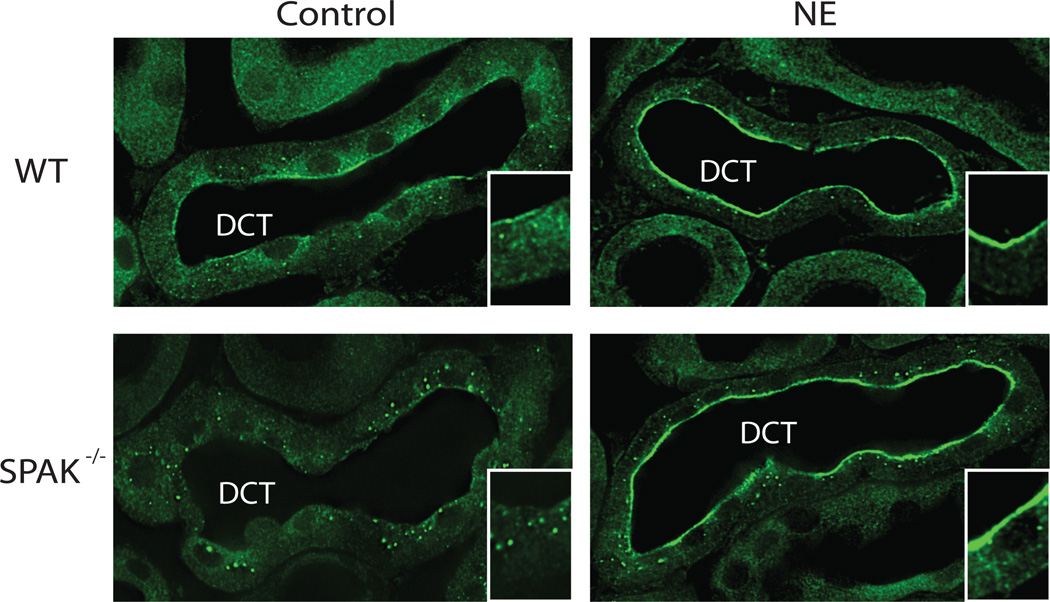
As OxSR1 has also been shown to phosphorylate NCC in vitro,28 and it is expressed along the DCT, we tested whether NE alters its cellular localization. In both wild type and SPAK−/− mice, NE strikingly altered cellular OxSR1 appearance by immunofluorescence (Fig 6). Treatment with NE caused a striking increase in apical OxSR1, which assumed a ribbon-like appearance near the apical membrane of the DCT. This was especially obvious in the SPAK−/− mice, because OxSR1 assumes a distinctive punctate pattern in SPAK−/− mice, at baseline26, 27, 29 (Supplemental Movie 1).
Figure 6. NE increased DCT OxSR1 apical localization in both wild-type and SPAK−/− mice.
Treatment with NE for 30 minutes increased the apical localization of OxSR1 in the DCT of both wild-type and SPAK−/− mice. Insets are high magnification images of the represented tubule sections. Staining was performed on 3 mice per group.
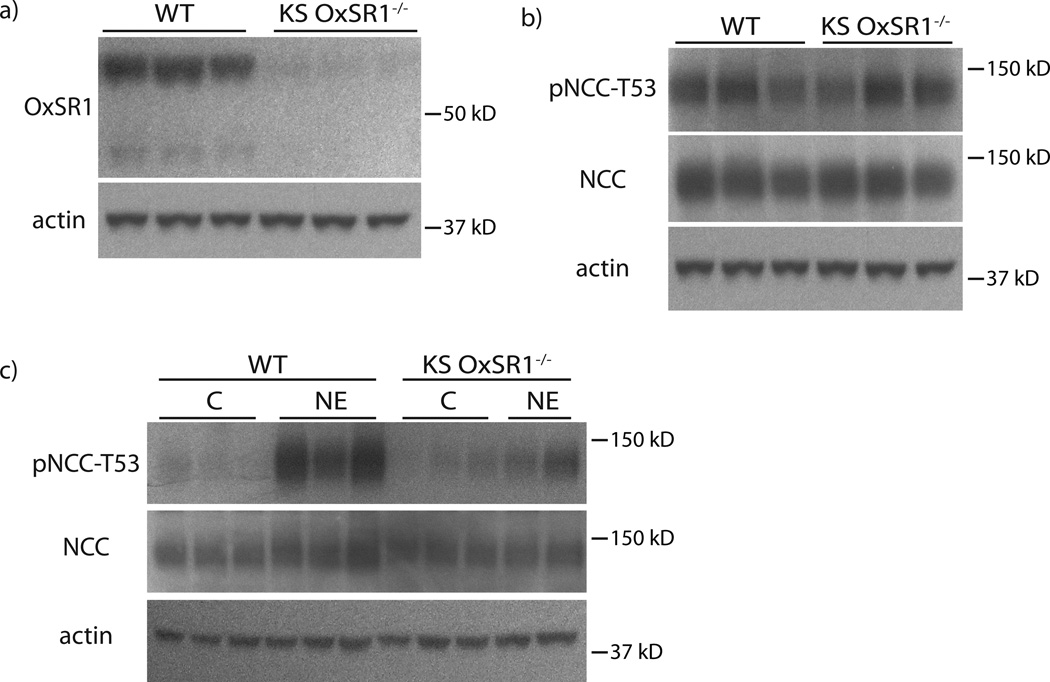
Although these results suggested a role for OxSR1 in mediating the effects of NE, they do not prove that it is essential. To determine whether OxSR1 is essential for increasing the abundance of pNCC, we generated doxycycline-inducible kidney-specific (KS) OxSR1−/− mice. Total body OxSR1 is embryonic lethal, so we deleted OxSR1 along the nephron, using the Pax8 LC1 system (Fig 7a, Supplemental Fig 5a). After deletion of OxSR1, these mice have normal NCC abundance (both total and phosphorylated) (Fig 7b, Supplemental Fig 5b, c). Thirty minutes after NE administration, pNCC abundance increased in these mice, but in contrast to the previously performed experiments, the increase was severely blunted (Fig 7c, Supplemental Fig 5d, e). This suggests that OxSR1 is essential for the full effect of NE on NCC.
Figure 7. KS OxSR1−/− mice exhibited a diminished response to NE.
Panel A: KS OxSR1−/− mice had significantly decreased abundance of OxSR1 in their kidneys compared with wild-type (WT) controls. n=3 per group. p<0.05 by unpaired t-test. Panel B: The abundance of NCC protein and phosphorylation at threonine-53 was unaltered in KS OxSR1−/− mice at baseline. n=3 per group. Panel C: KS OxSR1−/− mice exhibited a blunted response to a 30-minute treatment with NE. NCC phosphorylation at threonine-53 increased in these mice, but to a lesser degree than that observed in wild-type controls. p<0.05 by unpaired t-test. n=3 for WT per group mice and 4 for KS OxSR1−/− mice per group. C, Con, control. Representative images are shown. See online supplement for densiometry.
Discussion
The sympathetic nervous system plays a central role in human blood pressure control. Sympathetic nerves to the kidney contribute importantly to these effects by stimulating renal renin release, modulating renal vascular tone, and activating NaCl transporters.1 Ablating renal sympathetic nerves is an old approach to resistant hypertension that has recently regained favor, owing to technical advances, which make it easier and safer. In many animal and human studies, this approach has reduced arterial pressure substantially;12 yet a rigorous and large clinical trial recently could not confirm that renal denervation is superior to standard care. Thus, even though renal denervation reduces blood pressure in models of hypertension, its role in clinical medicine remains to be established.
Here, we confirmed that NE causes salt-sensitive hypertension in mice, in part, by activating salt reabsorption along the distal nephron via NCC. We show that the effects of NE on NCC activity occur rapidly and are largely mediated via β-adrenergic receptors; we also identify a novel role for OxSR1 in stimulating NCC in response to stress. While this inquiry has largely focused on the abundance of pNCC, our immunofluorescence data also suggest that NE may induce a translocation of NCC from the cytosol to the apical membrane (Fig 2b), as reported previously for angiotensin II.22 In our acute studies, we could not measure salt transport directly, but the abundance of pNCC is used widely as a surrogate for NCC activity.23, 30 Furthermore, Mu and colleagues reported that thiazide treatment reverses NE-induced hypertension,8 supporting a functional role for NCC activity in these effects. The finding that reducing salt intake re-lowers pressure during continued NE infusion8 further supports the importance of salt, and is consistent with effects of salt loading in a number other experimental models.31 The current results clarify the important role that NaCl reabsorption plays in the effects of NE on arterial pressure, and suggest one reason that SYMPLICITY HTN-3 may have failed to achieve its primary goal.
The nature of the direct and indirect effects of adrenergic stimulation on the kidney has been debated for many decades. Here, we show that the effect of NE to increase pNCC rapidly is fully preserved in mice lacking AT1A receptors, indicating that the effect is not mediated by renin or angiotensin II. The effect appears to be mediated instead through β-adrenergic receptors, as observed previously during chronic treatment.8 Yet, we also detected evidence for synergistic interactions between α- and β-adrenergic receptors to stimulate NCC, a finding compatible with previously contradictory results. Although we find that both β1- and β2-adrenergic receptor subtypes are expressed in the DCT, β1-receptors were highly enriched, suggesting that they play a dominant role. This is consistent with anatomical studies performed in the 1970s and 1980s by Barajas and colleagues.32, 33 Taken together, our results suggest that NCC can be modulated rapidly by direct β-adrenergic input to DCT cells, probably enhanced by concomitant α-adrenergic stimulation.
While many groups have observed that NE infusion increases blood pressure, especially in the setting of dietary NaCl loading,7, 8 the effects of this treatment on NCC and pNCC abundance, as well as WNK signaling cascade activity, have been more controversial. Our data here confirm that NE infusion chronically increases both NCC and pNCC and raises arterial pressure, as shown previously by Mu and colleagues.8 Uchida and colleagues7 confirmed the blood pressure effects of NE infusion with dietary salt loading, but could not detect the effects on NCC and WNK4. While reasons for these discrepancies are not clear, acute hypertension, or dietary salt loading, typically down-regulates NCC;34, 35 thus, the absence of this effect during NE infusion noted by Uchida and colleagues likely reflects abnormal NCC activation.
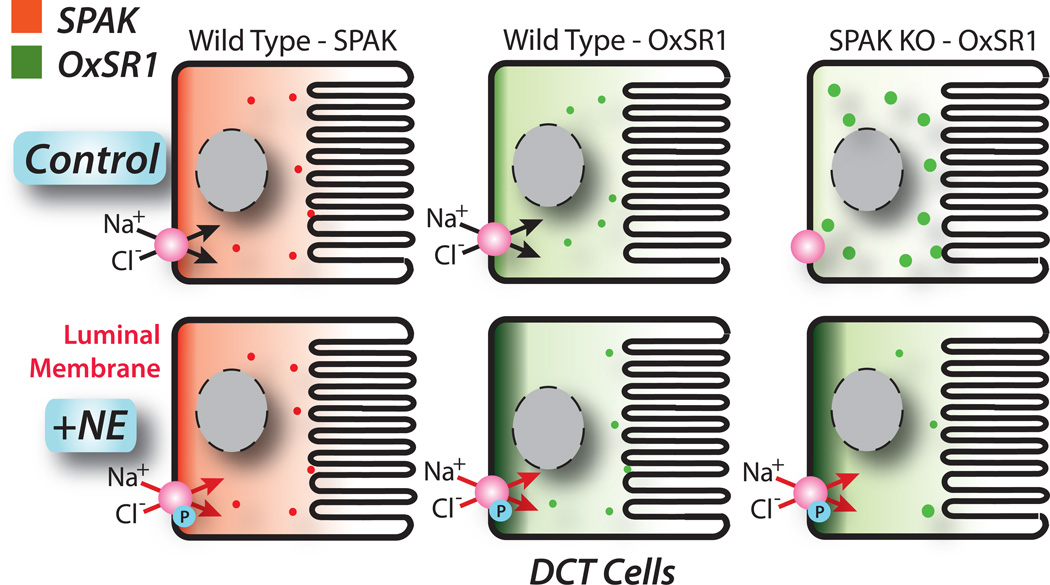
NCC, and the furosemide-sensitive Na-K-2Cl cotransporter, NKCC2, are stimulated by a signaling cascade that includes WNK kinases, SPAK and OxSR1. We found no change in WNK4 abundance upon NE stimulation, using an antibody validated against WNK4 knockout tissue, suggesting that WNK4 is not involved. Similarly, we found no apparent effect of NE on SPAK. SPAK deletion strikingly reduces the abundance of pNCC, in vivo;26, 27, 36,37 whereas OxSR1 deletion does not.38 This has led to a simple paradigm in which SPAK, and not OxSR1, was viewed as responsible for activating NCC.26, 38 Surprisingly, adrenergic stimulation, induced a shift in OxSR1 toward the apical membrane of DCT cells, where it might be expected to phosphorylate and activate NCC (Fig 8). This apical redistribution occurs whether or not SPAK is present, suggesting that this does not require SPAK/OxSR1 interactions. As OxSR1 is capable of phosphorylating cation chloride cotransporters in vitro,39 this suggests that OxSR1 plays an essential and non-redundant role in the adrenergic response. In confirmation of this, inducing OxSR1 deletion along the nephron, which did not affect basal pNCC abundance, strikingly blunted the ability of NE to increase pNCC.
Figure 8. Model of NE effects on SPAK and OxSR1 in the DCT.
Cartoon shows effects of NE on SPAK (left panels) and OxSR1 (middle panels) distribution in wild type mice. Note that OxSR1, but not SPAK, shifts to an apical location. The right panels show effects of NE on OxSR1 in SPAK knockout mice. Notice that SPAK knockout causes OxSR1 to shift to a more punctate appearance. Upon NE stimulation, OxSR1 shifts substantially toward the apical membrane. The NCC is phosphorylated (P), upon activation.
A role for OxSR1 in regulating NCC was suggested by Chiga et al., who reported that OxSR1 kinase activity is required for the full FHHt phenotype.40 They found that disrupting SPAK kinase activity did not completely ameliorate the FHHt phenotype. Instead, disruption of both OxSR1 and SPAK was necessary to return NCC phosphorylation, blood pressure, and plasma K+ back to normal. Grimm and colleagues27 postulated that OxSR1 requires SPAK to move to the apical membrane, where it can phosphorylate NCC. The current results indicate that this deficiency can be overcome by adrenergic stimulation, as apical OxSR1 is abundant following stimulation in SPAK−/− mice (Fig 8). Taken together, the results suggest complementary, but distinct, roles for SPAK and OxSR1 in regulating NCC. Clearly, SPAK is the dominant NCC-activating kinase under basal conditions, but OxSR1 appears to predominate when the kidney responds to adrenergic stimulation. As suggested by Grimm and colleagues,27 both kinases may be necessary to activate NCC to its fullest extent.
Guyton and colleagues41 suggested that, although the kidney is an especially potent regulator of arterial pressure, these actions are slow. It has been surprising, therefore, to find that NCC is modulated acutely, in a variety of circumstances. Acute hypertension, or ACE inhibition, shifts NCC out of the plasma membrane;22, 34, 42 acute KCl gavage rapidly reduces pNCC abundance;43 Here, we have shown that adrenergic stimulation activates NCC rapidly and substantially. This response is most likely a part of the fight-or-flight reflex, described by Cannon,44 in which maintenance of arterial pressure and essential organ perfusion provides short-term advantage. When this response is anomalously derived and maintained chronically, however, it likely contributes to pathological hypertension. There has been substantial disappointment that renal denervation did not prove effective in the large rigorous SYMPLICITY HTN-3 trial for the treatment of resistant hypertension.14 Some commentators have suggested that one difference between the SYMPLICITY HTN-3 trial and earlier trials is the intense focus on appropriate medical management in the SYMPLICITY HTN-3.45 This focus included aggressive use of thiazide-type diuretics and aldosterone antagonists, both which inhibit NCC.46 Thus, these drugs may have achieved, medically, some of the effects that nerve ablation would otherwise, rendering the intervention less effective.
Perspectives
The current work shows that adrenergic stimulation of β-receptors in DCT cells stimulates NCC, both acutely and chronically, contributing to salt-sensitive hypertension. This effect is mediated largely by OxSR1, rather than SPAK, identifying an essential and non-redundant role for OxSR1 in modulating NCC activity, a role that was previously unappreciated. The results suggest that renal nerves exert important effects on the kidney by activating salt transport pathways to increase arterial pressure; for this reason, the benefits of invasive procedures may not appear as great, when compared to a control group treated aggressively with drugs that block salt transport.
Supplementary Material
Novelty and Significance.
What is new?: Adrenergic stimulation rapidly activates NCC in the distal tubule via β-adrenergic receptors, which are enriched in distal convoluted tubule cells. The effect is not dependent on angiotensin II or SPAK, and is largely mediated by the kinase OxSR1. These effects appear to contribute importantly to salt-sensitive hypertension resulting from norepinephrine infusion.
What is relevant?: These findings help explain how excessive sympathetic nerve activity causes hypertension. They indicate that medical and device-based approaches to hypertension converge on common mechanisms.
Summary: Our data provide evidence for an in vivo β-adrenergic signaling cascade in the DCT. It is largely mediated by the kinase OxSR1 and is relevant in the generation of salt-sensitive hypertension.
Acknowledgements
AST and DHE are grateful to Virginia Brooks, Ph.D. for advice and support in the completion of this work. We wish to acknowledge Aurelie Snyder and the Advanced Light Microscopy Core at The Jungers Center, OHSU, for technical assistance.
Sources of Funding
This work was performed in partial fulfillment of the requirements for a Ph.D. by Andrew S. Terker. It was supported by a Predoctoral Fellowship from the American Heart Association (13PRE14090030), and by grants to DHE from the NIH and Department of Veterans Affairs (DK51496 and Merit Review) and, from NIH to ED (GM074771 and DK093501). It was also supported by Shared Instrumentation Grant Number S10-RR023432 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
This protein is often termed OSR1. As OSR1 is the official gene symbol for ODD-SKIPPED-RELATED1, we have used the term OxSR1, for clarity)
Conflicts of Interest/Disclosures
None.
References
- 1.DiBona GF. Physiology in perspective: The Wisdom of the Body. Neural control of the kidney. Am J Physiol Regul Integr Comp Physiol. 2005;289:R633–R641. doi: 10.1152/ajpregu.00258.2005. [DOI] [PubMed] [Google Scholar]
- 2.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Hanafiah KM, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foss JD, Fink GD, Osborn JW. Reversal of salt-sensitive hypertension by targeted symptathetic ablation. Hypertension. 2013;61:806–811. doi: 10.1161/HYPERTENSIONAHA.111.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kandlikar SS, Fink GD. Splanchnic sympathetic nerves in the development of mild DOCA-salt hypertension. American Journal of Physiology - Heart & Circulatory Physiology. 2011;301:H1965–H1973. doi: 10.1152/ajpheart.00086.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacob F, Clark LA, Guzman PA, Osborn JW. Role of renal nerves in development of hypertension in DOCA-salt model in rats: a telemetric approach. American Journal of Physiology - Heart & Circulatory Physiology. 2005;289:H1519–H1529. doi: 10.1152/ajpheart.00206.2005. [DOI] [PubMed] [Google Scholar]
- 6.Lohmeier TE, Tilman LJ, Carroll RG, Brown AJ, Guyton AC. Malignant hypertensive crisis induced by chronic intrarenal norepinephrine infusion. Hypertension. 1984;6:I177–I182. doi: 10.1161/01.hyp.6.2_pt_2.i177. [DOI] [PubMed] [Google Scholar]
- 7.Uchida S, Chiga M, Sohara E, Rai T, Sasaki S. Does a beta2-adrenergic receptor-WNK4-Na-Cl co-transporter signal cascade exist in the in vivo kidney. Nature medicine. 2012;18:1324–1325. doi: 10.1038/nm.2809. author reply 1325–1327. [DOI] [PubMed] [Google Scholar]
- 8.Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami-Mori F, Marumo T, Yatomi Y, Geller DS, Tanaka H, Fujita T. Epigenetic modulation of the renal beta-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nature medicine. 2011;17:573–580. doi: 10.1038/nm.2337. [DOI] [PubMed] [Google Scholar]
- 9.Ellison DH, Brooks VL. Renal nerves, WNK4, glucocorticoids, and salt transport. Cell Metab. 2011;13:619–620. doi: 10.1016/j.cmet.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greven J, Heidenreich O. A micropuncture study of the effect of isoprenaline on renal tubular fluid and electrolyte transport in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1975;287:117–128. doi: 10.1007/BF00510444. [DOI] [PubMed] [Google Scholar]
- 11.Morel F. Sites of hormone action in the mammalian nephron. AmJPhysiol. 1981;240:F159–F164. doi: 10.1152/ajprenal.1981.240.3.F159. [DOI] [PubMed] [Google Scholar]
- 12.Thukkani AK, Bhatt DL. Renal denervation therapy for hypertension. Circulation. 2013;128:2251–2254. doi: 10.1161/CIRCULATIONAHA.113.004660. [DOI] [PubMed] [Google Scholar]
- 13.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris G. A Controlled Trial of Renal Denervation for Resistant Hypertension. N Engl J Med. 2014 doi: 10.1056/NEJMoa1402670. In Press. [DOI] [PubMed] [Google Scholar]
- 15.Louis-Dit-Picard H, Barc J, Trujillano D, Miserey-Lenkei S, Bouatia-Naji N, Pylypenko O, Beaurain G, Bonnefond A, Sand O, Simian C, Vidal-Petiot E, Soukaseum C, Mandet C, Broux F, Chabre O, Delahousse M, Esnault V, Fiquet B, Houillier P, Bagnis CI, Koenig J, Konrad M, Landais P, Mourani C, Niaudet P, Probst V, Thauvin C, Unwin RJ, Soroka SD, Ehret G, Ossowski S, Caulfield M, Bruneval P, Estivill X, Froguel P, Hadchouel J, Schott JJ, Jeunemaitre X. KLHL3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nature genetics. 2012;44:456–460. S1–S3. doi: 10.1038/ng.2218. [DOI] [PubMed] [Google Scholar]
- 16.Pathare G, Hoenderop JG, Bindels RJ, San-Cristobal P. A molecular update on Pseudohypoaldosteronism type II. American journal of physiology Renal physiology. 2013 doi: 10.1152/ajprenal.00440.2013. In Press. [DOI] [PubMed] [Google Scholar]
- 17.Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B, Lifton RP. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482:98–102. doi: 10.1038/nature10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kotchen TA, Cowley AW, Jr, Frohlich ED. Salt in health and disease--a delicate balance. N Engl J Med. 2013;368:2531–2532. doi: 10.1056/NEJMc1305326. [DOI] [PubMed] [Google Scholar]
- 19.Freis ED, Wanko A, Wilson IM, Parrish AE. Chlorothiazide in hypertensive and normotensive patients. Ann N Y Acad Sci. 1958;71:450–455. doi: 10.1111/j.1749-6632.1958.tb46773.x. [DOI] [PubMed] [Google Scholar]
- 20.Shah S, Khatri I, Freis ED. Mechanism of antihypertensive effect of thiazide diuretics. Am Heart J. 1978;95:611–618. doi: 10.1016/0002-8703(78)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Freis ED, Reda DJ, Materson BJ. Volume (weight) loss and blood pressure response following thiazide diuretics. Hypertension. 1988;12:244–250. doi: 10.1161/01.hyp.12.3.244. [DOI] [PubMed] [Google Scholar]
- 22.Sandberg MB, Riquier AD, Pihakaski-Maunsbach K, McDonough AA, Maunsbach AB. ANG II provokes acute trafficking of distal tubule Na+-Cl cotransporter to apical membrane. Am J Physiol Renal Physiol. 2007;293:F662–F669. doi: 10.1152/ajprenal.00064.2007. [DOI] [PubMed] [Google Scholar]
- 23.Castaneda-Bueno M, Cervantes-Perez LG, Vazquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+:Cl− cotransporter by angiotensin II is a WNK4-dependent process. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7929–7934. doi: 10.1073/pnas.1200947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markadieu N, San-Cristobal P, Nair AV, Verkaart S, Lenssen E, Tudpor K, van Zeeland F, Loffing J, Bindels RJ, Hoenderop JG. A primary culture of distal convoluted tubules expressing functional thiazide-sensitive NaCl transport. American journal of physiology Renal physiology. 2012;303:F886–F892. doi: 10.1152/ajprenal.00114.2012. [DOI] [PubMed] [Google Scholar]
- 25.Picard N, Trompf K, Yang CL, Miller RL, Carrel M, Loffing-Cueni D, Fenton RA, Ellison DH, Loffing J. Protein Phosphatase 1 Inhibitor-1 Deficiency Reduces Phosphorylation of Renal NaCl Cotransporter and Causes Arterial Hypotension. J Am Soc Nephrol. 2013 doi: 10.1681/ASN.2012121202. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang C-L, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH. A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab. 2011;14:352–364. doi: 10.1016/j.cmet.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, Wade JB, Welling PA. SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. The Journal of biological chemistry. 2012;287:37673–37690. doi: 10.1074/jbc.M112.402800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121:675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- 29.Saritas T, Borschewski A, McCormick JA, Paliege A, Dathe C, Uchida S, Terker A, Himmerkus N, Bleich M, Demareetz S, Laghmani K, Delpire E, Ellison DH, Bachmann S, Mutig K. SPAK differentially mediates vascopressin effects on sodium cotransporters. J Am Soc Nephrol. 2013;24:407–418. doi: 10.1681/ASN.2012040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S. Molecular Pathogenesis of Pseudohypoaldosteronism Type II: Generation and Analysis of a Wnk4(D561A/+) Knockin Mouse Model. Cell Metab. 2007;5:331–344. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Andou K, Sato Y, Fujita T. Salt sensitiviy in hypertensive rats with angiotensin II administration. Am J Physiol Regul Integr Comp Physiol. 1990;259:R1012–R1016. doi: 10.1152/ajpregu.1990.259.5.R1012. [DOI] [PubMed] [Google Scholar]
- 32.Barajas L, Powers K, Wang P. Innervation of the renal cortical tubules: a quantitative study. The American journal of physiology. 1984;247:F50–F60. doi: 10.1152/ajprenal.1984.247.1.F50. [DOI] [PubMed] [Google Scholar]
- 33.Barajas L, Powers K, Wang P. Innervation of the late distal nephron: an autoradiographic and ultrastructural study. Journal of ultrastructure research. 1985;92:146–157. doi: 10.1016/0889-1605(85)90042-4. [DOI] [PubMed] [Google Scholar]
- 34.Lee DH, Riquier AD, Yang LE, Leong PK, Maunsbach AB, McDonough AA. Acute hypertension provokes acute trafficking of distal tubule Na-Cl cotransporter (NCC) to subapical cytoplasmic vesicles. Am J Physiol Renal Physiol. 2009;296:F810–F818. doi: 10.1152/ajprenal.90606.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. American journal of physiology Renal physiology. 2009;297:F704–F712. doi: 10.1152/ajprenal.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-Knockout Mice Manifest Gitelman Syndrome and Impaired Vasoconstriction. J Am Soc Nephrol. 2010;21:1868–1877. doi: 10.1681/ASN.2009121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanovic S, Jovanovic A, O'Shaughnessy KM, Alessi DR. Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med. 2010;2:63–75. doi: 10.1002/emmm.200900058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin SH, Yu IS, Jiang ST, Lin SW, Chu P, Chen A, Sytwu HK, Sohara E, Uchida S, Sasaki S, Yang SS. Impaired phosphorylation of Na(+)-K(+)-2Cl(−) cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and Bartter-like syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:17538–17543. doi: 10.1073/pnas.1107452108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. The Journal of biological chemistry. 2005;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- 40.Chiga M, Rafiqi FH, Alessi DR, Sohara E, Ohta A, Rai T, Sasaki S, Uchida S. Phenotypes of pseudohypoaldosteronism type II caused by the WNK4 D561A missense mutation are dependent on the WNK-OSR1/SPAK kinase cascade. Journal of cell science. 2011;124:1391–1395. doi: 10.1242/jcs.084111. [DOI] [PubMed] [Google Scholar]
- 41.Guyton AC. Blood pressure control-Special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 42.Sandberg MB, Maunsbach AB, McDonough AA. Redistribution of distal tubule Na+-Cl− cotransporter (NCC) in response to a high-salt diet. Am J Physiol Renal Physiol. 2006;291:F503–F508. doi: 10.1152/ajprenal.00482.2005. [DOI] [PubMed] [Google Scholar]
- 43.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int. 2013;83:811–824. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 44.Cannon WB. The Wisdom of the Body. W.W. Norton & Company; 1932. [Google Scholar]
- 45.Messerli FH, Bangalore S. Renal denervation for resistant hypertension. N Engl J Med. 2014;370 doi: 10.1056/NEJMe1402388. [DOI] [PubMed] [Google Scholar]
- 46.Abdallah JG, Schrier RW, Edelstein C, Jennings SD, Wyse B, Ellison DH. Loop diuretic infusion increases thiazide-sensitive Na(+)/Cl(−)-cotransporter abundance: role of aldosterone. J Am Soc Nephrol. 2001;12:1335–1341. doi: 10.1681/ASN.V1271335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.