Abstract
Fungal infections are one of the most significant causes of morbidity and mortality in immunocompromised patients. The incidence of invasive fungal infections, including those of the gastrointestinal tract, has increased significantly as numbers of immunocompromised patients have increased. The diagnosis of fungal infections in immunocompromised patients may be particularly problematic as these patients may present with atypical clinical features. Although Candida and Aspergillus species represent the majority of fungi diagnosed in the immunocompromised patient population, other fungi are emerging as increasingly common pathogens, and this review will focus on several important emerging fungal infections in immunocompromised patients.
Infections are one of the most common complications in immunocompromised patients, and the endemic mycoses are one of the most significant infectious causes of morbidity and mortality in this population (1-3). The incidence of invasive fungal infections, including those of the gastrointestinal tract, has increased significantly over the past 20 years as numbers of immunocompromised patients have increased. Despite advances in laboratory technology, particularly in the areas of serologic and molecular testing, the recognition, diagnosis, and classification of fungal infections in this patient population remains challenging.
Although primary transmural invasion by fungi cause some gastrointestinal infections, disseminated fungal disease (and recognition therof) is equally important. Signs and symptoms of gastrointestinal fungal infections include diarrhea, vomiting, melena, hemorrhage, abdominal pain, and fever, and are often similar regardless of the type of fungus involved. The diagnosis of fungal infections in immunocompromised patients may be particularly problematic as these patients may present with atypical clinical features, and it is important to remember that GI signs and symptoms may be the initial and only presenting features of a disseminated illness.
The term “immunocompromised” is primarily associated with underlying disorders such as AIDS, chemotherapy, and solid organ and bone marrow transplantation. However, many other forms of immunocompromise also result in susceptibility to fungal infections, including primary immunodeficiencies (e.g. common variable immunodeficiency), patients on chronic immunomodulatory therapy or steroids, very young or very elderly patients, diabetics, patients who are status-post splenectomy, and those with chronic alcoholism, malnutrition, or any chronic debilitating disease (4-7).
Although Candida and Aspergillus species represent the majority of fungi diagnosed in the immunocompromised patient population (5-6), other fungi are emerging as increasingly common serious pathogens. The organism to which any individual patient is susceptible varies with a number of factors, including the underlying disease, the degree of immunocompromise, and environmental factors such as where the patient lives and types and magnitude of exposure. Furthermore, the host is the sole source of the inflammatory response to the organism, and the specific deficits in the host immune system along with the patient's environment and exposure history create the differential diagnosis for any given mycosis. This review will focus on several important emerging fungal infections in immunocompromised patients.
Filamentous Fungi
Mucormycosis is a life-threatening infection caused by fungi of the order Mucorales (8-12). As noted above, Aspergillus species have long been recognized as the most commonly encountered filamentous fungus in the immunocompromised patient population. For reasons that remain poorly understood, however, the incidence of mucormycosis is increasing, particularly in patients with diabetes, hematologic malignancies, and bone marrow transplants (8). Recent reclassification abolished the order known as the Zygomycetes, and placed the order Mucorales in the subphylum Mucormycotina (13). Therefore, infection by these organisms is now referred to as “mucormycosis” rather than “zygomycosis.” Mucormycosis is associated with diabetes and other causes of metabolic acidosis, deferoxamine therapy, organ or bone marrow transplant, neutropenia, skin and soft tissue breakdown, intravenous drug use, neonatal prematurity, and malnourishment. Interestingly, HIV/AIDS does not appear to be a risk factor for this infection. The incidence appears to be increasing, especially in cancer patients. The mortality rate is quite high (over 40% in general, and even higher in patients with hematologic malignancies and those status post bone marrow transplant (9-12).
The major categories of disease caused by the Mucorales are sinonasal/rhinocerebral, pulmonary, cutaneous/subcutaneous, gastrointestinal, and disseminated infection (9-12). Sinonasal/rhinocerebral disease represents one-third to one-half of all cases, and is most often associated with diabetic ketoacidosis. Pulmonary disease is also common. Gastrointestinal zygomycosis (14-16) is relatively uncommon, but often fatal, with mortality reportedly approaching 40-50%. Although any portion of the alimentary tract can be affected, gastric and colonic involvement are the most frequent. Ulcers are the most common gross manifestation, often large, with rolled, irregular edges that may mimic malignancy. These fungi may also superinfect previously ulcerated tissues, and disseminated disease may arise from primary gastrointestinal infection.
The lesions caused by Mucor and related species are histologically very similar to those seen in aspergillosis (Figure 1) (17-18). Both Mucor and Aspergillus are angioinvasive (Figure 2), leading to thrombosis and areas of infarction and necrosis. The inflammatory response is variable, but neutrophils are usually prominent (Figure 3). Mucor produces broad, ribbon-like pauciseptate hyphae with irregular walls, which branch randomly at any angle (Figure 4). When cut at cross-section, they may appear optically clear. They do not produce spores. Segmented atypical forms can rarely be seen, which have a variable large, round to oval appearance and bear internal structures (Figure 3C).
Figure 1.
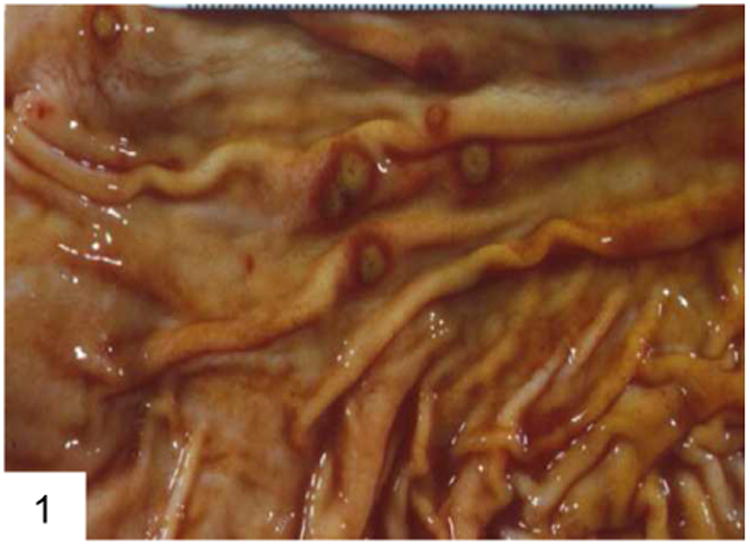
This case of gastric aspergillosis illustrates the classic “target lesions” produced by angioinvasive fungi, which occlude vessels and lead to surrounding infarction and necrosis (courtesy Dr. George F. Gray Jr.).
Figure 2.
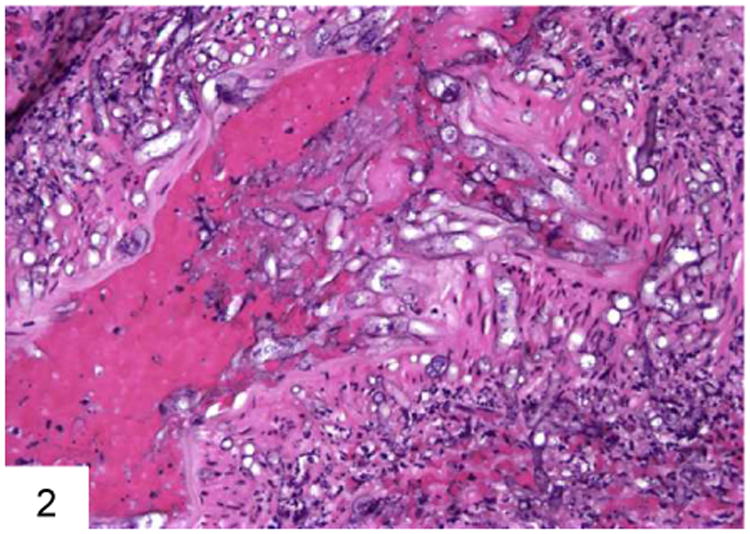
This blood vessel wall has been invaded by Mucor, with associated inflammation and necrosis. Note the large pauciseptate hyphae with optically clear centers on cross section.
Figure 3.
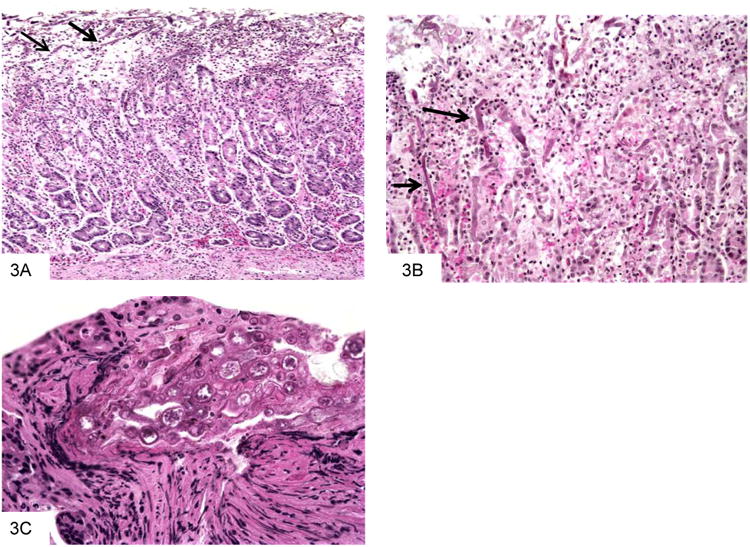
This gastric biopsy shows a predominantly neutrophilic infiltrate, with admixed fungal forms that are most easily seen near the mucosal surface (A). A higher power view shows the irregular, ribbon-like appearance of the hyphae (B). Segmented atypical forms are rarely seen, which have a variable large, round to oval appearance and bear internal structures (C).
Figure 4.
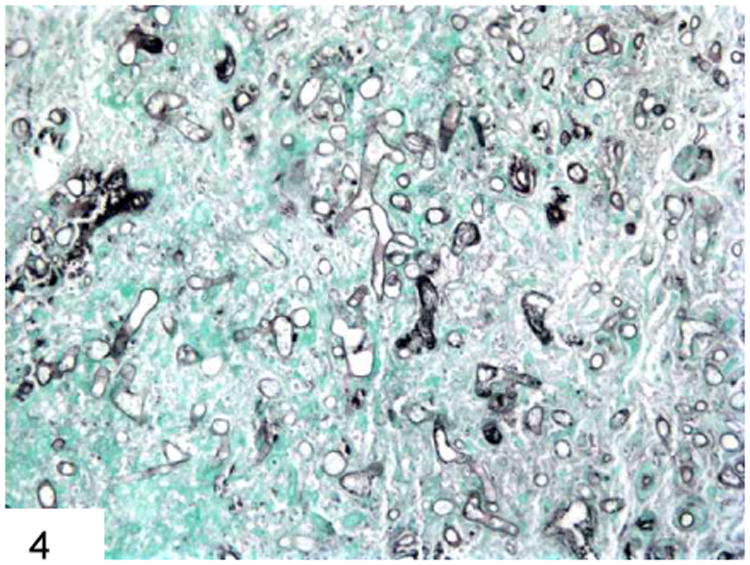
GMS stain shows the broad, pauciseptate ribbon-like hyphae of Mucor with irregular branches and optically clear centers, especially on cross-section.
Differential Diagnosis
The differential diagnosis of mucormycosis primarily includes other fungal infections (see Table 1). As above, aspergillosis and mucormycosis can closely mimic each other both clinically and morphologically (Figure 5) (17-18). In contrast to Mucor, however, Aspergillus has septate hyphae of uniform width that branch at acute angles. Fusarium, an emerging filamentous fungal pathogen that is also associated with neutropenia, closely mimics aspergillosis both clinically and radiographically, and is indistinguishable from Aspergillus on morphologic grounds alone (19).
Table 1. Morphologic Features of Filamentous Fungi in the GI Tract.
| Organism | Primary Geographic Distribution | Morphologic Features | Host Reaction | Major Differential Diagnoses |
|---|---|---|---|---|
| Aspergillus species (e.g. fumigatus, flavus, and niger) | Worldwide | Hyphae- Septate Uniform width Branching-Regular Acute angles Conidial head formation in cavitary lesions |
Ischemic necrosis with angioinvasion Acute inflammation Occasionally granulomatous |
Mucormycosis Fusarium |
| Candida albicans; Candida tropicalis | Worldwide | Mixture of budding yeast and pseudohyphae; occasional septate hyphae Yeast forms are Gram positive | Usually suppurative, with variable necrosis and ulceration Occasionally granulomatous Occasional angioinvasion |
Trichosporon (produces arthrocondia; disseminated disease in iron overload with immunosuppression especially AML; causes positive Cryptococcal latex agglutination test) |
| Candida (Torulopsis) glabrata | Worldwide | Budding yeast No hyphae No “halo” effect Yeast forms are Gram positive | Similar to other Candida species | Histoplasma Cryptococcus |
| Mucormycosis | Worldwide, associated with diabetics more than any other mycosis | Hyphae-Pauciseptate Ribbon-like Thin walls Branching-Haphazard | Similar to Aspergillus | Similar to Aspergillus |
| Basidiobolomycosis | Saudi Arabia, Africa, Parts of Asia; Arizona | Similar to mucor; fewer organisms, “cellophane ball” appearance | Eosinophilia, necrosis, granulomas, Splendore-Hoeppli reaction to organisms Produces mass | Mucor |
| Fusarium | Worldwide | Similar to Aspergillus; hyphae constricted at sites of origin | Similar to Aspergillus | Aspergillus Mucor |
| Phaeo-hyphomycosis (Dematiaceous Fungi) | Worldwide; associated with immuno-suppression | Pigmented; hyphae are closely septate and constructed at septations. Budding and vesicular swellings may be present. | Granulomatous inflammation with associated giant cells, necrosis, and dense fibrosis | Chromo-blastomycosis |
Figure 5.
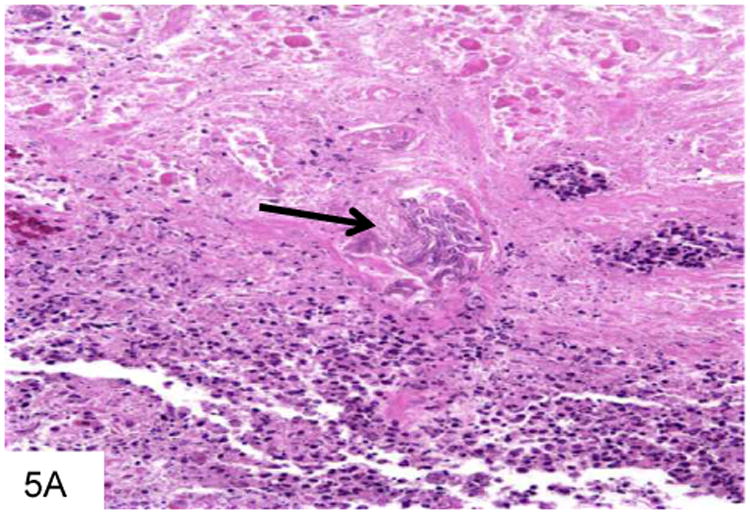
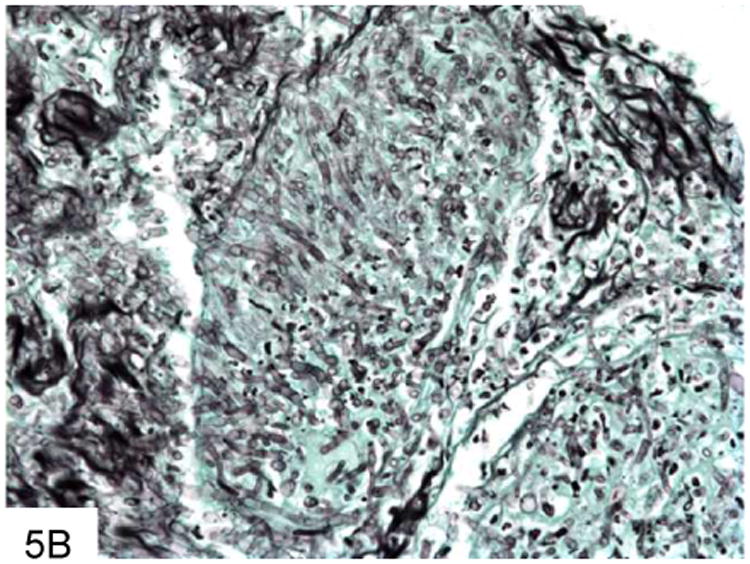
This gastric biopsy shows a vessel occluded with Aspergillus, with surrounding necrosis and infarction (A). The septate hyphae of Aspergillus are narrower than Mucor, as seen on this GMS stain, and branch at acute angles (B). Note the vessel occluded by fungi in the middle of the field.
Basidiobolomycosis
Basidiobolomycosis is another fungus in the differential diagnosis of mucormycosis that has recently gained attention as a gastrointestinal pathogen (20-27). The causative organism, Basidiobolus ranarum, is a zygomycete of the order Entomophthorales, and a worldwide soil saprophyte. This infection was originally described in Africa, Saudi Arabia, and Southeast Asia, but has recently gained attention in the United States, primarily in Arizona (24,26). Risk factors include pediatric age group, peptic ulcer disease, diabetes, pica, ranitidine use, and living in an endemic area. Of note, unlike many of the other filamentous fungi, typical causes of immunocompromise such as neutropenia, HIV/AIDS, and organ transplantation are not risk factors for this infection. Gastrointestinal infections may mimic malignancy or chronic idiopathic inflammatory bowel disease clinically, and pericolonic masses that are worrisome for colon cancer are common in this disease (27).
Although the fungi themselves are morphologically similar to Mucor, there are typically fewer of them on a given tissue section, and the fungi may have a “cellophane-ball” or crumpled appearance (Figure 6). In addition, Basidiobolus is not angioinvasive. The inflammatory response is also quite different from that of mucormycosis, featuring prominent eosinophilia, granulomas, and a Splendore-Hoeppli reaction to organisms (Figure 7).
Figure 6.
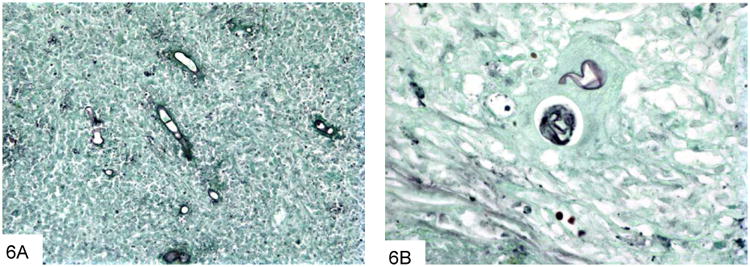
This GMS stain of basidiobolomycosis shows fewer organisms than typically seen with Mucor, rare septae, and optically clear centers on cross-section. There is very little branching (A). Basidiobolus may show a “crumpled cellophane ball” appearance as well.
Figure 7.
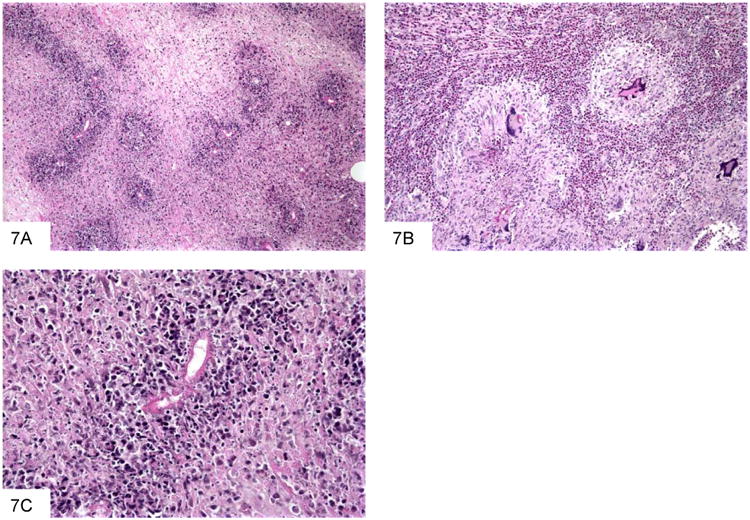
This section of a paracolonic mass due to B. ranarum shows extensive necrosis and scattered organisms highlighted by the bright pink protein deposition known as Splendore-Hoeppli phenomenon (A). Basidiobolomycosis often features granulomas with giant cells, and abundant eosinophils (B). This high power view shows a fungus outlined by bright pink proteinaceousmaterial (C).
Phaeohyphomycosis
Phaeohyphomycosis refers to infections caused by various (over 70 recognized species) dematiaceous or naturally pigmented fungi that develop as black molds in culture, and are visible as pigmented brown organisms in tissue sections. These fungi are worldwide saphrophytes present in soil, wood, and compost. Infections typically occur in immunocompromised hosts, and risk factors include neutropenia, bone marrow or solid organ transplantation, and exposure to dirt/soil through farming, gardening, or other outdoor work (28-34). Infections are most often subcutaneous, but gastrointestinal infections rarely occur, probably through colonization of pre-existing ulcers (35). The inflammatory reaction is mixed, and may consist of neutrophils, granulomas, or both. The organisms appear as variable sized, often branched fungi that are pigmented (Figure 8), although the pigment may be difficult to detect on H&E in some cases (17). Fontana Masson stains may be useful in accentuating the melanin pigment. (Table 1)
Figure 8.
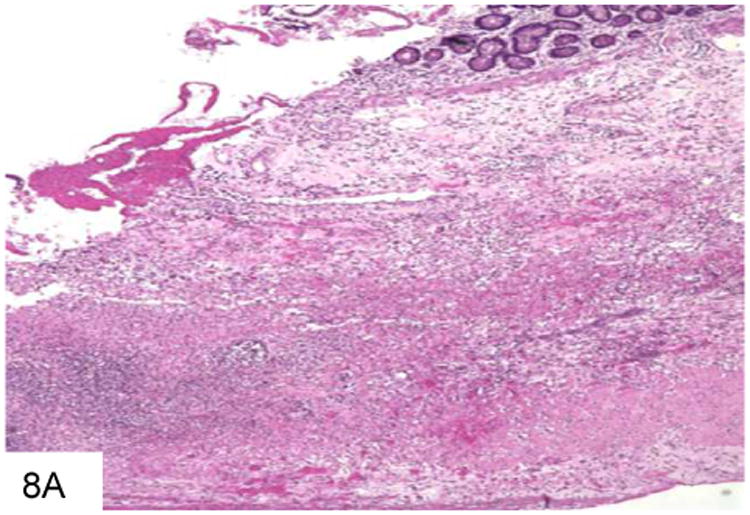
This duodenal ulcer with transmural inflammation and necrosis is from a plasma cell myeloma patient who presented with an acute abdomen (A). The necrotic ulcer debris contains numerous pigmented filamentous fungi, indicating an infection by phaeohyphomycosis (B).
Ancillary Tests
Accurate speciation of fungal infections is critical, because it significantly affects antifungal therapy. Culture remains the gold standard of diagnosis and speciation for fungal infections but it may require a substantial amount of time for cultures to grow and to achieve speciation. More importantly, because of the clinical setting, culture material is not always available. The use of 18S rRNA sequence to identify fungus directly by polymerase chain reaction and sequencing is increasingly available as well, and can be performed directly from either culture material or the paraffin tissue block. The phaeohyphomycoses cannot be speciated with certainty morphologically, and culture or molecular studies are required for definite classification. If no material is available for culture, then molecular assays should be attempted, or the patient should be re-biopsied so that cultures can be performed.
In addition to molecular testing, there are two serological laboratory tests (36) that are helpful for distinguishing between the various filamentous fungi that may be encountered on a slide. Galactomannan is a component of the Aspergillus cell wall that is released during growth. The galactomannan assay is a serologic test that is helpful in diagnosing invasive aspergillosis, as it is positive in invasive aspergillosis but negative in other invasive fungal infections. Thegalactomannan assay will cross-react with Penicillium marneffei, however(see below). The beta (1.3) D glucan assay is also helpful, as it is positive in virtually all disseminated fungal infections including Pneumocystis infection, but is negative inmucormycosis.
The Differential Diagnosis of Yeast
Candida species are what most often comes to mind when considering gastrointestinal infections with yeast forms, and Cryptococcus remains the most common cause of mortality due to fungal infection in the HIV population (37). However, many other less common but important yeasts are emerging as important pathogens in the immunocompromised patient population.
Penicillium marneffei is a dimorphic fungus that is endemic in Thailand, China, Hong Kong, Vietnam, and Indonesia. Patients with this infection who are encountered in the USA have almost always either traveled in these areas or lived there (38-42). Penicilliosisis emerging as one of the most common opportunistic infections in Asian patients with AIDS (39, 42). Patients with this infection who are HIV-negative often have other immunocompromising conditions such as hematologic malignancies, autoimmune diseases, malnutrition or other debilitating infections, and this infection has rarely been described in immunocompetent persons. P. marneffei most commonly involves the lungs and liver, followed by the GI tract. Dissemination can occur in a matter of a few weeks and be quickly fatal, especially in immunocompromised patients.
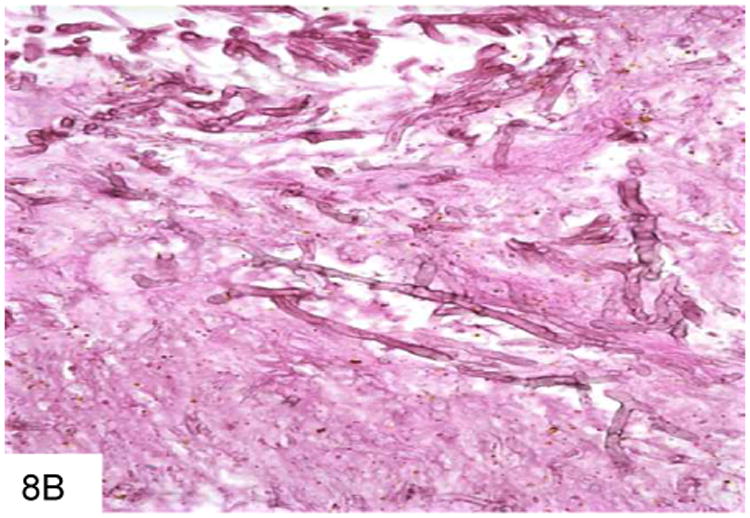
The organism infects the mononuclear phagocyte system, and multiplies within histiocytes that enlarge to accommodate increasing numbers of organisms. The inflammatory response may be granulomatous, suppurative, or mixed (Figure 9). The organisms themselves are spherical to ovoid, and pack and distend the involved macrophages. As the lesions expand, necrosis predominates, and the macrophages may lyse and release free organisms. The fungi are typically small (2-5 microns) and resemble histoplasma; however, occasional elongated and/or curved forms with a central septum and rounded ends (“pill capsule” form) may be present (Figure 10), and these may measure up to 20 microns. In addition, P. marneffei do not bud, as they divide at their central septum by fission.
Figure 9.
This colon biopsy is from a Vietnam veteran who developed diarrhea after undergoing chemotherapy. The mixed inflammatory infiltrate contains numerous macrophages that are distended by P. marneffei (A). This smear from a liver abscess in a Chinese patient shows numerous macrophages distended by P. marneffei, and surrounded by a mixed inflammatory infiltrate (B).
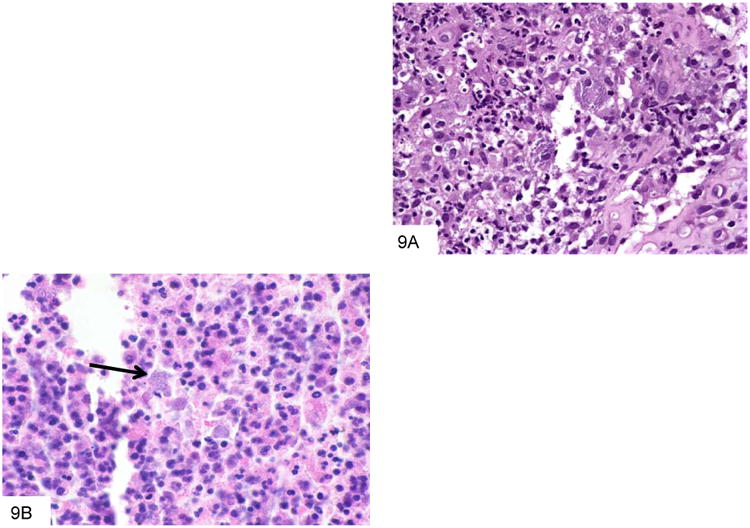
Figure 10.
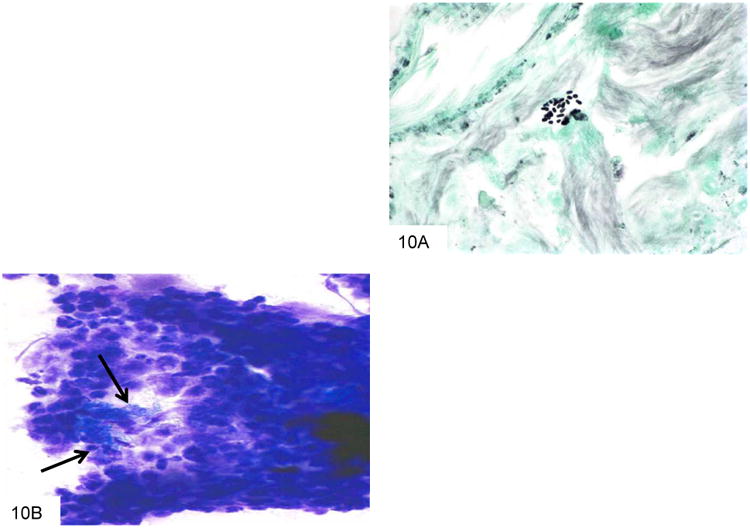
This GMS stain from the colon biopsy above shows P. marneffei that have been released from a lysed macrophage. The larger fungi have rounded ends, and some have a transverse septum at the point at which the fungus appears “pinched” in the middle (A). This Wright-Giemsa smear from a liver abscess shows a macrophage that has burst, and is releasing the numerous fungi that it contained (B).
Differential Diagnosis
Histoplasmosis
The major organism in the morphological differential diagnosis is Histoplasma capsulatum, as both may be intracellular and are of similar size (43-44). Histoplasma capsulatum is endemic to the central United States but has been described in many nonendemic areas as well. GI involvement occurs in more than 80% of patients with disseminated infection. Histologic findings include diffuse lymphohistiocytic infiltrates and nodules, usually involving the mucosa and submucosa, with associated ulceration (Figure 11). These lesions are often located over lymphoid aggregates. Discrete granulomas and giant cells are present in only a minority of cases. In immunocompromised patients, large numbers of organisms may be seen with virtually no tissue reaction. Whereas Histoplasma have easily identified buds at their pointed pole (Figure 12) P. marneffei does not bud and has a transverse septum. On H&E staining, Histoplasma with in macrophages also have a surrounding small “halo,” reflecting the thin, poorly stained cell wall in contrast to the basophilic cytoplasm. In addition, the areas in which these two yeasts are endemic are very distinct.
Figure 11.
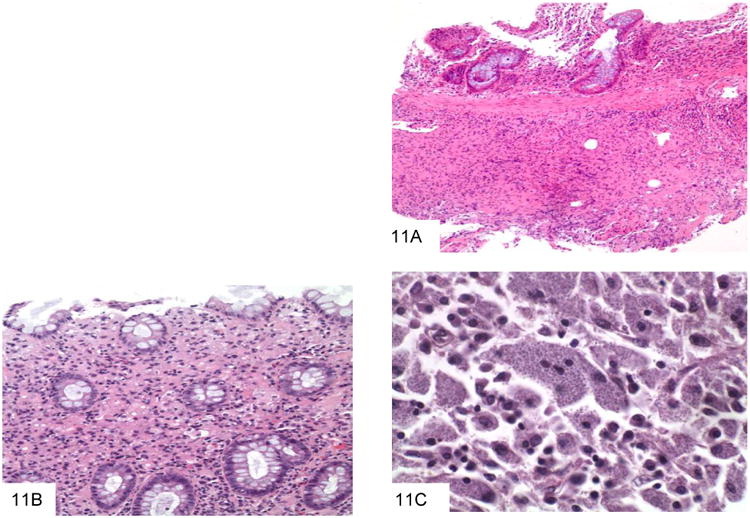
This colon biopsy shows a submucosal lymphohistiocytic infiltrate, typical of gastrointestinal histoplasmosis (A). A second colon biopsy shows a lymphohistiocytic infiltrate expanding the lamina propria. Small dot-like organisms with a surrounding halo can be appreciated within histiocytes (B). This high power view shows macrophages distended by Histoplasm capsulatum, which have a white halo surrounding each fungus (C).
Figure 12.
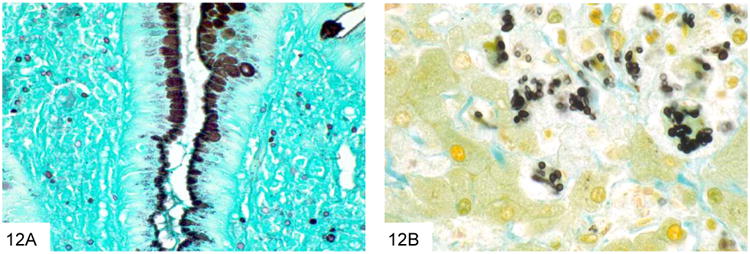
This GMS stain of the colon biopsy from Figure 11 shows small, similarly sized yeast within the lamina propria, characteristic of Histoplasma (A). A high power GMS stain shows that the yeast are pointed at one pole, which may give rise to narrow-based buds (B, courtesy Dr. A. Brian West).
Cryptococcosis
Cryptococcus neoformans is a very commonly encountered yeast in the immunocompromised patient population that is an unusual but important cause of GI infection (45-46). Virtually all patients with GI cryptococcosis have hematogenously disseminated disease with multisystem organ involvement, and most have associated pulmonary and meningeal disease. The inflammatory reaction is variable and depends on the immune status of the host, ranging from a suppurative, necrotizing inflammatory reaction, often with granulomatous features, to virtually no reaction such as in anergic hosts. Cryptococcus (measuring 4-7 microns) is larger than P. marneffei, typically shows considerable variation in size (Figure 13). Cryptococcus is round to oval and often has a “soap-bubble” area of clearing around the organism representing the poorly stained capsule on H&E sections. It also has frequent budding, and lacks the transverse septum of P. marneffei. Cryptococcus stains with Fontana-Masson and often with mucicarmine. Of note, capsule-deficient Cryptococcus neoformans and Cryptococcus gattii (an emerging variant of Cryptococcus that is particularly prominent in the Pacific Northwest [47-48]) will often be negative with mucicarmine staining, and require Fontana-Masson or other diagnostic methods for diagnosis (Figure 14).
Figure 13.
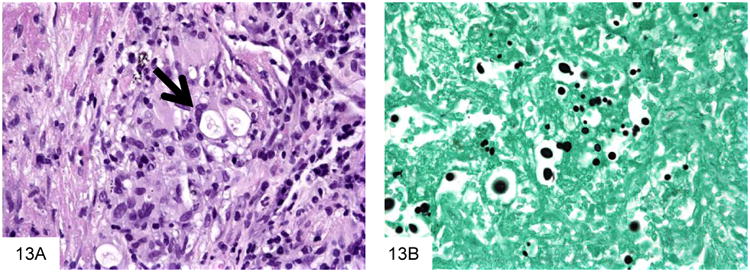
Cryptococcus neoformans are surrounded by a “soap bubble,” indicating the capsule, and then by a granulomatous reaction (A, courtesy Dr. Matthew Lindberg). This GMS stain shows that Cryptococcus are very pleomorphic in size, and have occasional narrow-based buds (B).
Figure 14.
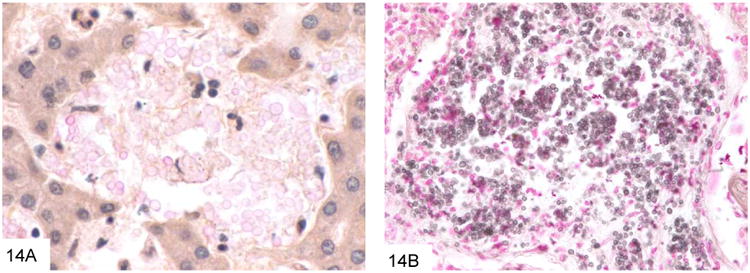
This example of capsule-deficient Cryptococcus neoformans in an AIDS patient shows that only rare fungi show significant mucin positivity (A). The Fontana-Masson stain (B) can be very useful in staining cryptococci, especially those that are capsule-deficient. Note the variation in size as well.
Pneumocystosis
Pneumocystis jiroveci (formerly carinii) is also an important entity in this patient population. Although the life cycle of this organism more closely resembles that of a protozoan, there is convincing molecular evidence indicating that P. jiroveci has greater homology with fungi. Extrapulmonary (including GI) involvement is not uncommon in the immuncompromised population (49-50), and in addition to patients with AIDS, Pneumocystis infection rarely has been reported in the context of organ transplant, hematologic malignancy, other immunodeficiency states, and steroid therapy. Pneumocystis infection has also been reported in association with infliximab therapy, an immunosuppressive treatment for Crohn's disease and rheumatoid arthritis (50). Microscopically, granular, foamy eosinophilic casts similar to those seen in pulmonary Pneumocystis infection may be seen in mucosal vessels or in the lamina propria, which helps differentiate this from other types of infection (Figure 15). As in the lung, a wide variety of inflammatory responses may occur, including granulomatous inflammation, prominent macrophage infiltrates, and necrosis. The organisms are 5-7 micron spherules that have cup or crescent shapes when collapsed (Figure 16), but lack the transverse septum of P. marneffei, and do not pack and distend macrophages. Many contain characteristic single or paired comma-shaped internal structures. Organisms stain with GMS and Toluidine blue. Pneumocystis does not bud, as well, which helps differentiate them from other similarly sized yeast.
Figure 15.
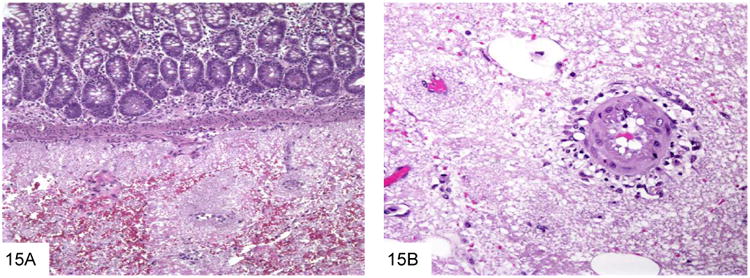
The foamy casts typical of Pneumocystis are seen in the submucosa of the small bowel in this AIDS patient with disseminated Pneumocystis infection (A, courtesy Dr. Henry Appelman). The foamy casts extend to surround vessels in the submucosa and mesentery (B).
Figure 16.
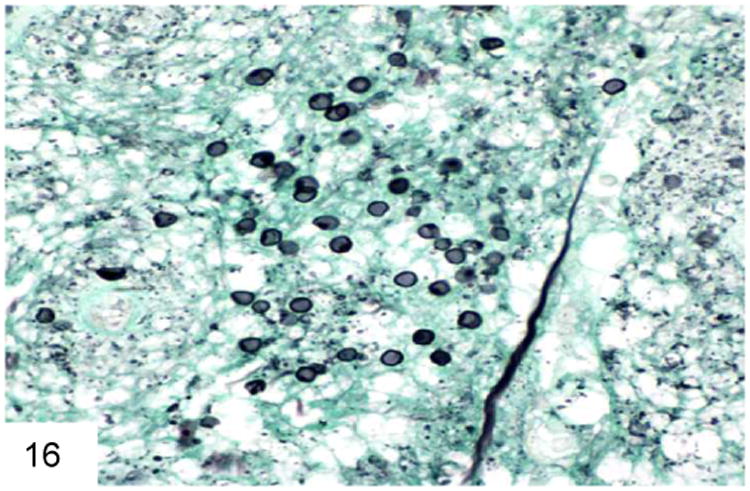
Pneumocystis has a cup-shaped or “deflated football” appearance when collapsed. Some of them contain a delicate internal dot structure (GMS). Note the lack of buds or a transverse septum.
Other entities in the differential diagnosis include Candida glabrata, which features tiny budding yeast forms of similar size to Histoplasma and P. marneffei, but does not produce hyphae or pseudohyphae (Figure 17) (17). C. glabrata also have more frequent buds than Histoplasma, are often extracellular, and lack the “halo” that Histoplasma have in tissue sections. C. glabrata does not typically pack and distend macrophages, and lacks the transverse septum of P. marneffei. Candida species yeast forms will also stain Gram positive on Gram stain.
Figure 17.
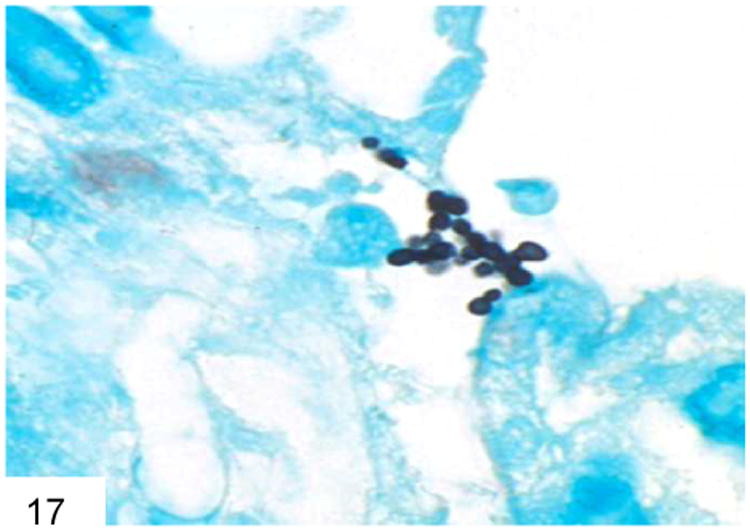
Candida glabrata does not form hyphae, only buds, and is often extracellular rather than within a macrophage (GMS).
Occasionally other types of organisms such as Leishmania or Toxoplasma may enter the differential diagnosis of the smaller yeasts. Leishmania have a characteristic kinetoplast, are GMS negative, and stain with Giemsa stain (Figure 18). Toxoplasma is also GMS negative and stains with Giemsa (Figure 19).
Figure 18.
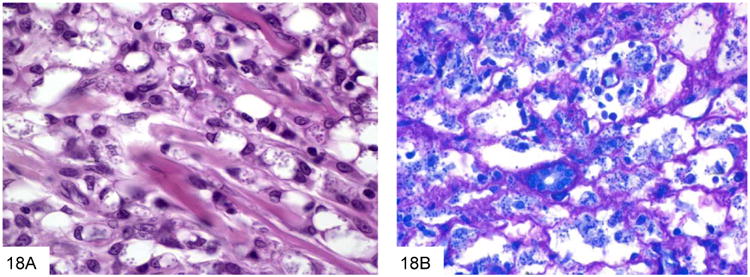
Leishmania have a characteristic kinetoplast, as seen here in this case of visceral leishmaniasis with numerous organisms within macrophages (A, courtesy Dr. Rhonda Yantiss). These parasitesare GMS negative, and stain with Giemsa stain (B).
Figure 19.
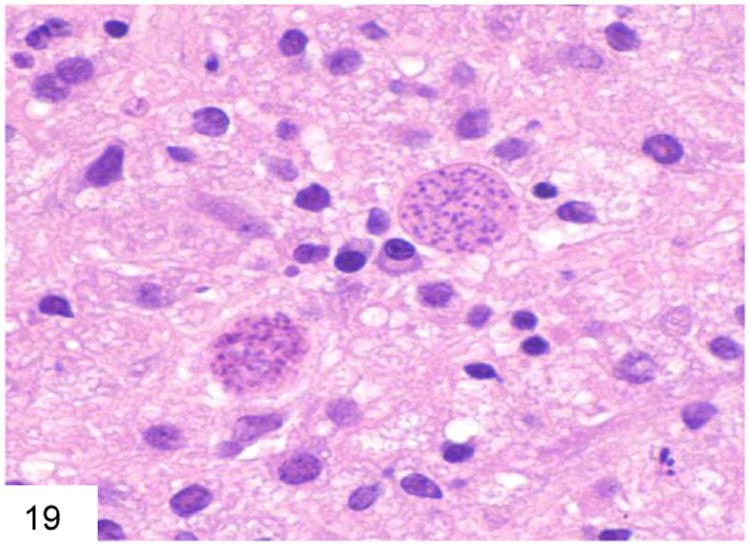
The cyst forms of Toxoplasma may mimic organisms within a macrophage as well. Toxoplasma is also GMS negative and Giemsa positive (courtesy Dr. Becky Wheeler).
Ancillary Tests
As with the filamentous fungi, accurate speciation of these invasive yeasts is critical, because it significantly affects therapy. When material is available, cultures are extremely helpful, although diagnostic features may be present on tissue sections that allow institution of therapy without waiting for mycological culture results. Molecular assays for many of these yeasts are increasingly widely available as well, and can often be performed directly from the paraffin block. Immunohistochemistry and in-situ hybridization assays are available for some of these entities, but are not widely available.
As mentioned above, serologic tests (36) may be helpful in this category of fungal infections as well. The galactomannan assay for detection of Aspergillus infection will cross-react with P. marneffei (51), which may have utility as the organisms appear quite different in tissue. The beta (1.3) D glucan assay is also helpful, as it is positive in Pneumocystis infection. (Table 2)
Table 2. Characteristics of Morphologically Distinct Yeast In Human Tissue.
| Organism | Exposure | Histology |
|---|---|---|
| Histoplasma capsulatum; Histoplasma duboisii | Ohio River Valley/Lower Mississippi River (NA); East Southern Cone (SA); Central Africa | Small, round to oval yeast (2 to 6 uM) with narrow based budding; “halo” effect |
| Penicillium marneffei | Southeast or Far East Asia | Small, round to oval yeast (2-5) with occasional large septated forms “pill form” |
| Cryptococcus neoformans (plus 36 additional species) | Worldwide distribution | Small, round to oval yeast (5 to 10 uM) with narrow based budding; pleomorphic in size; “soap bubble” Fontana-Masson positive |
| C. glabrata | Worldwide distribution | Budding yeast without hyphae; may be extracellular Gram positive |
Acknowledgments
Source of Funding:Dr. Danny Milner a) is currently receiving grant funds for Malaria research from the NIH, b) has been paid money by Up To Date for malaria and stool diagnostics, and c) has been paid money by Biosciences Solutions Group, LLC as a partial owner of laboratory consulting firm. Dr. Keith Lai has nothing to declare.
Footnotes
Conflicts of Interest: Dr. Laura Lamps receives royalties from Amirsys Publishing Company for chapter royalties;
Contributor Information
Laura W. Lamps, Dept. of Pathology, University of Arkansas for Medical Sciences, 4301 W. Markham Street, Slot 517, Little Rock, AR, 72205, 501-296-1458, Fax: 501-603-1479
Keith K.T. Lai, Dept. of Pathology, University of Arkansas for Medical Sciences, 4301 W. Markham Street, Slot 517, Little Rock, AR, 72205, 501-686-5182, Fax: 501-603-1479
Dr. Danny A. Milner, Jr., Dept. of Pathology, Brigham and Women's, Harvard Medical School, 75 Francis Street, MR-7, Boston, MA 02115, 617 -525- 7761.
References
- 1.Malcolm TR, Chin-Hong PV. Endemic mycoses in immunocompromised hosts. Curr Infect Dis Rep. 2013;15:536–43. doi: 10.1007/s11908-013-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis M. Invasive fungal infections: evolving challenges for diagnosis and therapeutics. MolImmunol. 2001;38:947–57. doi: 10.1016/s0161-5890(02)00022-6. [DOI] [PubMed] [Google Scholar]
- 3.Fleming RV, Walsh TJ, Anaissie EJ. Emerging and less common fungal pathogens. Infect Dis Clin N Am. 2002;16:915–33. doi: 10.1016/s0891-5520(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 4.Dave M, Purohit T, Razonable R, Loftus EV., Jr Opportunistic infections due to inflammatory bowel disease therapy. Inflamm Bowel Dis. 2013 Sep 18; doi: 10.1097/MIB.0b013e3182a827d2. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Parize P, Rammaert B, Lortholary O. Emerging invasive fungal diseases in transplantation. Curr Infect Dis Rep. 2012;14:668–75. doi: 10.1007/s11908-012-0296-y. [DOI] [PubMed] [Google Scholar]
- 6.Schwesinger G, Junghans D, Schroder G, et al. Candidosis and aspergillosis as autopsy findings from 1994-2003. Mycoses. 2005;48:176–80. doi: 10.1111/j.1439-0507.2005.01101.x. [DOI] [PubMed] [Google Scholar]
- 7.Bitar D, Van Cauteren D, Lanternier F, et al. Increasing incidence of zygomycosis (mucormycosis), France, 1997-2006. Emerg Infect Dis. 2009;15(9):1395–401. doi: 10.3201/eid1509.090334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dictar MO, Maiolo E, Alexander B, et al. Mycoses in the transplanted patient. Med Mycol. 2000;38(Suppl 1):251–8. [PubMed] [Google Scholar]
- 9.Gonzalez CE, Rinaldi MG, Sugar AM. Zygomycosis. Inf Dis Clin N Am. 2002;16:895–914. doi: 10.1016/s0891-5520(02)00037-5. [DOI] [PubMed] [Google Scholar]
- 10.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–53. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 12.Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556–69. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hibbett DS, Binder M, Bischoff JF, et al. A higher-level phylogenetic classification of the fungi. Mycol Res. 2007;111:509–47. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Lyon DT, Schubert TT, Mantia AG. Phycomycosis of the gastrointestinal tract. Am J Gastroenterol. 1979;72:379–94. [PubMed] [Google Scholar]
- 15.Thomson SR, Bade PG, Taams M, Chrystal V. Gastrointestinal mucormycosis. Brit J Surg. 1991;78:952–4. doi: 10.1002/bjs.1800780819. [DOI] [PubMed] [Google Scholar]
- 16.Radhakrishnan N, Yadav SP, Oberoi J, et al. Intestinal mucormycosis: a rare entity in pediatric oncology. Pediatr Hematol Oncol. 2013;30:178–83. doi: 10.3109/08880018.2013.769286. [DOI] [PubMed] [Google Scholar]
- 17.Chandler FW, Watts JC. Pathologic Diagnosis of Fungal Infections. Chicago: ASCP Press; 1987. [Google Scholar]
- 18.Young RC, Bennett JE, Vogel CL, et al. Aspergillosis: the spectrum of the disease in 98 patients. Medicine. 1970;49:147–73. doi: 10.1097/00005792-197003000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Martino P, Gastaldi R, Raccah R, Girmenia C. Clinical patterns of Fusarium infections in immunocompromised patients. J Infection. 1994;28(Suppl 1):7–15. doi: 10.1016/s0163-4453(94)95911-0. [DOI] [PubMed] [Google Scholar]
- 20.Smilack JD. Gastrointestinal basidiobolomycosis. Clin Infect Dis. 1998;27:663–4. doi: 10.1086/517154. [DOI] [PubMed] [Google Scholar]
- 21.El-Shabrawi MH, Kamal NM. Gastrointestinal basidiobolomycosis in children: an overlooked emerging infection? J Med Microbiol. 2011;60:871–80. doi: 10.1099/jmm.0.028670-0. [DOI] [PubMed] [Google Scholar]
- 22.Al Jarie A, Al-Mohsen I, Al Jumaah S, et al. Pediatric gastrointestinalbasidiobolomycosis. Pediatr Infect Dis J. 2003;22(11):1007–14. doi: 10.1097/01.inf.0000095166.94823.11. [DOI] [PubMed] [Google Scholar]
- 23.Khan ZU, Khoursheed M, Makar R, et al. Basidiobolus ranarum as an etiologic agent of gastrointestinal zygomycosis. J Clin Microbiol. 2001;39(6):2360–3. doi: 10.1128/JCM.39.6.2360-2363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC) Gastrointestinal basidiobolomycosis-Arizona, 1994-1999. MMWR Morb Mortal Wkly Rep. 1999 Aug 20;48(32):710–3. [PubMed] [Google Scholar]
- 25.Yousef OM, Smilack JD, Kerr DM, et al. Gastrointestinal basidiobolomycosis. Morphologic findings in a cluster of six cases. Am J Clin Pathol. 1999;112(5):610–6. doi: 10.1093/ajcp/112.5.610. [DOI] [PubMed] [Google Scholar]
- 26.Lyon GM, Smilack JD, Komatsu KK, et al. Gastrointestinal basidiobolomycosis in Arizona: clinical and epidemiological characteristics and review of the literature. Clin Infect Dis. 2001;32(10):1448–55. doi: 10.1086/320161. [DOI] [PubMed] [Google Scholar]
- 27.Nemenqani D, Yaqoob N, Khoja H, et al. Gastrointestinal basidiobolomycosis: an unusual fungal infection mimicking colon cancer. Arch Pathol Lab Med. 2009;133(12):1938–42. doi: 10.5858/133.12.1938. [DOI] [PubMed] [Google Scholar]
- 28.Adam RD, Paquin EA, et al. Phaeophyphomycosis caused by the fungal genera Bipolaris and Exserohilum: a report of 9 cases and review of the literature. Medicine. 1986;65:203–17. doi: 10.1097/00005792-198607000-00001. [DOI] [PubMed] [Google Scholar]
- 29.McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am AcadDermatol. 1983;8:1–16. doi: 10.1016/s0190-9622(83)70001-0. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Ami R, Lewis RE, Raad II, Kontoyiannis DP. Phaeohyphomycosis in a tertiary care cancer center. Clin Infect Dis. 2009;48(8):1033–41. doi: 10.1086/597400. [DOI] [PubMed] [Google Scholar]
- 31.Queiroz-Telles F, Nucci M, Colombo AL, Tobón A, Restrepo A. Mycoses of implantation in Latin America: an overview of epidemiology, clinical manifestations, diagnosis and treatment. Med Mycol. 2011;49(3):225–36. doi: 10.3109/13693786.2010.539631. [DOI] [PubMed] [Google Scholar]
- 32.Brandt ME, Warnock DW. Epidemiology, clinical manifestations, and therapy of infections caused by dematiaceous fungi. J Chemother. 2003;15(Suppl 2):36–47. doi: 10.1179/joc.2003.15.Supplement-2.36. [DOI] [PubMed] [Google Scholar]
- 33.Revankar SG, Patterson JE, Sutton DA, et al. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin Infect Dis. 2002;15:467–76. doi: 10.1086/338636. [DOI] [PubMed] [Google Scholar]
- 34.Rohwedder JJ, Simmons JL, Colfer H, et al. Disseminated Curvularia lunata infection in a football player. Arch Intern Med. 1979;139:940–1. [PubMed] [Google Scholar]
- 35.Woo PC, Ngan AH, Tsang CC, et al. Clinical spectrum of Exophiala infections and a novel Exophiala species, Exophialahongkongensis. J Clin Microbiol. 51(1):260–7. 2013. doi: 10.1128/JCM.02336-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. 2005;5:609–22. doi: 10.1016/S1473-3099(05)70238-3. [DOI] [PubMed] [Google Scholar]
- 37.Walsh TJ, Groll A, Hiemenz J, et al. Infections due to emerging and uncommon medically important fungal pathogens. Clin Microbiol Infect. 2004;10(Suppl 1):48–66. doi: 10.1111/j.1470-9465.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 38.Ko CI, Hung CC, Chen MY, Hsueh PR, Hsiao CH, Wong JM. Endoscopic diagnosis of intestinal Penicilliosis marneffei: report of three cases and review of the literature. Gastrointest Endosc. 1999;50(1):111–4. doi: 10.1016/s0016-5107(99)70359-7. [DOI] [PubMed] [Google Scholar]
- 39.Borradori L, Schmit JC, Stetzkowski M, et al. Penicilliosis marneffei infection in AIDS. J Am Acad Dermatol. 1994;31(5 Pt 2):843–6. doi: 10.1016/s0190-9622(94)70242-x. [DOI] [PubMed] [Google Scholar]
- 40.Wong KF. Marrow penicilliosis: a readily missed diagnosis. Am J Clin Pathol. 2010;134:214–18. doi: 10.1309/AJCPWVBQCW13DJLO. [DOI] [PubMed] [Google Scholar]
- 41.Ukarapol N, Sirisanthana V, Wongsawasdi L. Penicillium marneffei mesenteric lymphadenitis in human immunodeficiency virus-infected children. J Med Assoc Thai. 1998;81(8):637–40. [PubMed] [Google Scholar]
- 42.Bulterys PL, Le T, Quang VM, et al. Environmental predictors and incubation period of AIDS-associated penicillium marneffei. Clin Infect Dis. 2013;56:1273–9. doi: 10.1093/cid/cit058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamps LW, Molina CP, West AB, et al. The pathologic spectrum of gastrointestinal and hepatic histoplasmosis. Am J ClinPathol. 2000;113:64–72. doi: 10.1309/X0Y2-P3GY-TWE8-DM02. [DOI] [PubMed] [Google Scholar]
- 44.Cappell MS, Mandell W, Grimes MM, et al. Gastrointestinal histoplasmosis. Dig Dis Sci. 1988;33:353–60. doi: 10.1007/BF01535762. [DOI] [PubMed] [Google Scholar]
- 45.Washington K, Gottfried MR, Wilson ML. Gastrointestinal cryptococcosis. Mod Pathol. 1991;4711:707. [PubMed] [Google Scholar]
- 46.Bonacini M, Nussbaum J, Ahluwalia C. Gastrointestinal, hepatic, and pancreatic involvement with Cryptococcus neoformans in AIDS. J Clin Gastroenterol. 1990;12:295–7. doi: 10.1097/00004836-199006000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Harris JR, Lockhart SR, Debess E, et al. Cryptococcus gattii in the United States: clinical aspects of infection with an emerging pathogen. Clin Infect Dis. 2011;53:1188–95. doi: 10.1093/cid/cir723. [DOI] [PubMed] [Google Scholar]
- 48.Dixit A, Carroll SF, Quereshi ST. Cryptotoccus gattii: an emerging cause of fungal disease in North America. Interdiscip Perspect Infect Dis. doi: 10.1155/2009/840452. Epub 2009 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dieterich DT, Lew EA, Bacon DJ, et al. Gastrointestinal pneumocystosis in HIV-infected patients on aerosolized pentamidine: Report of five cases and review of the literature. Am J Gastroenterol. 1992;87:1763–1770. [PubMed] [Google Scholar]
- 50.Kaur N, Mahl TC. Pneumocystis jiroveci (carinii) pneumonia after infliximab therapy: a review of 84 cases. Dig Dis Sci. 2007;52:1481–4. doi: 10.1007/s10620-006-9250-x. [DOI] [PubMed] [Google Scholar]
- 51.Van Cutsem J, Meulemans L, van Gerven F, Stynen D. Detection of circulating galactomannan by Pastorex Aspergillus in experimental invasive aspergillosis. Mycoses. 1990;33:61–69. doi: 10.1111/myc.1990.33.2.61. [DOI] [PubMed] [Google Scholar]


