Abstract
Background
Subanesthetic doses of (R,S)-ketamine are used in the treatment of neuropathic pain and depression. In the rat, the antidepressant effects of (R,S)-ketamine are associated with increased activity and function of mammalian target of rapamycin (mTOR); however, (R,S)-ketamine is extensively metabolized and the contribution of its metabolites to increased mTOR signaling is unknown.
Methods
Rats (n = 3/time point) were given (R,S)-ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine and their effect on the mTOR pathway determined after 20, 30, and 60 minutes. PC-12 pheochromocytoma cells (n = 3/experiment) were treated with escalating concentrations of each compound and the impact on the mTOR pathway was determined.
Results
The phosphorylation of mTOR and its downstream targets was significantly increased in rat pre-frontal cortex tissue by more than ~2.5-, ~25- and ~2-fold, respectively, in response to a 60-min post-administration of (R,S)-ketamine, (R,S)-norketamine, and (2S,6S)-hydroxynorketamine (p<0.05, ANOVA analysis). In PC-12 pheochromocytoma cells, the test compounds activated the mTOR pathway in a concentration-dependent manner, which resulted in a significantly higher expression of serine racemase with ~2-fold increases at 0.05nM (2S,6S)-hydroxynorketamine, 10nM (R,S)-norketamine and 1000nM (R,S)-ketamine. The potency of the effect reflected antagonistic activity of the test compounds at the α7-nicotinic acetylcholine receptor.
Conclusions
The data demonstrate that (R,S)-norketamine and (2S,6S)-hydroxynorketamine have potent pharmacological activity both in vitro and in vivo and contribute to the molecular effects produced by sub-anesthetic doses of (R,S)-ketamine. The results suggest that the determination of the mechanisms underlying the antidepressant and analgesic effects of (R,S)-ketamine requires a full study of the parent compound and its metabolites.
Introduction
(R,S)-Ketamine is a rapid and long-lasting antidepressant agent used in the treatment of major depressive disorder and bipolar depression1–3. Data from studies in rats suggest that the rapid effect produced by (R,S)-ketamine is due to increased phosphorylation of the mammalian target of rapamycin (mTOR) and corresponding increases in the phosphorylated forms of the extracellular signal-regulated kinases (pERK1/ERK2), protein kinase B (pAkt), eukaryotic initiation factor 4E binding protein (p4E-BP1) and p70S6 kinase (pp70S6K)4 as well as a rise in the number and function of new spine synapses in the prefrontal cortex4,5.
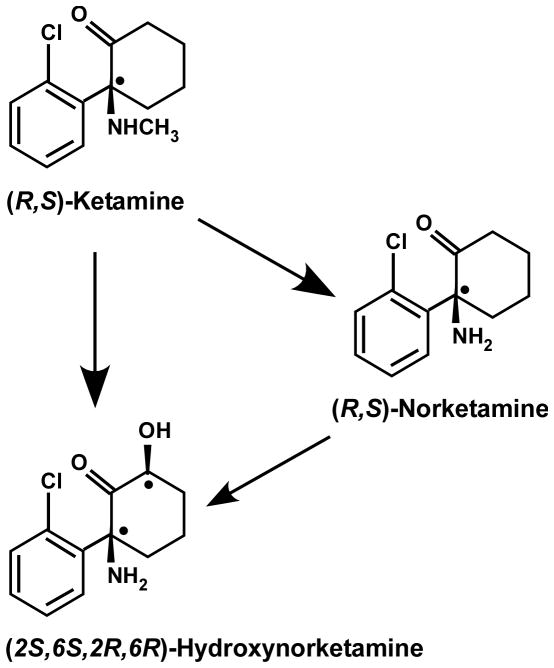
(R,S)-Ketamine is extensively transformed into the N-demethylated metabolite (R,S)-norketamine, two diastereomeric hydroxyketamines, a series of diastereomeric hydroxynorketamines, including (2S,6S;2R,6R)-hydroxynorketamine, and (R,S)-dehydronorketamine6–8; figure 1. In the rat, (R,S)-ketamine is rapidly converted to (R,S)-norketamine and (2S,6S;2R,6R)-hydroxynorketamine, which occurs within 2 min following an intravenous administration9. Similarly, administration of (R,S)-norketamine leads to the detection of both (R,S)-norketamine and (2S,6S;2R,6R)-hydroxynorketamine, whereas only (2S,6S;2R,6R)-hydroxynorketamine is present after intravenous administration of (2S,6S;2R,6R)-hydroxynorketamine9. The rapid and extensive metabolic transformation of (R,S)-ketamine is also observed in studies utilizing human microsomal preparations10 and in clinical studies2,3,11.
Figure 1.
Structures of (R,S)-ketamine, (R,S)-norketamine and (2S,6S;2R,6R)-hydroxynorketamine. (R,S)-Ketamine undergoes hepatic metabolism to be transformed into (2S,6S;2R,6R)-hydroxynorketamine directly and/or via (R,S)-norketamine as an intermediate.
(R,S)-Ketamine was developed as an anesthetic agent and initial pharmacodynamic studies demonstrated that both (R,S)-ketamine and (R,S)-norketamine produce anesthesia9, which is associated with non-competitive inhibition of the N-methyl-D-aspartate receptor (NMDAR)12. (2S,6S;2R,6R)-Hydroxynorketamine lacks anesthetic activity9 and the individual enantiomers of the compound, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine, are not active at the NMDAR13, α3β4-nicotinic acetylcholine receptor (α3β4-nAChR)13, or muscarinic and opioid receptors (unpublished data from competitive binding screen provided by Bryan Roth, M.D., Ph.D., NIMH Psychoactive Drug Screening, Chapel-Hill, North Carolina, 2013). However, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine are potent (IC50 <100 nM) and selective inhibitors of the α7-nAChR13. We have recently demonstrated that treatment of 1321N1 astrocytoma cells with the α7-nAChR selective inhibitor methyllycaconitine promotes the de novo protein synthesis of monomeric serine racemase (m-SR) due to increased levels of pERK1/2, pAkt and pmTOR14. A similar effect was observed with PC-12 cells14. Since (R,S)-norketamine, (2S,6S)-hydroxynorketamine and (2R,6R)-hydroxynorketamine are α7-nAChR inhibitors13, it is possible that the synaptogenesis and antidepressive effects observed in the rat after (R,S)-ketamine administration may be due, in part, to the activity of these metabolites. This possibility was examined in this study through the administration of (R,S)-ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine to male Wistar rats. The resultant brain tissue concentration profiles of the tested compounds and corresponding changes in the levels of pmTOR and related proteins were determined. The data were compared to the results obtained in PC-12 cells. The latter studies were undertaken to clarify the independent contribution of each of the tested compounds due to the rapid and significant in vivo conversion of (R,S)-ketamine into (R,S)-norketamine and (2S,6S;2R,6R)-hydroxynorketamine, and of (R,S)-norketamine to (2S,6S;2R,6R)-hydroxynorketamine. In the present study, (2S,6S)-hydroxynorketamine was utilized instead of (2R,6R)-hydroxynorketamine or the racemic mixture, a decision based on data indicating that this isomer was the most potent of the three compounds in the suppression of intracellular D-serine levels (unpublished data from preliminary in vitro study obtained following previously described protocol14 provided by Irving W. Wainer, Ph.D., DHC., Baltimore, Maryland, 2013) and its availability in our laboratory.
Material and Methods
Materials
(R,S)-Ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine were prepared as previously described11. Methyllycaconitine and (S)-nicotine were obtained from Sigma-Aldrich (St. Louis, MO). De-ionized water was obtained from a Milli-Q system (Millipore, Billerica, MA). All other chemicals used were of analytical grade.
Studies in the Wistar Rat
Animals
Male Wistar rats were obtained from Harlan (Livermore, CA) at 9–10 weeks of age and housed 1 to 3 per cage, in polycarbonate hanging cages. All rats (271 to 354 g of body weight) had ad libitum access to Harlan Teklad Certified Rodent Chow #2018C and reverse osmosis-purified water. Animal rooms were maintained at 68–73°F with 20–60% relative humidity and a 12-hr light/dark cycle. All animal procedures in this study were conducted in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals (1996) and the Animal Welfare Standards incorporated in 9 CFR Part 3, 1991. All study protocols were reviewed and approved by SRI’s Institutional Animal Care and Use Committee (SRI International, Menlo Park, CA).
Administration of (R,S)-ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine
The animals (n = 3 for each experiment) received either a single intraperitoneal injection of (R,S)-ketamine, 40 mg·kg−1 in saline (5 ml·kg−1), or a single intravenous injection of either (R,S)-norketamine, 20 mg·kg−1 in saline (5 ml·kg−1) or (2S,6S)-hydroxynorketamine, 20 mg·kg−1 in saline (5 ml·kg−1), administered via a jugular vein catheter. After (R,S)-ketamine injection, the rats were euthanized with an overdose of pentobarbital at 10, 30, 60 and 240 min whereas rats treated with (R,S)-norketamine and (2S,6S)-hydroxyketamine were euthanized with an overdose of pentobarbital at 10, 20, 60 and 240 min post-administration. In all cases, whole brains were promptly collected, rinsed with phosphate-buffered saline and stored frozen at −70°C until analysis.
Preparation of brain tissue samples
The frozen whole brains were thawed on ice and longitudinally dissected. One of the hemispheres was utilized for the determination of (R,S)-ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine concentrations. A portion of the prefrontal cortex of the other hemisphere was used for Western blotting. Brain tissue obtained from drug-free male Wistar rats was used as control tissue samples.
Analysis of brain tissue concentrations of (R,S)-ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine
The brain tissue sample was weighed (average weight 900 mg), suspended in 990 μl of water:methanol (3:2, v/v) and 10 μl of the internal standard 3,4,5,6-tetradeuterophenyl-(R,S)-ketamine HCl [10 μg·ml−1 in methanol] (Cerillant, Round Rock, TX). The mixture was homogenized on ice with a polytron homogenizer, centrifuged at 21,000 × g for 30 min and the supernatant collected. The analytes were isolated using 1 ml Oasis HLB solid phase extraction cartridges (Waters Corp., Waltham, MA). The cartridges were preconditioned with 1 ml of methanol, followed by 1 ml of water and then 1 ml of ammonium acetate [10 mM, pH 9.5]. The supernatants were added to the cartridges, followed by 1 ml of water and (R,S)-ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine were eluted with 1 ml of methanol. The eluent was transferred to an autosampler vial for analysis. The samples were assayed using a previously reported liquid chromatographic method utilizing mass spectrometric detection, which had been validated for use with clinical samples11. The method was cross-validated using whole brains obtained from drug-free Wistar rats and spiked drug concentrations ranging from 0.025 to 25 μM. The measured analyte concentrations were normalized using the weight of each tissue sample and reported as μM·g−1 tissue. Based upon 1 g of tissue, the lowest levels of quantification (LOQs) were 0.16 μM·g−1 tissue for (R,S)-ketamine, 0.18 μM·g−1 tissue for (R,S)-norketamine and 0.16 μM·g−1 tissue for (2S,6S)-hydroxynorketamine.
Western blotting
Cells and brain tissues (50 mg cortex) were lysed in radioimmunoprecipitation buffer (RIPA) containing ethylenediaminetetraacetic acid (EDTA) and ethylene glycol tetraacetic acid (EGTA) (Boston BioProducts, Ashland, MA) and supplemented with a protease inhibitor cocktail (Sigma-Aldrich) and phosphatase inhibitor cocktail sets I and II (EMD Millipore, Billerica, MA). Brain tissues were homogenized using PRO200 (PRO Scientific Inc., Oxford, CT) hand homogenizer for 15 sec on ice and soluble extracts were collected after centrifugation at 14000 × g for 20 min at 4 °C. Protein concentrations were determined in the clarified lysates using the bicinchoninic acid reagent (Thermo Fisher Scientific, Waltham, MA). Proteins (20 μg/well) were separated on 4 to 12% precast gels (Invitrogen, Carlsbad, CA) using SDS-polyacrylamide gel electrophoresis under reducing conditions and then electrophoretically transferred onto polyvinylidene fluoride membranes (Invitrogen). Western blotting experiments were performed according to standard methods, which involved a blocking step in 5% non-fat milk/0.1% Tween-20 in PBS and incubation with the primary antibody of interest, followed by incubation with a secondary antibody conjugated with the enzyme horseradish peroxidase. The detection of immunoreactive bands was performed by using the ECL Plus Western Blotting Detection System (GE Healthcare, Piscataway, NJ). The quantification of bands was done by volume densitometry using ImageJ software (National Institutes of Health, Bethesda, MD) and normalization to β-actin. The primary antibodies for the phosphorylated forms of ERK1/2 (pERK1/2; Thr202/Tyr204; cat. # 4376S), Akt (pAkt; Ser473; cat. # 9271S), mTOR (pmTOR; Ser2448; cat. # 2971), 4E-BP1 (p4E-BP1; Thr37/46; cat. # 2855), p70S6K (pp70S6K; Thr389; cat. # 9234), and total forms of ERK1/2 (cat. # 9108S), Akt (cat. # 4685S), 4E-BP1 (cat. # 9452), p70S6K (cat. # 2708) and mTOR (cat. # 2972) were obtained from Cell Signaling Technology (Beverly, MA). The primary antibodies for the determination of serine racemase (cat. # ab45434) and β-actin (cat. #ab6276) were purchased from Abcam, Inc. (Cambridge, MA). The antibodies were used at a dilution recommended by the manufacturers.
Studies in PC-12 cells
Maintenance of PC-12 cells
The PC-12 pheochromocytoma cell line derived from rat adrenal medulla was obtained from American Type Culture Collection (Manassas, VA). RPMI-1640, trypsin solution, phosphate-buffered saline, fetal bovine serum (FBS), sodium pyruvate (0.1 M), L-glutamine (0.2 M) and penicillin/streptomycin solution (containing 10,000 units·ml−1 penicillin and 10,000 μg·ml−1 streptomycin) were obtained from Quality Biological (Gaithersburg, MD), horse serum (heat inactivated) was purchased from Biosource (Rockville, MD) and HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer [1 M, pH 7.4] was obtained from Mediatech, Inc. (Manassas, VA). The PC-12 cells were maintained in RPMI-1640 supplemented with 1 mM HEPES buffer, 10% horse serum, 5% FBS, 1% sodium pyruvate, 1% L-glutamine and 1% penicillin/streptomycin.
Effect of (R,S)-ketamine, (R,S)-norketamine, (2S,6S)-hydroxynorketamine, methyllycaconitine and (S)-nicotine on monomeric SR expression in PC-12 cells
The studies were carried out using a previously described approach14. In brief, PC-12 cells were seeded on 100-mm tissue culture plates and maintained at 37 °C under humidified 5% CO2 in air until they reached >70% confluence. The original media was replaced with medium containing the test compounds and the plates were incubated for an additional 36 h. The medium was removed, and the cells were collected for analysis. The concentrations used for (R,S)-ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine were as indicated above. In some experiments, PC-12 cells were pre-incubated with (S)-nicotine (2 μM) for 1 h followed by the addition of vehicle, (R,S)-ketamine (1 μM), (R,S)-norketamine (10 nM), (2S,6S)-hydroxynorketamine (0.1 nM), or methyllycaconitine (50 nM) and the incubation continued for an additional 36 h14. Determination of m-SR protein level was carried out by Western blot analysis on one set of dishes, and the same experiment was repeated on three separate days (n = 3).
Effect of (R,S)-ketamine, (R,S)-norketamine, (2S,6S)-hydroxynorketamine, methyllycaconitine and (S)-nicotine on pmTOR, pAkt, pERK, p4E-BP1 and pp70S6K levels in PC-12 cells
PC-12 cells were seeded on 100-mm tissue culture dishes and maintained at 37 °C under humidified 5% CO2 in air until they reached >70% confluence. The original media was replaced with serum-free medium and the plates were incubated for an additional 12 h, unless otherwise indicated. Then, the media were replaced with serum-free medium containing the test compounds and the plates were incubated for an additional 1 h. (R,S)-Ketamine was used at the concentrations of 0 – 10 μM; (R,S)-norketamine at 0 – 1 μM and (2S,6S)-hydroxynorketamine at 0 – 0.1 μM. The effect of (S)-nicotine on (R,S)-ketamine-, (R,S)-norketamine-, (2S,6S)-hydroxynorketamine-and methyllycaconitine-induced changes in mTOR, Akt, ERK, 4E-BP1 and p70S6K phosphorylation levels was then investigated in PC-12 cells. In this experiment, PC-12 cells were incubated for 1 h in the presence of (R,S)-ketamine (1 μM), (R,S)-norketamine (10 nM), (2S,6S)-hydroxynorketamine (0.1 nM), or methyllycaconitine (50 nM) alone and combined with (S)-nicotine (2 μM)14. Determination of the expression of total and phosphorylated forms of the signaling proteins was carried out on one set of dishes, and the same experiment was repeated on three separate days (n = 3).
Statistical Analysis
Data are presented as ‘average relative change ± standard deviation (SD)’. All statistical analyses were performed using one-way analysis of variance (ANOVA) and the Dunnett’s test for post-hoc multiple comparisons. Graphpad Prism 4 software package (GraphPad Software, Inc., La Jolla, CA) was used to carry out statistical analyses. P-values of 0.05 or less were considered statistically significant.
Results
Brain tissue concentrations of (R,S)-ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine
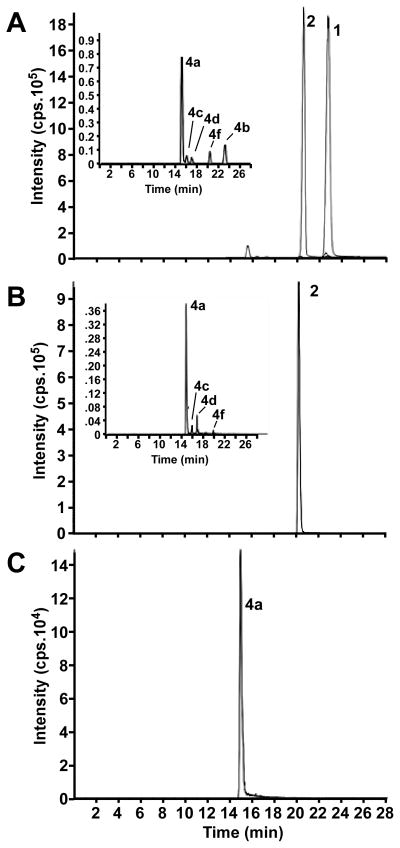
Following the intraperitoneal administration of (R,S)-ketamine, the brain tissue samples contained (R,S)-ketamine, (R,S)-norketamine and (2S,6S;2R,6R)-hydroxynorketamine as well as four additional diastereomeric hydroxynorketamines. A representative chromatogram from a 60-min sample is presented in figure 2A. The brain tissue concentration of (R,S)-ketamine peaked at 137 ± 6 μM·g−1 in the 10-min post administration sample and then rapidly declined to 0.6 ± 0.1 μM·g−1 in the 240-min sample, Table 1. In these samples, the concentration of (2S,6S;2R,6R)-hydroxynorketamine exceeded that of (R,S)-norketamine at the 60- and 240-min time points, Table 1.
Figure 2.
A representative chromatogram from a 60-min brain sample obtained after a single intraperitoneal injection of (R,S)-ketamine, 40 mg·kg−1 in saline (5 ml·kg−1) (A), after a single intravenous injection of (R,S)-norketamine, 20 mg·kg−1 in saline (5 ml·kg−1) (B), and after a single intravenous injection of (2S,6S)-hydroxynorketamine, 20 mg·kg−1 in saline (5 ml·kg−1) (C). The labeled peaks correspond to (R,S)-ketamine (1), (R,S)-norketamine (2), (2S,6S;2R,6R)-hydroxynorketamine (4a), (2S,6R;2R,6S)-hydroxynorketamine (4b), (2S,5S;2R,5R)-hydroxynorketamine (4c), (2S,4S;2R,5R)-hydroxynorketamine (4d) and (2S,4R;2R,4S)-hydroxynorketamine (4f).
Table 1.
Brain tissue concentrations of (R,S)-ketamine (Ket), (R,S)-norketamine (norKet) and (2S,6S;2R,6R)-hydroxynorketamine (HNK) after the administration of (R,S)-Ket and (R,S)-norKet to male Wistar rats. Brain tissue concentrations of (2S,6S)-HNK after the administration of (2S,6S)-HNK, n = 3 at each time point.
| Administered compound | Rat brain tissue conc. (μM·g−1) | |||
|---|---|---|---|---|
| Time (min) | (R,S)-Ket | (R,S)-norKet | (2S,6S;2R,6R)-HNK | |
| (R,S)-Ket | 10 | 137 ± 6 | 21 ± 8 | 30 ± 5 |
| 30 | 52 ± 44 | 34 ± 6 | 21 ± 8 | |
| 60 | 19 ± 9 | 16 ± 4 | 25 ± 5 | |
| 240 | 0.6 ± 0.1 | 1.3 ± 0.3 | 11 ± 5 | |
| (R,S)-norKet | 10 | - | 88 ± 8 | 16 ± 3 |
| 20 | - | 51 ± 4 | 16 ± 3 | |
| 60 | - | 20 ± 7 | 8.9 ± 0.7 | |
| 240 | - | 1.0 ± 0.1 | 2.1 ± 0.4 | |
| (2S,6S)-HNK | 10 | - | - | 127 ± 4* |
| 20 | - | - | 122 ± 17* | |
| 60 | - | - | 26 ± 9* | |
| 240 | - | - | 12 ± 14* | |
reported values are of (2S,6S)-HNK.
After the intravenous administration of (R,S)-norketamine, the brain tissue samples contained (R,S)-norketamine, (2S,6S;2R,6R)-hydroxynorketamine and three additional diastereomeric hydroxynorketamines, figure 2B. (R,S)-Norketamine concentrations peaked at 88 ± 8 μM·g−1 in the 10-min post administration sample and then rapidly declined to 1.0 ± 0.1 μM·g−1 in the 240-min sample, Table 1. (2S,6S;2R,6R)-Hydroxynorketamine was also present in the 10-min sample and the concentration of this metabolite exceeded that of (R,S)-norketamine in the 240-min sample, Table 1.
After intravenous administration of (2S,6S)-hydroxynorketamine, the brain tissue samples contained only this compound and no additional diastereomeric hydroxynorketamines were observed, figure 2C. The peak brain tissue concentration of (2S,6S)-hydroxynorketamine was 127 ± 4 μM·g−1 in the 10-min sample and was essentially maintained in the 20-min sample, with the 240-min sample retaining ~10% of the peak concentration, Table 1.
Effect of (R,S)-ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine on the in vivo phosphorylation of mTOR and related proteins in rat brain tissue
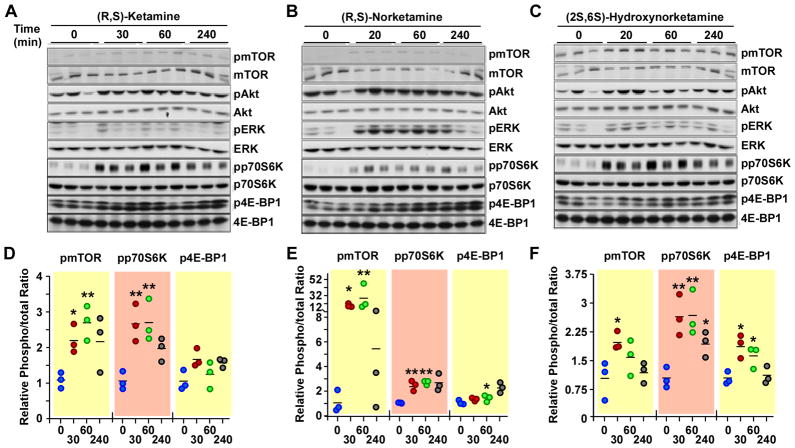
The phosphorylation of mTOR and related proteins was determined in rat brain tissue obtained 30, 60 and 240 min after the administration of (R,S)-ketamine, but not in the 10-min post-administration samples as the amount of tissue was insufficient to carry out these studies. Western blot analysis was performed using primary antibodies specific for the phosphorylated forms of mTOR, 4E-BP1, p70S6K, ERK1/2 and Akt. The results demonstrated that (R,S)-ketamine administration produced a time-dependent increase in the expression of pmTOR (~2.5-fold) and pp70S6K (~2.5-fold), which reached a maximum at the 30- and 60-min time-points, followed by a gradual decline (figure 3A). Similar findings were reported in a recent study where the increase in the phosphorylated forms of these proteins (~1.5-fold) peaked at 30–60 min post-administration of (R,S)-ketamine to Wistar rats, before returning to baseline levels4. Here, ANOVA analysis showed that administration of (R,S)-ketamine for 30 and 60 min led to significant increases in pmTOR and pp70S6K levels (p<0.05), while increases in the average expression of p4E-BP1 (~1.5-fold), pERK1/2 (~2-fold) and pAkt (~1.3-fold) did not reach statistical significance (figure 3A and Supplemental Digital Content 1 figure S1, which illustrates the ratios of phosphorylated to total Akt and ERK protein levels at various incubation periods with (R,S)-ketamine and its metabolites).
Figure 3.
Levels of phospho-active forms of mTOR, ERK1/2, Akt, 4E-BP1 and p70S6K in rat brain (cortex) tissues at different time intervals (0 – 240 min) after administration of either (R,S)-ketamine, (R,S)-norketamine or (2S,6S)-hydroxynorketamine. The mode of administration and dosage protocol of the test compounds are described in the legend of Figure 2. A–C, Representative immunoblots using the indicated primary antibodies. D–F, Scatter plots illustrating the relative levels of phosphorylated and total forms of mTOR, p70S6K and 4E-BP1 in response to (R,S)-ketamine (D), (R,S)-norketamine (E) and (2S,6S)-hydroxynorketamine (F) are shown (n= 3 independent experiments). *, **, P< 0.05, 0.01 (ANOVA) compared with controls.
Administration of (R,S)-norketamine produced significant increases in the levels of phosphorylated forms of mTOR, pp70S6K and p4E-BP1 in the 20- and 60-min samples (figure 3B). The 15- and 25-fold increases in the pmTOR levels were much higher than the ~2.5-fold increase observed after the administration of (R,S)-ketamine and may be a result of larger initial exposure to (R,S)-norketamine relative to the concentration of the compound produced by the administration of (R,S)-ketamine (figure 2A and B, Table 1). While the relative pAkt levels were not impacted, there was significant increase in the relative levels of pERK1/2 in response to (R,S)-norketamine administration (figure 3B and Supplemental Digital Content 1 figure S1). The ~6 fold increase in pERK level was also significantly greater than the ~2-fold (figure 3A) and 1.5-fold4 increases produced by (R,S)-ketamine.
Administration of (2S,6S)-hydroxynorketamine produced time-dependent increases in pmTOR (~2.0-fold), p4E-BP1 (~2.0-fold) and pp70S6K (~2.5-fold) levels, which reached significance in the 20- and 60-min samples (figure 3C). These effects were similar to that produced by (R,S)-ketamine (figure 3A). Even though the relative levels of pERK1/2 and pAkt were increased in the 20- and 60-min samples, they did not reach statistical significance (Supplemental Digital Content 1 figure S1).
Effect of (R,S)-ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine on the expression of m-SR in PC-12 cells
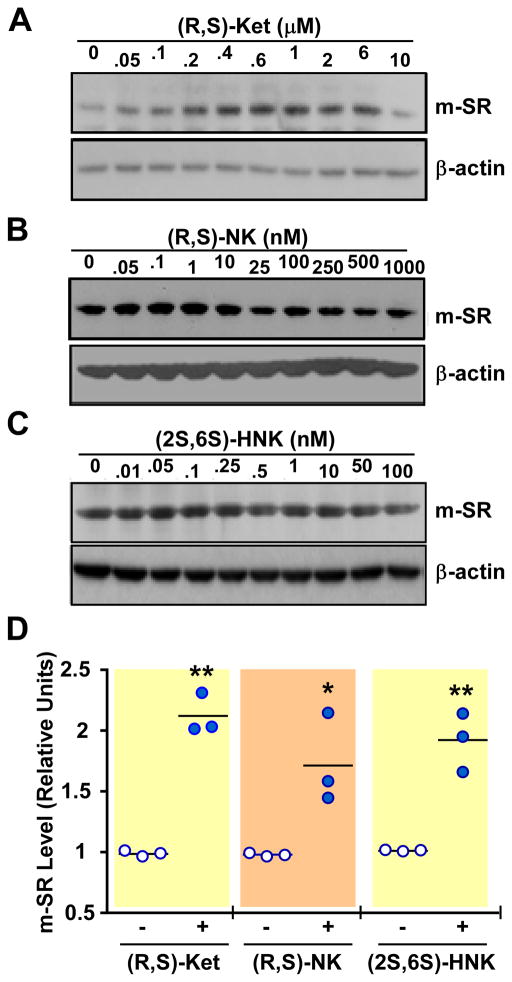
The treatment of PC-12 cells with (R,S)-ketamine (0 – 10,000 nM), (R,S)-norketamine (0 – 1000 nM) and (2S,6S)-hydroxynorketamine (0 – 100 nM) produced concentration-dependent increases in the expression of the m-SR protein, with maximum at 600, 10 and 0.05 nM, respectively, and gradual decline at higher concentrations of the test compounds (figure 4 and Supplemental Digital Content 1 figure S2, which illustrates m-SR protein levels at various concentrations of (R,S)-ketamine and its metabolites). ANOVA analysis showed significant increases at 600 and 1000 nM of (R,S)-ketamine, 10 and 25 nM of (R,S)-norketamine, and between 0.05 and 0.25 nM of (2S,6S)-hydroxynorketamine.
Figure 4.
Expression of monomeric serine racemase (m-SR) protein in PC-12 cells after 36 h incubation with different concentrations of (R,S)-ketamine (Ket, 0 – 10 μM) (A), (R,S)-norketamine (NK, 0 – 1 μM) (B), and (2S,6S)-hydroxynorketamine (HNK, 0 – 0.1 μM) (C). A–C, Representative Western blot analysis with primary antibodies raised against serine racemase and β-actin. D, Scatter plots illustrating the relative levels of m-SR in response to 600 nM (R,S)-ketamine (Ket), 10 nM (R,S)-norketamine (NK) and 0.05 nM (2S,6S)-hydroxynorketamine (HNK) after quantification and normalization with β-actin (n= 3 independent experiments). *, **, P< 0.05, 0.01 (ANOVA) compared with control cells.
Effect of (R,S)-ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine on the phosphorylation of mTOR and related proteins in PC-12 cells
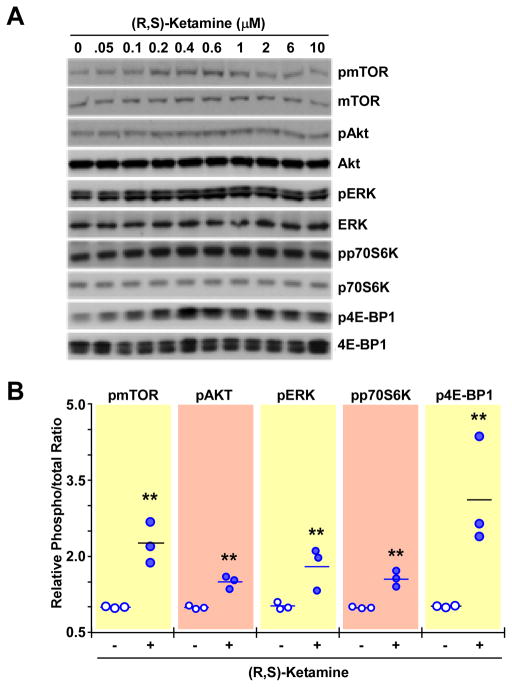
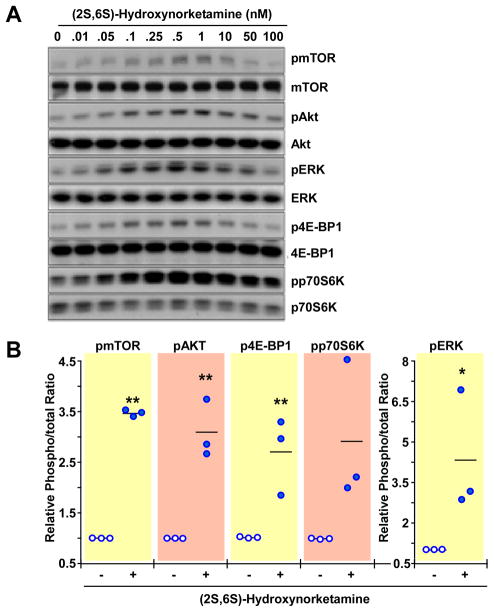
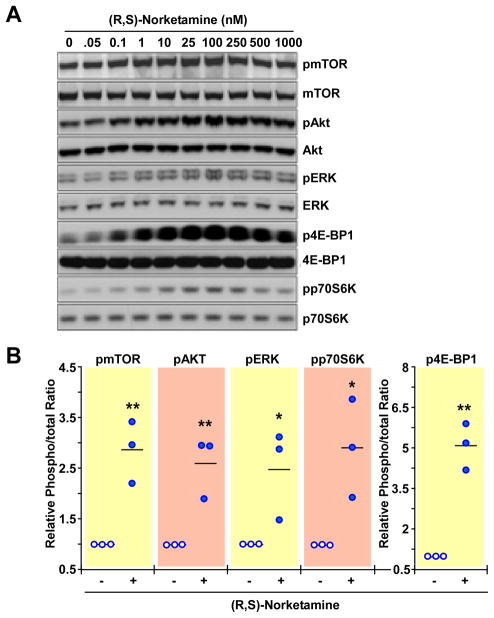
PC-12 cells were incubated for 60 min with a range of concentrations of (R,S)-ketamine (50–10,000nM), (R,S)-norketamine (0.05–1,000nM) and (2S,6S)-hydroxynorketamine (0.01–100nM). The incubation time was chosen based on data obtained in the in vivo studies in which significant increases in the levels of phosphorylated forms of mTOR, 4E-BP1, p70S6K, ERK1/2 and Akt were observed 60 min after administration of (R,S)-ketamine and its metabolites. All three compounds produced significant and concentration-dependent increases in the phosphorylation of these signaling intermediates, figures 5–7 and Supplemental Digital Content 1 figures S3-S5, which illustrate quantitative densitometric analyses of the relative phosphorylation levels of these signaling proteins at various concentrations of (R,S)-ketamine and its metabolites. Cell treatment with (R,S)-ketamine (400–600 nM) elicited significant increases in protein phosphorylation, which gradually lost significance at concentrations greater than 2000 nM (figure 5 and Supplemental Digital Content 1 figure S3). At 600 nM of (R,S)-ketamine, the relative increases in phosphorylation over baseline ranged from 1.5- to 3-fold (figure 5B). Incubation with 1 to 250nM of (R,S)-norketamine produced significant increases in pmTOR levels, which lost significance at concentrations greater than 250 nM (figure 6 and Supplemental Digital Content 1 figure S4). At 25 nM of (R,S)-norketamine, the relative increases in phosphorylation over baseline ranged from 2.5- to 5-fold (figure 6B). Lastly, treatment of PC-12 cells with 0.01 to 10nM of (2S,6S)-hydroxynorketamine resulted in significant increases in pmTOR levels, with marked reduction at concentrations greater than 1 nM (figure 7, Supplemental Digital Content 1 figure S5). At 0.50nM of (2S,6S)-hydroxynorketamine, the relative increases in phosphorylation over baseline ranged from ~2.8- to ~4.2-fold (figure 7B).
Figure 5.
Effect of (R,S)-ketamine on the levels of phospho-active forms of mTOR, Akt, ERK1/2, p70S6K and 4E-BP1 in PC-12 cells. A, Cells were treated with different concentrations of (R,S)-ketamine (0 – 10 μM) for 1 h and processed for Western blot analysis. (B), Scatter plots illustrating the relative ratio of phosphorylated versus total forms of mTOR, Akt, ERK1/2, p70S6K and 4E-BP1 in response to cell treatment with 600 nM of (R,S)-ketamine are shown (n = 3 independent experiments). ** P< 0.01 (ANOVA) compared with control cells.
Figure 7.
Effect of (2S,6S)-hydroxynorketamine on the levels of phospho-active forms of mTOR, Akt, ERK1/2, p70S6K and 4E-BP1 in PC-12 cells. A, Cells were treated with different concentrations of (2S,6S)-hydroxynorketamine (0 – 0.1 μM) for 1 h and processed for Western blot analysis. (B), Scatter plots illustrating the relative ratio of phosphorylated versus total forms of mTOR, Akt, ERK1/2, p70S6K and 4E-BP1 in response to cell treatment with 0.5 nM of (R,S)-hydroxynorketamine are shown (n = 3 independent experiments). *, **, P< 0.05, 0.01 (ANOVA) compared with control cells.
Figure 6.
Effect of (R,S)-norketamine on the levels of phospho-active forms of mTOR, Akt, ERK1/2, p70S6K and 4E-BP1 in PC-12 cells. A, Cells were treated with different concentrations of (R,S)-norketamine (0 – 1 μM) for 1 h and processed for Western blot analysis. (B), Scatter plots illustrating the relative ratio of phosphorylated versus total forms of mTOR, Akt, ERK1/2, p70S6K and 4E-BP1 in response to cell treatment with 25 nM of (R,S)-norketamine are shown (n = 3 independent experiments). *, ** P< 0.05, 0.01 (ANOVA) compared with control cells.
Effect of (S)-nicotine on the expression of m-SR and the phosphorylation of mTOR and related proteins in PC-12 cells treated with (R,S)-ketamine, (R,S)-norketamine, (2S,6S)-hydroxynorketamine and methyllycaconitine
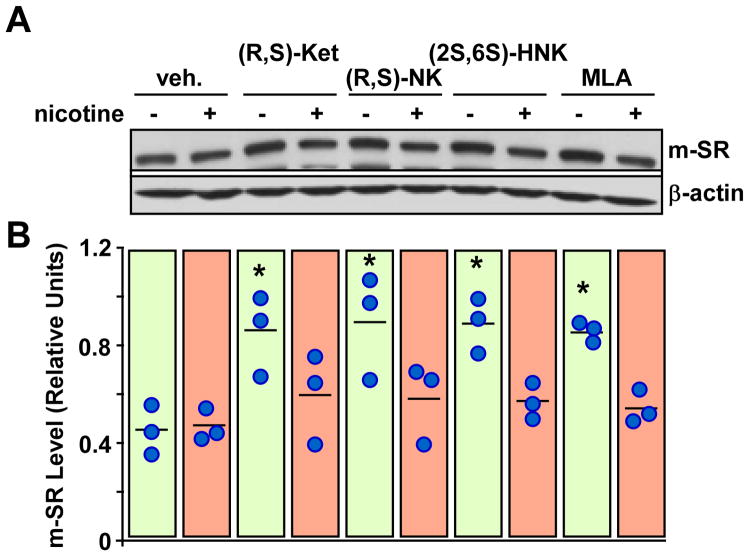
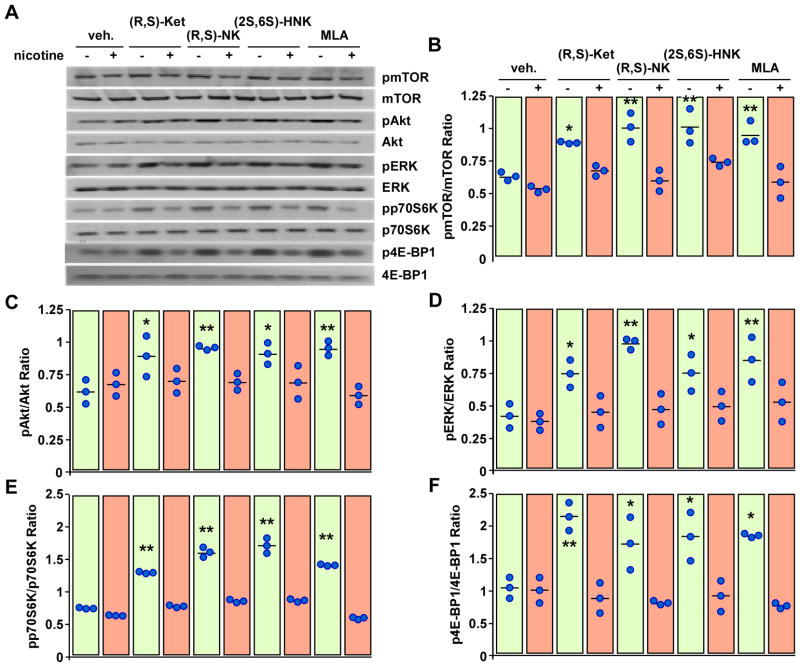
We have recently shown that the incubation of PC-12 cells with the selective α7-nAChR antagonist methyllycaconitine significantly increases the expression of m-SR, while treatment with the α7-nAChR agonist (S)-nicotine alone has no effect but can attenuate the response to methyllycaconitine14. Here, the data illustrates that (S)-nicotine attenuated the increase in m-SR expression elicited by (R,S)-ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine in PC-12 cells, figure 8. Moreover, (S)-nicotine markedly attenuated the response of (R,S)-ketamine, (R,S)-norketamine, (2S,6S)-hydroxynorketamine and methyllycaconitine toward pmTOR, p4E-BP1, pp70S6K, pERK1/2 and pAkt levels, figure 9. Treatment of PC-12 cells with (S)-nicotine alone had no effect on the phosphorylation of these signaling intermediates.
Figure 8.
Effects of (R,S)-ketamine (Ket, 1 μM), (R,S)-norketamine (NK, 10 nM), (2S,6S)-hydroxynorketamine (HNK, 0.1 nM) and methyllycaconitine (MLA, 50 nM) with or without nicotine (2 μM) on the levels of monomeric serine racemase (m-SR) protein in PC-12 cells. A, Representative immunoblots of m-SR and β-actin. B, Scatter plot illustrating the relative m-SR levels after quantification and normalization with β-actin (n = 3 independent experiments). *, P< 0.05 (ANOVA) compared with control cells.
Figure 9.
Effects of (R,S)-ketamine, (R,S)-norketamine, (2S,6S)-hydroxynorketamine and methyllycaconitine with or without nicotine on the levels of phospho-active forms of mTOR, Akt, ERK1/2, p70S6K and 4E-BP1 in PC-12 cells. A, Cells were treated with (R,S)-ketamine (Ket, 1 μM), (R,S)-norketamine (NK, 10 nM), (2S,6S)-hydroxynorketamine (HNK, 0.1 nM) or methyllycaconitine (MLA, 50 nM) with or without nicotine (2 μM) for 1 h and then processed for Western blot analysis. A, Representative immunoblots. B–F, Scatter plots illustrating the relative ratio of phosphorylated versus total forms of mTOR (B), Akt (C), ERK1/2 (D), p70S6K (E) and 4E-BP1 (F) are shown (n= 3 independent experiments). *, **, P< 0.05, 0.01 (ANOVA) compared with control cells.
Discussion
The initial studies of (R,S)-ketamine in the rat demonstrated that the compound was rapidly and extensively metabolized, forming (R,S)-norketamine, (2S,6S;2R,6R)-hydroxynorketamine and additional diastereomeric hydroxynorketamines6–9. This was also observed in the current study, figure 2A. In an earlier study, the anesthetic effects of (R,S)-ketamine, (R,S)-norketamine and (2S,6S;2R,6R)-hydroxynorketamine were examined in the rat using the determination of the duration of anesthesia and of spontaneous locomotor activity during the post-anesthetic recovery phase9. The results indicated that the anesthetic effects were primarily due to (R,S)-ketamine and that (R,S)-norketamine contributed also to this effect9. Moreover, the data indicated that (2S,6S;2R,6R)-hydroxynorketamine had no anesthetic effect and, on the basis of this observation, further investigations of the pharmacological activities of (2S,6S;2R,6R)-hydroxynorketamine and other post-norketamine metabolites have not been undertaken. This concept still persists even when sub-anesthetic doses of (R,S)-ketamine and (S)-ketamine are employed and the pharmacological endpoints are the treatment of pain and depression. The results from the current study demonstrate that (2S,6S)-hydroxynorketamine has pharmacological activity that contributes to the effects produced by the administration of sub-anesthetic doses of (R,S)-ketamine in the rat.
In this study, the administration of (R,S)-ketamine to male Wistar rats produced an increase in the relative levels of the phosphorylated forms of mTOR, Akt, ERK1/2, p70S6K and 4E-BP1 in the pre-frontal cortex, consistent with earlier observations4,5. The administration of (R,S)-norketamine and (2S,6S)-hydroxynorketamine produced similar effects. The data indicate that all 3 compounds activate the mTOR signaling pathway, which has been associated with the rapid antidepressant effects of (R,S)-ketamine, including the induction of synaptogenesis and behavioral response4,5. Although the effect of (R,S)-norketamine and (2S,6S)-hydroxynorketamine on synaptogenesis was not examined, studies utilizing PC-12 cells indicate that the activation of the mTOR signaling pathway by (R,S)-ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine results in an increase in the de novo synthesis of m-SR. Thus, the signaling process initiated by these compounds was successfully translated into increased protein expression.
We have recently reported that the incubation of 1321N1 astrocytoma cells with the α7-nAChR antagonists, methyllycaconitine and (R,S)-dehydroxynorketamine, increased de novo protein synthesis of m-SR via the mTOR pathway14. A similar effect on m-SR expression was observed in PC-12 cells, although the involvement of the mTOR pathway was not definitively established14. The previous study also demonstrated that the effect on m-SR expression in PC-12 cells was primarily due to inhibition of the α7-nAChR and not heteromeric αxβy-nAChRs; both subtypes are expressed in PC-12 cells. In the current study, we have demonstrated that incubation of PC-12 cells with (R,S)-ketamine, (R,S)-norketamine, (2S,6S)-hydroxynorketamine and methyllycaconitine produced significant increases in the levels of phosphorylated mTOR, 4E-BP1, p70S6K, ERK1/2 and Akt and that of m-SR protein. The concentrations required to produce the maximum effect on the mTOR pathway and m-SR expression were (2S,6S)-hydroxynorketamine (0.50nM) > (R,S)-norketamine (25nM) > (R,S)-ketamine (600nM). The fact that treatment with (S)-nicotine attenuated the response produced by ketamine and its metabolites supports the involvement of the nAChR. As indicated above, PC-12 cells express both the homomeric α7-nAChR and heteromeric nAChRs, in particular the α3β4-nAChR. The relative potencies of the test compounds reflect their activities at the α7-nAChR as (2S,6S)-hydroxynorketamine is a selective and potent inhibitor of the α7-nAChR with little effect on α3β4-nAChR, IC50 > 300μM13. (R,S)-Norketamine is also a potent inhibitor of the α7-nAChR, although weaker than (2S,6S)-hydroxynorketamine, and an effective inhibitor of the α3β4-nAChR with an IC50 of 9μM13. Lastly, (R,S)-ketamine is the weakest of the 3 compounds as it inhibits (S)-nicotine-induced currents in PC-12 cells (IC50 5μM15), which appears to be due to both the inhibition of α7-nAChR (IC50 20μM16) and α3β4-nAChR (IC50 3μM13). Thus, the data indicate that the observed pharmacological effect is initiated by inhibition of the α7-nAChR, which is consistent with the results from our previous study of nAChR antagonists in 1321N1 and PC-12 cells17.
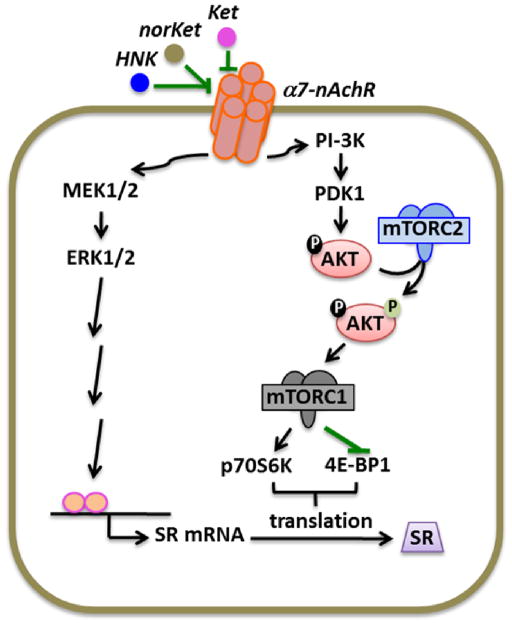
Inhibition of the homomeric α7-nAChR basal activity by (R,S)-ketamine and ketamine metabolites stimulates multiple signaling pathways, which, in turn, increases the translation of SR mRNA into monomeric SR protein. Activation of the PI3K/Akt pathway is key to mTORC1 activation and regulation of cap-dependent protein synthesis18. Here, (R,S)-ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine were found to promote mTORC1 function, as determined by the increased phosphorylation of the translation regulatory protein 4E-BP1 and the 70-kDa isoform of S6K1, which are frequently used as surrogate markers of mTOR kinase activity19. AKT phosphorylation on Thr308 and Ser473 contribute to the stimulation of mTORC1, and AKT phosphorylation on Ser473 is a recognized effector of mTORC2 whose activity is considered to be upstream of mTORC117. This process is depicted in Figure 10. Our results suggest that mTORC2 mediates the translational activity of (R,S)-ketamine and its metabolites because of their ability to promote AKT Ser473 phosphorylation, but how mTORC2 is regulated is unclear. Sin1 (stress-activated protein kinase-interacting protein 1) and rictor maintain mTORC2 integrity and mediates mTORC2 function18. It is possible that stimulation of mTORC2 by (R,S)-ketamine, (R,S)-norketamine and (2S,6S)-hydroxynorketamine might come from enhanced binding of rictor and Sin1 to the mTOR kinase.
Figure 10.
Schematic representation of the modulation of serine racemase expression functioning via mTOR and ERK pathways. Abbreviations: Ket, (R,S)-ketamine; NorKet, (R,S)-norketamine; HNK, (2S,6S)-hydroxynorketamine; nAchR, nicotinic acetylcholine receptor; mTORC1, mammalian target of rapamycin complex 1; mTORC2, mammalian target of rapamycin complex 2; SR, serine racemase.
A single subanesthetic dose of (R,S)-ketamine, 10 mg/kg, has been shown to relieve depression-like behaviors in rats suffering from neuropathic pain but not spared nerve injury-induced hypersensitivity20. The authors suggested that there are differences in the underlying mechanism of the antidepressant function of (R,S)-ketamine and its analgesic properties. Although the data from this study does not include behavioral studies of pain or depression, the results are consistent with the proposed hypothesis. A single low dose of (R,S)-ketamine increases the phosphorylated forms of the proteins associated with the mTOR pathway and subsequently stimulates de novo protein synthesis, a process that has been associated with the antidepressive activity of (R,S)-ketamine4,5. However, these effects of (R,S)-ketamine are likely to be time- and concentration-dependent. The results from this study suggest that the higher doses of (R,S)-ketamine required to achieve analgesia as well as the repetitive or continuous dosage protocols used in the treatment of neuropathic pain syndromes, such as Complex Regional Pain Syndrome, might negate or perhaps even decrease phosphorylation of the proteins associated with the mTOR pathway and protein expression. An alternative mechanism may lie in the regulation of SR activity rather than its expression. Indeed, previous studies in our laboratory have indicated that the incubation of PC-12 cells with increasing concentrations of methyllycaconitine or (R,S)-dehydroxynorketamine decreases the intracellular concentration of the NMDAR co-agonist D-serine, a product of SR-mediated racemization of L-serine, despite higher expression of m-SR14. A similar decrease in intracellular D-serine concentrations was observed after incubation of PC-12 cells with the voltage-gated calcium channel α2δ inhibitors gabapentin and (S)-pregabalin21, which are used in the treatment of neuropathic pain, without impacting on m-SR protein level. The inhibition of SR activity has also been associated with a decrease in NMDAR activity, and SR inhibitors are being explored for use in the treatment of some CNS disorders22,23.
The results of the study suggest that the therapeutic effects produced by sub-anesthetic doses of (R,S)-ketamine may be the result of a combination of independent but interrelated pharmacological effects at the α7-nAChR produced by the parent drug and its metabolites. One of the effects is increased protein expression via the mTOR pathway, which is initiated by antagonism of α7-nAChR, and is reflected by the observed increase in m-SR expression. The second effect is an “indirect” inhibition of NMDAR activity resulting from a reduction in the intracellular Ca+2 flux. These two inter-connected mechanisms are reflected in the previously observed and apparently contradictory effects produced by methyllycaconitine and (R,S)-dehydroxynorketamine in 1321N1 and PC-12 cells in which m-SR expression was increased while the m-SR function, expressed as intracellular D-serine concentration, was reduced17. The inter-relationship and importance of the effects produced by (R,S)-ketamine metabolites and the related mechanisms are under investigation and the results will be reported elsewhere.
Supplementary Material
Acknowledgments
Funding
This work was supported by funding from the Intramural Research Program of the National Institute on Aging/National Institute of Health (Baltimore, Maryland) and by National Institute on Aging Contract No. HHSN271201000008I.
Footnotes
Conflicts of Interest:
Irving W. Wainer, Ruin Moaddel, Michel Bernier and Marc C. Torjman are listed as co-inventors on a patent application for the use of ketamine metabolites in the treatment of bipolar disorder and major depression. They have assigned their rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. Rajib K. Paul, Nagendra S. Singh, Mohammed Khadeer, Mitesh Sanghvi, Carol E Green and Kathleen O’Loughlin declare no competing interests.
Contributor Information
Rajib K. Paul, Laboratory of Clinical Investigation, National Institute on Aging, National Institutes of Health, Baltimore, MD, USA.
Nagendra S. Singh, Laboratory of Clinical Investigation, National Institute on Aging, National Institutes of Health, Baltimore, MD, USA.
Mohammed Khadeer, Laboratory of Clinical Investigation, National Institute on Aging, National Institutes of Health, Baltimore, MD, USA
Ruin Moaddel, Laboratory of Clinical Investigation, National Institute on Aging, National Institutes of Health, Baltimore, MD, USA
Mitesh Sanghvi, Laboratory of Clinical Investigation, National Institute on Aging, National Institutes of Health, Baltimore, MD, USA
Carol E Green, SRI Biosciences, SRI International, Menlo Park, CA, USA.
Kathleen O’Loughlin, SRI Biosciences, SRI International, Menlo Park, CA, USA
Marc C. Torjman, Department of Anesthesiology, Cooper Medical School of Rowan University, Camden, NJ, USA
Michel Bernier, Translational Gerontology Branch, National Institute on Aging, National Institutes of Health, Baltimore, MD, USA
Irving W. Wainer, Laboratory of Clinical Investigation, National Institute on Aging, National Institutes of Health, Baltimore, MD, USA. Department of Anesthesiology, Cooper Medical School of Rowan University, Camden, NJ, USA.
References
- 1.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarate CA, Jr, Brutsche N, Laje G, Luckenbaugh DA, Ramamoorthy A, Moaddel R, Wainer IW. Relationship of Ketamine’s Plasma Metabolites with Response and Diagnosis, and Side Effects in Major Depression. Biol Psychiatry. 2012;72:331–8. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao X, Venkats SLV, Moaddel R, Luckenbaugh DA, Brutsche NE, Ibrahim L, Zarate CA, Jr, Mager DE, Wainer IW. Simultaneous population pharmacokinetic modeling of ketamine and three major metabolites in patients with treatment-resistant bipolar depression. Br J Clin Pharmacol. 2012;74:304–14. doi: 10.1111/j.1365-2125.2012.04198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li N, Lee B, Lui R-J, Banasr M, Dwyer JM, Iwata X-Y, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dwyer JM, Duman RS. Activation of mammalian target of rapamycin and synaptogenesis: Role in the actions of rapid-acting antidepressants. Biol Psychiatry. 1013;73:1189–98. doi: 10.1016/j.biopsych.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen ML, Chan S-L, Way WL, Trevor AJ. Distribution in the brain and metabolism of ketamine in the rat after intravenous administration. Anesthesiology. 1973;39:370–6. doi: 10.1097/00000542-197310000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Adams JD, Baille TA, Trevor AJ, Castagnoli N., Jr Studies on the biotransformation of ketamine: Identification of metabolites produced in vitro from rat liver microsomal preparations. Biomed Mass Spec. 1981;8:527–38. doi: 10.1002/bms.1200081103. [DOI] [PubMed] [Google Scholar]
- 8.Wolf T, Adams JD. Biotransformation of ketamine, (Z)-6-hydroxyketamine and (E)-6-hydroxyketamine by rat, rabbit, and human liver microsomal preparations. Xenobiotica. 1987;17:839–47. doi: 10.3109/00498258709043993. [DOI] [PubMed] [Google Scholar]
- 9.Leung LY, Baillie TA. Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)-6-hydroxynorketamine. J Med Chem. 1986;29:2396–9. doi: 10.1021/jm00161a043. [DOI] [PubMed] [Google Scholar]
- 10.Desta Z, Moaddel R, Ogburn ET, Xu C, Ramamoorthy A, Venkata SLV, Sanghvi M, Goldberg ME, Torjman MC, Wainer IW. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica. 2012;42:1076–87. doi: 10.3109/00498254.2012.685777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moaddel R, Venkata SLV, Tanga MJ, Bupp JE, Green CE, LaIyer L, Furimsky A, Goldberg ME, Torjman MC, Wainer IW. A parallel chiral-achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta. 2010;82:1892–904. doi: 10.1016/j.talanta.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol. 1997;333:99–104. doi: 10.1016/s0014-2999(97)01116-3. [DOI] [PubMed] [Google Scholar]
- 13.Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, Rosenberg A, Tran T, Xiao Y, Zarate CA, Jr, Wainer IW. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;698:228–34. doi: 10.1016/j.ejphar.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh NS, Paul RK, Ramamoorthy A, Torjman MC, Moaddel R, Bernier M, Wainer IW. Nicotinic acetylcholine receptor antagonists alter the function and expression of serine racemase in PC-12 and 1321N1 cells. Cell Signal. 2013;25:2634–45. doi: 10.1016/j.cellsig.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki T, Andoh T, Watanabe I, Kamiya Y, Itoh H, Higashi T, Matsuura T. Nonstereoselective inhibition of neuronal nicotinic acetylcholine receptors by ketamine isomers. Anesth Analg. 2000;91:741–8. doi: 10.1097/00000539-200009000-00046. [DOI] [PubMed] [Google Scholar]
- 16.Coates KM, Flood P. Ketamine and its preservative benzethonium chloride both inhibit human recombinant α7 and α4β2 neuronal nicotinic acetylcholine receptors in Xenopus oocytes. Br J Pharmacol. 2001;134:871–9. doi: 10.1038/sj.bjp.0704315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): Conducting the cellular signaling symphony. J Biol Chem. 2010;285:14071–7. doi: 10.1074/jbc.R109.094003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–70. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Goffer Y, Xu D, Tukey DS, Shamir DB, Eberle SE, Zou AH, Blanck TJJ, Ziff EB. A single subanesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology. 2011;115:812–21. doi: 10.1097/ALN.0b013e31822f16ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh NS, Paul RK, Torjman MC, Wainer IW. Gabapentin and (S)-pregabalin decrease intracellular d-serine concentrations in PC-12 cells. Neurosci Lett. 2013;535:90–4. doi: 10.1016/j.neulet.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sethuraman R, Lee TL, Tachibana S. D-Serine regulation: A possible therapeutic approach for central nervous diseases and chronic pain. Mini Rev Med Chem. 2009;9:813–9. doi: 10.2174/138955709788452630. [DOI] [PubMed] [Google Scholar]
- 23.Jirásková-Vanícková J, Ettrich R, Vorlová B, Hoffman H, Lepšík M, Jansa P, Konvalinka J. Inhibition of human serine racemase, an emerging target for medicinal chemistry. Curr Drug Targets. 2011;12:1037–55. doi: 10.2174/138945011795677755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.