Abstract
Background
It is uncertain whether gender differences in outcome after primary percutaneous coronary intervention (PCI) are only attributable to different baseline characteristics or additional factors.
Methods
Databases of two German myocardial infarction network registries were combined with a total of 1104 consecutive patients admitted with acute ST-elevation myocardial infarction (STEMI) and treated according to standardized protocols.
Results
Approximately 25% of patients were females. Mean age (69 vs 61 years), incidence of diabetes (28% vs 20%), hypertension (68 vs 58%) and renal insufficiency (26% vs 19%) was significantly higher compared to males. Mean prehospital delay was numerically longer in females (227 vs 209 min) as was in hospital delay (35 vs 30 min). PCI was finally performed in 92% of females and 95% of males with comparable procedural success (95% vs 97%). Use of drug eluting stents (55% vs 68%) and application of GP 2b 3a blockers (75% vs 89%) was significantly less frequent in women. At discharge, prescription of beta blockers and lipid lowering drugs was also significantly lower in females (84% vs 90% and 71% vs 84%). Unadjusted in-hospital mortality was significantly higher in females (10% vs 5%) without attenuation after 12 months. Adjusted mortality however did not differ significantly between genders.
Conclusion
Higher unadjusted mortality in females after primary PCI was accompanied by significant differences in baseline characteristics, interventional approach and secondary prophylaxis in spite of the same standard of care. Lower guideline adherence seems to be less gender specific but rather a manifestation of the risk-treatment paradox.
Keywords: Primary PCI, Myocardial infarction network, Gender differences
Background
Gender differences in outcome after acute ST-elevation myocardial infarction (STEMI) are well known [1-5]. This holds true irrespective of performance of mechanical reperfusion with primary percutaneous coronary intervention (PCI) in most large observations [6,7], although there are single contradictory reports [8]. There is some uncertainty whether higher mortality in females is only attributable to different baseline characteristics or additional factors such as delayed diagnosis and reperfusion, under-treatment or genuine gender specific differences in therapeutic susceptibility [1-9]. Female STEMI patients usually present at higher mean age than males and therefore at higher risk. Interactions between higher risk and less intensive treatment, the risk-treatment paradox, have been described [10-12].
Our objective was to compare indicators of guideline adherent therapy in a large cohort of consecutive STEMI patients according to gender in the defined setting of a myocardial infarction network aiming at primary PCI for all STEMI patients according to the same treatment algorithm. For this purpose we combined data from two German myocardial infarction network registries. A previous observation in one of the networks had shown that more than 90% of the regional STEMI population received primary PCI after network implementation. In the cohort of patients who did not receive any revascularization attempt, mainly because of age and comorbidities, the proportion of females was reduced from 77% to 44% [13].
Methods
Network structures
Both networks aim at reperfusion therapy with primary PCI for all regional STEMI patients according to a uniform, regional treatment protocol during 24h/7d a week in one interventional centre.
Network A is located in the North-eastern Germany and comprises both an urban and a rural catchment area with a population of approximately 415.000 inhabitants. The diameter of the network area is up to 120 km. At the time of data collection there were eight hospitals in the network area, seven of them without cathlabs and one with a high-volume interventional facility and a 24h/7d primary PCI service. Emergency medical services (EMS) transferred STEMI patients to the nearest hospital without announcement. After admission local emergency departments alarmed the interventional team and organized direct transfer to the cathlab.
Network B is located in the South-western Germany and comprises a rural catchment area with a population of approximately 350.000 inhabitants. The diameter of the network area is up to 70 km. At the time of data collection there were six hospitals in the network area, five of them without cathlabs and one with a high-volume interventional facility and a 24h/7d primary PCI service. Network structures included 12-lead ECG in the ambulance, ECG telemetry to the intensive care unit of the invasive facility, a structured phone call between EMS and the intensive care physician on call and preparation of the cathlab before patient arrival. STEMI patients were intended to be directly admitted to the cathlab, irrespective of the presence of cardiogenic shock or resuscitation.
Primary PCI protocol
All patients were treated with 250–500 mg aspirin intravenously and received a weight adjusted unfractionated heparin dose of 70 IU/kg by EMS or the emergency department. A clopidogrel loading dose of 600 mg was administered either by EMS or the emergency department in most cases. Otherwise, it was given directly before or immediately after the intervention. Operators of patients in shock were encouraged to treat all presumably hemodynamically relevant non-target lesions. Thrombectomy, periprocedural GP2b3a blockers (predominantly abciximab) and drug eluting stents were used at the discretion of the operator. Full dose anticoagulation with heparin was stopped after PCI, unless there was a high thromboembolic risk (e.g. atrial fibrillation or mechanical heart valves).
Study population
Consecutive STEMI patients admitted for primary PCI were prospectively included in their respective registries, in network A from 2001 to 2003 (n = 603) and in network B from 2005 to 2007 (n = 501).
Definitions
The diagnosis of ST-elevation acute myocardial infarction was based on the presence of chest pain lasting > 20 min and of significant ST-segment elevation (>0.1 mV in two adjacent leads if leads I-III, aVF, aVL, V4-V6, and ≥ 0.2 mV in leads V1-V3), as recorded in the first ECG obtained. Patients with persistent angina and presumably new left bundle branch block were included in the registry if myocardial infarction was subsequently confirmed. Cardiogenic shock was defined clinically by the presence of hypotension (systolic blood pressure < 90 mm Hg for ≥30 minutes or need for vasopressors to maintain systolic blood pressure >90 mm Hg) and tachycardia (heart rate >90 beats/min) with evidence of end-organ hypo-perfusion [14]. Thrombolysis In Myocardial Infarction (TIMI) flow grades were assessed in the culprit vessel before and after the PCI procedure. No reflow was defined as TIMI 0 flow after successful interventional treatment of the culprit lesion.
Major bleeding was defined according to the TIMI major bleeding definition as intracerebral bleeding, bleeding requiring surgical intervention, bleeding requiring transfusion or loss of more than 5 g% haemoglobin [15].
As indicators of guideline adherent therapy we analysed pre- and in-hospital delays, procedural success of primary PCI, stent use, peri-interventional antiplatelet management, medication at discharge and medication at 12 months [16]. Procedural success was defined as residual stenosis < 30% of the culprit lesion.
For outcomes we analysed mortality, re-infarction rate, TLR and TVR until 12 month.
Data collection and follow-up
All patients were prospectively documented in a dedicated database. Follow-up was obtained from telephone interviews and questionnaires at 6 and 12 months. Complete follow-up concerning mortality was obtained from state registries.
The registry was approved by the Freiburg Ethics Commission International. All patients were asked for their written informed consent for the extension of our routine follow-up.
Statistical methods
Data was analyzed according to established standards of descriptive statistics. Categorical variables were compared by χ2 test. Continuous variables are reported as mean ± standard deviation or median with interquartile ranges. For comparisons, the t test or the two-tailed Mann–Whitney U test was used as appropriate. Odds ratios and 95% confidence intervals were provided where appropriate. A p value of less than 0.05 was considered significant.
A multivariate logistic regression analysis (stepwise forward model) with gender as a fixed parameter was performed to determine independent factors predicting 12-month mortality. The following 6 variables were identified: age, beta-blocker medication at discharge, diabetes, lipid lowering medication at discharge, shock and renal impairment. The logistic model showed a good predictive value (C-statistic = 0.85), and good calibration characteristics using the Hosmer-Lemeshow test (p = 0.78).
Mortality at 12 months was adjusted for covariates and for propensity score alone, as well as for the covariates with propensity score added as an additional covariate.
Results
One thousand one hundred and four consecutive patients (n = 1104) with the diagnosis of acute STEMI were prospectively included in the combined registries: 281 women and 823 men.
Mean age (69 vs 61 years, p < 0.01), incidence of diabetes (28% vs 20%, p < 0.01), hypertension (68% vs 58%, p < 0.01) and renal insufficiency (26% vs 19%, p < 0.01) was significantly higher in females compared to males. However, significantly more males were smokers (23% vs 46%, p < 0.01). 9% of patients in both groups were in cardiogenic shock. 6% of females and 9% of males were admitted after resuscitation (p = 0.2) (Table 1).
Table 1.
Baseline clinical characteristics according to gender
| Women (n = 281) | Men (n = 823) | p value | |
|---|---|---|---|
| Age (yrs.) |
69 ± 11 |
61 ± 12 |
< 0.01 |
| Diabetes |
28% |
20% |
< 0.01 |
| Current smoker |
23% |
46% |
< 0.01 |
| Arterial hypertension |
68% |
58% |
< 0.01 |
| Hyperlipidemia |
41% |
46% |
0.21 |
| Creatinine clearance < 60 ml/min |
26% |
19% |
< 0.01 |
| Previous myocardial infarction |
7% |
11% |
0.12 |
| Previous PCI |
5% |
8% |
0.06 |
| Peripheral artery disease |
3% |
5% |
0.31 |
| Previous TIA/stroke |
7% |
4% |
0.09 |
| Cardiogenic shock |
10% |
10% |
0.96 |
| Post CPR |
6% |
9% |
0.15 |
| Systolic blood presssure |
134 ± 3 |
131 ± 1 |
0.14 |
| Diastolic blood pressure |
74 ± 2 |
75 ± 1 |
0.77 |
| Heart rate | 81 ± 2 | 76 ± 1 | <0.01 |
Data presented as mean value ± SD or percentage of patients.
CPR: cardiopulmonary resuscitation, PCI: percutaneous coronary intervention.
Mean transfer distances were nearly identical in both groups (21 km). EMS escorted 55% and 56% of female and male patients as announced STEMI to the primary PCI centre. Mean pre-hospital delay was insignificantly longer in females (227 vs 209 min, p = 0.2) as was in-hospital delay (35 vs 30 min, p = 0.4). PCI was finally performed in 92% of females and 95% of males (p = 0.1) with comparable procedural success (95% vs 97%, p = 0.1). Use of drug eluting stents (55% vs 68%, p = 0.03) and application of GP2b3a blockers (75% vs 89%, p < 0.01) was significantly less frequent in women (Table 2).
Table 2.
Reperfusion delays and primary PCI details according to gender
| Women (n = 281) | Men (n = 823) | p value | |
|---|---|---|---|
| Pre-hospital delay (min)* |
169 (104;296) |
158 (90;278) |
0.08 |
| In-hospital delay (min)* |
18 (7;39) |
15 (6,31) |
0.07 |
| Announced EMS escorted transfer |
55% |
56% |
0,75 |
| No coronary artery stenosis > 50% |
1% |
2% |
0.97 |
| Multivessel disease |
48% |
48% |
0.97 |
| Culprit vessel LAD |
41% |
43% |
0.70 |
| Culprit vessel LMS |
1% |
0% |
0,70 |
| PCI performed |
92% |
95% |
0.07 |
| Multivessel PCI performed |
5% |
4% |
0,80 |
| Further staged PCI |
19% |
21% |
0,51 |
| Staged CABG |
1% |
3% |
0,12 |
| Average number of stents implanted§ |
1.41 |
1.45 |
0,69 |
| Stent length (mm)§ |
29.2 |
30.9 |
0.36 |
| Minimal stent diameter (mm)§ |
2.9 |
3.2 |
0.15 |
| Drug eluting stent |
55% |
68% |
0,03 |
| Peri- or intraprocedural GP2b3a blocker |
75% |
89% |
<0.01 |
| Residual diameter stenosis after PCI < 30% |
95% |
97% |
0.12 |
| Pre-procedural TIMI 0/3 flow§ |
60%/17% |
60%/17% |
0.99 |
| Post-procedural TIMI 0/3 flow§ | 6%/81% | 7%/82% | 0,23 |
*Data analysed in the 216 females and 708 males where symptom onset could be clearly assigned to the 12h period before hospital admission; pre-hospital delay: symptom onset until admission to interventional hospital; in-hopital delay: admission until start of angiography.
§Complete data only available from network B.
Data presented as median with interquartile ranges or percentage of patients.
CABG; coronary artery bypass grafting; EMS: emergency medical services; LAD: left anterior descendant; LMS: left main stem; PCI: percutaneous coronary intervention.
In spite of lesser use of GP2b3a blockers major bleeding was encountered significantly more often in females (6% vs 2%; p < 0.01) (Table 3).
Table 3.
In-hospital bleeding complications according to gender
| Women (n = 281) | Men (n = 823) | P value | |
|---|---|---|---|
| Major bleeding* |
6% |
2% |
< 0.01. |
| Minor bleeding§ |
4% |
7% |
0.22 |
| Insignificant bleeding$ | 19% | 14% | 0.21 |
Data presented as percentage of patients.
*intra-cerebral bleeding, bleeding requiring surgical intervention or transfusion, loss of haemoglobin ≥ 5g%; §haematuria, haematemesis, loss of haemoglobin ≥ 3g% and <5 g% with or ≥ 4g% and < 5g% without identifiable source of bleeding; $bleedings not fulfilling the criteria of a major or minor bleeding.
At discharge prescription of beta-blockers and lipid lowering drugs was also significantly lower in females (84% vs 90%, p < 0.01 rsp. 71% vs 84%; p < 0.01). These differences were more pronounced in network A. Numerical differences in prescription persisted at 12 months (data only available for network B) (Table 4).
Table 4.
Medication at discharge and 12 months according to gender
| |
At discharge |
At 12 months* |
||||
|---|---|---|---|---|---|---|
|
Women |
Men |
p value |
Women |
Men |
p value | |
| (n = 253) | (n = 795) | (n = 97) | (n = 375) | |||
| ASS |
91% |
94% |
0.05 |
56% |
65% |
0.07 |
| Clopidogrel |
88% |
93% |
0.02 |
31% |
40% |
0.10 |
| Anticoagulation |
10% |
9% |
0.80 |
8% |
5% |
0.20. |
| Triple therapy |
8% |
8% |
0.69 |
0% |
2% |
0.20. |
| Beta-Blocker |
84% |
90% |
<0.01 |
51% |
61% |
0.06 |
| ACE inhibitor |
76% |
79% |
0.18 |
50% |
58% |
0.15 |
| Lipid lowering drug | 71% | 84% | <0.01 | 53% | 61% | 0.14 |
Data presented as percentage of patients.
*12-month data only available for network B.
Unadjusted in-hospital mortality was significantly higher in females (10% vs 5%, p < 0.01). Difference persisted during the first year without attenuation (15% vs 7%, p < 0.01). Re-infarction, target lesion revascularisation and target vessel revascularisation rates were numerically lower in females within the first year after the index event (Table 5).However, after adjustment by propensity score or covariates, female gender failed to be predictive as explanatory variable for 12 month mortality (Figure 1).
Table 5.
Major adverse cardiac events until 12 months according to gender
| Women | Men | p value | |
|---|---|---|---|
| Mortality: |
|
|
|
| In-hospital |
10.0% |
4.5% |
< 0.01 |
| 6-month |
14.2% |
6.9% |
< 0.01 |
| 12-month |
14.9% |
6.9% |
<0-01 |
| STEMI* |
1.9% |
3.8% |
0.31 |
| NSTEMI* |
1.9% |
2.0% |
0.90 |
| Clinically driven TLR* |
3,7% |
6.6% |
0.28 |
| Clinically driven TVR* |
5.6% |
7.4% |
0.54 |
| Definite stent thrombosis*§ | 0.9% | 2.3% | 0.46 |
Data presented as percentage of patients.
NSTEMI: non ST-elevation myocardial infarction; STEMI: ST-elevation myocardial infarction; TLR: target lesion revascularisation; TVR: target vessel revascularisation.
*Complete data only available from network B.
§ARC definition.
Figure 1.
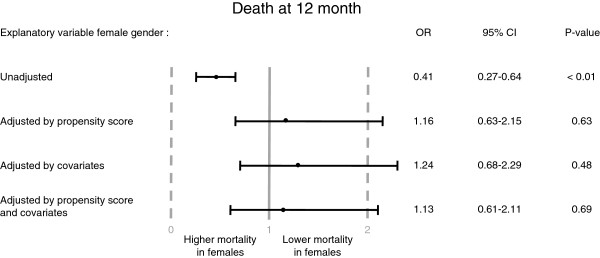
Death at 12 months. Explanatory variable female gender: unadjusted, adjusted by propensity score, covariates or by covariates with propensity score as additional covariate (details under “Statistical methods”).
Discussion
The analysis of 1104 consecutive STEMI patients admitted for primary PCI according to the uniform standard of care of a myocardial infarction network, showed that early and late mortality in females was more than double the mortality of males. This was accompanied by significant differences in baseline characteristics as has been described in a number of previous observations [1-5]. Mean age of women presenting with STEMI was approximately eight years older compared to men. Furthermore incidence of diabetes, hypertension and renal impairment was significantly higher in the female cohort.
Comparable gender differences in mortality have been found in large registries [1-5]. Single observations reported, however, similar unadjusted mortality of males and females after primary PCI [8]. This might be due to different patient selection.
Under network conditions more than 90% of all regional STEMI patients are treated with primary PCI rendering the analysis highly representative for an unselected STEMI population suitable for revascularisation. We have shown that in the remaining subset of predominantly elderly patients who are not scheduled for any revascularisation attempt females were not overrepresented [13].
A unisex standard of care for STEMI patients reflects current guidelines [16,17] with primary PCI being the preferred reperfusion strategy for both genders. It has been demonstrated that primary PCI is equally effective in men and women [18,19].
Although standard of care in the network regions was not gender specific, actual treatment showed relevant differences. There was a trend to more frequent abortion of intended PCI in females, although the proportion of patients without significant coronary artery disease and patients with multi-vessel disease was quite comparable in both cohorts. In contrast to our finding, a significantly higher proportion of non-obstructive coronary artery disease has been previously described in female compared to male ACS patients in a large meta-analysis [1]; this difference was however smallest in the subset of ACS patients presenting with STEMI. The use of drug eluting stents and GP2b3a blockers was significantly lower in females whereas pre- and in-hospital delays before primary PCI were only numerically longer and the immediate result of primary PCI comparable. Reduction of system related time delays before primary PCI is obviously a primary objective of myocardial infarction networks and has been successfully proven [13,20-23]. Attempts to intervene on patient related time delays showed no similar success [24]. A comparable pattern of slightly increased system delays and lesser use of drug eluting stents and GP blockers seen in females has also been shown in elderly network patients [25].
More strikingly, was the significantly lower prescription of beta-blockers and lipid lowering drugs as well as the numerically lower prescription of anti-platelets in females at discharge. The differences in anti-platelet medication cannot be fully explained by the slight differences in the need for anticoagulation, but rather by the significantly higher incidence of major bleedings in females during hospitalisation in spite of less aggressive peri-interventional platelet management. Higher bleeding rates after primary percutaneous intervention in females have been described in many observations [26-28]. The lesser prescription of betablockers in females cannot explained by lower blood pressure or heart rate on admission. Numerical differences in recommended secondary prophylaxis persisted over 12 months. Thus adherence to guideline recommended therapy was lower in females than males. Again, this pattern of less guideline adherent secondary prophylaxis in females has also been observed in elderly patients [25].
Multivariate analysis suggested that both the different baseline characteristics as well as the lesser use of recommended secondary prophylaxis had an independent influence on mortality. Interestingly, female gender failed to be predictive as explanatory variable for mortality after adjustment by propensity score or covariates. So there was no implication of gender differences in susceptibility to primary PCI or of a gender specific general under use of therapy in this analysis. The observed differences in guideline adherence with respect to secondary prophylaxis might be more related to the different baseline characteristics which clearly attributed a higher risk to females.
The risk-treatment paradox has been previously described [10-12]. Actually, myocardial infarction networks counteract this paradox by aiming at higher reperfusion rates also in high risk patients (especially the elderly and shock patients) [13,20-23]. This raises the question if the reluctant use of recommended secondary pharmacological prophylaxis in the subset of high risk elderly women reflects a residual under use of justified therapy or a truly higher prevalence of contra-indications to this therapy. At least with respect to antiplatelet therapy, increased bleeding risk seemed to be a limiting factor.
Our data also confirmed the previous description of a lower target lesion and target vessel revascularisation rate during long term follow up in females compared to males [29]. It is however questionable if this is primarily attributable to gender specific biological reactions as this phenomenon was also described for the comparison of octogenarians with younger patients after stenting in an all-comer population [30].
Limitations
A major limitation is the lack of external monitoring of the registries which is an inherent weakness of many investigator-driven observational studies.
A further limitation of our registry is that we only included primary PCI patients and not all regional STEMI patients. Therefore our analysis cannot be extended to the complete STEMI population. Preceding analyses, however, showed that in the setting of a myocardial infarction network more than 90% of the STEMI population were scheduled for mechanical reperfusion.
Conclusions
Higher unadjusted mortality in females after primary PCI was accompanied by significant differences in baseline characteristics, interventional approach and secondary prophylaxis in spite of a gender neutral standard of care which enabled similar reperfusion rates and attenuation of time delays before primary PCI. Lower guideline-adherence in females in this setting seemed to reflect predominantly a residual risk-treatment paradox and not a gender specific under-treatment. Adjusted mortality showed a favourable trend for females.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RB, HS, AR, IA, SK, LP, WJ, HI and CAN participated in treating the patients in the cathlab/ICU and in acquisition of data. RB and JE performed the statistical analysis. RB, HS and CAN drafted the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Ralf Birkemeyer, Email: rbirkemeyer@t-online.de.
Henrik Schneider, Email: henrik.schneider@med.uni-rostock.de.
Andreas Rillig, Email: arillig5@yahoo.de.
Juliane Ebeling, Email: jule.ebeling@web.de.
Ibrahim Akin, Email: ibrahim.akin@med.uni-rostock.de.
Stefan Kische, Email: stefan.kische@med.uni-rostock.de.
Liliya Paranskaya, Email: liliya.paranskaya@med.uni-rostock.de.
Werner Jung, Email: werner.jung@sbk-vs.de.
Hueseyin Ince, Email: hueseyin.ince@med.uni-rostock.de.
Christoph A Nienaber, Email: christoph.nienaber@med.uni-rostock.de.
Acknowledgements
We gratefully acknowledge the study teams of the two interventional centres for database management.
References
- Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW, Simes RJ, White HD, Van de Werf F, Topol EJ, Hochman JS, Newby LK, Harrington RA, Califf RM, Becker RC, Douglas PS. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302:874–882. doi: 10.1001/jama.2009.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfredsson J, Stenestrand U, Wallentin L, Swahn E. Gender differences in management and outcome in non-ST-elevation acute coronary syndrome. Heart. 2007;93:1357–1362. doi: 10.1136/hrt.2006.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Flather MD, Devlin G, Brieger D, Gurfinkel EP, Steg PG, Fitzgerald G, Jackson EA, Eagle KA. Global Registry of Acute Coronary Events investigators. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the global registry of acute coronary events. Heart. 2009;95:20–26. doi: 10.1136/hrt.2007.138537. [DOI] [PubMed] [Google Scholar]
- D’Ascenzo F, Gonella A, Quadri G, Longo G, Biondai-Zoccai G, Moretti C, Omedè P, Sciuto F, Gaita F, Sheiban I. Comparison of mortality rates in women versus men presenting with ST-segment elevation myocardial infarction. Am J Cardiol. 2011;107:651–654. doi: 10.1016/j.amjcard.2010.10.038. [DOI] [PubMed] [Google Scholar]
- Milcent C, Dormont B, Durand-Zaleski I, Steg PG. Gender differences in hospital mortality and use of percutaneous coronary intervention in acute myocardial infarction: microsimulation analysis of the 1999 nationwide French hospitals database. Circulation. 2007;115:833–839. doi: 10.1161/CIRCULATIONAHA.106.664979. [DOI] [PubMed] [Google Scholar]
- Benamer H, Tafflet M, Bataille S, Escolano S, Livarek B, Fourchard V, Caussin C, Teiger E, Garot P, Lambert Y, Jouven X, Spaulding C. CARDIO-ARHIF Registry investigators. Female gender is an independent predictor of in-hospital mortality after STEMI in the era of primary PCI: insights from the greater paris area PCI registry. Eurointervention. 2011;6:1073–1079. doi: 10.4244/EIJV6I9A187. [DOI] [PubMed] [Google Scholar]
- Jackson EA, Moscucci M, Smith DE, Share D, Dixon S, Greenbaum A, Grossman PM, Gurm HS. The association of sex with outcomes among patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction in the contemporary era: insights from the blue cross blue shield of Michigan cardiovascular consortium (BMC 2) Am Heart J. 2011;161:106–112. doi: 10.1016/j.ahj.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Mehilli J, Kastrati A, Dirschinger J, Pache J, Seyfarth M, Blasini R, Hall D, Neumann FJ, Schömig A. Sex-based analysis of outcome in patients with acute myocardial infarction treated predominantly with percutaneous coronary intervention. JAMA. 2002;287:210–215. doi: 10.1001/jama.287.2.210. [DOI] [PubMed] [Google Scholar]
- Anderson RD, Pepine CJ. Gender differences in the treatment for acute myocardial infarction: bias or biology? Circulation. 2007;115:823–826. doi: 10.1161/CIRCULATIONAHA.106.685859. [DOI] [PubMed] [Google Scholar]
- Alexander KP, Newby LK, Armstrong PW, Cannon CP, Gibler WB, Rich MW, Van de Werf F, White HD, Weaver WD, Naylor MD, Gore JM, Krumholz HM, Ohman EM. American Heart Association Council on Clinical Cardiology; Society of Geriatric Cardiology. Acute coronary care in the elderly, part II: ST-segment-elevation myocardial infarction: a scientific statement for healthcare professionals from the american heart association council on clinical cardiology: in collaboration with the society of geriatric cardiology. Circulation. 2007;115:2570–2589. doi: 10.1161/CIRCULATIONAHA.107.182616. [DOI] [PubMed] [Google Scholar]
- Mehilli J, King L. Risk-treatment paradox in women with symptomatic coronary artery disease. Clin Res Cardiol Suppl. 2013;8:20–24. doi: 10.1007/s11789-013-0052-3. [DOI] [Google Scholar]
- McAlister FA, Oreopoulos A, Norris CM, Graham MM, Tsuyuki RT, Knudtson M, Ghali WA. Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) Investigators. Exploring the treatment-risk paradox in coronary disease. Arch Intern Med. 2007;167:1019–1025. doi: 10.1001/archinte.167.10.1019. [DOI] [PubMed] [Google Scholar]
- Birkemeyer R, Rillig A, Koch A, Miljak T, Kunze M, Meyerfeldt U, Steffen W, Soballa M, Ranke C, Prassler R, Benzing A, Jung W. Primary angioplasty for any patient with ST-elevation myocardial infarction? guideline-adherent feasibility and impact on mortality in a rural infarction network. Clin Res Cardiol. 2010;99:833–840. doi: 10.1007/s00392-010-0196-9. [DOI] [PubMed] [Google Scholar]
- White HD, Assmann SF, Sanborn TA, Jakobs AK, Webb JG, Sleeper LA, Wong CK, Stewart JT, Aylwart PE, Wong SC, Hochman JS. Comparison of percutaneous coronary intervention and coronary artery bypass grafting after acute myocardial infarction complicated by cardiogenic shock. Results from the should We emergently revascularize occluded coronaries for cardiogenic shock (SHOCK) trial. Circulation. 2005;112:1992–2001. doi: 10.1161/CIRCULATIONAHA.105.540948. [DOI] [PubMed] [Google Scholar]
- Serebruany VL, Atar D. Assessment of bleeding events in clinical trials – proposal of a new classification. Am J Cardiol. 2007;99:288–290. doi: 10.1016/j.amjcard.2006.07.091. [DOI] [PubMed] [Google Scholar]
- Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fenandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Vaglimigli M, Van’t Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:33–619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz CB, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso JE, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;127:529–555. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- Glaser R, Herrmann HC, Murphy SA, Demopoulos LA, DiBattiste PM, Cannon CP, Braunwald E. Benefit of an early invasive management strategy in women with acute coronary syndromes. JAMA. 2002;288:3124–3129. doi: 10.1001/jama.288.24.3124. [DOI] [PubMed] [Google Scholar]
- Mehilli J, Ndrepepa G, Kastrati A, Nekolla SG, Markwardt C, Bollwein H, Pache J, Martinoff S, Dirschinger J, Schwaiger M, Schömig A. Gender and myocardial salvage after reperfusion treatment in acute myocardial infarction. J Am Coll Cardiol. 2005;45:828–831. doi: 10.1016/j.jacc.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Kalla K, Christ G, Karnik R, Malzer R, Norman G, Prachar H, Schreiber W, Unger G, Glogar HD, Kaff A, Laggner AN, Maurer G, Mlczoch J, Slany J, Weber HS, Huber K. The STEMI Vienna Registry Group. Implementation of guidelines improves the standard of care: the viennesse registry on reperfusion strategies in ST-elevation myocardial infarction (Vienna STEMI Registry) Circulation. 2006;113:2398–2405. doi: 10.1161/CIRCULATIONAHA.105.586198. [DOI] [PubMed] [Google Scholar]
- Widimsky P, Zelizko M, Jansky P, Tousek F, Holm F. Aschermann M on behalf of the CZECH investigators. The incidence, treatment strategies and outcomes of acute coronary syndromes in the “reperfusion network” of different hospital types in the Czech Republic: Results of the Czech evaluation of acute coronary syndromes in hospitalized patients (CZECH) registry. Int J Cardiol. 2007;119:212–219. doi: 10.1016/j.ijcard.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Saia F, Marrozzini C, Ortolani P, Palmerini T, Guastaroba P, Cortesi P, Pavesi PC, Gordini G, Pancaldi LG, Taglieri N, di Pasquale G, Branzi A, Marzocchi A. Optimization of therapeutic strategies for ST-elevation acute myocardial infarction: the impact of a territorial network on reperfusion therapy and mortality. Heart. 2009;95:370–376. doi: 10.1136/hrt.2008.146738. [DOI] [PubMed] [Google Scholar]
- Schneider H, Ince H, Rehders T, Körber T, Weber F, Kische S, Chatterjee T, Nienaber CA. Drip & Ship-Netzwerk. Treatment of acute ST elevation myocardial infarction in a regional network. Herz. 2007;32:635–640. doi: 10.1007/s00059-007-3061-5. [DOI] [PubMed] [Google Scholar]
- Diercks DB, Owen KP, Kontos MC, Blomkalns A, Chen AY, Miller C, Wiviott S, Peterson ED. Gender differences in time to presentation for myocardial infarction before and after a national women’s cardiovascular awareness campaign: a temporal analysis from the Can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation (CRUSADE) and the national cardiovascular data registry acute coronary treatment and intervention outcomes network-Get with the guidelines (NCDR ACTION registry-GWTG) Am Heart J. 2010;160:80–87. doi: 10.1016/j.ahj.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Birkemeyer R, Rillig A, Treusch F, Kunze M, Meyerfeldt U, Miljak T, Kostin D, Koch A, Jung W, Oster P, Bahrmann A. Outcome and treatment quality of transfer primary percutaneous intervention in older patients with acute ST-elevation myocardial infarction (STEMI) Arch Gerontol Geriatr. 2011;53(3):e259–e262. doi: 10.1016/j.archger.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Alexander KP, Chen AY, Newby LK, Schwartz JB, Redberg RF, Hochman JS, Roe MT, Gibler WB, Ohman EM, Peterson ED. Sex differences in major bleeding with glycoprotein IIb/IIIa inhibitors: results from the CRUSADE (Can rapid risk stratification of unstable angina patients suppress ADverse outcomes with early implementation of the ACC/AHA guidelines) initiative. Circulation. 2006;114:1380–1387. doi: 10.1161/CIRCULATIONAHA.106.620815. [DOI] [PubMed] [Google Scholar]
- Hochholzer W, Wiviott SD, Antman EM, Contant CF, Guo J, Giugliano RP, Dalby AJ, Montalescot G, Braunwald E. Predictors of bleeding and time dependence of association of bleeding with mortality: insights from the trial to assess improvement in therapeutic outcomes by optimizimg platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38 (TRITON-TIMI 38) Circulation. 2011;123:2681–2689. doi: 10.1161/CIRCULATIONAHA.110.002683. [DOI] [PubMed] [Google Scholar]
- Lansky AJ, Hochman JS, Ward PA, Mintz GS, Fabunmi R, Berger PB, New G, Grines CL, Pietras CG, Kern MJ, Ferrell M, Leon MB, Mehran R, White C, Mieres JH, Moses JW, Stone GW, Jacobs AK. American College of Cardiology Foundation; American Heart Association. Percutaneous coronary intervention and adjunctive pharmacotherapy in women: a statement for healthcare professionals from the american heart association. Circulation. 2005;111:940–953. doi: 10.1161/01.CIR.0000155337.50423.C9. [DOI] [PubMed] [Google Scholar]
- Mehilli J, Kastrati A, Bollwein H, Dibra A, Schühlen H, Dirschinger J, Schömig A. Gender and restenosis after coronary stenting. Eur Heart J. 2003;24:1523–1530. doi: 10.1016/S0195-668X(03)00320-8. [DOI] [PubMed] [Google Scholar]
- Marcolino MS, Simsek C, de Boer SP, van Domburg RT, van Geuns RJ, de Jaegere P, Akkerhuis KM, Daemen J, Serruys PW, Boersma E. Short- and long-term outcomes in octogenarians undergoing percutaneous coronary intervention with stenting. Eurointervention. 2012;8:920–928. doi: 10.4244/EIJV8I8A141. [DOI] [PubMed] [Google Scholar]


