Abstract
Tick-borne encephalitis virus (TBEV) causes acute central nervous system disease. Here, we investigated the roles of the TNF-α, IL-10 and other cytokines in appropriate KO mice following infection with Oshima and Sofjin strains of TBEV. Following infection with the Oshima strain, mortality rates were significantly increased in TNF-α KO and IL-10 KO mice compared with wild type (WT) mice. These results suggested that TNF-α and IL-10 play protective roles against fatal infection due to Oshima strain infection. However, viral loads and proinflammatory cytokine levels in the brain of TNF-α KO andIL-10 KO mice were not significantly different compared with those of WT mice. On the other hand, all WT, TNF-α KO and IL-10 KO mice died following infection with Sofjin strain. Interestingly, Sofjin-infected mice did not exhibit an up-regulated mRNA level of IL-2 in the spleen in all groups of mice, whereas Oshima-infected mice showed significantly increased level of IL-2 compared with mock-infected mice. From these results, we suggest that TNF-α, IL-10 and IL-2 are key factors for disease remission from fatal encephalitis due to infection with Oshima strain of TBEV.
Tick-borne encephalitis virus (TBEV), which is a member of the genus Flavivirus in the family Flaviviridae, causes acute central nervous system (CNS) disease in humans1,2. TBEV is distributed widely throughout Europe and Asia and is genetically divided into three closely-related subtypes (European-, Siberian-, and Far Eastern-subtypes), which correspond to the geographical distribution of Ixodes tick species1,3.
In human cases, typical clinical features of tick-borne encephalitis (TBE) are characterized by a biphasic course with a primary phase when symptoms such as fever, muscle pain, headache and malaise are observed in patients and a secondary phase when neurological symptoms including meningitis, meningoencephalitis and meningoencephalomyelitis are present4,5. Death usually occurs within 5 to 7 days from the onset of neurological signs. The clinical symptoms of TBE are nonspecific and vary from mild to severe disease, thus laboratory diagnosis of TBE is required.
The laboratory mouse model has been commonly employed to study the pathogenesis of encephalitic flaviviruses including Japanese encephalitis virus (JEV), West Nile virus (WNV) and TBEV. The pathologic changes in mouse brains due to encephalitic flavivirus are similar to those observed in human cases and the variety of disease prognosis is observed in TBEV-infected mice6,7,8. CNS pathology is the consequence of viral infection of the affected cells and the resulting inflammatory responses in the CNS. Flavivirus variants may induce different degrees of pathology. For example, some TBEV strains, such as Sofjin, cause early disease development and high mortality in mice following peripheral infection; whereas, other strains such as Oshima, cause late disease development and low mortality6,9.
Host immune response is likely to be a more critical determinant of clinical outcome due to encephalitic flavivirus infection. We previously showed the increase of TNF-α and IL-10 levels in mouse brains of severe cases infected with JEV10. We further showed that the mortality rates of TNF-α knockout (KO) and IL-10 KO mice were significantly increased compared with that of WT mice and that immunopathological effects contribute to severe neuronal degeneration resulting in fatal disease10. Thus, we suggested that these cytokines play a protective role against fatal infection due to JEV.
In a mouse model of TBEV infection, we previously suggested that the variation of fatal outcome in individual mice appeared to be due to variation in individual host responses6. Furthermore, we showed that the levels of TNF-α were increased in the brain and serum of dying mice following infection with Oshima strain. However, the role of TNF-α in the disease progression during TBEV infection was not elucidated. Thus, the purpose of present study was to investigate the roles of TNF-α and IL-10 in severe cases by using appropriate KO mice.
Results
Mortality following infection with Oshima and Sofjin strains of TBEV in TNF-α KO and IL-10 KO mice
We first compared the disease courses of TNF-α KO, IL-10 KO and immunocompetent wild type (WT) B6 mice following subcutaneous infection with the Oshima and Sofjin strains of TBEV.
Following infection with the Oshima strain, TNF-α KO and IL-10 KO mice exhibited significant increase in mortality (100% and 61.9%, respectively) compared with WT mice (42.9%) (Figure 1a). In particular, no mice were observed to survive among the TNF-α KO mice until the 19 days post-infection (pi). Mice started to exhibit clinical signs such as weight loss, slow movement, ataxia, piloerection and anorexia at 6 days pi, but apparent paralysis was not observed. Onset of disease such as weight loss was not significantly different between the three groups (Figure 1b). Mean survival times (MSTs) of fatal cases were 12.5 ± 1.6, 11.5 ± 1.06 and 14.9 ± 1.73 days in TNF-α KO, IL-10 KO and WT mice, respectively. However, these MSTs were not significantly different between the three groups. These observations indicate that TNF-α and IL-10 protected a significant proportion of mice from fatal infection by the Oshima strain, and that TNF-α had a particularly pronounced protective effect.
Figure 1. Survival rates (a and c) and ratios of weight change (b and d) in WT, IL-10 KO and TNF-α KO mice following subcutaneous infection with 104 pfu of Oshima and Sofjin strains (n = 12 to 21).
Mice were monitored daily for the averages ratio of weight change of living mice at the time points compared with those of day 0. Error bars represent standard deviations.
On the other hand, following infection with the Sofjin strain, all mice died within the period of observation (Figure 1c). MSTs were 8.83 ± 0.25, 9.00 ± 0.32, and 9.40 ± 0.41 days for WT, IL-10 KO and TNF-α KO mice, respectively, but were not significantly different between these groups of mice. Mice started to exhibit clinical signs at 5 days pi, and the clinical disease course was similar between the three groups (Figure 1D). The Sofjin strain in comparison with the Oshima strain caused rapid and high mortality in all the three groups of mice. However, protective effect of TNF-α and IL-10 against disease severity or their enhancing effect was not confirmed in Sofjin-infected mice.
Viral loads in TNF-α KO and IL-10 KO mice infected with TBEV
We next compared viral loads in the spleen and different parts of the CNS (brain cortex, thalamus, brainstem cerebellum and spinal cord) of WT, IL-10 KO and TNF-α KO groups of mice at 5 and 9 days pi.
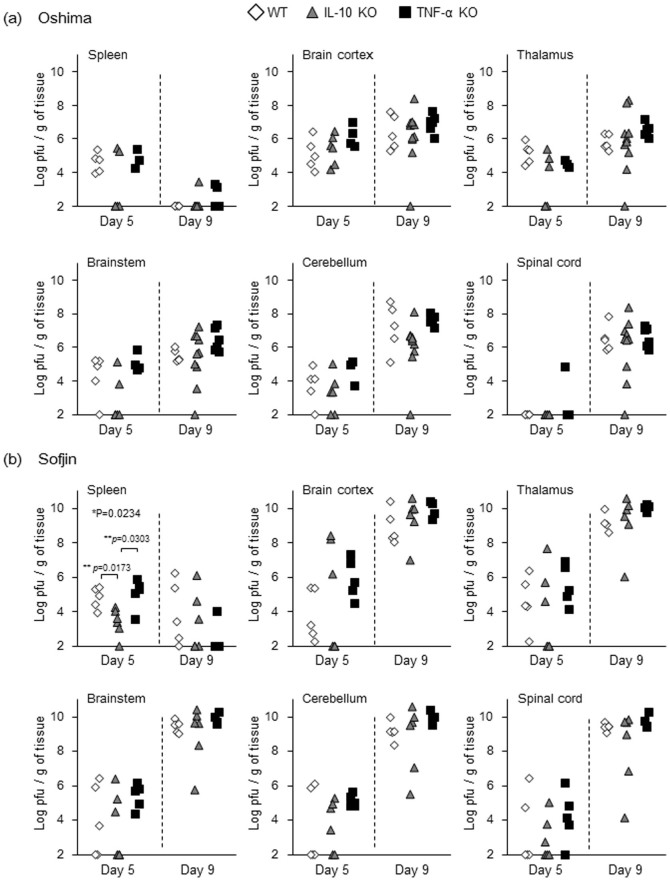
Following infection with the Oshima strain, viral load in the spleen decreased at 9 days pi compared with at 5 days pi in all WT, IL-10 KO and TNF-α KO groups, whereas CNS infection increased during this period in all the groups (Figure 2a). However, there was no significant difference in the viral load in the spleen and in the CNS between the three groups at 5 days pi nor 9 days pi, suggesting that the increased mortality in IL-10 KO and TNF-α KO mice was not simply correlated with the increased viral loads in the peripheral and CNS tissues.
Figure 2. Viral loads in the spleen and CNS of WT, IL-10 KO and TNF-α KO mice (n = 4 to 10) at 5 and 9 days pi after subcutaneous infection with 104 pfu of Oshima (a) and Sofjin (b).
Following infection with the Sofjin strain, IL-10 KO mice exhibited slightly lower viral load in the spleen compared with WT and TNF-α KO mice at 5 days pi (Figure 2b). However, there was no significant difference in the viral load in the different areas of the CNS of WT, IL-10 KO and TNF-α KO mice at 5 days pi nor 9 days pi (Figure 2b). Of note, all Sofjin-infected mice regardless of the grouping showed significantly higher viral load in every CNS component at 9 days pi compared with Oshima-infected mice (Supplementary figure 1). Increase of viral load in the spleen was also observed in Sofjin-infected WT mice compared with Oshima-infected WT mice (Supplementary figure 1). Thus, it is suggested that higher mortality due to infection with Sofjin strain could be due to the viral factors i.e. viral gene differences between Sofjin and Oshima strains.
mRNA levels of proinflammatory cytokines in the brain of TNF-α KO and IL-10 KO mice infected with TBEV
We then examined the mRNA transcript levels of proinflammatory cytokines IFN-γ, IL-2, IL-4, IL-6 in the brain of mice (WT, IL-10 KO and TNF-α KO groups) following Oshima and Sofjin infection.
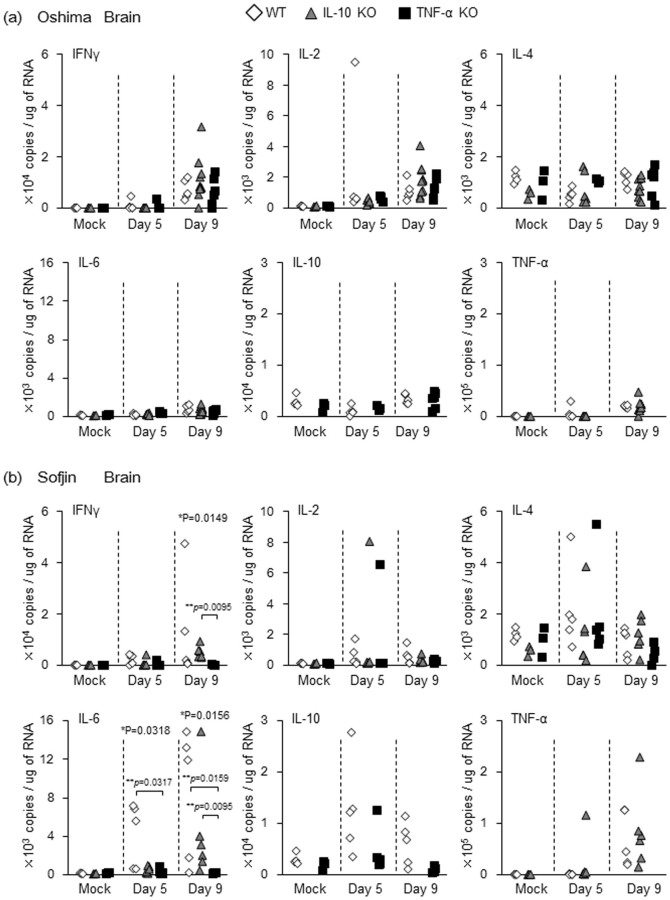
The following levels were observed in the brain of specific group of Oshima-infected mice as compared with that of the mock-infected mice (Supplementary Figure 2a): (i) significantly higher levels of IFN-γ and of IL-2 at 9 days pi or at both 5 and 9 days pi in each of the three groups of mice, (ii) significantly higher level of IL-6 at 9 days pi in WT and IL-10 KO mice but not in TNF-α KO mice, (iii) significantly higher level of TNF-α in WT at both 5 and 9 days pi and in IL-10 KO mice at 9 days pi, and (iv) no significant difference in IL-4 level among the three groups nor in IL-10 level in WT and TNF-α KO groups at both 5 and 9 days pi. Of note, the levels of each cytokine observed were not significantly different between WT, IL-10 KO and TNF-α KO groups of mice after Oshima infection (Figure 3a). These observations suggest that inflammatory responses due to these cytokines in the brain could not be responsible to the increase of mortality observed in IL-10 KO and TNF-α KO mice.
Figure 3. mRNA levels of IFN-γ, IL-2, IL-4, IL-6, IL-10 and TNF-α quantified by real time PCR in the brain of WT, IL-10 KO and TNF-α KO mice (n = 4 to 10) infected with 104 pfu of Oshima (a) and Sofjin (b) at 5 and 9 days pi.
Following infection with Sofjin strain as compared with mock infection, the levels of IFN-γ and IL-6 were significantly increased at 5 and 9 days pi in the brain of WT and IL-10 KO mice but not of TNF-α KO mice (Supplementary Figure 2b). Accordingly, the levels of IFN-γ and IL-6 were higher or significantly higher in WT and IL-10 KO mice than in TNF-α KO mice at 5 and/or 9 days pi (Figure 3b). When these cytokine levels were compared between Oshima- and Sofjin-infected mice, IFN-γ level in TNF-α KO mice was higher at 9 days pi following infection with Oshima but not with Sofjin (Supplementary figure 3). On the other hand, IL-6 level in WT mice was higher after 5 days pi following infection with Sofjin but not with Oshima (Supplementary figure 3). Thus, it indicates that immune response such as IFN-γ and IL-6 induction in the brain are different between Oshima and Sofjin infection.
TNF-α level was significantly increased in WT at 9 days pi and IL-10 KO mice at 5 and 9 days pi following infection with Sofjin strain (Supplementary Figure 2b). IL-2 level in IL-10 KO mice at 9 days pi was increased and IL-10 level in TNF-α KO mice was increased at 5 days rather than at 9 days pi after infection with Sofjin strain (Supplementary Figure 2b). IL-4 level was not increased following Sofjin infection in the three mouse groups (Supplementary Figure 2b). However, the levels of IL-2, IL-4, IL-10 and TNF-α were not significantly different between WT, IL-10 KO and TNF-α KO groups at 5 and 9 days pi (Figure 3b).
mRNA levels of proinflammatory cytokines in the spleen of TNF-α KO and IL-10 KO mice infected with TBEV
We next examined the mRNA levels of IFN-γ, IL-2, IL-4, IL-6, IL-10 and TNF-α in the spleen in WT, IL-10 KO and TNF-α KO groups of mice infected with Oshima and Sofjin strains.
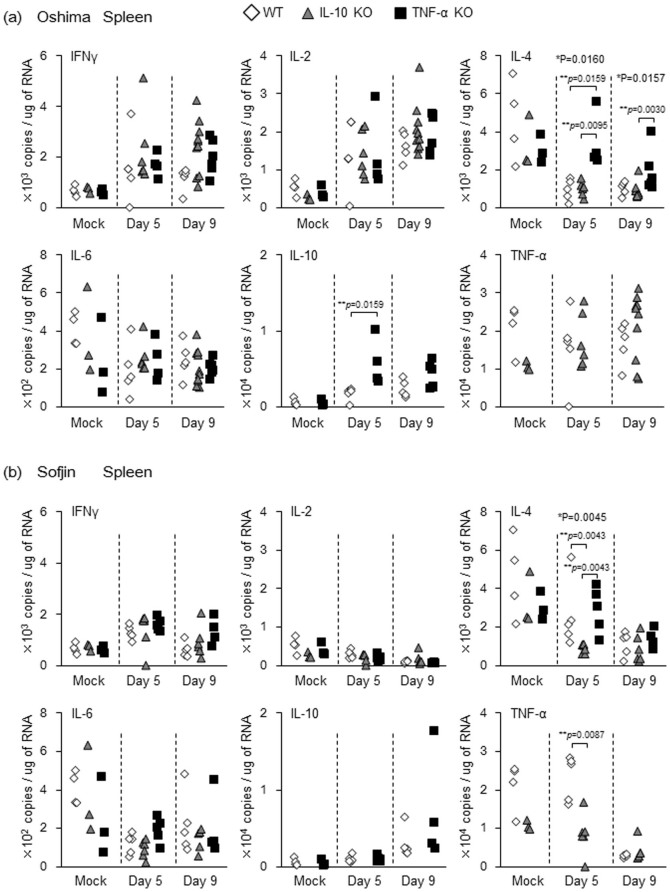
Following infection with Oshima strain in comparison with mock infection, the following levels in cytokines were observed in the spleen of mice: (i) significant increase in the levels of IFN-γ and IL-2 in IL-10 KO and in TNF-α KO mice at 5 and/or 9 days pi (Supplementary Figure 4a), but no significant difference in the levels of each cytokine between the three groups at 5 days pi nor at 9 days pi (Figure 4a), (ii) significant increase in IL-10 level in TNF-α KO mice at 9 days pi but not in WT mice (Supplementary Figure 4a) and comparing the level of this cytokine between these two groups of mice, significantly higher IL-10 level in the former mice at 5 days pi (Figure 4a), (iii) significant decrease in IL-4 level in WT and IL-10 KO mice after infection (Supplementary Figure 4a) with the level of this cytokine in TNF-α KO mice significantly higher than that in WT and IL-10 KO mice at 5 and 9 days pi (Figure 4a), and (ix) absence of significant up- or down-regulation in IL-6 and TNF-α levels in each mouse group (Supplementary Figure 4a) and no significant difference in the levels of these cytokines between the three groups (Figure 4a).
Figure 4. mRNA levels of IFN-γ, IL-2, IL-4, IL-6, IL-10 and TNF-α quantified by real time PCR in the spleen of WT, IL-10 KO and TNF-α KO mice (n = 4 to 10) infected with 104 pfu of Oshima (a) and Sofjin (b) at 5 and 9 days pi.
Interestingly, following Sofjin infection, the level of IL-2 was not increased in IL-10 KO and TNF-α KO mice and was decreased in WT mice (Supplementary Figure 4b). These IL-2 levels were not significantly different among all the groups (Figure 4b). Accordingly, IL-2 level due to Oshima infection was significantly higher than that due to Sofjin infection in all groups of mice (Supplementary figure 5). It indicates that Sofjin infection did not up-regulate the mRNA level of IL-2 in the spleen, implying that IL-2 response was related to the pathological difference due to Oshima and Sofjin infection.
Following Sofjin infection, IL-4 level was decreased in WT mice at 9 days pi and IL-10 KO mice at 5 days pi (Supplementary Figure 4b), and accordingly the level in IL-10 KO mice was lower than that in WT and TNF-α KO mice at 5 days pi (Figure 4b). Decrease of IFN-γ level was observed in WT mice between 5 and 9 days pi and IL-6 level was also decreased in WT and IL-10 KO mice at 5 days following Sofjin infection (Supplementary Figure 4b). However, there was no significant difference in the levels of these cytokine among the three groups (Figure 4b). Increase of IL-10 level was observed in WT mice after Sofjin infection (Supplementary Figure 4b), but there was no significant difference in the level between WT and TNF-α KO groups (Figure 4b). TNF-α level was decreased in WT and IL-10 KO mice after Sofjin infection (Supplementary Figure 4b) and the level in WT mice was higher than in IL-10 KO mice (Figure 4b).
When these cytokine levels in the spleen were compared between Oshima- and Sofjin-infected mice, spleen from the latter mice specifically in TNF-α KO mice had significantly higher level of IL-10 at 5 days pi (Supplementary figure 5). Interestingly, TNF-α level due to Sofjin infection was significantly lower in WT mice at 9 days pi and in IL-10 KO mice at 5 and 9 days pi compared with that due to Oshima infection (Supplementary figure 5). Thus, it indicates that TNF-α response in the spleen could have an effect on the different pathogenesis due to Oshima and Sofjin infections.
TBEV specific antibody responses elicited in TNF-α KO and IL-10 KO mice infected with TBEV
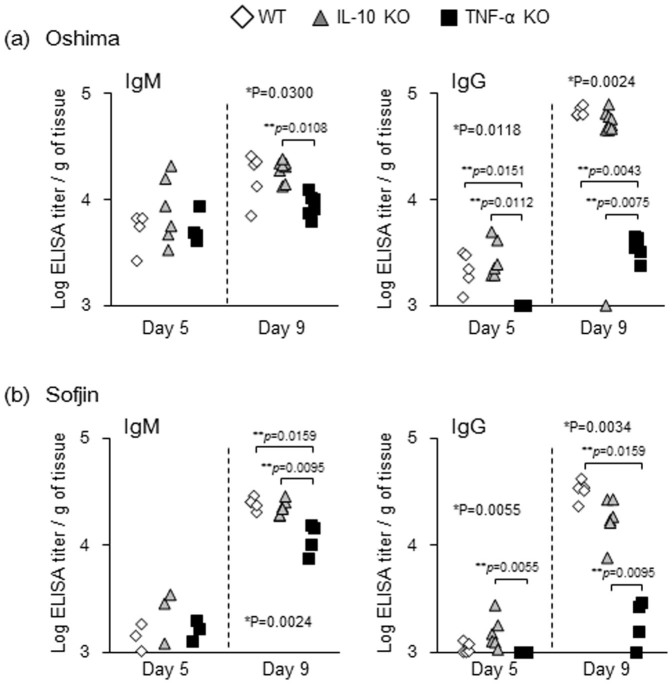
Antibody responses during Oshima and Sofjin infection were compared between WT, IL-10 KO and TNF-α KO groups. Following Oshima virus infection, titer of IgM at 9 days pi and of IgG at 5 and 9 days pi was lower significantly in TNF-α KO mice compared with that of WT and/or IL-10 KO mice (Figure 5a). Following Sofjin infection, TNF-α KO mice also exhibited significantly lower levels of IgM titer at 9 days pi and of IgG titer at 5 and 9 days pi compared with either or both of the other groups of mice (Figure 5b). These observations suggest that TNF-α contributed to the increase in IgM and IgG production at early phase following infection with TBEV.
Figure 5. Titers of TBEV-specific antibody in WT, IL-10 KO and TNF-α KO mice (n = 4 to 10) infected with Oshima (a) and Sofjin (b).
The appearance of TBEV-specific IgM and IgG from serum was determined at 5 and 9 days pi by enzyme-linked immunosorbent assay with purified TBEV antigen as the assay antigen.
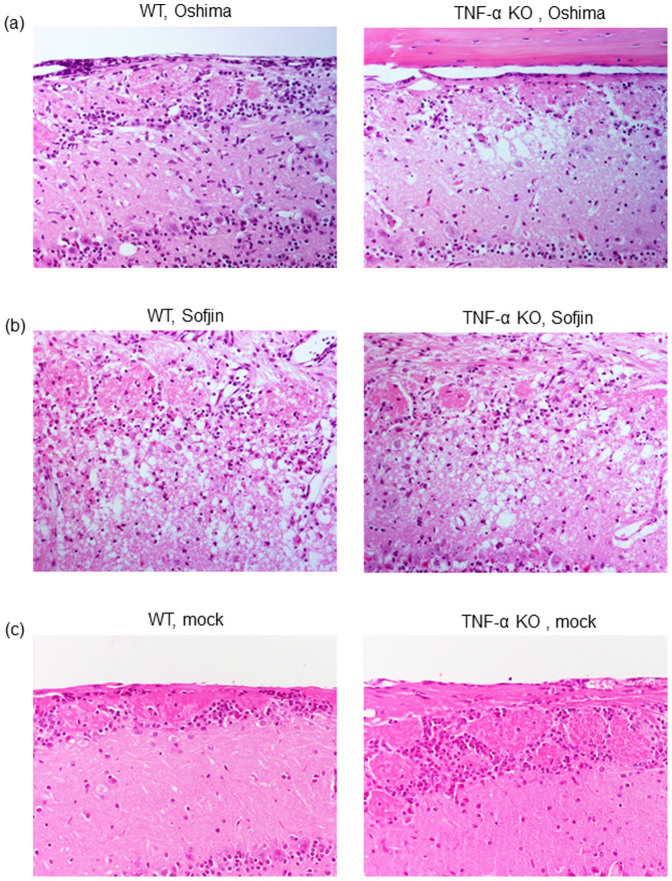
Histopathological features of the brains of TNF-α KO mice infected with TBEV
To confirm whether severe disease resulting in 100% mortality in TNF-α KO mice was attributed to severe neuronal damage, histopathological features of the brains of WT and TNF-α KO mice infected with Oshima and Sofjin strains were compared.
Following Oshima infection, TNF-α KO mice exhibited severe neuronal loss in several areas of the brain cortex when compared with WT mice (Figure 6a). Following Sofjin infection, more severe neuronal damage was observed in both of WT and TNF-α KO mice (Figure 6b). Accordingly, the degree of neuronal damage on Sofjin-infected WT and TNF-α KO mice was more extensive than that of Oshima-infected mice (Figure 6a and 6B). Mock-infected mice showed none of the neuronal destruction (Figure 6c). These observations suggest that severe neuronal damage indeed correlate with the increase in mortality and that TNF-α deficiency enhanced severe neuronal damage resulting in 100% mortality in TBEV-infected mice.
Figure 6. Histopathological features of the brain cortex of WT and TNF-α KO mice infected with Oshima (a) and Sofjin (b) at 104 pfu and those of mock-infected mice (c).
Sacrifice of mice was done at 9 days pi. Each figure represents three to five mice.
Discussion
In this study, we provided evidences by using a mouse model that TNF-α and IL-10 have protective roles from fatal disease due to infection with the Oshima strain of TBEV. We showed that increase in mortality and severe neuronal loss were more pronounced in TNF-α KO mice. Furthermore, we showed that mRNA levels of IL-2 in the spleen were not up-regulated following infection with more virulent Sofjin strain that caused 100% mortality, whereas these levels were significantly up-regulated in Oshima-infected mice. Thus, we suggest that TNF-α, IL-10 and IL-2 are key factors for disease remission from severe encephalitis due to TBEV infection.
To examine the cytokine levels in TBEV-infected mice, we preliminary tried to detect and compare the expression levels of these proteins in the serum, spleen or brain by using commercial bead based Analyte Detection System or Western blot. We first examined several cytokines (IL-1α, IL-2, IL-5, IL-6, IL-10, IFN-γ, TNF-α, GM-CSF, IL-4 and IL-17) in the serum of TBEV-infected and mock-infected mice by using Mouse Th1/Th2 10plex kit (eBioscience). However, only a few samples showed positive detection levels and most samples were under the detection limit of the proteins. Thus, the cytokine levels through this technique could not be compared; no apparent correlation of the cytokine levels between the sample groups was observed. We next tried to detect and compare the protein levels of some proinflamatory cytokines such as IFN-γ and IL-2 in the spleen and brain of TBEV-infected and mock-infected mice by western blot. However, we failed to detect them from homogenized tissue samples. Failures of these preliminary experiments could be due to technical difficulty or low sensitivity of the assays. Thus, we examined the mRNA levels of proinflammatory cytokines by real-time PCR referred to in previous studies11,12. We summarized the data of mRNA levels in the brain and spleen of mice infected with Oshima and Sofjin strains in the Supplementary table.
In our previous study on JEV, similar observations were obtained in TNF-α and IL-10 KO mice infected with the JaOArS982 strain that exhibited similar mortality pattern to Oshima strain of TBEV6,10. Following infection with JaOArS982, TNF-α KO and IL-10 KO mice exhibited significant increase in mortality rates when compared with WT mice. High inflammatory responses were observed in the CNS of TNF-α KO mice and IL-10 KO mice, thus we suggested that immunopathological effects contribute to the severe neuronal degeneration and fatal disease in those KO mice. However, in the current study using the Oshima strain such exaggerated inflammatory responses were not observed in the CNS of the same KO mice when compared with WT mice. Therefore, we consider that the mechanism of neuronal damage and increased mortality in TNF-α KO mice is different between infections with JaOArS982 strain of JEV and Oshima strain of TBEV, although TNF-α appears to provide protective effect on disease severity due to both of these viruses.
Interestingly, IL-2 level in the spleen was not increased after infection with Sofjin strain in all WT, TNF-α and IL-10 KO groups. Infection with Sofjin strain caused extremely higher viral replication in the brain compared with that of Oshima strain, suggesting that severity of Sofjin strain attributes to the viral infection and replication in the brain. However, IL-2 level in the brain was not significantly different between infections with Sofjin and Oshima strains. Although it remains unclear how IL-2 response in the spleen relates to the viral replication in the brain, we raise the possibility that IL-2 response in the spleen indirectly affects the immune response in the brain.
IL-2 affects T cell proliferation. Following peripheral infection with TBEV, primary T cell activation occurs in the peripheral tissues such as lymph nodes and spleen. Then, after TBEV enters the CNS, T cells infiltrate the infected site in the brain. We previously showed that patterns of T cell repertoire were different between spleen and brain of Oshima-infected mice and that specific T cells in the brain may contribute to the disease severity in mice13. Based on our current ongoing experiment, we suggest that T cell repertoire in the brain is possibly different between Sofjin- and Oshima-infected mice and suppressive effect to virus replication in the brain may be different. Therefore, we consider that Sofjin infection may have an effect on the down regulation of IL-2 in the spleen and that the T cells harboring weak suppressive effect infiltrate the brain. Further investigations will be required to resolve this hypothesis to understand the mechanism(s) of IL-2 response owing to Oshima and Sofjin infections which could provide valuable insights into the TBE pathogenesis.
Sofjin strain used in this study had the same origin with Sofjin-HO strain used in previous studies9,14. The nucleotide homology between Sofjin and Oshima strains is 96% and there are 44 amino acid differences in the coding region, and 17 nucleotide differences and a deletion of 207 nucleotides in the non-coding region between those strains. Sakai et al. recently reported by using a mouse model that the variable region of the 3′ non-coding region (NCR) is a critical factor for the virulence of Sofjin strain14. This region has an effect on the increase in mortality and on higher viral load in the brain of Sofjin-infected mice compared with those of Oshima-infected mice14. However, how and whether this region affects the immune response including IL-2 induction was not investigated. Thus, it is interesting to examine whether the 3′NCR of TBEV affects IL-2 responses in the spleen of infected mice; further investigation will be required to answer this question.
In our previous study on JEV, we also showed that more virulent JaTH160 strain of JEV caused a significant increase on CNS infection and higher mortality in mice compared with JaOArS982. Interestingly, IL-2 level was also not significantly elevated in the spleen of JaTH160-infected mice similar to the one observed in Sofjin infection in the present study10. Therefore, the low level of IL-2 in the spleen may be a common factor of severe pathogenesis due to high virulent strains of JEV and TBEV.
It has been reported that TNF-α KO mice show developmental defects in the humoral immune system including a lack of primary B cell follicles15. TNF-α KO mice infected with either Oshima or Sofjin strain used in this study did not show a significant difference in the level of anti-TBEV IgM response on day 5. A previous report showed that an absence of TNF-R1 did not affect the magnitude or quality of early antibody response after WNV infection16. However, our data showed that IgG levels were decreased in TNF-α KO mice compared with WT and IL-10 KO mice. Thus, an absence of TNF-α could affect the level of antibody response after TBEV infection. The decreased levels of antibody response in TNF-α KO mice could be due to the lack of B cells priming, although it is unclear whether the responses are related with reduced incidence of mortality in TNF-α KO mice.
Compensation with cytokines such as TNF-α, IL-10 and IL-2 may provide valuable clue to the effective treatment for TBE. Although we did not perform this experiment in the current study, we previously tried it in JEV- and in TBEV-infected mice. However, in our preliminary experiments we failed to observe the functional compensation after injecting cytokines, agonist, antagonist or antibody in mice. We consider that technical improvement on the amount, frequency or administration route of compensating factors will be required for their delivery to the effective cells in order to obtain beneficial effects on the resolution of the disease. In particular, if these cytokines have effects in the CNS, delivery by injection is difficult, because in general intravenous or intraperitoneal administration is performed for external supplementation. We are currently trying to make technical improvement, but we have not yet obtained convincing evidence. We believe that these attempts are important priorities to enable the development of effective treatment strategies for TBE.
Methods
Cells and viruses
The baby hamster kidney (BHK) cells were maintained in minimum essential medium (MEM) supplemented with 10% fetal calf serum (FCS). The cells were allowed to grow at 37°C with 5% CO2. Stock virus of TBEV Oshima strain and Sofjin strain were prepared in BHK cells. All experiments using live TBEV were performed in a biosafety level 3 laboratory of the Institute of Tropical Medicine, Nagasaki University according to the standard BSL3 guidelines.
Mice
C57BL/6j wild type mice were purchased from Japan SLC Cooperation (Shizuoka, Japan). B6 background IL-10 KO mice were purchased from the Jackson Laboratory, USA. TNF-α KO mice were kindly provided by Yoichiro Iwakura, Research Institute for Biomedical Sciences, Tokyo University of Science. These mice were mated in the facility of Nagasaki University. Five to six week old mice were subcutaneously inoculated with 104 PFU of TBEV diluted in MEM containing 2% FCS. Mock-infected mice were inoculated with MEM containing 2%FCS. Mice were weighed daily up to 21 days and observed for clinical signs. The animal experiments were carried out in accordance with the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology. The experimental protocols were approved by the Animal Care and Use Committee of the Nagasaki University (approval number: 091130-2-7/0912080807-7).
Virus titration
Following TBEV infection, mice were sacrificed and the blood was collected. After perfusion with cold phosphate-buffered saline (PBS), spleens, brains and spinal cords were collected. Brains were divided into four parts: brain cortex, thalamus, cerebellum and brain stem. They were kept at −80°C until further use. Each brain part was homogenized in ten volumes of PBS containing 10%FCS and diluted with MEM containing 2%FCS. By using BHK cells, virus titers were determined by plaque forming assays and were expressed as PFU/g tissue.
Measurement of TBEV-specific antibodies
Following TBEV infection, mice were sacrificed and the blood was collected. Blood was centrifuged and plasma was collected. TBEV IgM and IgG indirect ELISAs were done following modified procedures of previous studies17. Purified TBEV (Oshima strain) was applied as assay antigen. The test plasma was diluted at 1:100 and 1:1,000 for the detection of IgM and IgG respectively. HRPO-conjugated goat anti-mouse IgM and IgG (American Qualex) were diluted at 1:2,000. The substrate solution consisted of OPD with hydrogen peroxide. The reaction was stopped by the addition of 1 N sulphuric acid. A standard curve was prepared using TBEV positive control serum starting with a 100- and 1,000-fold dilutions for IgM and IgG, respectively. The IgM and IgG titers of mice sera were determined from respective standard curve.
Quantification of inflammatory cytokines and real-time PCR
Following TBEV infection, mice were sacrificed and spleens and brains were collected after perfusion. Those tissues were immediately submerged in RNAlater (Ambion). Total RNA was extracted using an RNeasy Lipid Tissue Mini Kit (Qiagen).
Transcribed mRNA levels of IFN-γ, IL-2, IL-4, IL-6, IL-10 and TNF-α were examined by SYBR real-time PCR as done previously10,11. Absolute copy numbers of unknown samples were calculated by comparing the threshold cycle with the corresponding standard curve. The relative quantification was expressed as a ratio between unknown samples and internal control for which glyceraldehyde-3-phosphate dehydrogenase (GADPH) was used. Results were normalized to a calibrator sample, and the relative transcribed mRNA level was obtained, providing indirect information on target mRNA levels and taking into account corrections for experimental variations in different samples18,19.
Histopathological examination
Mice inoculated with TBEV were anesthetized and perfused with 10% phosphate-buffered formalin. Fixed tissues were routinely embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Statistical analyses
Kruskal-Wallis test and Mann Whitney test were used for statistical analysis to assess the significant differences in viral loads and mRNA levels of genes. Gehan-Breslow-Wilcoxon Test was performed to assess the survival curves of TBEV-infected groups of mice.
Author Contributions
D.H. designed the study; M.M.N.T., K.A., M.S., K.S. and D.H. performed the experiments; M.M.N.T., K.A., R.S., K.M. and D.H. analyzed the data; M.M.N.T., C.C.B. and D.H. wrote the paper.
Supplementary Material
Supplementary figures
Acknowledgments
We thank Ikuo Takashima (Graduate School of Veterinary Medicine, Hokkaido University) for providing TBEV Oshima strain, and Yoichiro Iwakura (Center for Experimental Medicine, Institute of Medical Science, University of Tokyo; address in current position: Division of Experimental Animal Immunology, Research Institute of Biomedical Science/Graduate School of Biological Science, Tokyo University of Science) for kindly providing TNF-α KO mice. This work was supported by JSPS KAKENHI Grant Numbers 25304045, 25660229, 23658243 and from Japan Society for the Promotion of Science; Health and Labour Sciences Research Grant on Emerging and Re-emerging Infectious Diseases from the Japanese Ministry of Health, Labour and Welfare; Research on International Cooperation in Medical Science (Japan-US Cooperative Program), Health and Labour Sciences Research Grants; the Cooperative Research Grant(s) of NEKKEN, 2014 and the Japan Initiative for Global Research Network on Infectious Diseases.
References
- Dumpis U., Crook D. & Oksi J. Tick-borne encephalitis. Clin Infect Dis 28, 882–890 (1999). [DOI] [PubMed] [Google Scholar]
- Lindquist L. & Vapalahti O. Tick-borne encephalitis. Lancet 371, 1861–1871 (2008). [DOI] [PubMed] [Google Scholar]
- Ecker M., Allison S. L., Meixner T. & Heinz F. X. Sequence analysis and genetic classification of tick-borne encephalitis viruses from Europe and Asia. J Gen Virol 80, 179–185 (1999). [DOI] [PubMed] [Google Scholar]
- Holzmann H. Diagnosis of tick-borne encephalitis. Vaccine 21 Suppl 1, S36–40 (2003). [DOI] [PubMed] [Google Scholar]
- Mandl C. W. Steps of the tick-borne encephalitis virus replication cycle that affect neuropathogenesis. Virus research 111, 161–174 (2005). [DOI] [PubMed] [Google Scholar]
- Hayasaka D. et al. Mortality following peripheral infection with tick-borne encephalitis virus results from a combination of central nervous system pathology, systemic inflammatory and stress responses. Virology 390, 139–150 (2009). [DOI] [PubMed] [Google Scholar]
- Kimura T., Sasaki M., Okumura M., Kim E. & Sawa H. Flavivirus encephalitis: pathological aspects of mouse and other animal models. Vet Pathol 47, 806–818 (2010). [DOI] [PubMed] [Google Scholar]
- Larena M. & Lobigs M. in FLAVIVIRUS ENCEPHALITIS (ed Ruzek, D.) Ch. 16, 317–338 (InTech, 2011). [Google Scholar]
- Chiba N. et al. Pathogenicity of tick-borne encephalitis virus isolated in Hokkaido, Japan in mouse model. Vaccine 17, 779–787 (1999). [DOI] [PubMed] [Google Scholar]
- Hayasaka D. et al. TNF-alpha Acts as an Immunoregulator in the Mouse Brain by Reducing the Incidence of Severe Disease Following Japanese Encephalitis Virus Infection. PLoS One 8, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y. et al. Accumulation of T-cells with selected T-cell receptors in the brains of Japanese encephalitis virus-infected mice. Jpn J Infect Dis 61, 40–48 (2008). [PubMed] [Google Scholar]
- Ida-Hosonuma M. et al. The alpha/beta interferon response controls tissue tropism and pathogenicity of poliovirus. J Virol 79, 4460–4469 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y. et al. T-cell clones expressing different T-cell receptors accumulate in the brains of dying and surviving mice after peripheral infection with far eastern strain of tick-borne encephalitis virus. Viral Immunol 24, 291–302 (2011). [DOI] [PubMed] [Google Scholar]
- Sakai M. et al. The variable region of the 3′ untranslated region is a critical virulence factor in the Far-Eastern subtype of tick-borne encephalitis virus in a mouse model. J Gen Virol 95, 823–835 (2014). [DOI] [PubMed] [Google Scholar]
- Pasparakis M., Alexopoulou L., Episkopou V. & Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med 184, 1397–1411 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B., Zhang B., Purtha W. E., Klein R. S. & Diamond M. S. Tumor necrosis factor alpha protects against lethal West Nile virus infection by promoting trafficking of mononuclear leukocytes into the central nervous system. J Virol 82, 8956–8964 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S. et al. Evaluation of a dengue IgG indirect enzyme-linked immunosorbent assay and a Japanese encephalitis IgG indirect enzyme-linked immunosorbent assay for diagnosis of secondary dengue virus infection. Vector-Borne Zoonot- 10, 143–150 (2010). [DOI] [PubMed] [Google Scholar]
- Stordeur P. et al. Cytokine mRNA quantification by real-time PCR. J Immunol Methods 259, 55–64 (2002). [DOI] [PubMed] [Google Scholar]
- Vernel-Pauillac F. & Merien F. Proinflammatory and immunomodulatory cytokine mRNA time course profiles in hamsters infected with a virulent variant of Leptospira interrogans. Infect Immun 74, 4172–4179 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures