Abstract
We experimentally examined the effects of elevated O3 and whitefly herbivory on tomato volatiles, feeding and oviposition preferences of whiteflies and behavioural responses of Encarsia formosa to these emissions on two tomato genotypes, a wild-type (Wt) and a jasmonic acid (JA) defence-enhanced genotype (JA-OE, 35S). The O3 level and whitefly herbivory significantly increased the total amount of volatile organic compounds (VOCs), monoterpenes, green leaf volatiles (GLVs), and aldehyde volatiles produced by tomato plants. The 35S plants released higher amount of total VOCs and monoterpene volatiles than Wt plants under O3+herbivory treatments. The feeding and oviposition bioassays showed that control plants were preferred by adult whiteflies whereas the 35S plants were not preferred by whiteflies. In the Y-tube tests, O3+herbivory treatment genotypes were preferred by adult E. Formosa. The 35S plants were preferred by adult E. formosa under O3, herbivory and O3+herbivory treatments. Our results demonstrated that elevated O3 and whitefly herbivory significantly increased tomato volatiles, which attracted E. formosa and reduced whitefly feeding. The 35S plants had a higher resistance to B. tabaci than Wt plant. Such changes suggest that the direct and indirect defences of resistant genotypes, such as 35S, could strengthen as the atmospheric O3 concentration increases.
Plants are a source of a large diversity of volatile organic compounds (VOCs), such as monoterpenes, sesquiterpenes and homoterpenes1. These VOCs can serve as semiochemicals that can be detected by other organisms to reveal the host presence2, to signify availability of food and mating sites3,4 and also to aid in navigating to food sources5,6.
Insect feeding influences the nutrient condition7; secondary metabolite production, i.e., jasmonic acid (JA is a plant hormone involved in regulating secondary metabolite production among other things)8,9,10; and the type and amount of herbivore-induced plant volatiles (HIPVs) released by the plant11,12. HIPVs can function as plant defences by directly repelling herbivores from ovipositing and host-seeking13 and by attracting herbivore enemies, such as parasitic wasps, predatory arthropods and possibly even insectivorous birds14,15,16. For example, the monoterpene volatiles of Chrysanthemum morifolium repel ovipositing females of the diamondback moth (Plutella xylostella), a lepidopteran that does not normally lay eggs on C. morifolium17. Volatiles, such as the aphid alarm pheromone from transgenic Arabidopsis thaliana, repelled the green peach aphid (Myzus persicae) from feeding, although wild-type Arabidopsis does not normally produce this pheromone18. Laboratory and field studies have shown that HIPVs attract parasitoids and/or predators to many plant species that reduce the risk of damage from herbivorous insects19,20,21. Predatory mites preferred the odour of Lima bean plants infested with two-spotted spider mites (Tetranychus urticae) to that of uninfested plants11. The pea leafminer, Liriomyza huidobrensis, caused an increased emission of volatiles in tomato plants and consequently increased the attraction of the parasitoid Opius dissitus22. Enhancement of these interactions between the HIPVs and the natural enemies of herbivores can aid the development and delivery of integrated pest management programs12,22.
Recently, the global atmospheric concentration of ozone (O3) has risen from less than 10 ppb (parts per billion) a century ago to 40 ppb today and is projected to continue to increase at an annual rate of 1–2%23,24 to 68 ppb by the year 205025. Elevated O3 changes plant biochemistry, including the activation of many plant defence responses26,27,28. Elevated O3 is also changing the atmospheric life times of phytogenic VOCs, including the HIPVs29. Due to these alterations, the behavioural and life history parameters of herbivorous insects are also influenced30,31. O3 stress can increase VOC emissions from plants, including terpenoids and green leaf volatiles (GLVs)32,33. For example, Blande et al. reported increased emissions of some monoterpenes (α-pinene and/or β-pinene, (E)-β-ocimenes) and higher total monoterpene emissions from hybrid aspens (Populus tremula × tremuloides Michx.) grown under moderately elevated O312. Furthermore, O3 fumigation has been reported to promote the emission of herbivore-induced volatiles11. Differences in the quality and quantity of the volatile mixtures induced by a high O3 concentration will affect tritrophic interactions among plants, herbivorous insects and natural enemies34. However, little is known regarding HIPVs induction by piercing-sucking insects and the effects of herbivores and their natural enemies under elevated levels of O3.
Tomato, Solanum lycopersicum, is an economically important vegetable worldwide and a commonly used model plant for biologists35,36. It is also an O3-sensitive species37. Bemisia tabaci Gennadius (Homoptera: Aleyrodidae)38 is one of the most noxious insect pests on field and greenhouse crops worldwide39,40. Encarsia formosa is the main parasitoid of B. tabaci, and the use of E. formosa for whitefly management is now universally accepted as a biological control agent41. Previous research indicated that several JA-overexpression mutants exhibit greater resistance against insects than wild-type plants42,43,44. Wei et al. (2011) showed that there are ecological trade-offs between JA-dependent direct and indirect defences in genetically modified plants22. Our previous research indicated that the JA-overexpression tomato mutant 35S was resistant to B. tabaci under a high O3 concentration and whitefly infestation and there was a reduction in the fitness of conspecific B. tabaci that fed on three previously infested tomato genotypes that differed in the JA pathway45,46. However, the mechanism(s) by which VOCs affect whitefly feeding and oviposition and the behavioural responses of E. formosa induced by whitefly infestation remain unclear under elevated O3 conditions.
Here, we hypothesise that elevated O3 levels and whitefly infestation will affect the type and amount of tomato volatiles produced, which will affect the feeding and oviposition preferences of whiteflies and their interaction with E. formosa. To test this hypothesis, the effects of elevated O3 and whitefly infestation on two tomato genotypes, including the wild-type and a JA-defence-enhanced genotype (35S), in association with the phloem feeder B. tabaci Gennadius biotype B and a natural enemy, E. Formosa, were examined in open-top chambers in the field. Our specific objectives were to determine: (1) whether elevated O3 levels alter the emission of whitefly-induced volatiles, (2) the effects of VOCs on the feeding and oviposition preferences of B. tabaci and (3) the effects of VOCs on the behavioural responses of E. formosa.
Results
Volatile emission rate
O3 level, whitefly herbivory, tomato genotypes and the interactions between the whitefly herbivory and tomato genotypes significantly affected the total amount of VOCs, monoterpene volatile emissions, GLVs and aldehyde production. The interactions between O3 and whitefly herbivory significantly affected the total amount of VOCs, monoterpenes and GLVs. The interactions between O3 and tomato genotypes significantly affected the total amount of VOCs and monoterpene volatile emissions. Ozone level × tomato genotype × whitefly herbivory significantly affected the total amount of VOCs, monoterpene volatile emissions and aldehyde volatiles (Table 1).
Table 1. ANOVA analyses on the effects of the O3 level, whitefly herbivory and tomato genotypes on the volatile emission rate of tomato plants.
| Measured Indices F(P) | ||||
|---|---|---|---|---|
| Factor | Monoterpene volatile emissions | GLVsa | Aldehyde volatiles | VOCsb |
| O3 | <0.001 | <0.001 | <0.001 | <0.001 |
| Herbivory | <0.001 | <0.001 | <0.001 | <0.001 |
| Genotype | <0.001 | <0.001 | <0.001 | <0.001 |
| O3 × herbivory | <0.001 | <0.001 | 0.41 | <0.001 |
| O3 × genotype | <0.001 | 0.19 | 0.2 | <0.001 |
| Herbivory × genotype | <0.001 | <0.001 | <0.001 | <0.001 |
| O3 × herbivory × genotype | <0.001 | 0.5 | 0.03 | <0.001 |
aGreen leaf volatiles.
bTotal amount of volatile organic compounds.
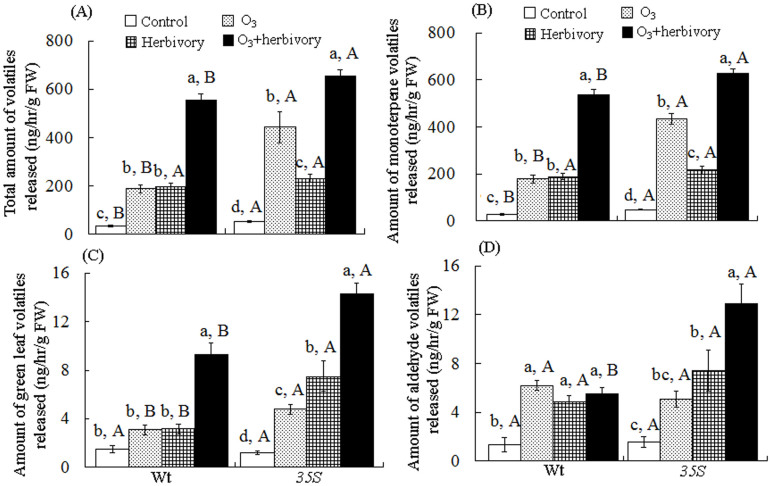
The monoterpene volatile emissions included terpinolene, (+)-α-pinene, (+)-3-carene, D-limonene, α-phellandrene, ocimene and β-phellandrene in the two tomato genotypes. The GLVs were Z-3-hexanol and E-2-hexanol. The aldehyde volatiles referred to nonanal and octanal (Fig. 1A, B, C, D).
Figure 1. Emission rate1 of volatile organic compounds (VOCs) from tomato genotypes grown under ambient and elevated O3 with and without Bemisia tabaci of herbivore preconditining after three weeks.
Different lowercase letters within a row indicate significant differences among the four treatments in a specific tomato cultivar (LSD test: P < 0.05); Different uppercase letters indicate significant differences among tomato genotypes within the same O3 and whitefly treatment (LSD test: P < 0.05).1 Emission rate = ng of compound released per g (fresh weight) of leaves per hour. Each value represents the average (±SE) of 4 replicates.
Elevated O3 levels increased the VOC emissions 4.85-fold in the Wt genotype (F3, 12 = 382.234, P = 0.000) and 7.48-fold in the 35S genotype (F3, 12 = 195.509, P = 0.000) (Table 1, Fig. 1A). Elevated O3 levels enhanced the monoterpene emissions 5.12-fold in the Wt genotype (F3, 12 = 372.234, P = 0.000) and 7.80-fold in the 35S genotype (F3, 12 = 198.668, P = 0.000) (Table 1, Fig. 1B).
Whitefly herbivory increased the total amount of VOC emissions 5.12-fold in the Wt genotype (F3, 12 = 382.234, P = 0.000) and 3.41-fold in the 35S genotype (F3, 12 = 195.509, P = 0.000) (Table 1, Fig. 1A). Whitefly herbivory increased the monoterpene emissions 5.56-fold in the Wt genotype (F3, 12 = 372.234, P = 0.000) and 3.38-fold in the 35S genotype (F3, 12 = 198.668, P = 0.000) (Table 1, Fig. 1B).
For the two plant genotypes, the total amount of VOCs is highest under O3+herbivory treatment. The sum of the peak areas show the total amount of VOCs from the 35S plants was 656.75 ± 22.85, whereas that from Wt plants was 554.53 ± 56.65. The 35S plants released higher levels of monoterpene volatiles than the Wt plants under control, O3 and O3+herbivory treatments (Fig. 1A, B).
Feeding and Oviposition Preferences of B. tabaci
O3 level, whitefly herbivory and tomato genotypes significantly affected the feeding and oviposition preferences of B. tabaci.
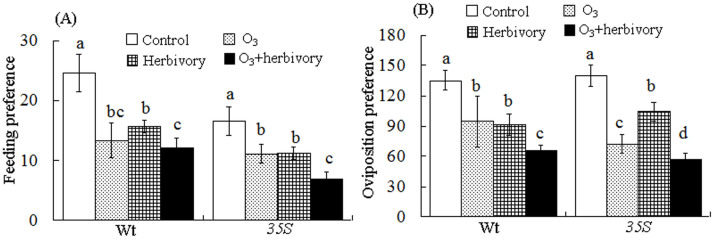
Adult whiteflies more often preferred the control plants for feeding (Wt: X2 = 87.063, P< 0.001; 35S: X2 = 62.349, P< 0.001) and oviposition (Wt: X2 = 385.914, P< 0.001; 35S: X2 = 660.616, P< 0.001) (Fig. 2A, B).
Figure 2. Effects of elevated O3 concentration and whitefly infestation on the feeding and oviposition preferences of whiteflies after three weeks on the two tomato genotypes.
Each value represents the average (±SE) of 15 replicates. Different lowercase letters indicate significant differences between the four treatments for a specific tomato cultivar (X2 test: P < 0.05).
Adult whiteflies preferred Wt plants for feeding under control (X2 = 11.645, P = 0.001), O3 (X2 = 25.712, P = 0.000) and herbivory (X2 = 3.939, P = 0.047) treatments. Adult whiteflies preferred Wt plants for oviposition under four treatments (control: X2 = 177.888, P = 0.000; O3: X2 = 200.166, P = 0.000; herbivory: X2 = 50.797, P = 0.000; O3+herbivory: X2 = 5.69, P = 0.017). Adult whiteflies did not prefer 35S plants for feeding and oviposition under the four treatments (Fig. 3A, B).
Figure 3. Effects of the tomato genotypes on the feeding and oviposition preferences of feeding whiteflies after three weeks under four treatments.
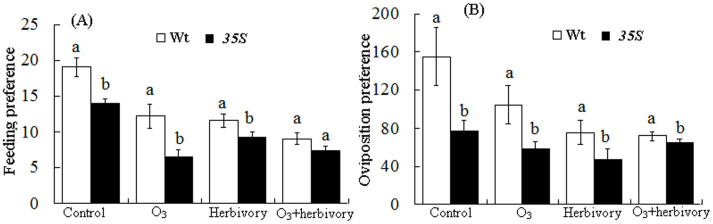
Each value represents the average (±SE) of 15 replicates. Different lowercase letters indicate significant differences between the tomato cultivars under four treatments (X2 test: P < 0.05).
The behavioural responses of E. formosa to the odors of two tomato genotypes in various treatments
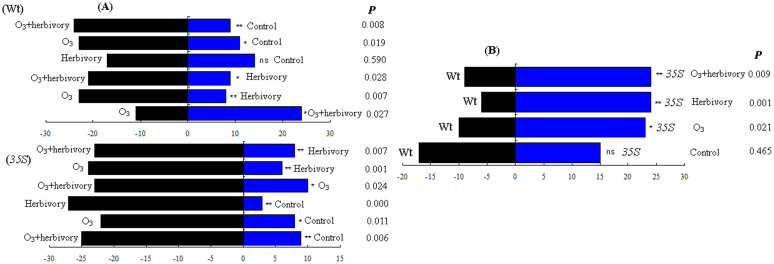
In a two-choice behavioural assay, O3+herbivory treatment plants were preferred by adult E. formosa for the two tomato genotypes in the Y-tube tests (Fig. 4A). Control treatment plants were not preferred by adult E. formosa for the two tomato genotypes in the Y-tube tests (Fig. 4A).
Figure 4.
Effects of elevated O3 levels and whitefly infestation after three weeks on the behavioural responses of E. formosa to the two host tomato genotypes (A). Effects of the tomato genotypes on the behavioural responses of E. formosa after three weeks under four treatments (B). X2 test was used for differences between the numbers of E. Formosa in each arm (*, P < 0.05; **, P < 0.01; ns, non significant).
To determine the preference of E. formosa for volatiles emitted by different tomato genotypes, behavioural responses were investigated in a dual-choice olfactometer. Behavioural assays in the Y-tube olfactometer showed that the 35S plants were preferred by adult E. formosa under O3, herbivory and O3+herbivory treatments (Fig. 4B). Adult parasitoid preferences for the two tomato genotypes were not significantly different under the control treatment (Fig. 4B).
Discussion
The herbivorous insect/plant interaction has proven to be a complex process that extends beyond compromising plant fitness to include directly inducing defensive responses by the plant to the herbivore and indirect effects involving VOC emissions by the plant that affect natural enemies9,47. Elevated O3 levels can alter nutritional quality, secondary metabolites and the resistance of plants as well as result in significantly increased VOC emissions45,48. Elevated O3 concentrations (100 ppb up to 250 ppb) resulted in increased monoterpene emissions of 3-year-old Quercus ilex L. plants49. Either the acute ozone-exposure or spider mite-infestation could induce the volatile emission of the Lima bean, but under elevated ozone condition, spider mite-damaged plants could release significantly higher amount of volatile emission11. Our previous research showed that elevated O3 levels altered the nutritional content and secondary metabolites in tomato plants45. In this study, we report that elevated O3 concentrations and whitefly infestation significantly increased the total amount of VOCs, monoterpene emissions, GLVs and aldehyde volatile productions of the tomato plants. The monoterpene volatile emissions remain the dominant product among the VOCs. Moreover, the tomato VOC emissions in the O3+herbivory treatment are the highest among the four treatments. The results indicated that the O3 factor and the whitefly infestation factor are related, suggesting that elevated O3 levels significantly abet the emission of whitefly-induced volatiles.
VOC release following elevated O3 levels; herbivore and mechanical damage can repel herbivores13,22,50. For example, volatiles from the infested transgenic A. thaliana repelled the green peach aphid (Myzus persicae) in comparison to volatiles from control transgenic plants18. Cannon (1990) found that O3-induced VOCs from red spruce needles repel spruce budworm larvae51. Some studies showed that monoterpenes are biogenic volatile organic compounds that play an important role in repelling herbivore oviposition and feeding17,48. Our results found that adult whiteflies preferred feeding and oviposition on control plants to three other O3 and herbivory treatments, suggesting that the increasing VOCs (especially monoterpenes) following elevated O3 levels and herbivore exposure will repel the whitefly.
VOC release following elevated O3 levels, herbivore and mechanical damage can also attract herbivore enemies13,14,32. The predatory mite Phytoseiulus persimilis prefers volatiles from the Lima bean leaves of plants injured by spider mites to volatiles from uninfested Lima bean leaves52. The aphid Myzus persicae caused an increased emission of volatiles triggered by spider mites in pepper plants and consequently increased the attraction of predators53. Pinto et al. (2007) found that VOCs, such as terpenes and GLVs of Brassica oleracea, induced by elevated O3 levels are crucial for the orientation of the wasps Cotesia plutellae48. Previous studies showed that monoterpenes and GLVs are important cues for natural enemies in host finding54,55,56. Our results showed that O3+herbivory treatment plants were preferred by adult E. formosa compared to control treatment plants in the two tomato genotypes in the Y-tube tests. The enrichment of VOCs in the O3+herbivory tomato plant treatment significantly attracted E. formosa.
JA-overexpression tomato genotype 35S has higher resistance to sucking insects than Wt plant and thus is considered one of the best plant genotype for insect resistance22,43. Cui et al. (2012a and 2012b) showed that the 35S has the highest resistance to B. tabaci under elevated O3 and whitefly infestation45,46. We report here that the 35S tomato genotypes released higher VOC emissions (including the total amount of VOCs, monoterpene volatile emissions, GLVs and aldehyde volatiles) than the Wt tomato plants. The 35S plants were not preferred by adult whiteflies for feeding or oviposition under four treatments, while the 35S plants were preferred by adult E. formosa under O3, herbivory and O3+herbivory treatments. Moreover, the differences between the two tomato genotypes were accentuated under elevated O3. These results showed that JA-overexpression tomato genotype 35S has a significantly higher resistance to the whitefly under elevated O3.
To our knowledge, this is the first systematic study of the responses to elevated O3 levels on tritrophic interactions among plants, herbivorous insects and natural enemies. Our results indicate that elevated O3 and whitefly infestation significantly increased the VOC emissions, especially the monoterpene volatile emissions. The tomato VOC emissions in the O3+herbivory treatment were the highest among the four treatments. 35S tomato genotypes released higher VOC emissions, especially monoterpene volatile emissions, than did the Wt tomato plants. The 35S plants had a higher repellent effect on the whitefly and a higher attraction of E. formosa. Such changes suggest that the direct and indirect defence of resistant genotypes, such as 35S, would be further strengthened as the atmospheric concentration of O3 continues to increase.
Methods
Open-top chambers and O3 Treatment
Experiments were conducted using eight octagonal open-top chambers (OTCs), each 2.2 m in height and 2 m in diameter, at the Observation Station for Global Change Biology at the Institute of Zoology of the Chinese Academy of Science, in Xiaotangshan County, Beijing, China (40°11′N, 116°24′E). Four OTCs were used for each O3 concentration treatment. In the elevated O3 treatment, O3 was generated from ambient air by an O3 generator (3S-A15, Tonglin Technology, Beijing, China) and then transported to the entrances of the OTCs using a fan (HB-429, 4.1 m3 min−1, Ruiyong Mechanical and Electrical Equipment Company). Mixed air (O3 and ambient air) was ventilated into each OTC through columniform polyvinyl chloride pipes (inner diameter 11 cm, outer diameter 16 cm). O3 concentrations were monitored at both the fan output (Shenzhen Yiyuntian Electronic CO. LTD) and within the OTCs (AQL-200, Aeroqual). From July 28th to August 21th, 2010, except for 2 rainy days, the OTCs were ventilated with air daily from 9:00 a.m. to 5:00 p.m. through a hemispherical stainless steel sprayer (diameter = 30 cm) situated 0.5 m above the canopy at a rate of approximately 4.1 m3 min−1, resulting in approximately 0.59 air changes per minute in each OTC. Gas concentrations were measured once every hour in each chamber receiving O3 treatment45.
The two O3 concentration treatments employed were as follows: (1) current ambient atmospheric O3 levels (average value from 9:00 a.m. to 5:00 p.m. on all air-treated days of 37.3 nmol mol−1) and (2) twice the current ambient O3 levels (average value from 9:00 a.m. to 5:00 p.m. on all air-treated days of 72.2 nmol mol−1)45.
Herbivory Treatment
Individuals of B. tabaci biotype B were collected on 5 April 2010 from cabbage growing at the Beijing Academy of Agriculture and Forestry. The offspring of these whiteflies were reared on tomatoes. For the infestation treatments, three leaves of each intact plant were encased in a mesh gauze bag and infested with 90 male whiteflies (to avoid the production of offspring) that were replaced every three days from July 28th to August 21th, 2010.
Host Plants
Two tomato genotypes were selected for the present study: wild-type (Wt) tomato plants (Solanum esculentum cv. Castlemart) and 35S::prosystemin transgenic tomato plants (35S). Professor C. Li of the Institute of Genetics and Developmental Biology, Chinese Academy of Science provided these plants. L. esculentum cv. Castlemart was the Wt parent for the 35S transgenic plants. The 35S::prosystemin (35S) JA-biosynthesis mutant transgenic plants overexpress prosystemin, which constitutively activates the defence system in unwounded plants and results in a stronger and more rapidly induced resistance43. After being grown in sterilised soil for two weeks, the tomato seedlings were individually transplanted into small plastic pots (14 cm diameter, 12 cm height) containing sterilised loamy field soil. Plants that were approximately 40 days old with heights of 20–30 cm were moved to the OTCs on 27 July 2010.
Feeding and Oviposition Preferences of B. tabaci
One control plant and one herbivore-preconditioned plant of each tomato genotype were placed into the same cage (dimensions = 60 × 60 × 60 cm) on 7 August 2010 in the open cylinders. They were control Wt plant/preconditioned Wt plant and control 35S plant/preconditioned 35S plant, respectively. The experiment had 15 replicates. For the preconditioning treatment, a cohort of 90 male whiteflies was established and replaced every three days on the lateral 3 leaves to provide a continuous infestation for three weeks in the open cylinders. After three weeks, 50 pairs of adult whiteflies were put into each cage, and three days later, the feeding and oviposition preference of each whitefly was recorded.
The second experiment was prepared in the same manner as the above mentioned experiment. The differences were each of 15 cages containing two control plants (one Wt and one 35S plant). Each of the other 15 cages contained two preconditioned treatment plants (one preconditioned Wt and one preconditioned 35S plant).
Adult parasitoid Encarsia formosa preferences for two tomato genotypes
Y-tube experiments A Y-tube olfactometer was used to investigate the behavioural responses of E. formosa to the volatile blends from different treatments within a tomato cultivar and different tomato cultivars within a same treatment. Each parasitoid E. formosa was placed in the olfactometer and observed for up to 5 minutes. A ‘no choice' outcome was recorded when the adults remained inactive during the testing period. A ‘first choice' outcome was recorded when the adults moved > 5 cm onto either arm (visually assessed by a line marked on each arm). Each experiment involved at least 30 E. formosa that made a choice.
Collection and quantification of plant volatiles
Volatiles were collected from one randomly selected plant from each combination of O3 concentration, tomato genotype and whitefly herbivory treatment in each chamber (8 individuals from each tomato genotype × four treatments). The headspace volatiles were collected according to Turlings et al57. The shoots and leaves of each plant, except for the stem extending 4 to 5 cm from the soil surface, were sealed in a plastic bag (40 cm wide and 46 cm long). Purified air was pumped (Beijing Institute of Labor Instruments, China) into the bag through a freshly activated charcoal trap (Beijing Chemical Company) and then withdrawn through a glass cartridge (3.0 mm internal diameter and 12.6 cm long) packed with 100 mg of the adsorbent Porapak Q (80–100 mesh, Supelco, Bellefonte, PA, USA); the flow rate was 0.2 L/min. Volatile compounds were rinsed from the Porapak Q with 1000 ml of n- pentane (HPLC grade, Sigma-Aldrich, USA) containing internal standards (200 ng of ethyl heptanoate) for quantification. The aeration extracts were stored at −20°C until analysed. Immediately after headspace volatiles were collected, the fresh weights of the plant leaves were measured.
Volatiles were quantified and identified using a gas chromatography-mass spectrometry (GC-MS) system (Hewlett Packard 6890N GC model coupled with 5973 MSD) equipped with a HP-5MS column (60 m long, 0.25 mm inner diameter, and 0.25 mm film thickness; Agilent Technologies, Palo Alto, CA, USA). The initial oven temperature was kept at 35°C for 1 min, which was increased to 250°C at a rate of 5°C/min. Volatile compounds were identified by comparing their retention times and spectra with those of compounds in the NIST02 library (Scientific Instrument Services, Inc., Ringoes, NJ, USA) and those of pure standards (terpinolene, (+)-3-carene, (+)-α-pinene, D-limonene, α-phellandrene, ocimene, β-phellandrene, Z-3-hexanol, E-2-hexanol, nonanal and octanal were purchased from Sigma-Aldrich (St. Louis, MO) and Fluka (Buchs, Switzerland) and the purity of them exceeds 95%).
Statistical Analyses
To study the impacts of elevated O3 on the volatile emission rate, we used a split-split plot design, with O3 and block (a pair of ambient and elevated OTCs) as the main effects, whitefly herbivory as the subplot effect, and tomato genotypes as the sub-subplot effect according to the following model:
Xijklm = μ+ Oi+B(O)j(i)+Hk+OHik+HB(O)kj(i)+Tl+OTil+TB(O)lj(i)+HTB(O)klj(i) +em(ijkl)
where O is the O3 treatment (i = 2), B is the block (j = 4), H is the herbivore treatment (k = 2), and T is the tomato genotypes (l = 2). Xijklm represents the error because of the smaller scale differences between samples and variability within blocks (SPSS 13.0, USA). Effects were considered significant if P < 0.05. The effect of block and the interactive effects of block and other factors were not significant (P > 0.45), and the effect of block and its interaction with other factors are not presented to simplify the presentation. LSD's multiple range tests were used to separate means when ANOVAs were significant. X2 tests were used to analyse adult whitefly feeding and oviposition preference. The chi-squared test was used to examine the significance of differences between the numbers of parasitoids choosing each olfactometer arm58,59.
Author Contributions
C.H.Y. performed the experiments and wrote the main manuscript text. G.F. designed the experiments. S.J.W., W.J.N. and H.Y.J. helped interpret the data. All of the authors read and approved the final manuscript.
Acknowledgments
We are grateful to Prof. Marvin Harris from Texas A&M University for reviewing the draft of this manuscript. This project was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences(XDB11050400), National Nature Science Fund of China (No. 31221091 and No. 31370438) and the R&D Special Fund for Public Welfare Industry (Agriculture 201303019).
References
- Pichersky E., Noel J. P. & Dudareva N. Biosynthesis of plant volatiles: nature's diversity and ingenuity. Science 311, 808–811 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicke M., Vanpoecke R. M. P. & Deboer J. G. Inducible indirect defence of plants: from mechanisms to ecological functions. Basic Appl. Ecol. 4, 27–42 (2003). [Google Scholar]
- Jervis M. A., Kidd N. A. C., Fitton M. G., Huddleston T. & Dawah H. A. Flower-visiting by hymenopterous parasitoids. J. Nat. Hist. 27, 67–105 (1993). [Google Scholar]
- Jonsson M., Lindkvist A. & Anderson P. Behavioural responses in three ichneumonid pollen beetle parasitoids to volatiles emitted from different phenological stages of oilseed rape. Entomol. Exp. Appl. 115, 363–369 (2005). [Google Scholar]
- Patt J. M., Hamilton G. C. & Lashomb J. H. Response of two parasitoid wasps to nectar odors as a function of experience. Entomol. Exp. Appl. 90, 1–8 (1999). [Google Scholar]
- Wackers F. L. Assessing the suitability of flowering herbs as parasitoid food sources: flower attractiveness and nectar accessibility. Biol. Control 29, 307–314 (2004). [Google Scholar]
- Larson K. C. & Whitham T. G. Manipulation of food resources by a gall-forming aphid: the physiology of sink-source interactions. Oecologia 88, 15–21 (1991). [DOI] [PubMed] [Google Scholar]
- Petersen M. K. & Sandstrom J. P. Outcome of indirect competition between two aphid species mediated by responses in their common host plant. Funct. Ecol. 15, 525–534 (2001). [Google Scholar]
- Sanchez-Hernandez C., Lopez M. G. & Delano-frier J. P. Reduced levels of volatile emissions in jasmonate-deficient spr2 tomato mutants favour oviposition by insect herbivores. Plant Cell Environ. 29, 546–557 (2006). [DOI] [PubMed] [Google Scholar]
- Zarate S. I., Kempema L. A. & Walling L. L. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 143, 866–875 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorinen T., Nerg A. M. & Holopainen J. K. Ozone exposure triggers the emission of herbivore-induced plant volatiles, but does not disturb tritrophic signalling. Environ. Pollut. 131, 305–311 (2004). [DOI] [PubMed] [Google Scholar]
- Blande J. D., Tiiva P., Oksanen E. & Holopainnen J. K. Emission of herbivore-induced volatile terpenoids from two hybrid aspen (Populus tremula × tremuloides) clones under ambient and elevated ozone concentrations in the field. Global Change Biol. 13, 2538–2550 (2007). [Google Scholar]
- Unsicker S. B., Kunert G. & Gershenzon J. Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 12, 479–485 (2009). [DOI] [PubMed] [Google Scholar]
- Walling L. L. The myriad plant responses to herbivores. J. Plant Growth Regul. 19, 195–216 (2000). [DOI] [PubMed] [Google Scholar]
- Heil M. Indirect defence via tritrophic interactions. New Phytol. 178, 41–61 (2008). [DOI] [PubMed] [Google Scholar]
- Amo L., Jansen J. J., van Dam N. M., Dicke M. & Visser M. E. Birds exploit herbivore-induced plant volatiles to locate herbivorous prey. Ecol. Lett. 16, 1348–1355 (2013). [DOI] [PubMed] [Google Scholar]
- Wang H., Guo W. F., Zhang P. J., Wu Z. Y. & Liu S. S. Experience-induced habituation and preference towards non-host plant odors in ovipositing females of a moth. J. Chem. Ecol. 34, 330–338 (2008). [DOI] [PubMed] [Google Scholar]
- Beale M. H. et al. Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior. Proc. Natl. Acad. Sci. USA 103, 10509–10513 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A. & Baldwin I. T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 291, 2141–2144 (2001). [DOI] [PubMed] [Google Scholar]
- Zhang P. J. et al. Whiteflies interfere with indirect plant defense against spider mites in Lima bean. PNAS 106, 50 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicke M. Behavioural and community ecology of plants that cry for help. Plant Cell Environ. 32, 654–665 (2009). [DOI] [PubMed] [Google Scholar]
- Wei J. N. et al. Ecological trade-offs between jasmonic acid-dependent direct and indirect plant defences in tritrophic interactions. New Phytol. 189, 557–567 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinzargan R. A review of surface ozone background levels and trends. Atmos. Environ. 38, 3431–3442 (2004). [Google Scholar]
- Jaffe D. & Ray J. Increase in surface ozone at rural sites in the western US. Atmos. Environ. 41, 5452–5463 (2007). [Google Scholar]
- Wilkinson S. & Davies W. J. Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ. 33, 510–525 (2010). [DOI] [PubMed] [Google Scholar]
- Andersen C. P. Source-sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol. 157, 213–228 (2003). [DOI] [PubMed] [Google Scholar]
- Ashmore M. R. Assessing the future global impacts of ozone on vegetation. Plant Cell Environ. 28, 949–964 (2005). [Google Scholar]
- Pinto D. M., BlandeL J. D., Souza S. R., Nerg A. M. & Holopainen J. K. Plant Volatile Organic Compounds (VOCs) in Ozone (O3) Polluted Atmospheres: The Ecological Effects. J. Chem. Ecol. 36, 22–34 (2010). [DOI] [PubMed] [Google Scholar]
- Yuan J. S., Himanen S. J., Holopainen J. K., Chen F. & Stewart C. N. Smelling global climate change: mitigation of function for plant volatile organic compounds. Trends Ecol. Evol. 24, 323–331 (2009). [DOI] [PubMed] [Google Scholar]
- Kainulainen P., Holopainen J. & Holopainen T. Combined effects of ozone and nitrogen on secondary compounds, amino acids, and aphid performance in Scots pine seedlings. J. Environ. Qual. 29, 334–342 (2000). [Google Scholar]
- Jondrup P. M., Barnes J. D. & Port G. R. The effect of ozone fumigation and different Brassica rapa lines on the feeding behaviour of Pieris brassicae larvae. Entomol. Exp. Appl. 104, 143–151 (2002). [Google Scholar]
- Himanen S. J. et al. Effects of elevated carbon dioxide and ozone on volatile terpenoid emissions and multitrophic communication of transgenic insecticidal oilseed rape (Brassica napus). New Phytol. 181, 174–186 (2009). [DOI] [PubMed] [Google Scholar]
- Beauchamp J. et al. Ozone induced emissions of biogenic VOC from tobacco: relationships between ozone uptake and emission of LOX products. Plant Cell Environ. 28, 1334–1343 (2005). [Google Scholar]
- Gate I. M., Mcneill S. & Ashmore M. R. Effects of air pollution on the searching behaviour of an insect parasitoid. Wat. Air Soil Pollut. 85, 1425–1430 (1995). [Google Scholar]
- Browse J. Jasmonate: an oxylipin signal with many roles in plants. Plant Hormones 72, 431–456 (2005). [DOI] [PubMed] [Google Scholar]
- Schilmiller A. L. & Howe G. A. Systemic signaling in the wound response. Curr. Opin. Plant Biol. 8, 369–377 (2005). [DOI] [PubMed] [Google Scholar]
- Oguntimehin I., Eissa F. & Sakugawa H. Simultaneous ozone fumigation and fluoranthene sprayed as mists negatively affected cherry tomato (Lycopersicon esculentum Mill). Ecotox. Environ. Safe. 73, 1028–1033 (2010). [DOI] [PubMed] [Google Scholar]
- Gennadius P. Disease of tobacco plantations in Trikonia: the aleurodid of tobacco. Ellenike Georgia 5, 1–3 (1889). [Google Scholar]
- Bird T. L. & Kruger K. Response of the polyphagous whitefly Bemisia tabaci B-biotype (Hemiptera: Aleyrodidae) to crop diversification-influence of multiple sensory stimuli on activity and fecundity. Bull. Entomol. Res. 96, 15–23 (2006). [DOI] [PubMed] [Google Scholar]
- Bardin M., Fargues J. & Nicot P. C. Compatibility between biopesticides used to control grey mould, powdery mildew and whitefly on tomato. Biol. Control 46, 476–483 (2008). [Google Scholar]
- Birkett M. A. et al. Volatiles from Whitefly-Infested Plants Elicit a Host-Locating Response in the Parasitoid, Encarsia formosa. J. Chem. Ecol. 29, 1589–1600 (2003). [DOI] [PubMed] [Google Scholar]
- Ellis C., Karafylldis I. & Turner J. G. Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol. Plant Microbe Interact. 15, 1025–1030 (2002). [DOI] [PubMed] [Google Scholar]
- Li C. et al. The tomato suppressor of prosystemin-mediated responses2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. Plant Cell 15, 1646–1661 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempema L. A., Cui X. P., Holzer F. M. & Walliing L. L. Arabidopsis Transcriptome Changes in Response to Phloem-Feeding Silverleaf Whitefly Nymphs. Similarities and Distinctions in Responses to Aphids. Plant Physiol. 143, 849–865 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H. Y. et al. Elevated O3 reduces the fitness of Bemisia tabaci via enhancement of the SA-dependent defense of the tomato plant. Arthropod Plant Interact. 6, 425–437 (2012a). [Google Scholar]
- Cui H. Y., Sun Y. C., Su J. W., Li C. Y. & Ge F. Reduction in the fitness of conspecific Bemisia tabaci fed on three previously infested tomato genotypes differing in the JA pathway. Environ. Entomol. 41, 1443–1453 (2012b). [DOI] [PubMed] [Google Scholar]
- Ohgushi T. Indirect interaction webs: herbivore-induced effects through trait change in plants. Annu. Rev. Ecol. Evol. Syst. 36, 81–105 (2005). [Google Scholar]
- Pinto D. M., Nerg A. M. & Holopainen J. K. The role of zone-reactive compounds, terpenes, and green leaf volatiles (GLVs), in the orientation of Cotesia plutellae. J. Chem. Ecol. 33, 2218–2228 (2007). [DOI] [PubMed] [Google Scholar]
- Loreto F., Pinelli P., Manes F. & Kollist H. Impact of ozone on monoterpene emissions and evidence for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiol. 24, 361–367 (2004). [DOI] [PubMed] [Google Scholar]
- Ras M. R., Marce R. M. & Borrull F. Characterization of ozone precursor volatile organic compounds in urban atmospheres and around the petrochemical industry in the Tarragona region. Sci. Total Environ. 407, 4312–4319 (2009). [DOI] [PubMed] [Google Scholar]
- Cannon W. N. Olfactory response of eastern spruce budworm larvae to red spruce needles exposed to acid-rain and elevated levels of ozone. J. Chem. Ecol. 16, 3255–3261 (1990). [DOI] [PubMed] [Google Scholar]
- Dicke M., Sabelis M. W., Takabayashi J., Bruin J. & Posthumus M. A. Plant strategies of manipulating predator-prey interactions through alMochemicals: prospects for application in pest control. J. Chem. Ecol. 16, 3091–3118 (1990). [DOI] [PubMed] [Google Scholar]
- Moayeri H. R. S., Ashouri A., Poll L. & Enkegaard A. Olfactory response of a predatory mirid to herbivore induced plant volatiles: Multiple herbivory vs. single herbivory. J. Appl. Entomol. 131, 326–332 (2007). [Google Scholar]
- Reddy G. V. P., Holopainen J. K. & Guerrero A. Olfactory responses of Plutella xylostella natural enemies to host pheromone, larval frass, and green leaf cabbage volatiles. J. Chem. Ecol. 28, 131–143 (2002). [DOI] [PubMed] [Google Scholar]
- Ibrahim M. A., Nissinen A. & Holopainen J. K. Response of Plutella xylostella and its parasitoid Cotesia plutellae to volatile compounds. J. Chem. Ecol. 31, 1969–1984 (2005). [DOI] [PubMed] [Google Scholar]
- Ren Q. et al. Volatile Emissions from the Invasive Weed Eupatorium adenophorum Induced by Aphis gossypii Feeding and Methyl Jasmonate Treatment. Weed Sci. 58, 252–257 (2010). [Google Scholar]
- Turlings T. C. J. et al. The induction of volatile emissions in maize by three herbivore species with different feeding habits: possible consequences for their natural enemies. Biol. Control 11, 122–129 (1998). [Google Scholar]
- Wei J. N. & Kang L. Electrophysiological and behavioral responses of a parasitic wasp to plant volatiles induced by two leaf miner species. Chem. Senses 31, 467–477 (2006). [DOI] [PubMed] [Google Scholar]
- Wei J. et al. Plants attract parasitic wasps to defend themselves against insect pests by releasing hexenol. PLoS ONE 2, e852 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]