Abstract
Dapper, Dishevelled-associated antagonist of β-catenin (DACT), is involved in Xenopus embryonic development. Human DACT2 is localized on chromosome 6q27, a region of frequent loss of heterozygosity (LOH) in human cancers. However, the function and regulation of DACT2 in human lung cancer remain unclear. DNA sequencing, methylation-specific PCR (MSP), semi-quantitative RT-PCR, western blotting, and xenograft models were employed in this study. Eight lung cancer cell lines, 106 cases of primary lung cancer, four specimens of normal lung from patients without cancer, and 99 blood samples from healthy individuals were examined. We found that while there was no SNP related to lung cancer, the DACT2 promoter region is frequently methylated in human lung cancer. DACT2 is silenced by promoter region hypermethylation and re-expressed by 5-aza-2′-deoxyazacytidine treatment of lung cancer cell lines. Methylation of DACT2 was associated with poor differentiation of lung cancer. Loss of DACT2 expression was associated with promoter region hypermethylation in primary lung cancer, and was associated with increased β-catenin expression. Restoration of DACT2 expression suppressed tumour proliferation both in vitro and in vivo. DACT2 expression was down-regulated by siRNA knockdown in H727 cells. DACT2 inhibited T-cell factor/lymphoid enhancer factor (TCF/LEF) and its downstream genes. In conclusion, DACT2 methylation is a potential lung cancer detection marker. DACT2 is regulated by promoter region hypermethylation. DACT2 inhibits lung cancer proliferation by suppressing the Wnt signalling pathway in lung cancer.
Keywords: DACT2, chromosome 6q27, SNP, DNA methylation, epigenetics, lung cancer
Introduction
Lung cancer is the leading cause of cancer death in the world [1,2]. Cigarette smoking is the major cause but genetic and epigenetic factors also affect susceptibility [3,4]. Deletions of chromosome 6q are among the most frequent chromosome aberrations in multiple human tumours including lung cancer [5,6]. LOH within 6q22–27 occurs in 30–55% of tumours [5]. 6q27 was linked to both risk of NSCLC in a Chinese population via gene scanning and lung function [7,8]. Dapper2, a Dishevelled-associated antagonist of β-catenin homolog 2 (DACT2), is a member of the DACT family and is located on human chromosome 6q27 within this region [9,10].
Dapper was first isolated from Xenopus by Cheyette et al in a screen for proteins interacting with Dishevelled (Dvl), a key factor in the Wnt signalling pathway [11]. DACT2 was reported to promote Dvl degradation in a lysosome-dependent pathway, and inhibits LEF1 binding to β-catenin [12]. Human DACT1 and DACT2 were characterized by Katoh and Katoh in 2003 [9]. Human DACT3 was identified by Fisher et al through human genome and EST databases in 2006 [10]. DACT1 is located on human chromosome 14q22.3 and DACT3 is located on human chromosome 19q13.32. DACT1 was reported to be frequently methylated in hepatocellular carcinoma, while DACT3 was reported to be regulated by histone modification in colorectal cancer [13,14]. These studies suggest the potential involvement of DACT genes in cellular transformation, but the role of DACT2 in human tumours has not been extensively examined. To explore the function and regulation of DACT2 in lung cancer, we analysed genetic and epigenetic changes of DACT2, and determined the potential involvement of this gene as a regulator of Wnt signalling and in lung carcinogenesis.
Materials and methods
Human tissue samples and cell lines
One hundred and six cases of primary human lung cancer with 44 cases of matched adjacent non-neoplastic tissues, 99 samples of blood from healthy individuals, and four cases of normal lung tissue from patients without cancer were collected from the Chinese PLA General Hospital in Beijing, China. The median age of cancer patients was 59.5 years (range 36–76 years), with the ratio of males/females being 2:1. All cancer samples were classified according to the TNM (UICC 2009) staging system, including 37 cases of stage I, 43 cases of stage II, 19 cases of stage III, and seven cases of stage IV. All samples were collected under the guidelines approved by the Chinese PLA General Hospital’s institutional review board and with informed consent from patients.
Eight lung cancer cell lines (NCI-H1299, U-1752, NCI-H446, NCI-H460, 95D, A549, NCI-H23, and NCI-H727) were previously established from primary lung cancer and maintained in 90% RPMI 1640 (Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum.
5-Aza-2′-deoxyazacytidine treatment
Lung cancer cell lines were split to a low density (30% confluence) 12 h before treatment. Cells were treated with 5-aza-2′-deoxyazacytidine (5-AZA) (Sigma, St Louis, MO, USA) at a concentration of 2 μM. Growth medium, conditioned with 5-AZA at 2 μM, was exchanged every 24 h for a total of 96 h of treatment.
RNA isolation and semi-quantitative RT-PCR
Total RNA was isolated by the Trizol reagent (Life Technologies, Gaithersburg, MD, USA). First-strand cDNA was synthesized according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). DACT2 primers were as follows: 5′-GGCTGAGACAACAGGACATCG-3′ (F) and 5′-GACCGTCGCTCATCTCGTAAAA-3′ (R). Thirty-three cycles were amplified for each RT-PCR. As an internal control, GAPDH was amplified with 25 cycles to ensure cDNA quality and quantity. GAPDH primers were as follows: 5′-GACCACAGTCCATGCCATCAC-3′ (F) and 5′-GTCCACCACCCTGTTGCTGTA-3′ (R). Amplified products were analysed on 1.5% agarose gels.
Bisulfite modification, methylation-specific PCR (MSP), and bisulfite sequencing (BSSQ)
DNA was prepared by the proteinase K method. Bisulfite treatment was carried out as previously described [15]. MSP primers were designed according to genomic sequences around transcription start sites (TSS) and synthesized (Invitrogen, Beijing) to detect unmethylated (U) and methylated (M) alleles. MSP primers were as follows: 5′-GCGCGTGTAGATTTCGTTTTTCGC-3′ (MF); 5′-AACCCCACGAACGACGCCG-3′ (MR); 5′-TTGGGGTGTGTGTAGATTTTGTTTTTTGT-3′ (UF); and 5′-CCCAAACCCCACAAACAACACCA-3′ (UR). The size of the unmethylation PCR product was 161 bp and of the methylation PCR product 152 bp. Bisulfite-treated DNA was amplified using BSSQ primers flanking the targeted regions, including MSP products and the transcription start site. Sequencing primers were as follows: 5′-GGGGGAGGTYGYGGTGATTT-3′ (F) and 5′-ACCTACRACRATCCCAACCC-3′ (R). Bisulfite sequencing was performed as previously described [16].
Immunohistochemistry (IHC)
Rabbit anti-DACT2 antibody (OriGene Tech, MD, USA) and mouse anti-β-catenin antibody (ZSGB Biotech, Beijing, China) were employed. IHC was performed as previously described [16]. The expression of DACT2 and β-catenin was evaluated according to a previous report [17].
Construction of expression vectors
Full-length DACT2 cDNA (GenBank accession number NM_214462) was cloned into a pCMV6 vector (OriGene Tech, MD, USA). Genomic fragment of Homo sapiens miRNA precursors were amplified and cloned into pcDNA3.0 vector (Invitrogen, Carlsbad, CA). 3′-UTR of DACT2 was generated according to a previous report and cloned into a pGL3 vector (Promega, Madison, WI, USA) immediately downstream of the stop codon of the luciferase reporter gene [18].
Transfection assay
Transient transfection was performed by using Lipo-fectamine 2000 (Invitrogen, Carlsbad, CA) or FuGENE HD (Roche Applied Science, Indianapolis, IN, USA) according to the manufacturer’s instructions.
Colony formation assay
DACT2 expression or the empty vector was transfected into NCI-H23 cells according to the manufacturer’s instructions. After 36 h, cells were reseeded at 1500 cells per well in six-well plates in triplicate. Growth medium, conditioned with G418 (Invitrogen, Carlsbad, CA) at 450 μg/ml, was exchanged every 24 h. Clones were counted by 14 days after being fixed with 75% ethanol for 30 min and stained with 0.2% crystal violet.
DACT2 knockdown by siRNA
Two selected siRNAs targeting DACT2 and RNAi Negative Control Duplex were used in this study. The sequences were as follows: siRNA duplex 1# (sense: 5′-CCAGCUGUCCUGAGUCUAATT-3′; anti-sense: 5′-UUAGACUCAGGACAGCUGGTT-3′); siRNA duplex 2# (sense: 5′-GUCGGUUGAUGAGACUACUTT-3′; antisense: 5′-AGUAGUCUCAUCAACCGACTT-3′); RNAi negative control duplex (sense: 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense: 5′-ACGUGACACGUUCGGAGAATT-3). RNAi oligonucleotide or RNAi negative control duplex (Gene Pharma Co, Shanghai, China) was transfected into H727 cells according to the manufacturer’s instructions.
Flow cytometry assay
Lung cancer cells were collected and analysed by quantification of cellular DNA content with propidium iodide (PI) staining according to the manufacturer’s instructions (KeyGen Biotech, Jiangsu, China). The ratio of G0/G1, G2/M, and S-phase cells was evaluated by Modfit software.
Evaluating the effect of DACT2 on mice xenograft lung cancer
2 × 106 DACT2 expressed or unexpressed (empty vector) H23 cells were diluted in 0.1 ml of PBS and injected subcutaneously into the right armpit of each 4-week-old female Bal b/c nude mouse (6 per group). The diameter of tumours was measured every three days. Tumour volume (mm3) was estimated by the formula tumour volume = (length) × (width)2/2 [19]. Mice were sacrificed at 4 weeks. Tumour size and weight were measured after dissection. All procedures were approved by the Animal Ethics Committee of the Chinese PLA General Hospital.
Luciferase reporter assay
Cells were seeded at 5 × 104 cells per well in 24-well culture plates 24 h before transfection. The pGL3-OT construct is a TCF/LEF-responsive reporter containing three consensus TCF binding sites. The pRL-TK vector (Promega, Madison, WI) was used as a system control [20,21]. Both 100 ng/well pGL3-OT vectors and 10 ng/well pRL-TK vectors were transfected into NCI-H23 cell lines as a basic luciferase reporter system [20]. Either the 150 ng/well wild-type or the Ser45 mutation β-catenin vector was transfected into the NCI-H23 cell line as a wild-type or mutant β-catenin expression group, respectively [22]. In order to explore the effect of DACT2 on β-catenin/TCF luciferase reporter activity, either 100 ng/well DACT2 or DACT2 (100 ng/well) + Dvl2 (100 ng/well) vectors were transfected into the wild-type or mutant β-catenin expression NCI-H23 cells. To further determine the effect of DACT2 on β-catenin/TCF luciferase reporter activity, the wild-type or mutant β-catenin expression vector was co-transfected into NCI-H23 cells with 150 or 250 ng/well.
125 ng of pGL3-DACT2-3′-UTR luciferase reporter constructs, 10 ng of pRL-TK control vectors, and 375 ng of miRNA expression vectors or empty vectors were co-transfected into NCI-H23 cells, respectively. These miRNAs included pcDNA3.0-miRNA-18a, 18b, 182, 424, 497, 553, 609, and 671.
Relative luciferase activity was detected 48 hs after transfection by a GLOMAX luminometer (Promega, Madison, WI). Normalized luciferase activity (the ratio of firefly luciferase to renilla luciferase) served as the system background (Promega, Madison, WI). Each experiment was repeated in triplicate.
Western blot
Rabbit DACT2 (Abcam, MA, USA), cyclin D1 (Bioworld Tech, MN, USA), c-Myc (Bioworld Tech, MN, USA) or mouse β-actin (Beyotime Biotech, China) antibodies were applied in this study. Forty-eight hours after transfection, cell collecting and western blotting were performed according to a previous report [19].
Statistical analysis
We evaluated the relationship between DNA methylation in human lung cancers and clinicopathological characteristics using the Pearson chi-squared test and Fisher’s exact test for independence for dichotomous variables and Student’s t-test for continuous variables. Results were judged to be statistically significant at p< 0:05. All analyses were carried out using STATA statistical package version 10.0 (College Station, TX, USA).
Results
Single nucleotide polymorphisms (SNPs) of DACT2 in lung cancers
Since genomic loss of 6p27 is common in lung cancer and includes the DACT2 gene, we first sequenced DACT2 for the presence of genetic variation. After sequencing the full-length cDNA and genomic DNA in eight lung cancer cell lines, three SNPs were found in four different cell lines (U-1752, NCI-H727, A549, and 95D), all within DACT2 exon 4, which encodes several protein-interacting regions, including the PDZ-binding motif (Dvl binding region). No other mutation or SNP was found in the other exons in our lung cancer cell lines. To further explore the association of SNPs in this region with lung cancer, 106 cases of primary lung cancer and 99 blood samples from healthy individuals were included in this study. Although three more SNPs were found in these samples, no additional mutations were discovered. The respective SNPs observed in the lung cancer patients versus those in the healthy individuals were as follows: A/G (rs6925614) 14.15% versus 25.25%; T/C (rs79931308) 4.72% versus 4.04%; A/C (rs10945501) 33.96% versus 30.30%; G/C (rs10945500) 15.09% versus 17.17%; T/C (rs10945499) 22.64% versus 23.23%; G/T (rs73789362) 5.66% versus 1.01%. No significant difference was found in lung cancer patients and healthy individuals (p> 0:05), suggesting that sequence variants of DACT2 were not associated with the development of lung cancer.
DACT2 is silenced by promoter regional hypermethylation in lung cancer cell lines
We explored the possibility that DACT2 was epigenetically regulated in human lung cancer by first using semi-quantitative RT-PCR to detect DACT2 expression in eight human lung cancer cell lines. Complete loss of DACT2 expression was observed in one cell line (NCI-H23), while weak expression of DACT2 was found in four cell lines (NCI-H1299, U-1752, 95D, and A549). DACT2 was expressed in three cell lines (NCI-H446, NCI-H460, and NCI-H727) (Figure 1A). Second, we examined DACT2 methylation using methylation-specific PCR (MSP) in these cell lines. The results demonstrated that DACT2 was completely methylated in NCI-H23 cell lines and partially methylated in NCI-H1299, U-1752, 95D, and A549 cell lines. No DNA methylation was found in NCI-H446, NCI-H460, and NCI-H727 cell lines (Figure 1B). These results suggest that loss or reduction of DACT2 is correlated with promoter region methylation in human lung cancer cell lines. Third, we performed bisulfite sequencing as shown in Figure 1C to provide a detailed map of the presence of DNA methylation. The size of the PCR product was 255 bp and included the transcriptional start site (−114 bp to +141 bp). Bisulfite sequencing confirmed the MSP findings, with DACT2 being densely methylated at the promoter in NCI-H23, partially methylated in NCI-H1299, and unmethylated in NCI-H727 cell lines. The scattered methylation observed in each allele of NCI-H1299 cells suggests an incomplete level of epigenetic silencing rather than subpopulations of cells with differing methylation status. Although scattered areas of incomplete methylation were observed adjacent to the transcriptional start site in NCI-H23 cells, the MSP primer binding sites were heavily methylated. These data indicate that the location of the MSP primers is properly situated for promoter methylation detection. To further determine whether DACT2 expression was regulated by promoter region methylation, 5-AZA was employed in this study. 5-AZA, a DNA methylation transferase (DNMTs) inhibitor, induces re-expression of methylated genes through demethylation [23,24]. Re-expression of DACT2 was induced in NCI-H23, and increased expression was also observed in NCI-H1299, U-1752, 95D, and A549 cell lines, cell lines with low levels of expression and a partially methylated promoter region. No change in expression was found in the unmethylated NCI-H446, NCI-H460, and NCI-H727 cell lines (Figure 1A). These results further indicate that DACT2 expression is regulated by promoter region methylation.
Figure 1.
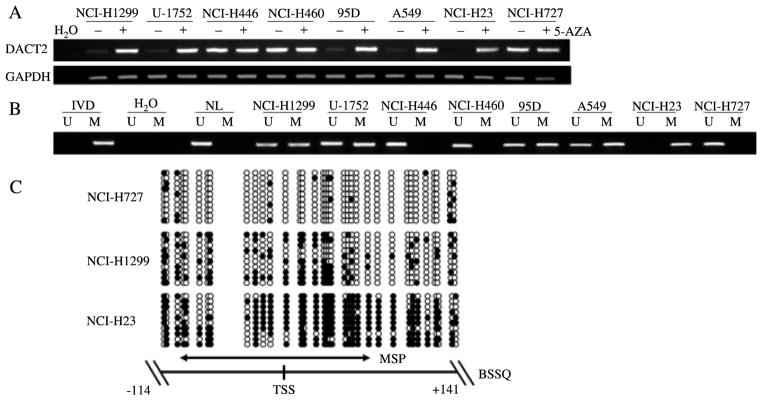
Expression of DACT2 was silenced by DNA methylation in lung cancer cell lines. (A) Expression of DACT2 was analysed by semi-quantitative RT-PCR in lung cancer cell lines. DACT2 was expressed in NCI-H446, NCI-H460, and NCI-H727; weakly expressed in NCI-H1299, U-1752, 95D, and A549; and unexpressed in NCI-H23. Re-expression or increased expression was induced by 5-AZA treatment. (−) untreated; (+) 5-AZA treated; H2O: double distilled water. GAPDH was used as an internal control. (B) MSP results of DACT2 in lung cancer cell lines (NCI-H1299, U-1752, NCI-H446, NCI-H460, 95D, A549, NCI-H23, and NCI-H727). IVD: in vitro methylated DNA; NL: normal lymphocyte DNA; M: methylated alleles; U: unmethylated alleles. (C) DACT2 BSSQ in lung cancer cell lines. DACT2 is methylated in NCI-H23, partially methylated in NCI-H1299, and unmethylated in NCI-H727 cell lines. The region amplified by MSP is indicated by a double-headed arrow. Filled circles represent methylated CpG sites and open circles denote unmethylated CpG sites. TSS: transcription start site.
DACT2 is frequently methylated in human primary lung cancer
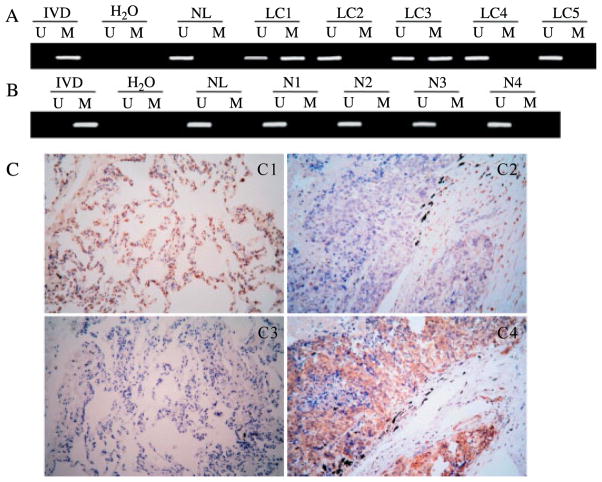
Methylation of DACT2 was examined in 106 cases of primary lung cancer and four cases of normal lung tissue. Methylation was observed in 41% (43/106) of DACT2 in primary lung cancer (Figure 2A). No methylation was found in the four normal lung tissues (Figure 2B). As shown in Table 1, DACT2 methylation was associated with poor differentiation of lung cancer (p = 0:0431).
Figure 2.
Expression and methylation of DACT2 in human primary lung cancer and non-cancerous tissue. (A) Representative MSP results of DACT2 in primary lung cancer tissue (LC). (B) MSP result of DACT2 in normal lung tissue (N1, N2, N3, and N4). (C) IHC staining of DACT2 and β-catenin in lung cancer and adjacent tissue (original magnification x 200). (C1) Expressed DACT2 in adjacent tissue. (C2) No DACT2 expression in primary lung cancer. (C3) No β-catenin expression in adjacent tissue. (C4) β-Catenin was stained in cytoplasm of primary lung cancer.
Table 1.
Clinical factors and DACT2 methylation in 106 cases of lung cancer
| Clinical factor | No | DACT2 methylation status
|
p value* | |
|---|---|---|---|---|
| Methylated n = 43 (40.6%) | Unmethylated n = 63 (59.4%) | |||
| Age (years) | ||||
| <50 | 25 | 9 | 16 | 0.5948 |
| ≥50 | 81 | 34 | 47 | |
| Gender | ||||
| Male | 71 | 29 | 42 | 0.9336 |
| Female | 35 | 14 | 21 | |
| Alcohol abuse | ||||
| Negative | 72 | 30 | 42 | 0.7370 |
| Positive | 34 | 13 | 21 | |
| Smoking | ||||
| Negative | 56 | 21 | 35 | 0.4963 |
| Positive | 50 | 22 | 28 | |
| Tumour size (cm) | ||||
| <5 | 79 | 31 | 48 | 0.6345 |
| ≥5 | 27 | 12 | 15 | |
| Differentiation | ||||
| Poor | 41 | 21 | 20 | 0.0431 < 0.05 |
| Moderate | 59 | 22 | 37 | |
| Well | 6 | 0 | 6 | |
| Tumour stage | ||||
| I | 37 | 14 | 23 | 0.9249 |
| II | 43 | 19 | 24 | |
| III | 19 | 7 | 12 | |
| IV | 7 | 3 | 4 | |
| Metastasis | ||||
| Negative | 60 | 23 | 37 | 0.5929 |
| Positive | 46 | 20 | 26 | |
| Pathological type | ||||
| Small cell carcinoma | 3 | 3 | 0 | 0.1870 |
| Squamous cell carcinoma | 38 | 17 | 21 | |
| Adenocarcinoma | 53 | 20 | 33 | |
| Large cell carcinoma | 3 | 1 | 2 | |
| Others | 9 | 2 | 7 | |
DACT2 methylation is associated with poor differentiation of lung cancer (p = 0:0431).
p values were obtained from the chi-square test; significant difference, p<0:05.
Loss of DACT2 expression and increased β-catenin expression are related to promoter regional hypermethylation in human primary lung cancer
DACT2 expression was evaluated by IHC in 44 cases of primary lung cancer and their matched non-neoplastic adjacent tissues. DACT2 staining was observed predominantly in the cytoplasm as expected. Positive DACT2 staining was found in nine cases of lung cancer tissue and in 34 tissues adjacent to lung tumours (Figure 2C1). Negative DACT2 staining was found in 35 cases of lung cancer tissue and in ten tissues adjacent to lung tumours (Figure 2C2). Among the cancer samples examined for expression, 21 cases were methylated and 23 cases were unmethylated. But in the non-neoplastic adjacent tissues, only nine cases were methylated and 35 cases were unmethylated. DACT2 staining was inversely associated with promoter region methylation (p = 0:0001). This indicates that DACT2 expression may be regulated by promoter region hypermethylation in primary lung cancer. To determine whether the silencing of DACT2 was associated with Wnt pathway activation, β-catenin expression was also evaluated by IHC in the primary lung cancers and adjacent non-neoplastic tissues. Negative β-catenin staining of the nucleus, cytoplasm or membrane was observed in five cases of cancer tissue and 38 cases of adjacent tissue (Figure 2C3). In the primary lung cancers, positive nuclear β-catenin staining was found in six cases, while positive cytoplasmic or membranous staining was found in 38 cases (Figure 2C4). In adjacent tissues, there was only one case of positive nuclear β-catenin staining, and only six cases of positive cytoplasmic or membranous staining were observed. These results indicate that expression of DACT2 and β-catenin are inversely associated in lung cancer (p = 0:0001).
Colony formation was inhibited by restoration of DACT2 expression
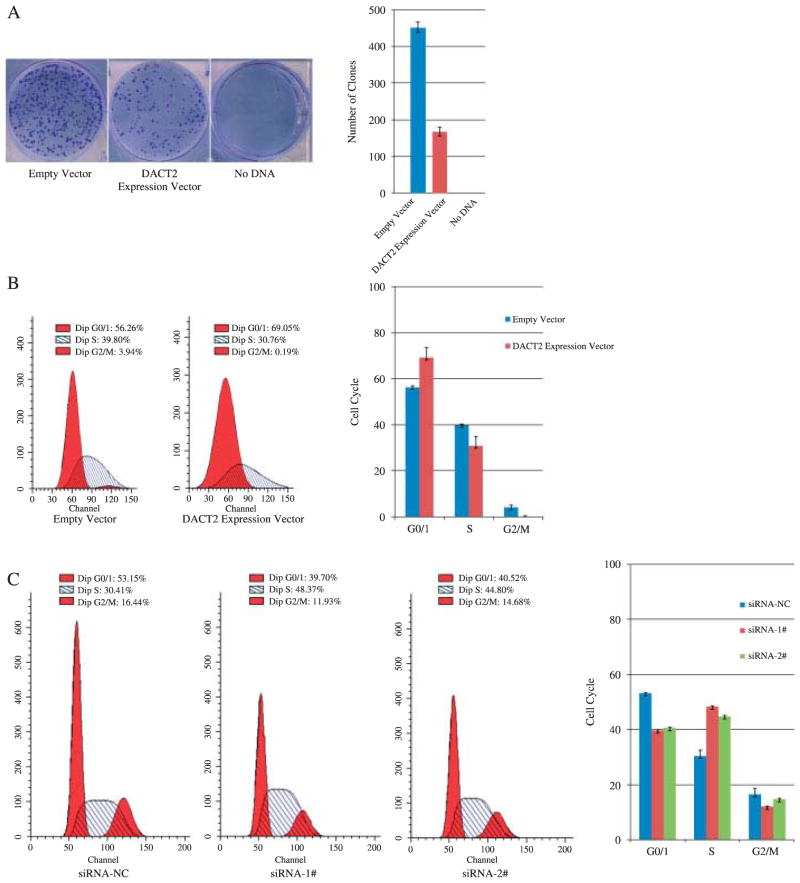
To evaluate the effect of DACT2 expression on the clonogenicity of lung cancer cells, a colony formation assay was employed using NCI-H23 cells. The number of clones was greatly reduced in the DACT2-expressing cells (168:0 ± 11:8 clones) compared with a control plasmid without DACT2 expression (452:3 ± 14:6 clones) in NCI-H23 cells (Figure 3A). These data demonstrate that DACT2 inhibits lung cancer cell proliferation.
Figure 3.
The effect of DACT2 on cell proliferation. (A) Clone number is reduced by DACT2 expression. No clone was found in the absence of anti-neomycin vector after G418 screening. Average number of tumour clones represented by bar diagram. Each experiment was repeated three times. (B) Cell cycle was analysed by flow cytometry. The distribution of cell phase in empty or DACT2 expression vector-transfected NCI-H23 cells. The ratio is represented by bar diagram. Each experiment was repeated three times. (C) Cell cycle was analysed by flow cytometry. The distribution of cell phase in siRNA negative control and siRNA duplex 1# and 2#-transfected NCI-H727 cells. The ratio is represented by bar diagram. Each experiment was repeated three times.
DACT2 expression alters the G1/S check point and inhibits S-phase entry
The effect of DACT2 on the cell cycle was evaluated using flow cytometry. In NCI-H23 cells without exogenous DACT2 expression, the percentage of G0/1 cells was 56:26 ± 0:58%, S phase was 39:80 ± 0:47%, and G2/M cells was 3:94 ± 1:05%. In contrast, for NCI-H23 cells in which DACT2 was re-expressed, the ratio of G0/1 cells was 69:05 ± 4:43%, S phase was 30:76 ± 4:16%, and G2/M cells was 0:19 ± 0:27% (Figure 3B). This demonstrates a reduction in the percentage of cells in the S phase and G2/M phase, with a retention of cells in the G0/1 phase with restoration of DACT2 expression in NCI-H23 cells (p = 0:0389).
To further examine the effect of DACT2 expression on cell cycle regulation, we reduced the expression of this gene in a cell line (NCI-H727) which expresses DACT2 using siRNA. DACT2 expression was reduced using two separate siRNA duplexes, 1# and 2# (Figure 5B). Using a negative control siRNA transfected into NCI-H727 cells, the following measures were obtained: 53:15 ± 0:15% cells were in the G0/1 phase; 30:41 ± 2:09% were in the S phase; and 16:44 ± 2:15% were in the G2/M phase. In contrast, for siRNA duplex (1#)-transfected NCI-H727 cells, 39:70 ± 0:23% was G0/1 phase; 48:37 ± 0:16% was S phase; and 11:93 ± 0:21% was G2/M phase (p = 0:0238). For siRNA duplex (2#)-transfected NCI-H727 cells, 40:52 ± 0:31% was G0/1 phase; 44:80 ± 0:29% was S phase; and 14:68 ± 0:31% was G2/M phase (p = 0:0498) (Figure 3C). Thus, both targeted siRNAs decreased the number of cells in the G0/1 phase, while significantly increasing the fraction of cells in the S phase, suggesting that loss of DACT2 expression increases cellular proliferation.
Figure 5.
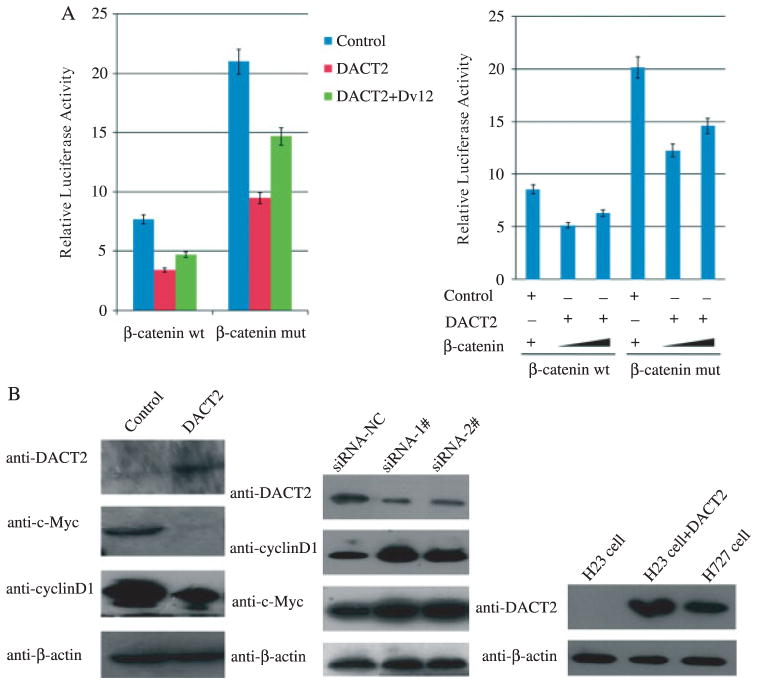
Wnt/β-catenin signalling was inhibited by DACT2. (A) Results of TCF/LEF luciferase reporter assay. Relative luciferase activity (the ratio of firefly luciferase to renilla luciferase) was suppressed by DACT2. Increased luciferase activity was induced by co-transfection of DACT2 with Dvl2. Inhibition of β-catenin/TCF luciferase reporter activity was tempered by co-transfection of β-catenin and DACT2 in a dose-dependent manner. The experiment was repeated three times. (B) Expression of c-Myc and cyclin D1 was inhibited by re-expression of DACT2 in NCI-H23 cells. Expression of c-Myc and cyclin D1 was increased in NCI-H727 cells by knocking down DACT2. Re-expression of DACT2 was found in DACT2-transfected NCI-H23 cells. NCI-H727 cells were the DACT2 expression control.
DACT2 inhibits lung cancer growth in mice xenografts
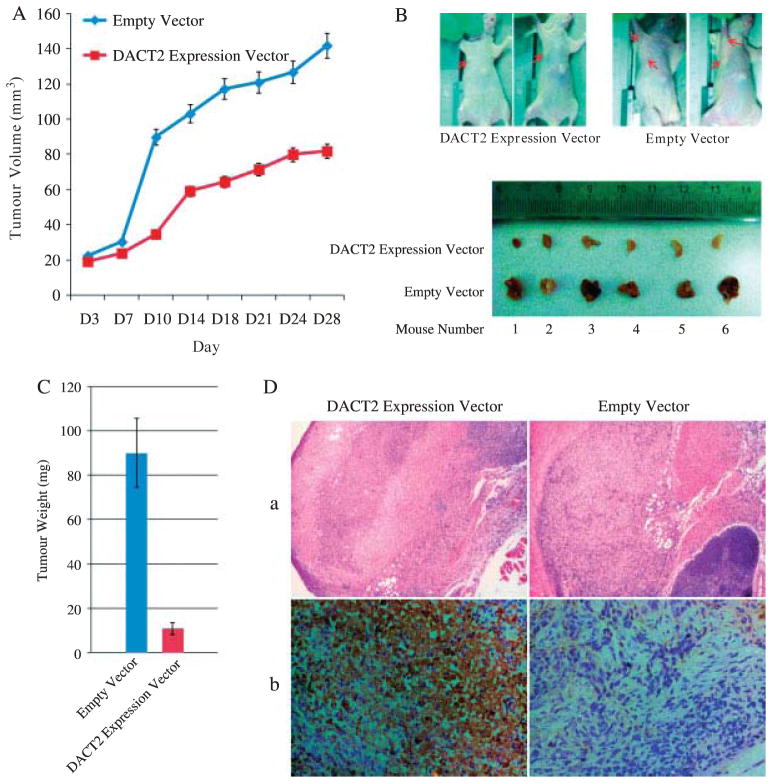
We further confirmed the impact of DACT2 by examining the effect of altered expression of this gene on xenograft tumour growth (Figure 4A). The tumour growth was significantly reduced in NCI-H23 cells which expressed DACT2, compared with the parental line, which does not express DACT2 (81:82 ± 35:74 mm3 versus 141:79 ± 35:90 mm3, p = 0:0158), a finding confirmed by measurements of tumour weight (DACT2-expressing tumours’ mean weight of 10:97 ± 2:70 mg versus 90:22 ± 15:57 mg in DACT2-negative tumours, p = 0:0001) (Figures 4B and 4C). H&E and IHC staining confirmed DACT2 expression and revealed increased tumour necrosis in the xenografts with NCI-H23 cells expressing DACT2 (Figure 4D). These results suggest that DACT2 inhibits lung cancer growth in mice xenografts.
Figure 4.
Lung cancer xenograft mice inoculated with DACT2 re-expressed and unexpressed NCI-H23 cells. (A) Tumour growth curve in DACT2-expressed and -unexpressed groups. (B) Representative burdened nude mice and isolated tumour in DACT2-expressed and -unexpressed groups. Red arrows show the position of subcutaneous tumours. (C) Average weight of tumours from DACT2-expressed or -unexpressed group represented by histogram. (D) H&E staining (original magnification 40x) and IHC staining (original magnification 200x) of DACT2-expressed or -unexpressed xenografted lung cancer.
DACT2 is a Wnt/β-catenin signalling pathway inhibitor in lung cancer
To explore the effect of DACT2 on β-catenin/TCF luciferase reporter activity, DACT2 or DACT2 + Dvl2 vectors were transfected into NCI-H23 cells with either wild-type or mutant β-catenin expression. As shown in Figure 5A, the activity of TCF/LEF was inhibited in both wild-type and mutant β-catenin expression cell lines by the re-expression of DACT2. The activity of TCF/LEF was increased in both the wild-type and the mutant β-catenin expression groups when re-expression of DACT2 and overexpression of Dvl2 occurred simultaneously. Inhibition of β-catenin/TCF luciferase reporter activity was tempered by co-transfection of β-catenin and DACT2 in a dose-dependent manner. In the canonical Wnt/β-catenin pathway, increased levels of mutant β-catenin can evade the cytoplasmic degradation complex, allowing increased β-catenin to enter the nucleus and activate TCF/LEF and downstream genes’ transcription [21,22]. In our study, DACT2 inhibited TCF/LEF activity in both wild-type and mutant β-catenin expression groups. The potential explanation is that DACT2 promotes Dvl degradation in a lysosome-dependent pathway and inhibits LEF1 binding to β-catenin [12]. Kivimäe et al reported that DACT2 directly binds to β-catenin strongly [25]. A combined DACT2 and β-catenin complex may reduce the activity of the Wnt reporter induced by β-catenin, including the mutant type. Overexpression of Dvl2 can neutralize the inhibition effect of DACT2 on the Wnt signalling pathway.
To explore the effect of DACT2 on the Wnt signalling pathway, DACT2 expression construct or a control empty vector was transfected into NCI-H23 cells. As shown in Figure 5B, the expression of c-Myc and cyclin D1, the TCF/LEF downstream genes, was decreased in DACT2-expressing NCI-H23 cells. Further evidence for the role of DACT2 in Wnt target gene regulation was provided by the observed increase in the expression of c-Myc and cyclin D1 with knockdown of DACT2 in NCI-H727 cells (Figure 5B).
DACT2 expression is not regulated by miRNAs in lung cancer
To explore whether DACT2 expression was regulated by other mechanisms including miRNAs, various algorithms were employed to identify likely inhibitory miRNAs, including TargetScan (http://www.targetscan.org/), miRGEN Target (http://www.diana.pcbi.upenn.edu/cgi-bin/miRGen/v3/Targets.cgi), and miRBase (http://www.microrna.org/) [26]. MiRNAs predicted to target the 3′-UTR of DACT2 mRNA, which were identified in these three databases, were selected for further study (Figure 6A). Candidate miRNAs were as follows: miR-18a, miR-18b, miR-182, miR-424, miR-497, miR-553, miR-609, and miR-671. Luciferase reporter assays were used to screen candidate miR-NAs in the NCI-H23 cell line. Relative luciferase activity was decreased in the miR-182, miR-424, and miR-609 transfected group (Figure 6B). To then determine whether DACT2 was indeed regulated by miRNA, miRNA mimics or controls were transfected into NCI-H727 cells. Western blot was used to determine any effect of miR-182, miR-424, and miR-609 on DACT2 protein expression. No change in the level of DACT2 protein was observed using these three miRNAs (Figure 6C), suggesting that DACT2 was not regulated by miRNAs in lung cancer cell lines.
Figure 6.
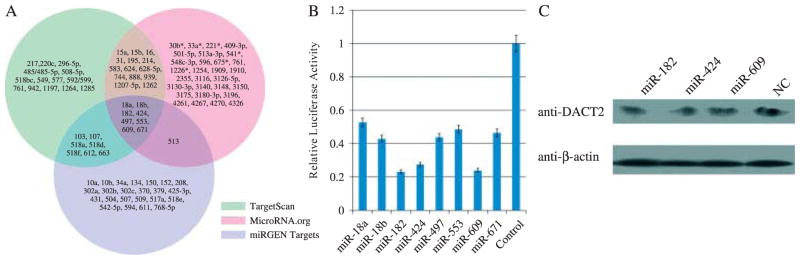
The regulation of DACT2 by microRNAs. (A) Predicted miRNAs targeting DACT2 by TargetScan, miRGEN Target, and miRBase websites. (B) Results of luciferase reporter assay. pGL3-DACT2-3′-UTR reporter plasmid and different predicting miRNA expression vectors were co-transfected into NCI-H23 cells separately. Each experiment was repeated three times. Average level of relative luciferase activity is shown by bar diagram. (C) Selected miRNA mimics or control (NC) were transfected into DACT2 expression cells (NCI-H727). No change in DACT2 expression was found 48 h after transfection. β-Actin was used as an internal control.
Discussion
Both genetic and epigenetic changes play important roles in lung cancer carcinogenesis. Gefitinib is an example of a treatment for cancer based on genetic changes [27]. Frequent methylation of Wnt signalling genes in lung cancer was reported by Licchesi et al [28], suggesting an important role for the regulation of this pathway in lung tumour formation.
DACT2 is located on human chromosome 6q27, a region frequently associated with loss of heterozygosity in human cancers [7,8]. Our study suggests that genetic changes in the DACT2 gene may not play an important role in human lung cancer, and no any changes of DACT2 expression was found due to miRNAs. It was that DACT2 expression was regulated by promoter region hypermethylation in both lung cancer cell lines and primary cancer.
A number of different approaches were utilized to examine the importance of alteration of DACT2 expression in lung cancer growth. Re-expression of DACT2 inhibited colony formation as well as cell proliferation in a human lung cancer cell line. The growth of lung cancer xenografts was suppressed by re-expression of DACT2 in NCI-H23 cells. These results suggest that DACT2 is a tumour suppressor in human lung cancer. It has been reported that DACT2 is involved in the Wnt signalling pathway during Xenopus laevis and mouse embryonic development [11,29]. To understand the mechanism by which DACT2 is involved in Wnt signalling in lung cancer, the effect of DACT2 on the Wnt/β-catenin signalling pathway was analysed by TCF/LEF luciferase activity assay and western blotting. TCF/β-catenin activity and its downstream targets, c-Myc and cyclin D1, were inhibited by re-expression of DACT2 in the lung cancer cell line NCI-H23, and up-regulated after knocking down DACT2 in NCI-H727 cells. The DACT gene family encodes a small group of intracellular proteins in vertebrates that can regulate intercellular signalling pathways by a conserved leucine zipper motif near the N-terminus and a binding motif for the PDZ (Post synaptic density-95/Discs large/Zonula occludens-1) domain at the C-terminus. The initial study assessing DACT’s effect on the Wnt signalling pathway was based on Xenopus laevis development [11]. The function of DACT2 in human cancer was previously not comprehensively examined. Our study indicates that DACT2 is a Wnt signalling inhibitor in human lung cancer. It has also been reported that DACT2 binds to the TGF-β receptors ALK5 and ALK4, and accelerates the lysosomal degradation of these receptors in zebrafish [30]. Recent studies further indicate that DACT proteins and ALK5 form a weak complex in murine models. But in HEK293T cells, no such complex formed between DACT2 protein and either ALK4 or ALK5. Instead, DACT2 exhibited a strong binding affinity with β-catenin or δ-catenin coIP in HEK293T cells [25]. DACT2 also formed very strong complexes with CDK1δ/ε, Dvl, and Vangl family members. These reports also support our hypothesis that DACT2 is a Wnt signalling pathway inhibitor in human lung cancer.
In conclusion, DACT2 is frequently methylated in human lung cancer, and methylation of DACT2 leads to loss of expression and induces β-catenin expression in human primary lung cancer. Wnt signalling pathway activation is associated with DACT2 methylation in human lung cancer. DACT2 expression may suppress lung cancer proliferation both in vitro and in vivo. Based on the above results and human chromosomal localization, DACT2 is a candidate tumour suppressor gene in human lung cancer which is inactivated primarily through epigenetic, and not genetic, mechanisms.
Acknowledgments
This work was supported by grants from the National Basic Research Program (973 Program No. 2012CB934002, 2010CB912802, and 2009CB521801), the National High-tech R&D Program (863 Program No. SS2012AA02A209, SS2012AA020821, and SS2012AA020303), the National Key Scientific Instrument Special Programme of China (grant No. 2011YQ03013405), and the National Science Foundation of China (grant No. 81121004, 81071953, and 81161120432). We thank Dr Qiang Yu (Genome Institute of Singapore) for kindly providing the Dvl2 construct.
Abbreviations
- 5-AZA
5-aza-2′-deoxycytidine
- DACT2
Homo sapiens dapper, antagonist of β-catenin, homolog 2
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- H&E
haematoxylin and eosin
- IVD
in vitro methylated DNA
- LEF
lymphoid enhancer factor
- miRNA
microRNA
- MSP
methylation-specific polymerase chain reaction
- NL
normal lymphocyte DNA
- RT-PCR
reverse-transcription polymerase chain reaction
- TCF
T-cell factor
- UTR
untranslated region
Footnotes
Conflict of interest statement: JGH is a consultant to MDxHealth. The other authors declare no conflicts of interest.
Author contribution statement
MG, YJ, MVB, and JGH were involved in writing the paper and had final approval of the submitted and published versions. QZ conceived data interpretation. YJ and YY conceived experiments and analysed data. MG and JGH conceived the study design.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Soria JC, Mok TS, Cappuzzo F, et al. EGFR-mutated oncogene-addicted non-small cell lung cancer: current trends and future prospects. Cancer Treat Rev. 2012 Aug;38(5):416–430. doi: 10.1016/j.ctrv.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Yu Y, Yin D, Hoque MO, et al. AKT signaling pathway activated by HIN-1 methylation in non-small cell lung cancer. Tumour Biol. 2012 Apr;33(2):307–314. doi: 10.1007/s13277-011-0266-2. [DOI] [PubMed] [Google Scholar]
- 5.Girard L, Zochbauer-Muller S, Virmani AK, et al. Genome-wide allelotyping of lung cancer identifies new regions of allelic loss, differences between small cell lung cancer and non-small cell lung cancer, and loci clustering. Cancer Res. 2000;60:4894–4906. [PubMed] [Google Scholar]
- 6.Steinemann D, Gesk S, Zhang Y, et al. Identification of candidate tumor-suppressor genes in 6q27 by combined deletion mapping and electronic expression profiling in lymphoid neoplasms. Genes Chromosomes Cancer. 2003;37:421–426. doi: 10.1002/gcc.10231. [DOI] [PubMed] [Google Scholar]
- 7.Gao H, Wang Q, Wang B, et al. Genescan analysis of non-small cell lung cancer in the long arm of chromosome 6. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2002;19:14–16. [PubMed] [Google Scholar]
- 8.Wilk JB, DeStefano AL, Joost O, et al. Linkage and association with pulmonary function measures on chromosome 6q27 in the Framingham Heart Study. Hum Mol Genet. 2003;12:2745–2751. doi: 10.1093/hmg/ddg311. [DOI] [PubMed] [Google Scholar]
- 9.Katoh M, Katoh M. Identification and characterization of human DAPPER1 and DAPPER2 genes in silico. Int J Oncol. 2003;22:907–913. [PubMed] [Google Scholar]
- 10.Fisher DA, Kivimae S, Hoshino J, et al. Three Dact gene family members are expressed during embryonic development and in the adult brains of mice. Dev Dyn. 2006;235:2620–2630. doi: 10.1002/dvdy.20917. [DOI] [PubMed] [Google Scholar]
- 11.Cheyette BN, Waxman JS, Miller JR, et al. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell. 2002;2:449–461. doi: 10.1016/s1534-5807(02)00140-5. [DOI] [PubMed] [Google Scholar]
- 12.Teran E, Branscomb AD, Seeling JM. Dpr acts as a molecular switch, inhibiting Wnt signaling when unphosphorylated, but promoting Wnt signaling when phosphorylated by casein kinase Idelta/epsilon. PLoS One. 2009;4:e5522. doi: 10.1371/journal.pone.0005522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yau TO, Chan CY, Chan KL, et al. HDPR1, a novel inhibitor of the WNT/beta-catenin signaling, is frequently downregulated in hepatocellular carcinoma: involvement of methylation-mediated gene silencing. Oncogene. 2005;24:1607–1614. doi: 10.1038/sj.onc.1208340. [DOI] [PubMed] [Google Scholar]
- 14.Jiang X, Tan J, Li J, et al. DACT3 is an epigenetic regulator of Wnt/beta-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell. 2008;13:529–541. doi: 10.1016/j.ccr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman JG, Graff JR, Myohanen S, et al. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia Y, Yang Y, Liu S, et al. SOX17 antagonizes WNT/beta-catenin signaling pathway in hepatocellular carcinoma. Epigenetics. 2010;5:743–749. doi: 10.4161/epi.5.8.13104. [DOI] [PubMed] [Google Scholar]
- 17.Han CP, Lee MY, Tzeng SL, et al. Nuclear Receptor Interaction Protein (NRIP) expression assay using human tissue microarray and immunohistochemistry technology confirming nuclear localization. J Exp Clin Cancer Res. 2008;27:25. doi: 10.1186/1756-9966-27-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Fu H, Wang Y, et al. MicroRNA-101 regulates expression of the v-fos FBJ murine osteosarcoma viral oncogene homolog (FOS) oncogene in human hepatocellular carcinoma. Hepatology. 2009;49:1194–1202. doi: 10.1002/hep.22757. [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Li X, Chu ES, et al. Paired box gene 5 is a novel tumor suppressor in hepatocellular carcinoma through interaction with p53 signaling pathway. Hepatology. 2011;53:843–853. doi: 10.1002/hep.24124. [DOI] [PubMed] [Google Scholar]
- 20.Shih IM, Yu J, He TC, et al. The beta-catenin binding domain of adenomatous polyposis coli is sufficient for tumor suppression. Cancer Res. 2000;60:1671–1676. [PubMed] [Google Scholar]
- 21.Korinek V, Barker N, Morin PJ, et al. Constitutive transcriptional activation by a beta-catenin–Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 22.Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin–Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths EA, Gore SD. DNA methyltransferase and histone deacetylase inhibitors in the treatment of myelodysplastic syndromes. Semin Hematol. 2008;45:23–30. doi: 10.1053/j.seminhematol.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin T, Jelinek J, Si J, et al. Mechanisms of resistance to 5-aza-2′-deoxycytidine in human cancer cell lines. Blood. 2009;113:659–667. doi: 10.1182/blood-2008-02-140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kivimæ S, Yang XY, Cheyette BN. All Dact (Dapper/Frodo) scaffold proteins dimerize and exhibit conserved interactions with Vangl, Dvl, and serine/threonine kinases. BMC Biochem. 2011;12:33. doi: 10.1186/1471-2091-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis BP, Shih IH, Jones-Rhoades MW, et al. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 27.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 28.Licchesi JD, Westra WH, Hooker CM, et al. Epigenetic alteration of Wnt pathway antagonists in progressive glandular neoplasia of the lung. Carcinogenesis. 2008;29:895–904. doi: 10.1093/carcin/bgn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kettunen P, Kivimae S, Keshari P, et al. Dact1-3 mRNAs exhibit distinct expression domains during tooth development. Gene Expr Patterns. 2010;10:140–143. doi: 10.1016/j.gep.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su Y, Zhang L, Gao X, et al. The evolutionally conserved activity of Dapper2 in antagonizing TGF-beta signaling. FASEB J. 2007;21:682–690. doi: 10.1096/fj.06-6246com. [DOI] [PubMed] [Google Scholar]